Rap1 GTPase is an important regulator of RPE cell junctions, required for maintenance of barrier function.
Abstract
Purpose.
To determine whether the small GTPase Rap1 regulates the formation and maintenance of the retinal pigment epithelial (RPE) cell junctional barrier.
Methods.
An in vitro model was used to study RPE barrier properties. To dissect the role of Rap1, two techniques were used to inhibit Rap1 function: overexpression of RapGAP, which acts as a negative regulator of endogenous Rap1 activity, and treatment with engineered, adenovirally-transduced microRNAs to knockdown Rap1 protein expression. Transepithelial electrical resistance (TER) and real-time cellular analysis (RTCA) of impedance were used as readouts for barrier properties. Immunofluorescence microscopy was used to visualize localization of cadherins under steady state conditions and also during junctional reassembly after calcium switch. Finally, choroidal endothelial cell (CEC) migration across RPE monolayers was quantified under conditions of Rap1 inhibition in RPE.
Results.
Knockdown of Rap1 or inhibition of its activity in RPE reduces TER and electrical impedance of the RPE monolayers. The loss of barrier function is also reflected by the mislocalization of cadherins and formation of gaps within the monolayer. TER measurement and immunofluorescent staining of cadherins after a calcium switch indicate that junctional reassembly kinetics are also impaired. Furthermore, CEC transmigration is significantly higher in Rap1-knockdown RPE monolayers compared with control.
Conclusions.
Rap1 GTPase is an important regulator of RPE cell junctions, and is required for maintenance of barrier function. This observation that RPE monolayers lacking Rap1 allow greater transmigration of CECs suggests a possible role for potentiating choroidal neovascularization during the pathology of neovascular age-related macular degeneration.
Neovascular age-related macular degeneration (AMD) is a leading cause of legal blindness in the United States and worldwide.1,2 The most severe vision loss occurs in neovascular AMD that is initiated when choroidal endothelial cells (CECs) originating from the choriocapillaris are activated to migrate through Bruch's membrane and then across the retinal pigment epithelium (RPE). Once across the RPE barrier, choroidal neovascularization (CNV) within the neurosensory retina can occur. CNV in the neurosensory retina can leak and bleed, causing vision loss. Thus, preventing CNV from entering the neurosensory retina is one important way to reduce blindness associated with neovascular AMD.
Under normal conditions, it is believed that the RPE can effectively limit CNV by forming a physical barrier and by appropriate compartmentalization of both proangiogenic (e.g., VEGF)3 and antiangiogenic factors (PEDF, endostatin, and thrombospondin 1),4,5 (reviewed in Ref. 6). The combination of polarized secretion of these factors, and then maintenance of the resulting chemotactic gradient owing to the barrier properties of the RPE is thought to play a critical role in preventing CNV development in the neurosensory retina.7 However, in aging eyes, metabolic stresses, hypoxia, and inflammation can all increase angiogenesis and cause RPE barrier compromise (reviewed in Ref. 8). We have previously shown that increased contact between CECs and the RPE can induce RPE barrier breakdown9 and facilitate CEC transmigration across the RPE.10 One mechanism for CEC transmigration is age-dependent upregulation of the RPE-derived VEGF189 isoform and subsequent Rac1 GTPase activation within CECs.11 There is evidence that this activation of Rac1 in CECs leads to increased generation of reactive oxygen species, which in turn causes further upregulation of VEGF expression by the RPE, resulting potentially in a positive feedback loop.12 Rac1 also has well-defined roles in promoting cell motility and migration in a wide variety of cell types.13 However, increased migratory capability of CECs notwithstanding, CNV in the neurosensory retina also requires RPE barrier disruption. Thus, better understanding of the proteins that regulate the RPE barrier may also improve our understanding of why CNV occurs and lend insight into mechanisms to reduce its occurrence.
Signaling molecules such as the small guanosine triphosphatases (GTPases) of the Rho family have been implicated in cell-cell junctional assembly, disassembly, and maintenance (reviewed in Refs. 14, 15), as well as regulation of actin cytoskeleton remodeling during dynamic events, including cell migration.16 Most recently, we have become interested in another GTPase, Rap1, which is a member of the Ras superfamily.17 Like all GTPases, Rap1 acts as a molecular switch, cycling between an active (GTP-bound) and an inactive (GDP-bound) form. GTP binding and subsequent activation of GTPases is facilitated by guanine nucleotide exchange factors (GEFs), whereas inactivation occurs by hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP) and is catalyzed by GTPase-activating proteins (GAPs).18 Several GEFs for Rap1 have been identified including Epac1/2, PDZGEF-1/-2, and C3G; some have been specifically implicated in Rap1 activation during endothelial cell junctional regulation.19–22 GAPs that inactivate Rap1 include Spa-1 and RapGAP.23,24 Rap1 has been previously shown to be involved in regulating the assembly and permeability of both endothelial25–27 and epithelial cell junctions.28,29 Interestingly, Rap1 activation and subsequent junctional strengthening have also been implicated as mechanisms for inhibiting monocyte transendothelial migration.25
In this work, we sought to determine whether Rap1 GTPase is involved in the regulation of RPE barrier integrity, and whether loss of Rap1 in RPE subsequently impacts CEC transmigration. Using a microRNA-based knockdown strategy and/or expression of a negative regulator of Rap1, RapGAP, we performed transepithelial electrical resistance (TER) and real-time cell electrical impedance analysis (RTCA) measurements to determine barrier properties of RPE under these conditions. Additionally, CEC transmigration across Rap1-deficient RPE was examined. Our results indicate that Rap1 is an important signaling protein involved in RPE barrier function, and suggest the possibility that taking steps to enhance the RPE barrier, for example by using mechanisms to activate Rap1, may limit choroidal endothelial cell transmigration in CNV.
