Abstract
Protection from the parasite Leishmania major is mediated by CD4 T cells. BALB/c mice are susceptible to L. major and show a nonprotective immunodominant CD4 T cell response to Leishmania homolog of activated receptor for c-kinase (LACK) 158–173. Host genes that underlie BALB/c susceptibility to L. major infections are poorly defined. DM, a nonclassical MHC class II molecule, due to its peptide editing properties has been shown to 1) edit the repertoire of peptides displayed by APC, and 2) focus the display of epitopes by APC to the immunodominant ones. We tested the hypothesis that deficiency of DM, by causing presentation of a different array of epitopes by infected APC than that presented by DM-sufficient APC, may change the course of L. major infection in the susceptible BALB/c mice. We show herein that unlike their susceptible wild-type counterparts, BALB/c mice deficient in DM are protected from infections with L. major. Furthermore, DM-deficient mice fail to display the immunodominant LACK 158–173 on infected APC. In its place, infected DM−/− hosts show elicitation of CD4 T cells specific for newer epitopes not presented by wild-type L. major-infected APC. Protection of BALB/c DM−/− mice is dependent on IFN-γ. DM is thus a host susceptibility gene in BALB/c mice, and Ag processing in the absence of DM results in elicitation of a protective T cell response against L. major infections. This report suggests a novel mechanism to trigger host resistance against pathogens.
Protection from Leishmania major, an obligate intracellular parasite, is mediated by CD4+ T cells (1–3). BALB/c mice are susceptible to L. major and are characterized by production of high levels of IL-4 and IL-10 in response to the infections with this parasite. In contrast, C57BL/6 mice are resistant to L. major infections and typically produce high levels of IFN-γ but no IL-4 and IL-10 (2–5). Although multiple avenues have been explored to elucidate mechanisms of host susceptibility of BALB/c mice, it still remains unknown whether events in Ag processing pathway can change the course of disease in L. major infections in BALB/c mice. It is also unclear why most CD4 T cells elicited in the susceptible BALB/c mice recognize a single epitope 158–173 of one protein, Leishmania homolog of receptor for activated c-kinase (LACK)3 (6–9), when the parasite is known to express ~10,000 different proteins. It can be postulated that a stringent selection of MHC class II-associated epitopes must occur during pathogen processing within the Ag processing pathway (10–13).
Besides catalyzing the exchange of class II invariant chain peptide (CLIP) with antigenic peptides within the groove of MHC class II molecules, DM, itself a nonclassical MHC class II molecule, functions as a peptide editor in selecting the repertoire of peptides displayed by MHC class II molecules (10–15). Such selection greatly influences the repertoire of responding CD4 T cells (10, 11). The default pathway of Ag-specific T cell responses in normal mice is characteristically focused on recognition of a limited number of immunodominant epitopes (11, 16). However, we have shown before that the specificity of responding T cells in the absence of DM-driven epitope editing in DM-deficient (DM−/−) mice expands to additional epitopes not recognized by T cells elicited in responses in the DM-sufficient mice (10, 11). We therefore hypothesized that T cells recognizing newer epitopes, not displayed by infected APC in the wild-type mice, could be qualitatively different in their ability to elicit the type of effector (e.g., TH1 or TH2 or TH17 type) response (17, 18), and thus could lead to a change in the outcome of infection in DM−/− BALB/c mice.
We tested this hypothesis by using the recently constructed DM−/− BALB/c mouse (10, 19) (note that BALB/c embryonic stem cells were used to construct DM−/− mice (19). Importantly, the consequences of DM peptide editing function on immunity against pathogens have remained unexplored primarily because the DM-deficient mice were initially constructed in a C57BL/6 (H-2b) haplotype (19, 20). Allelic properties of Ab MHC (unlike Ad, Ed, or Ak MHC) encode a very tight CLIP-MHC association such that in the absence of DM, all Ab molecules expressed by APC exclusively display CLIP (19–21). This attribute of Ab MHC precludes the use of C57BL/6 mice for an exploration of the role of DM peptide editing function in immunity (10, 22). However, Ad and Ed alleles can largely spontaneously dissociate from CLIP without the assistance of DM (10, 19), and this allowed us to examine the consequences of DM epitope editing in DM−/− BALB/c mice during L. major infections.
