Abstract
Bioassay guided fractionation of the ethanol extract of a new endemic species of the genus Astrotrichilia led to the isolation of the new antiproliferative 3-(4′-hydroxy-2′,3′-dihydroprenyl)-4,6-dimethoxy-5-methylcoumarin, named astrotricoumarin (8) with an IC50 value of 6.8 μM against the A2780 cell line. The structure of compound 8 was elucidated on the basis of its physical and spectroscopic data, including extensive 1D- and 2D-NMR analysis
Keywords: Meliaceae, dihydroprenyl methylcoumarin, astrotricoumarin, antiproliferative, A2780
As part of our ongoing International Cooperative Biodiversity Group (ICBG) program aimed at discovering compounds with antiproliferative activity from plants originating in Madagascar, we selected the ethanol extract of the stems of a plant identified as a new endemic species of the genus Astrotrichilia for investigation. Astrotrichilia is a genus endemic to Madagascar [2], and the extract had antiproliferative activity against A2780 ovarian cancer cells, with an IC50 value of 16 μg/mL. Previous investigation of A. voamatata led to the isolation of the limonoid derivatives voamatins A-D (1-4), while a limonoid named astrotrichilin (5) and two dammarane-type triterpenes (6,7) have been isolated from A. asterotricha [3-6].
Liquid-liquid partition of the ethanol extract using hexane, EtOAc and H2O afforded an active EtOAc fraction with an IC50 value of 3.7 μg/mL. The latter was purified by a bioguided separation using High Performance Liquid Chromatography (HPLC) to afford the new antiproliferative dihydroprenyl methylcoumarin 8 as the major bioactive constituent of the initial extract.
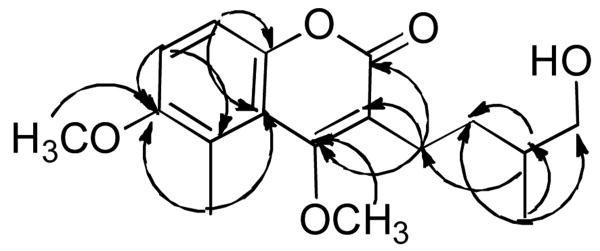
Positive electrospray ionization mass spectroscopy of compound 8 exhibited a quasi-molecular ion peak at m/z 307.1543 [M+H]+, corresponding to the molecular formula C17H22O5 (C17H23O5 requires m/z 307.1545). The IR spectrum displayed bands for a hydroxyl group at 3455 cm-1, the carbonyl group of an α,β-unsaturated lactone at 1708 cm-1, and aromatic methine hydrogens at 3050 cm-1. The 1H NMR spectrum displayed resonances of two ortho-coupled aromatic protons (δ 7.20 and 7.24, each doublets, J = 9.0 Hz), a methyl attached to an aromatic ring (δ 2.60 singlet, 3H), a secondary methyl group (δ 1.01 doublet, J= 6.7 Hz, 1H), an oxymethylene group (δ 3.40 and 3.47, each a doublet of doublets, J = 11.0, 6.0 Hz), one methine multiplet at δ 1.37, two methylenes (δ 2.60 and 1.65, each multiplets) and two methoxyl groups (δ 3.85 and 3.87, each a singlet). The 13C NMR spectrum (Table 1) showed seventeen resonances due to a tetrasubstituted aromatic ring (δC 115.7, 115.8. 117.3, 123.8, 148.6 and 155.6), a lactone carbonyl (δ 165.3), a conjugated quaternary olefinic carbon bearing an oxygen atom (δ 167.5), two methoxyl signals (δ 56.9 and 62.3), one of which was attached to the aromatic ring, and signals for five carbons ascribable to a 4′-hydroxy-2′,3′-dihydroprenyl group (δ 23.2, 32.8, 36.7, 67.7, 16.9). The above data suggested that compound 8 is a 4′-hydroxy-2′,3′-dihydroprenylated methyl coumarin [4]. The attachment of the 4′-hydroxy-2′,3′-dihydroprenyl group, the two methoxyl groups, and the aromatic methyl groups were substantiated by careful analysis of HSQC, COSY and HMBC spectra. The COSY spectrum demonstrated the presence of the spin systems from H-1′ to H-5′ and from H-1′ to H-4′. In the HMBC spectrum, clear long range correlations were observed from the methylene at δ 2.65 to the lactone carbonyl (δ 165.3) and the oxygen-bearing carbon atom at δ 167.5, corroborating the attachment of the 4′-hydroxy-2′,3′-dihydroprenyl group at C-3 and the oxygen-bearing conjugated quaternary olefinic carbon at C-4. The position of the aromatic methyl (δ 2.60) was deduced by the observation of cross-peaks from the signal at δ 2.60 to C-5 (δ 117.3), C-6 (δ 155.6) and C-10 (δ 123.8). The methoxyl groups were placed at C-4 (δ 167.5) and C-6 due to the observation of long-range correlations from the signal at δ 3.85 to C-4 and the signal at δ 3.87 to C-6. The assignments of the ortho-coupled protons were deduced by the HMBC correlations shown in Fig. 2.