Methods
Cell Culture
ARPE-19 cells (RPE) were obtained from ATCC (Rockville, MD) and grown in DMEM/F-12 plus 10% FBS, and used from passage 15–20, while epithelioid properties were present.30 Primary human choroidal ECs (CECs) were isolated from donor eyes obtained from the North Carolina Eye Bank as described in detail previously.31 CECs were grown in endothelial growth media (EGM-2, from Lonza, Walkersville, MD) supplemented with 10% FBS and used from passage 2–4.
Adenovirus Experiments
Generation of Knockdown Adenoviruses.
Engineered microRNA adenoviral constructs were engineered using an expression vector system (BLOCK-iT Pol II miR RNAi; Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Double-stranded oligonucleotides were designed to form an engineered pre-miRNA sequence structure that targets unique sequences in human Rap1A and Rap1B (Rap1 RNAi). Synthesized oligonucleotides were annealed and ligated into pcDNA 6.2-GW/ EmGFP-miR. As a negative control (Neg RNAi) we used the pcDNA6.2-GW/± EmGFP-miR-neg control plasmid (Invitrogen), which contains an insert that is processed into a mature miRNA, but is not predicted to target any known vertebrate gene. The EmGFP-miRNA cassette from these constructs was subsequently cloned (pAd-CMV-Dest Gateway vector kit).
Adenovirus Production.
Virus was produced in a packaging cell line (293A) with an expression system (ViraPower Adenoviral Expression System; Invitrogen) using the manufacturer's recommended protocol. Briefly, the cells were transfected with vector (PacI-digested pAd-CMV-Dest) containing the desired RNAi cassette using transfecting reagent (Lipofectamine 2000; Invitrogen). Mature viral particles were harvested by collecting the cells/media, and subjecting to multiple freeze-thaw cycles, then centrifugation. Cocistronically expressed EmGFP serves as a marker for knockdown cells. Viral vectors for expression of GFP and RapGAP were prepared and used as described previously.25
Infection of RPE with RNAi Adenovirus and Analysis of Knockdown
RPE were infected with adenovirus (GFP, RapGAP, Neg RNAi, or Rap1 RNAi) for times indicated in the figure legends. Efficient knockdown was usually attained 48 to 72 hours post virus addition. Knockdown was confirmed regularly by Western blot analysis of cell lysates using a polyclonal antibody that recognizes total Rap1 (Santa Cruz Biotechnology, Santa Cruz, CA). Expression of GFP and GFP-RapGAP was confirmed visually by fluorescence microscopy.
Rap1 Activity Assays
8CPT 2′OMe-cAMP (Biolog, Hayward, CA) is a compound commonly used as a means to induce Rap1 activation in cells.32 To confirm activation of Rap1 in RPE after this treatment, we performed standard Rap1 activity assays as described in detail previously.33 In brief, lysates were incubated with a GST-fusion protein of the Ras/Rap binding domain of the effector protein RalGDS (GST-RalGDS-RBD), immobilized on glutathione sepharose beads. This fusion protein specifically recognizes and binds only to active GTP-bound Rap1.34 The amount of active Rap1(from the pulldown) and total Rap1 (from a sample of reserved total cell lysate) was determined by Western blot analysis with a polyclonal anti-Rap1 antibody (Santa Cruz Biotechnology) which recognizes both isoforms of Rap1, followed by an HRP-conjugated anti-rabbit secondary antibody (Chemicon, Billerica, MA).
Barrier Function Assays
Transepithelial Electrical Resistance (TER) Experiments.
ARPE-19 were plated at confluent density (1.8 × 105 cells) on 12 mm diameter, 0.4 μm pore size Transwell filters and cultured for the duration indicated in each figure legend, with media replaced daily. TER was measured at given time points using an Endohm-12 Transwell Chamber connected to an EVOM Voltohmmeter according to the manufacturer's instructions. Uninfected or 0 hour TER readings were those taken immediately before virus addition. For calcium switch experiments, ARPE-19 cells were grown to confluency on Transwell filters, and then transduced with either a negative control or Rap1 knockdown virus for another 2 days before performing the calcium switch assay. In these experiments, “steady state” TER represented the measurement taken immediately before EGTA addition. Next, 4 mM EGTA was added for 30 minutes to disrupt junctions. After EGTA washout and readdition of calcium-containing media, TER was again measured after 0.5, 1, 2, 24, and 48 hours to determine the recovery of TER values during junctional reassembly. Data for these experiments was graphed as absolute change in TER (in ohms per cm2) relative to the initial steady state value.
Real-Time Cell Analysis (RTCA) Experiments.
Barrier properties of RPE were also measured using a commercially available system (xCELLigence Real-Time Cell Analyzer [RTCA]; Acea Biosciences/Roche Applied Science, Basel, Switzerland). This technology measures electrical impedance as a readout for the barrier status of cells grown directly on biocompatible microelectrode coated surfaces. Cells grown on these surfaces have comparable cell growth rate, proliferation, adherence, and other parameters as determined by traditional assay methods.35 Changes in impedance (represented as Cell Index) reflect changes in barrier function and permeability.36 ARPE-19 were pre-infected with GFP or GFP-RapGAP virus for 24 hours before plating an equal cell number onto microelectrode-coated surface of the wells of a disposable cell-based assay devise (E-Plate 16; Roche Applied Science). Cells were counted with an automated cell counter (Nexcelom Bioscience, Lawrence, MA). Impedance readings were taken automatically every 15 minutes until the end of the experiment and plotted as Cell Index ± SD. For experiments using the Rap-activating compound, 8CPT-2′OMe-cAMP, drug (250 μM) or vehicle alone (PBS) was added at the time of plating into the microelectrode plates. Confirmation of equal seeding density was obtained for every experiment by plating in parallel an equivalent number of cells into a 24-well dish and staining and counting nuclei at the end of the experiment (data not shown).