Invariant chain (Ii) is another molecule, a chaperone, expressed within the Ag processing pathway and is required for trafficking of MHC class II from the endoplasmic reticulum to the endocytic pathway within APC. Ii-deficient (Ii−/−) mice show a substantial quantitative decrease (unrelated to epitope editing) in epitope display as a result of altered trafficking and decreased class II expression on APC. BALB/c Ii−/− mice were therefore included in our studies to contrast the DM-mediated constraints in epitope display vs quantitative change in presentation of epitopes in Ii−/− mice (23, 24). Interestingly, Ii−/− mice also show allele-specific differential outcomes for Ag presentation in different haplotype mice. Thus, although C57BL/6 Ii−/− (23) mice show severe Ag presentation defects and marked decreases in the numbers of mature CD4 T cells in the thymus and the periphery, BALB/c Ii−/− mice show only limited defects in Ag presentation function and have relatively efficient maturation of CD4 T cells in the periphery (19, 23, 24). Thus, H-2d alleles can allow MHC class II trafficking and peptide loading via an Ii-independent pathway. Furthermore, doubly deficient DM−/−Ii−/− mice (19) were used to examine whether DM deficiency can influence the outcome of disease when not only is there a substantial quantitative decrease in epitope display but, additionally, peptide loading of class II takes place in the absence of DM.
The results presented herein indicate that DM-deficient (DM−/− and DM−/−Ii−/−) mice are resistant to infection with L. major. We show that the resistance of BALB/c DM−/− and DM−/−Ii−/− mice is attributable to elicitation of a distinctly different group of T cells recognizing a newer array of epitopes that are not displayed by APC in the infected wild-type BALB/c mice. The data also show that presence of T cells specific for the newer epitopes in DM-deficient mice leads to production of protective levels of IFN-γ accompanied by an absence of secretion of IL-4 or IL-10 through the entire course of disease. This report thus suggests that protective responses can be elicited in BALB/c mice infected with L. major when Ag processing takes place in the absence of DM.
Materials and Methods
Mice
BALB/c/J and C57BL/6 mice were purchased from The Jackson Laboratory. Homozygous mutant strains DM−/−, Ii−/−, and DM−/−Ii−/− (10, 19) and the wild-type B10.D2 mice were bred in a specific pathogen-free facility. All mice were maintained under American Association for the Accreditation of Laboratory Animal Care-approved conditions.
Parasites, infections, Ags, and peptides
L. major MHOM/IL/80/Friedlin (a gift from Dr. David Sacks, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health) was cultured in M199 medium containing 20% FCS, and metacyclic promastigotes were purified from stationary phase cultures using peanut agglutinin as described (25). Mice were infected in the left hind footpad with 0.5 × 106 metacyclics in 10–20 µl.
Soluble Leishmania Ag (SLA) was prepared by homogenizing stationary phase parasites (5 days in culture), using seven freeze-thaw cycles, and centrifuged at 10,000 rpm for 10 min at 4°C. Protein content was quantified using the BCA protein assay reagent (Pierce), with freeze-thaw lysates of 100 × 106 parasites yielding 77 µg of protein on average. Filter-sterilized (0.22 µm) lysate (SLA) was stored in 1-ml aliquots at −80°C.
Peptide 158–173 was custom synthesized and purified by Macromolecular Resources as described before (10).
Estimation of parasite load in infected tissues
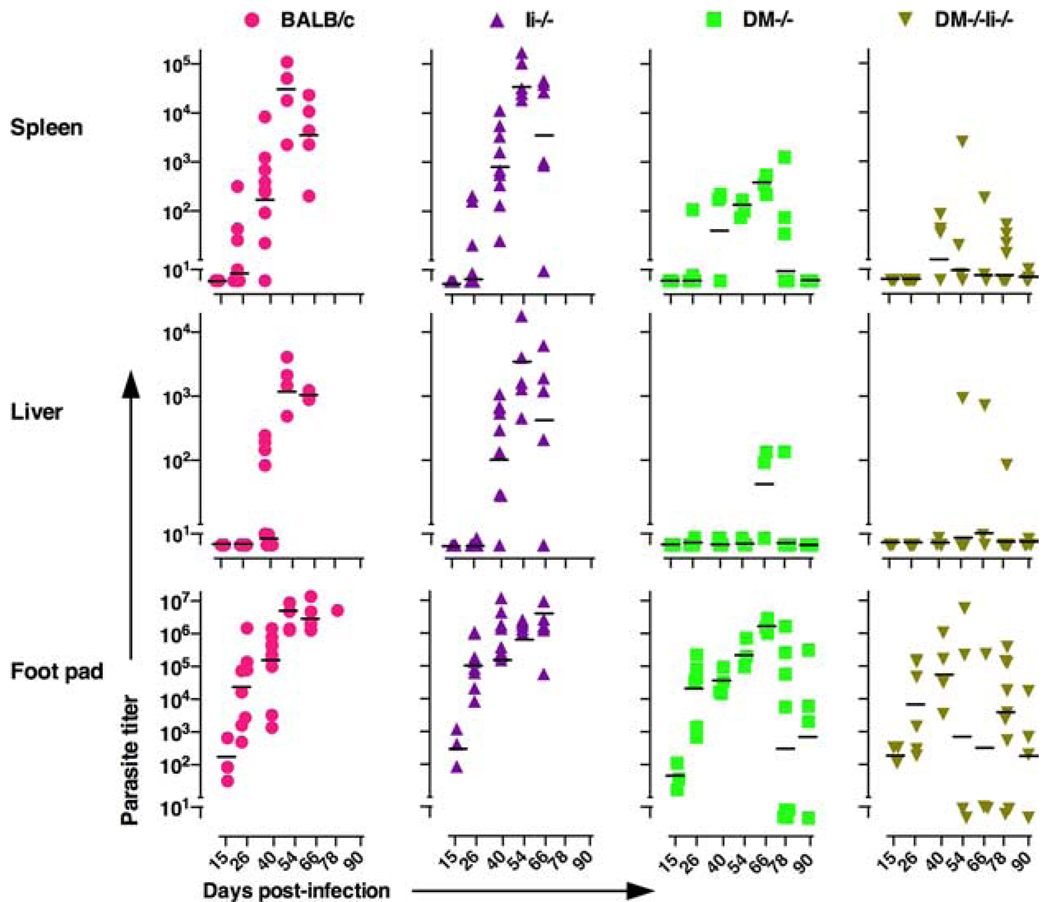