Table 1. 1H and 13C NMR data for 8 and 1H NMR data for 9 (500 MHz for 1H NMR and 125 MHz for 13C NMR).
| Position | 1H (8); in CD3OD | 1H (9); in CDCl3 | 13C (8): in CD3OD |
|---|---|---|---|
| 2 | 165.3 | ||
| 3 | 120.0 | ||
| 4 | 167.5 | ||
| 5 | 117.3 | ||
| 6 | 155.6 | ||
| 7 | 7.20 d (9.0) | 7.08 d | 115.7 |
| 8 | 7.24 d (9.0) | 7.15 d | 115.8 |
| 9 | 148.6 | ||
| 10 | 123.8 | ||
| 11 | 2.60 s | 2.61 s | 12.5 |
| 1′ab | 2.65 m | 23.2 | |
| 2′ab | 1.65 m | 32.8 | |
| 3′ | 1.37 m | 36.7 | |
| 4′a | 3.40 dd, (11, 6) | 67.7 | |
| 4′b | 3.47 dd, (11, 6) | ||
| 5′ | 1.01 d (6.7) | 16.9 | |
| 4-OMe | 3.85 s | 62.3 | |
| 6-OMe | 3.87 s | 3.85 s | 56.9 |
Figure 2. Selected HMBC correlations observed in 8.
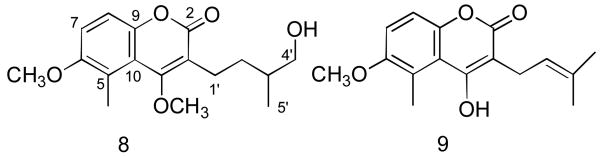
The structure of 8 was thus concluded to be the new compound 3-(4′-hydroxy-2′,3′-dihydroprenyl)-4,6-dimethoxy-5-methylcoumarin, named astrotricoumarin (Fig. 3).
Figure 3.
Structures of astrotricoumarin (8) and 9.
A related compound was isolated by Bohlmann and Zdero from Bothriocline laxa (Compositae) [7] and assigned the structure 9; the location of the methoxyl group was confirmed by synthesis [8]. The relevant 1H NMR data of the synthetic compound 9 agreed well with the corresponding data of 8 (Table 1).
Plants from the family Meliaceae are well known for producing limonoids [4,6,9,10]. Mulholland and co-workers suggested the close relationship between the genus Astrotrichilia and Ekebergia after the isolation of compound 5 from A. asterotricha, a compound which is related to a constituent of Ekebergia capensis, ekebergin (10) [10]. Only a few papers have reported the presence of coumarins in the family [11,12]. Ekersenin (11) was the first coumarin isolated from plants in the Meliaceae family (Ekebergia senegalensis) [12]. In 1997, Mulholland and her co-workers discovered the presence of five methyl coumarins in Ekebergia pterophylla, which added the list of coumarins in the Meliaceae family [11]. The isolation of 8 in the present paper increases the number of the species containing coumarins in the Meliaceae family and suggests that the genera Astrotrichilia and Ekebergia share the same ancestors.
Compound 8 was modestly active against the A2780 ovarian cancer cell line, with an IC50 value of 6.8 μM.
Experimental
General experimental procedures
Optical rotations were recorded on a JASCO P-2000 polarimeter. IR and UV spectra were measured on MIDAC M-series FTIR and Shimadzu UV-1201 spectrophotometers, respectively. 1H and 13C NMR spectra were recorded on a JEOL Eclipse 500 and Bruker Avance 600 spectrometers in CD3OD with TMS as internal standard. Mass spectra were obtained on a JEOL JMS-HX-110 instrument and a Finnigan LTQ LC/MS. Preparative HPLC was performed using Shimadzu LC-10AT pumps coupled with a semipreparative Varian Dynamax C18 column (5 μm, 250×10 mm), a Shimadzu SPD M10A diode array detector (DAD) and a SCL-10A system controller.