Immunofluorescence Microscopy
ARPE-19 grown on glass coverslips were fixed and permeabilized with 3.7% formaldehyde (30 minutes at room temperature), and 0.2% Triton X-100/TBS (5 minutes at room temperature). To detect all cadherin isoforms in the RPE, a pan-cadherin monoclonal antibody (clone CH-19) was used (Sigma, St. Louis, MO), followed by an Alexa 594-conjugated anti-mouse secondary antibody (Molecular Probes). Actin was visualized by staining with Texas Red-labeled phalloidin (Invitrogen). Fluorescence images were obtained with a microscope (Axiovert 200M; Zeiss, Thornwood, NY) equipped with a digital camera (Hamamatsu ORCA-ERAG, Bridgewater, NJ) and acquired using a high-end software package (Metamorph Workstation; Universal Imaging Corp., Sunnyvale, CA).
CEC Transmigration Assays
Transmigration of CECs across the RPE monolayer was measured as previously described.31 ARPE-19 cells were preinfected with negative control or Rap1 knockdown virus for 48 hours, and then plated onto the underside of 8 μm pore size Transwells (Costar/Corning) for another 24 hours. Uninfected ARPE-19 were plated in the bottom of each well to provide additional chemoattractant.31 Primary human CECs were fluorescently labeled with dye-delivery solution (Vybrant Dio Cell-labeling solution; Invitrogen) following the manufacturer's protocol (Molecular Probes), and then plated inside the inserts. Forty-eight hours after adding the CECs, the underside of the filters were trypsinized to collect CECs that had completed transmigration (i.e., those CECs which had migrated across the filter and/or the RPE), and counted using a hemacytometer and fluorescence microscopy.
Statistical Analysis
Statistical significance was determined by Student's t-test (one-tail, equal variance) using the average values obtained from at least triplicate Transwells in a given experiment. Graphs are representative of results from three independent experiments. A P value of < 0.05 was considered statistically significant. For RTCA experiments, one representative trace of at least 3 independent experiments is shown, graphed as the average Cell Index value from wells plated in triplicate or quadruplicate. Immunofluorescence data and Western blot analyses are also representative of multiple independent experiments.
Results
Inhibiting Rap1 Activity by Expressing RapGAP in RPE Decreases Plateau Levels of TER
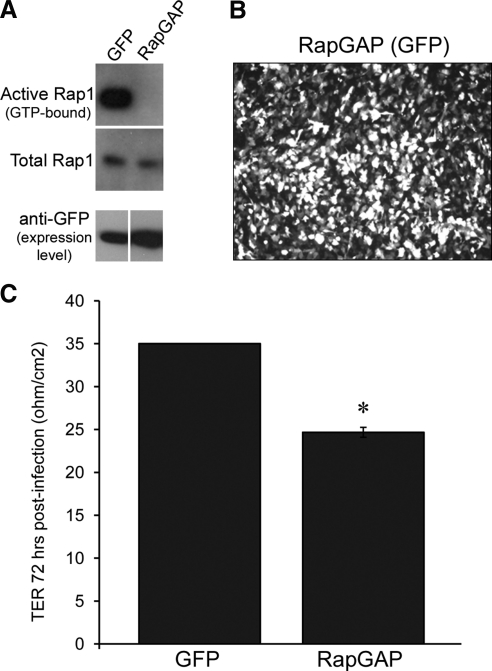
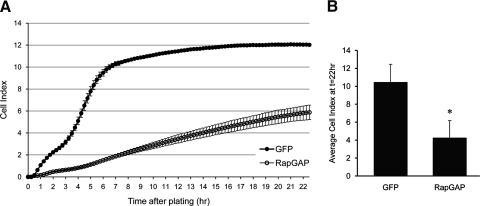
Rap1 GTPase has been implicated as a signaling protein involved in regulating cell-cell adhesion events involved in barrier function in many cell types, such as endothelia.25–27 We set out to test whether this is true for RPE cells, which do express Rap1 protein (data not shown). As an initial means to inhibit Rap1 activity, we exogenously expressed the protein RapGAP,37 a GTPase activating protein (GAP) that acts as a negative regulator of Rap1 activity by promoting the hydrolysis of GTP to GDP.38 Within 24 hours of transduction with an adenoviral expression vector, Rap1 activity assays revealed that RPE expressing RapGAP had reduced Rap1 activity as shown by decreased pull-down of Rap1-GTP compared with cells expressing GFP only (Fig. 1A). In ARPE-19 cells, adenoviral infection efficiency of RapGAP approaches 100% as determined by visualization of a cocistronically expressed GFP marker (Fig. 1B). We then measured the TER of ARPE-19 cells grown on Transwell filters after expression of either GFP-RapGAP or GFP alone for 72 hours. Figure 1C shows that 72 hours after expression and Transwell plating, the average TER of GFP control cells is 35 ohm/cm2, while the TER of RapGAP-expressing cells is significantly lower, at 24.67 ± 0.58 ohms per cm2 (*P = 0.0000032). As a second means of assessing barrier function in cultured RPE, we took advantage of real-time cell analysis (RTCA), an electrical impedance-based system that can be setup to take automatic, noninvasive measurements of barrier function in real-time.36 Cells pretreated so as to begin expressing either GFP alone, or RapGAP for 24 hours before the experiment were replated at equal cell density into microelectrode coated wells of the RTCA E-Plate. Impedance measurements were recorded every 15 minutes for up to 22 hours. A representative trace demonstrating decreased impedance with RapGAP expression is shown in Figure 2A. The average Cell Index of GFP or RapGAP-expressing cells from 3 independent experiments at the 22-hour time point is presented in Figure 2B. Expression of RapGAP significantly reduced the plateau Cell Index values (*P = 0.0093) compared with GFP control cells (average Cell Index of GFP control, 10.43 ± 2.02 vs. RapGAP, 4.23 ± 1.95). A confirmatory experiment was performed using hfRPE cells, and a similar pattern of reduced Cell Index on expression of RapGAP was observed (data not shown). Taken together, Figures 1 and 2 show two independent experimental methods (TER, RTCA) to indicate that activation of Rap1 is important for the acquisition of RPE barrier function.