Antiproliferative Bioassay
The A2780 ovarian cancer cell line assay was performed at Virginia Polytechnic Institute and State University as previously reported [13]. The A2780 cell line is a drug-sensitive ovarian cancer cell line [14].
Plant material
Stems of Astrotrichilia sp. (collection number Rakotonandrasana & al. 1091) were collected in the Diana region of Madagascar, 2 km west of the village of Ambolobozobe, in the Ankonahona forest, 12°31′26″S 049°31′29″E, on January 27, 2007. The sample collected was from a tree 8 m tall, with white petals, orange anthers, and white stigmatas. This collection represents a new endemic species from the northern dry forests (Ambolobozobe, Daraina and Sahafary) that will be described elsewhere. The identification of the plant was made by Gregory Wahlert and Peter B. Phillipson from the Missouri Botanical Garden (MO). Voucher specimens have been deposited in herbaria at the Parc Botanique and Zoologique de Tsimbazaza (TAN), at the Centre National d'Application des Recherches Pharmaceutiques in Antananarivo, Madagascar (CNARP), at the Missouri Botanical Garden in St. Louis, Missouri (MO), and at the Muséum National d'Histoire Naturelle in Paris, France (P).
Extraction and Isolation
Dried stems of Astrotrichilia sp. (276 g) were ground in a hammer mill, then extracted with EtOH by percolation for 24 hours at room temperature to give the crude extract MG 4292 (8.7 g), of which 2.7 g was available at Virginia Polytechnic Institute and State University (VPISU) for evaluation. The crude EtOH extract (1 g) was dissolved in MeOH and extracted with n-hexane (3 × 200 mL) to afford 126.3 mg of residue after evaporation of the hexane soluble fraction. The MeOH layer was then evaporated, suspended in H2O (300 ml) and extracted with EtOAc (3 × 200 mL) to yield 230 mg of EtOAc soluble fraction. The EtOAc fraction was found to be cytotoxic (IC50 3.7 μg/mL) and subjected to HPLC on a C-18 column as described above (gradient solvent system from 70% aqueous MeOH to 80% aqueous MeOH during 10 min, 80% to 90% at 10 min to 15 min, 90% to 95% from 15 min to 20 min and 95% to 100% from 20 min to 25 min and then continue 100% MeOH until 35 min) to yield compound 8 (1.2 mg, tR: 15.50 min). MG4292
Figure 1.
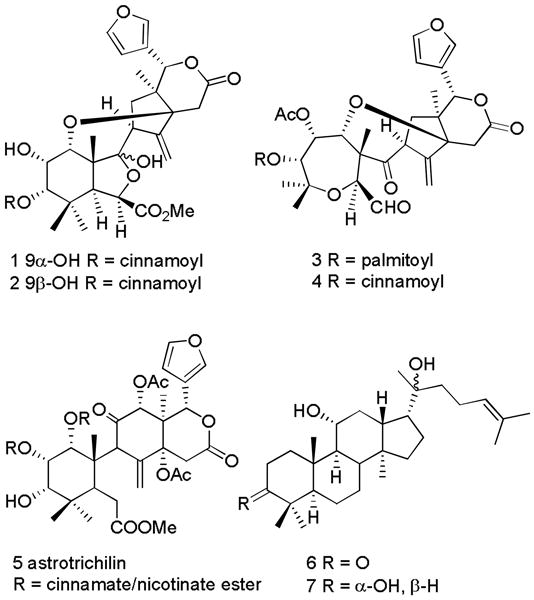
Structures of compounds 1 - 7.
Figure 4. Structures of ekebergin (10) and ekersenin (11).