Figure 1.
Loss of Rap1 GTPase activity by expressing RapGAP reduces plateau TER in ARPE-19 cells. (A) Comparison of active (GTP-bound) Rap1 in lysates of ARPE-19 cells expressing either GFP control or RapGAP and assayed. Active (GTP-bound) pool of Rap1 is shown in top row; middle row confirms equivalent loading using total Rap1 from whole cell lysates and bottom row indicates equal amount of expressed protein. (B) Immunofluorescence microscopy of a Transwell-grown monolayer of ARPE-19 72 hours postinfection to confirm that adenoviral infection efficiency of RapGAP approaches 100% (visualized by a cocistronically expressed GFP marker). (C) ARPE-19 cells grown on Transwell filters were infected with adenovirus to express either RapGAP or GFP only as a control. TER measurements were taken 72 hours post virus addition. Shown are data from one of three independent experiments. TER is significantly reduced when RapGAP has been expressed for 72 hours (*P = 0.0000032, n = average of 3 filters per condition).
Figure 2.
Electrical impedance of the RPE cell monolayer is decreased on expression of RapGAP. ARPE-19 cells expressing either GFP or RapGAP were plated at equal cell density into microelectrode-coated wells of an E-Plate 16 and electrical impedance was measured by RTCA. (A) Representative RTCA trace showing that Cell Index is lower in cells expressing RapGAP compared with GFP alone. Data points represent the average Cell Index ± SD from at least 3 separate wells. (B) Combined data from 3 independent experiments showing the average Cell Index ± SD at the 22-hour time point. RapGAP expression significantly decreases Cell Index at this time point (*P ≤ 0.01, n = 3 independent experiments).
Activating Rap1 Strengthens the RPE Cell Barrier
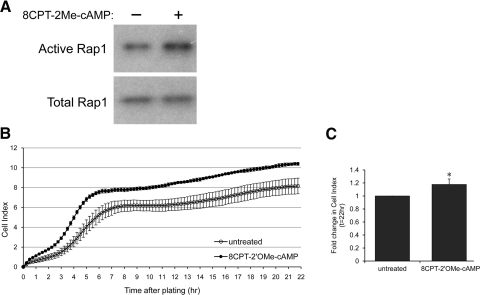
Having determined that inhibition of Rap1 activity by RapGAP expression decreases RPE monolayer barrier properties, we next wanted to determine whether, conversely, Rap1 activation enhances barrier function. To do this, we used the Rap GTPase-activating drug 8CPT-2′OMe-cAMP. This cAMP analog has been shown to specifically activate Rap1 via effects on upstream exchange factors (Epac1 and Epac2), but importantly does not activate other cAMP-responsive pathways involving protein kinase A or other downstream pathways, e.g., Ras and ERK.32 We first confirmed that treatment with 250 μM 8CPT-2′OMe-cAMP activates Rap1 in ARPE-19 cells. We used a standard GST pulldown method that utilizes the Ras/Rap binding domain of the effector protein RalGDS (GST-RalGDS-RBD) to selectively detect the active GTP-bound form of Rap1.34 The Rap1 Western blot analysis of a typical assay shows that the pool of active Rap1 is increased after treatment (Fig. 3A). Next we performed RTCA experiments in which RPE were treated at the time of plating with 250 μM 8CPT-2′OMe-cAMP or vehicle control phosphate buffered saline (PBS) to determine whether Rap1 activation affected monolayer impedance. A representative Cell Index trace (Fig. 3B) shows that Rap activation by drug treatment increases Cell Index compared with vehicle (Fig. 3B). Furthermore, combined data from 3 independent RTCA experiments (Fig. 3C) show that the increase is statistically significant (*P = 0.0113). Thus, we conclude that activating Rap1 has positive effects on RPE cell barrier properties.
Figure 3.
Rap1 can be activated with the drug 8CPT-2′OMe-cAMP in ARPE-19, and this increases electrical impedance. (A) Confluent ARPE-19 cells were treated with vehicle (PBS) or with the Rap1-activating drug 8CPT-2′OMe-cAMP before harvesting lysates for determination of Rap1 activity. Active (GTP-bound) Rap1 is shown in top row, and bottom row confirms equivalent loading using total Rap1 from whole cell lysates. (B) ARPE-19 cells were treated with vehicle (PBS) or drug, and electrical impedance was measured using RTCA. Graph shows a typical RTCA trace from one representative experiment. (C) Combined data from 3 independent experiments showing the fold change in Cell Index ± SD, 22 hours after 8CPT-2′OMe-cAMP treatment, normalized to untreated control. Rap1 activation increases Cell Index, indicating enhanced barrier properties (*P = 0.01, n = 3 independent experiments).
Specific Knockdown of Rap1 by RNAi: Effects on RPE Barrier Properties
While expression of RapGAP and treatment with 8CPT-2′OMe-cAMP are two commonly used methods for experimentally modulating the activity of Rap GTPases,37,39 these approaches may not be the most specific means to do so, because both treatments can also affect other closely related GTPases such as Rap2.32,40 To more specifically study the role of Rap1 and its two isoforms, Rap1a and Rap1b, on RPE barrier function, we turned to an adenovirally-encoded engineered microRNA knockdown approach. We have previously used this method to successfully target other GTPases, for example, RhoA.41 We generated adenoviral vectors that expressed miRNA for knockdown of total Rap1 (Rap1 RNAi) (both Rap1a and Rap1b isoforms), as well as a vector with a nontargeting negative control sequence (Neg RNAi). Importantly, Western blot analysis of RPE cell lysates 3 days after infection shows loss of Rap1 protein expression in Rap1-knockdown cells compared with negative control (Fig. 4).
Figure 4.
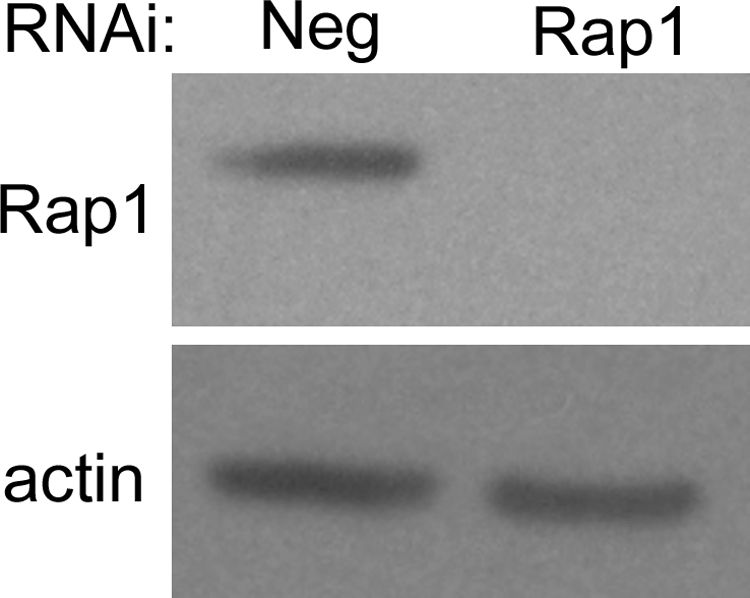
Efficient RNAi-mediated knockdown of Rap1 expression in ARPE-19 using an engineered microRNA. ARPE-19 cells were infected with adenovirally-delivered, nontargeting control (Neg), or Rap1-knockdown virus. Three days later, cells were harvested and lysates were analyzed for Rap1 protein by Western blot analysis. Loss of Rap1 protein is evident in Rap1 knockdown lane compared with Neg control lane. Blot was reprobed with an antibody against β-actin as a control to confirm equal loading.
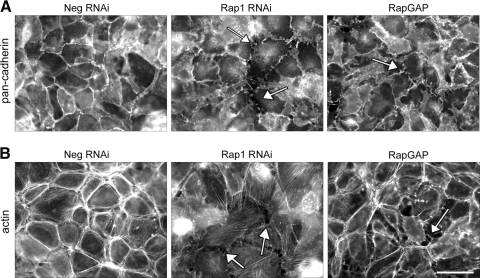
With the tools to efficiently knockdown Rap1 protein expression, we first analyzed the localization of the adherens junction proteins, the cadherins, after loss of Rap1 protein. Immunofluorescence microscopy reveals that Rap1 knockdown disrupts cadherin localization. Compared with negative control cell monolayers, which exhibit a continuous junctional staining pattern with no discontinuities or gaps, the Rap1 knockdown cells show decreased junctional localization of cadherin and loss of cell-cell contact, producing numerous gaps in the monolayer (Fig. 5A; examples of gaps indicated by arrows). This was also observed after Rap1 inactivation by RapGAP expression (Fig. 5A, far right panel). We next looked at the morphology of the F-actin cytoskeleton after knockdown of Rap1 or expression of RapGAP. RPE monolayers infected with the negative control knockdown construct have a prominent perijunctional actin ring, characteristic of epithelial monolayers with mature cell-cell adhesions (reviewed in Ref. 42). This peripheral actin ring is absent after Rap1 knockdown (Fig. 5B, middle panel), and we also observe the presence of cytoplasmic stress fibers and cellular retraction. In RPE expressing RapGAP, the perijunctional actin ring was also reduced, and there was obvious cell retraction and/or rounding, producing large gaps in the monolayer (Fig. 5B, right panel).
Figure 5.
Loss of Rap1 protein by knockdown or inhibition of Rap1 activity by expression of RapGAP disrupts cadherin and F-actin localization in ARPE-19 monolayers. ARPE-19 cells infected with the indicated RNAi viruses (Neg, Rap1), or RapGAPexpressing virus (RapGAP) were grown for 2 days and then replated onto coverslips for another 24 hours before fixation and processing for immunofluorescence microscopy. (A) Loss of Rap1 protein by knockdown or inhibition of its activity by RapGAP expression disrupts junctional localization of cadherins when compared with control (Neg) cells. The cell monolayer exhibits discontinuous cadherin staining and loss of cell-cell contact, producing gaps (examples indicated by arrows) in the normally confluent monolayer. (B) Knockdown of Rap1 or expression of RapGAP cause changes in morphology of the F-actin cytoskeleton. In control monolayers (Neg) actin staining is present in a perijunctional ring, however, knockdown of Rap1 results in the formation of cytoplasmic stress fibers and cell retraction, similar to expression of RapGAP. Scale bar, 50 μm.
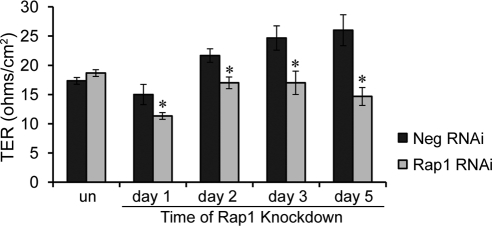
Morphologic analysis thus supported the idea that loss of Rap1 protein or inhibition of its activity negatively impacts RPE cell barrier properties. We next confirmed this by performing TER measurements on RPE monolayers treated with Rap1 RNAi virus compared with negative control. As shown in Figure 6, Rap1 knockdown significantly decreased TER relative to negative control (*P ≤ 0.01).
Figure 6.
Knockdown of Rap1 GTPase decreases plateau TER levels. ARPE-19 cells were plated on Transwell filters for 24 hours before infecting with virus to induce expression of either negative control or Rap1 RNAi constructs. TER was measured before addition of virus (un), and then at times indicated for a total period of 5 days. Data are shown from one representative of three independent experiments. Cells with Rap1 knockdown have significantly lower TERs at all time points after knockdown. Bars represent average TER ± SD from n = 3 Transwells per condition (*P < 0.01).