Acknowledgments
This International Cooperative Biodiversity Group project was supported by the Fogarty International Center, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart Lung and Blood Institute, the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, the National Institute of General Medical Sciences, the Biological Sciences Directorate of the National Science Foundation, and the Office of Biological and Environmental Research of the U.S. Department of Energy under Cooperative Agreement U01 TW00313 with the International Cooperative Biodiversity Groups. This project was also supported by the National Research Initiative of the Cooperative State Research, Education and Extension Service, USDA, Grant #2008-35621-04732. These supports are gratefully acknowledged. Work at Virginia Polytechnic Institute and State University was supported by the National Science Foundation under Grant CHE-0619382 for the purchase of the Bruker Avance 600 NMR spectrometer and Grant CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Mr. B. Bebout for obtaining the mass spectra. Fieldwork essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d'Application des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts). We thank S. Randrianasolo, R. Rakotondrajaona, R. L. Andriamiarisoa, V. Benjara, and M. Velonjara for assistance with the plant collection.
Footnotes
Astrotricoumarin (8): [α]D: +12.2° (c 0.6, methanol).
IR (film): 3455, 3050, 1708, 1598, 1564, 1449, 1257, 1046 cm-1.
UV/Vis λmax (MeOH) nm (log ε): 234 (3.80), 280 (4.52), 324 (3.45).
1H NMR (500 MHz) and 13C NMR (125 MHz) in CD3OD: see Table 1.
HRESIMS: m/z [M + H+] calcd for C17H23O5: 307.1545; found: 307.1543.
References
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 48. For Part 47, see Pan E, Cao S, Brodie PJ, Callmander M, Randrianaivo R, Rakotonandrasana S, Rakotobe E, Rasamison VE, TenDyke K, Shen Y, Suh EM, Kingston DGI. Isolation and synthesis of antiproliferative eupolauridine alkaloids of Ambavia gerrardii from the Madagascar dry forest. Journal of Natural Products. 2011;74 doi: 10.1021/np200093n. in press.
- 2.Mabberley DJ. The Plant-Book A Portable Dictionary of the Higher Plants. Cambridge, England: Cambridge University Press; 1997. [Google Scholar]
- 3.Mulholland DA, Nair JJ, Taylor DAH. Dammaranes from Astrotrichilia asterotricha. Phytochemistry. 1994;35:542–544. [Google Scholar]
- 4.Mulholland DA, Nair JJ, Taylor DAH. Astrotrichilin, a limonoid from Astrotrichilia asterotricha. Phytochemistry. 1996;42:1239–1241. [Google Scholar]
- 5.Mulholland DA, Randrianarivelojosia M, Lavaud C, Nuzillard JM, Schwikkard SL. Limonoid derivatives from Astrotrichilia voamatata. Phytochemistry. 2000;53:115–118. doi: 10.1016/s0031-9422(99)00488-4. [DOI] [PubMed] [Google Scholar]
- 6.Mulholland DA, Schwikkard SL, Randrianarivelojosia M. Limonoid from Astrotrichilia voamatata. Phytochemistry. 1999;52:705–707. doi: 10.1016/s0031-9422(99)00488-4. [DOI] [PubMed] [Google Scholar]
- 7.Bohlmann F, Zdero C. Neue 5-alkylcumarine und chromone aus Bothriocline laxa. Phytochemistry. 1977;16:1261–1263. [Google Scholar]
- 8.Bohlmann F, Wienhold C. Synthese naturlich vorkommender 5-methylcumarine und -chromone. Chemische Berichte. 1979;112:2394–2401. [Google Scholar]
- 9.Murphy BT, Brodie P, Slebodnick C, Miller JS, Birkinshaw C, Randrianjanaka LM, Andriantsiferana R, Rasamison VE, TenDyke K, Suh EM, Kingston DGI. Antiproliferative limonoids of a Malleastrum sp. From the Madagascar rainforest. Journal of Natural Products. 2008;71:325–329. doi: 10.1021/np070487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor DAH. Ekebergin, a limonoid extractive from Ekebergia capensis. Phytochemistry. 1981;20:2263–2265. [Google Scholar]
- 11.Mulholland DA, Iourine SE, Taylor DAH, Dean FM. Coumarins from Ekebergia pterophylla. Phytochemistry. 1998;47:1641–1644. [Google Scholar]
- 12.Okogun JI, Enyenihi VU, Ekong DEU. Spectral studies on coumarins and determination of constitution of ekersenin by total synthesis. Tetrahedron. 1978;34:1221–1224. [Google Scholar]
- 13.Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. Antiproliferative xanthones of Terminalia calcicola from the Madagascar rain forest. Journal of Natural Products. 2007;70:679–681. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Research. 1985;45:2110–2115. [PubMed] [Google Scholar]