Junctional Dynamics: Reassembly after Calcium Switch
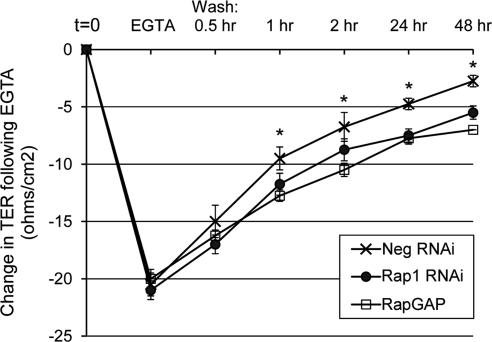
The ability of cellular junctions to respond to stimuli in a dynamic manner is paramount for appropriate regulation of paracellular permeability and the transmigration of other cell types, such as endothelial cells or immune cells, across the barrier presented by the monolayer. Related to the progression of AMD, the RPE monolayer must be able to respond to age-induced stresses such as hypoxia, inflammation, and oxidative stress, to maintain barrier function.8 To study the ability of Rap1 in RPE to dynamically regulate cell-cell junctions, we used the calcium switch protocol, which measures junctional assembly. The calcium switch assay is based on the observation that cell-cell junctions are disrupted when cells are switched to medium containing EGTA to chelate extracellular calcium,43 due to the loss of calcium-dependent cadherin-mediated adhesion. Subsequent washout of EGTA and restoration of physiological levels of calcium results in the synchronous de novo assembly of cell junctions.44 A representative calcium switch experiment is shown in Figure 7. On EGTA treatment, as expected, there was a sharp decrease in TER from starting levels (t = 0) for all conditions. However, after EGTA washout, recovery of TER is significantly delayed in RPE with Rap1 knockdown or RapGAP expression compared with control cells, indicating that Rap1 is required for optimal junctional reassembly.
Figure 7.
TER recovery after calcium switch is impaired after Rap1 knockdown or expression of RapGAP. ARPE-19 cells grown on Transwell filters were infected with adenovirus to express negative control RNAi, Rap1 RNAi, or RapGAP protein. Initial (t = 0) TER measurements were taken 48 hours post virus addition. Cells were then treated with EGTA for 30 minutes to disrupt cell-cell junctions, followed by washout and readdition of calcium-containing media for the indicated times to allow for junctional reassembly. The graph shows the average change in TER (ohms per cm2), relative to control cells at t = 0. Data points are the average ± SD from n = 4 wells (*P < 0.02, comparing both Rap1-knockdown and RapGAP-expressing cells to control). Shown is one representative from three independently performed experiments.
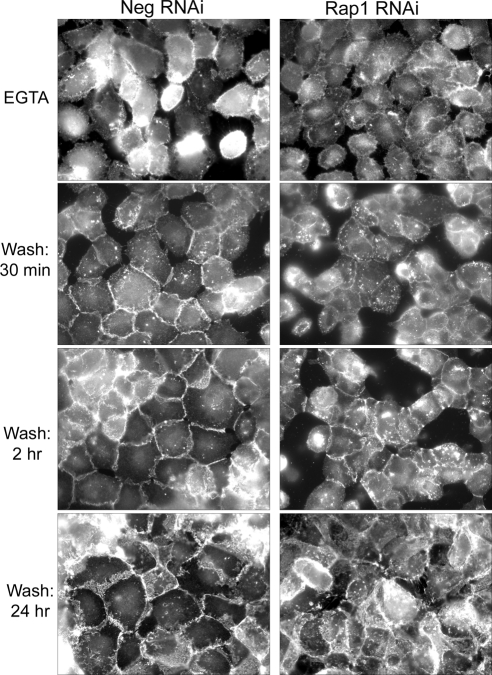
To better visualize what happens to junctional proteins during the calcium switch protocol, we set up, in parallel, coverslips plated with ARPE-19 that had also been infected with negative control or Rap1 RNAi. Coverslips were fixed at various time points after EGTA treatment and washout and processed for immunofluorescent detection of cadherins. As shown in Figure 8, EGTA treatment disrupts cadherin staining (compare with Fig. 5A, Neg RNAi panel) equally in control RPE and RPE with Rap1 knockdown. Thirty minutes after EGTA washout, the control cells begin to re-establish linear cadherin staining at cell-cell contacts, and this pattern increases over time, with complete cadherin localization at cell junctions within 24 hours of calcium readdition. However, in cells lacking Rap1, junctional cadherin relocalization is impaired. The delayed relocalization of cadherins after EGTA washout in this experiment mirrors the delay in TER recovery (Fig. 7). Thus, in ARPE-19, Rap1 GTPase is needed for dynamic junctional reassembly, indicating its significance for barrier regulation.
Figure 8.
Knockdown of Rap1 inhibits junctional localization of cadherin during junctional reassembly after calcium switch. ARPE-19 cells were plated onto coverslips at confluent density, and then treated with negative control or Rap1 RNAi for 48 hours. Cells were then treated with EGTA for 30 minutes to disrupt cell-cell junctions, followed by washout and readdition of calcium-containing media for indicated times. Coverslips were fixed and processed for immunofluorescent detection with a pan-cadherin antibody to visualize cell-cell junctions. EGTA treatment completely disrupts the cell monolayer of both control and Rap1 knockdown. On washout of EGTA, control cell monolayers regain junctional cadherin localization and intact monolayer appearance faster than Rap1 knockdown cells.
Enhanced Transmigration of CECs across Rap1-Knockdown ARPE
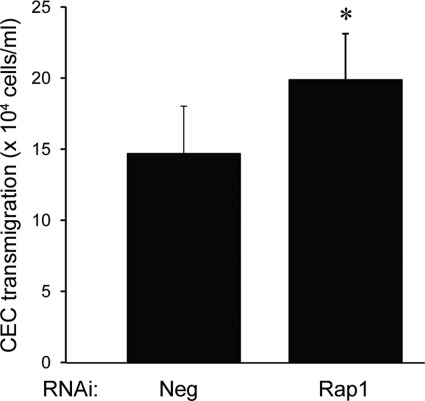
Ultimately, the RPE serves as a barrier to the neurosensory retina to migrating CECs during CNV. We have previously reported data from coculture and transmigration assays that relate to pathways important in human neovascular AMD.9–11,31,45 We therefore tested whether the observed junctional dysfunction that occurs with loss of Rap1 activity correlates functionally with CEC transmigration across the RPE monolayer. RPE infected with Rap1 RNAi or negative control RNAi were grown on the underside of Transwell inserts as described previously and in Methods.31 Fluorescently-labeled CECs were then plated within the insert, and after 48 hours, transmigrated CECs were collected and quantified. Figure 9 shows a representative experiment demonstrating that the number of CECs undergoing transmigration is significantly higher across the RPE monolayer that is deficient in Rap1 (P = 0.01886). These results reveal that the barrier-disruptive effects of Rap1 knockdown can increase CEC transmigration, a key step in the development of CNV.
Figure 9.
CEC transmigration is higher across Rap1 knockdown ARPE-19 cell monolayers. ARPE-19 cells preinfected with control or Rap1 knockdown virus were plated on inverted Transwells. Fluorescently-labeled CECs were added and allowed to transmigrate for 48 hours before quantification. Data are shown as number of transmigrated CECs × 104 cells/mL (mean of n = 5 Transwells ± SD). Graph shows one representative of three independent experiments (*P = 0.018862).
Discussion
In this study we have identified the small GTPase Rap1 as a novel regulator of RPE cell junctions. We found that RPE barrier function was negatively impacted when Rap1 protein function was inhibited, either by RNAi knockdown, or expression of RapGAP, a negative regulator of Rap1. Multiple readouts of junctional integrity were affected; both TER and cellular impedance were decreased, and mislocalization of cadherins and the loss of the perijunctional actin ring were observed. The junctional defects after Rap1 inhibition not only involved maintenance of junctions, but also impaired the dynamic regulation of RPE cell junctions, as evidenced by the delayed junctional reassembly kinetics observed after calcium switch. Furthermore, loss of Rap1 in RPE resulted in increased CEC transmigration in our in vitro model of CNV.
The ability to regulate junctions dynamically is important during metabolism. As age-related changes occur in the RPE, impairment of this function may potentially manifest as ill-defined leakage of fluorescein dye, such as that seen during fluorescein angiography in occult forms of CNV. Loss of the RPE's ability to dynamically regulate its junctions may also interfere with compartmentalization of angiogenic agonists, normally secreted from the basal aspect of the RPE,3 and inhibitors secreted from the apical aspect, by permitting movement of factors to opposite compartments. Taken together with increased RPE expression of angiogenic factors like VEGF, this change in growth factor concentrations may lead to activated, motile CECs, such as through activation of Rac112 and provide chemoattractants in the neurosensory retinal space for activated CECs to transmigrate the RPE. Activation of Rap1 in the RPE cells may be a protective mechanism to maintain the integrity of the RPE junctions, and thus serve to contain occult CNV from breaking through to the neurosensory retina. Our in vitro data are in line with this possibility, because activation of Rap1 with a Rap-activating drug (8CPT-2′OMe-cAMP) enhanced the barrier properties (electrical impedance) as measured by RTCA, whereas inhibition of Rap1 function in RPE facilitated CEC transmigration across an RPE monolayer.
Our in vitro experiments make use of the nontransformed human RPE cell line ARPE-19. In early publications, ARPE-19 formed polarized monolayers with high TERs.46 Also, ARPE-19 cells were reported to have expression profiles similar to native human RPE when grown on plastic.47 With repeated passages, however, the barrier properties of the monolayers were reduced.30 Also, ARPE-19 cells have been reported to lack claudin-19, which is expressed in human RPE.48 Still, ARPE-19, when used in a consistent manner make a good RPE model for AMD for several reasons. First, diseases in which the RPE barrier is compromised such as in AMD, may in fact be better represented by a cell type, which also exhibits lower TER.30 We also found that ARPE-19 express increased levels of VEGF compared with other RPE types,30 better reflecting human neovascular AMD in which inhibition of VEGF positively and profoundly affects the natural progression.49 In this regard, ARPE-19 may be likened to “stressed” RPE in vivo under conditions that favor development of neovascular AMD. We have corroborated our findings with ARPE-19 in an experiment using hfRPE in short-term culture, in which overexpression of RapGAP similarly reduced monolayer impedance when assayed using RTCA (data not shown). Finally, when choosing an in vitro model system, one must also acknowledge that the use of human fetal RPE in research has similar ethical considerations to those that have been raised in the fetal RPE transplantation field.50
Our observation that Rap1-knockdown RPE monolayers allow for greater transmigration of CECs suggests that taking steps to enhance the RPE barrier by using mechanisms to activate Rap1, may limit CEC transmigration in neovascular AMD. The therapeutic use of specific Rap1-activating compounds such as 8CPT 2′OMe-cAMP warrants investigation, as does gene therapy involving targeted expression of activated Rap1 in the RPE layer of the eye. Further study with in vivo models will be important to determine the potential of this approach. Rap1 exists as two nearly identical isoforms Rap1A, and Rap1B; knockout mouse models for each isoform exist.51–53 Currently it is not clear if these isoforms have overlapping or unique functions in the RPE. Thus, it will be informative to determine individual isoform effects on RPE barrier properties and on laser-induced CNV formation in vivo.
In conclusion, our findings identify Rap1 GTPase as a novel regulator of RPE junctional barrier function, and suggest the idea that selectively activating Rap1 in some AMD patients, perhaps with occult forms of CNV, might be a mechanism to limit vision loss from neurosensory retinal CNV and improve clinical outcomes.
Footnotes
Supported by R01 EY017011 (NEI/NIH) (MEH), American Heart Association Scientist Development Grant 10SDG3430042 (ESW), R01-GM029860, and ARRA 3-R01-GM029860-28S.
Disclosure: E.S. Wittchen, None; M.E. Hartnett, None
References
- 1. Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 3. Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becerra SP, Fariss RN, Wu YQ, Montuenga LM, Wong P, Pfeffer BA. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp Eye Res. 2004;78:223–234 [DOI] [PubMed] [Google Scholar]
- 5. Bhutto IA, Uno K, Merges C, Zhang L, McLeod DS, Lutty GA. Reduction of endogenous angiogenesis inhibitors in Bruch's membrane of the submacular region in eyes with age-related macular degeneration. Arch Ophthalmol. 2008;126:670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234 [DOI] [PubMed] [Google Scholar]
- 7. Sonoda S, Sreekumar PG, Kase S, et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY). 2009;2:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D'Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77:593–599 [DOI] [PubMed] [Google Scholar]
- 10. Peterson LJ, Wittchen ES, Geisen P, Burridge K, Hartnett ME. Heterotypic RPE-choroidal endothelial cell contact increases choroidal endothelial cell transmigration via PI 3-kinase and Rac1. Exp Eye Res. 2007;84:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H, Geisen P, Wittchen ES, et al. The role of RPE cell-associated VEGF189 in choroidal endothelial cell transmigration in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monaghan-Benson E, Hartmann J, Vendrov AE, et al. The role of vascular endothelial growth factor-induced activation of NADPH oxidase in choroidal endothelial cells and choroidal neovascularization. Am J Pathol. 2010;177:2091–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62 [DOI] [PubMed] [Google Scholar]
- 14. Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–199 [DOI] [PubMed] [Google Scholar]
- 15. Braga VM. Cell-cell adhesion and signalling. Curr Opin Cell Biol. 2002;14:546–556 [DOI] [PubMed] [Google Scholar]
- 16. Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514 [DOI] [PubMed] [Google Scholar]
- 17. McCormick F. Ras-related proteins in signal transduction and growth control. Mol Reprod Dev. 1995;42:500–506 [DOI] [PubMed] [Google Scholar]
- 18. Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179 [DOI] [PubMed] [Google Scholar]
- 19. Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972 [DOI] [PubMed] [Google Scholar]
- 20. Sakurai A, Fukuhara S, Yamagishi A, et al. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sehrawat S, Cullere X, Patel S, Italiano J, Jr, Mayadas TN. Role of epac1, an exchange factor for rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birukova AA, Zagranichnaya T, Fu P, et al. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsukamoto N, Hattori M, Yang H, Bos JL, Minato N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J Biol Chem. 1999;274:18463–18469 [DOI] [PubMed] [Google Scholar]
- 24. Su L, Hattori M, Moriyama M, et al. AF-6 controls integrin-mediated cell adhesion by regulating Rap1 activation through the specific recruitment of Rap1GTP and SPA-1. J Biol Chem. 2003;278:15232–15238 [DOI] [PubMed] [Google Scholar]
- 25. Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–11682 [DOI] [PubMed] [Google Scholar]
- 26. Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955 [DOI] [PubMed] [Google Scholar]
- 27. Fukuhara S, Sakurai A, Yamagishi A, Sako K, Mochizuki N. Vascular endothelial cadherin-mediated cell-cell adhesion regulated by a small GTPase, Rap1. J Biochem Mol Biol. 2006;39:132–139 [DOI] [PubMed] [Google Scholar]
- 28. Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–35132 [DOI] [PubMed] [Google Scholar]
- 29. Hogan C, Serpente N, Cogram P, et al. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;6690–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geisen P, McColm JR, King BM, Hartnett ME. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr Eye Res. 2006;31:739–748 [DOI] [PubMed] [Google Scholar]
- 31. Geisen P, McColm JR, Hartnett ME. Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res. 2006;82:608–619 [DOI] [PubMed] [Google Scholar]
- 32. Enserink JM, Christensen AE, de Rooij J, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906 [DOI] [PubMed] [Google Scholar]
- 33. Wittchen ES, Burridge K. Analysis of low molecular weight GTPase activity in endothelial cell cultures. Methods Enzymol. 2008;443:285–298 [DOI] [PubMed] [Google Scholar]
- 34. Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. Embo J. 1997;16:252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solly K, Wang X, Xu X, Strulovici B, Zheng W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2:363–372 [DOI] [PubMed] [Google Scholar]
- 36. Atienza JM, Yu N, Kirstein SL, et al. Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay Drug Dev Technol. 2006;4:597–607 [DOI] [PubMed] [Google Scholar]
- 37. Rubinfeld B, Munemitsu S, Clark R, et al. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell. 1991;65:1033–1042 [DOI] [PubMed] [Google Scholar]
- 38. Scheffzek K, Ahmadian MR. GTPase activating proteins: structural and functional insights 18 years after discovery. Cell Mol Life Sci. 2005;62:3014–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375 [DOI] [PubMed] [Google Scholar]
- 40. Janoueix-Lerosey I, Polakis P, Tavitian A, de Gunzburg J. Regulation of the GTPase activity of the ras-related rap2 protein. Biochem Biophys Res Comm. 1992;189:455–464 [DOI] [PubMed] [Google Scholar]
- 41. Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lampugnani MG. Endothelial adherens junctions and the actin cytoskeleton: an ‘infinity net’? J Biol. 2010;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J Cell Biol. 1986;102:1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169 [DOI] [PubMed] [Google Scholar]
- 47. Tian J, Ishibashi K, Handa JT. The expression of native and cultured RPE grown on different matrices. Physiol Genomics. 2004;17:170–182 [DOI] [PubMed] [Google Scholar]
- 48. Peng S, Rao VS, Adelman RA, Rizzolo LJ. Claudin-19 and the barrier properties of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:1392–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870 [DOI] [PubMed] [Google Scholar]
- 50. Gieser JP. Ethics and human fetal retinal pigment epithelium transplantation. Arch Ophthalmol. 2001;119:899–900 [DOI] [PubMed] [Google Scholar]
- 51. Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Yan J, De P, et al. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]