Numbers of live parasites were quantified as described before (25). Briefly, at various times after infection, infected footpads, a pool of draining popliteal, inguinal, and periaortic lymph nodes (LN), liver, and spleen were isolated and either weighed (in the case of foot pads and livers) or dissociated for lymphocyte count (in the case of LN and spleen). Homogenized tissue samples were plated in 96-well round-bottom plates at 25°C. For limiting dilution analysis, four to eight replicates per sample were cultured in 7–11 serial dilutions (5- to 10-fold each dilution) in M199 medium containing 20% FCS. Visible parasite colonies were scored under the microscope at 3 wk. The parasite number was calculated from minimum χ2 analysis of the Poisson distribution relationship between dilution of homogenate and percentage of negative wells (i.e., wells that did not grow out any parasites). We assumed that a dilution that had 37% negative wells equated to at least one L. major CFU (26). Each symbol in Fig. 2 represents data from one mouse.
FIGURE 2.
DM−/− and DM−/−Ii−/− BALB/c mice are protected from systemic parasite spread and clear parasite load in FP. Age-matched, BALB/c/J wild-type, DM−/−, Ii−/−, and DM−/−Ii−/− were infected in one hind FP as described in Fig. 1 and in Materials and Methods. Three to five mice from each group were sacrificed at the time points indicated postinfection. The data shown are from individual mice from two to three different experiments. FP, spleens, and livers were homogenized and cultured in limiting dilution and the parasite numbers were calculated as described before (25, 26) and in the supplemental materials. Horizontal bars represent the geometric means of data at a given time point. All wild-type and the Ii−/− mice had to be sacrificed at day 52 due to their large FP sizes except one rare wild-type mouse that we were able to analyze on day 78 postinfection. Note that the DM−/− and DM−/−Ii−/− BALB/c mice have cleared parasites in spleens and livers and have controlled parasite numbers to between 100 and 800 in the FP by day 90 postinfections. Wild-type (●), Ii−/− (▲), DM−/− (■) and DM−/−Ii−/− (▼).
Generation of L. major-specific hybridomas from DM−/− BALB/c mice
Draining LN T cells from 4-wk-infected DM−/− BALB/c mice were cultured at 5 × 106 cells per well in 24-well flat bottom plates with 100 µg of SLA. Five days later the T cell blasts were harvested and fused with BW5147α-β-hybridoma partner cells using standard methodology as described before (except that the current hybridomas were not subcloned) (27). Ag specificity of hybridomas was assessed by IL-2 secretion response following overnight culture of hybridomas with 24-h L. major infected or uninfected DM−/− bone marrow-derived dendritic cells (BMDC).
Cytokine analyses
LN or spleen cells were cultured at 5 × 106 cells per well in 24-well flat bottom plates (Costar) in the presence or absence of 100 µg of SLA. Culture supernatants obtained after 7 days (or after indicated times as in data presented in supplemental Fig. 44 for time kinetics) were analyzed for IL-4, γ-IFN, IL-10, and IL-17 by sandwich ELISA using commercial kits (R&D Systems) and as described before (11).
Infection of BMDC with L. major
Bone marrow (BM) cells from wild-type and mutant BALB/c mice were harvested from long bones of thighs. Cells were frozen at 5 × 106 cells/ml and stored in liquid nitrogen until needed. BMDC were grown using a variation of a previously published protocol (28). BM cells were thawed and plated at 1 million cells per ml per well in 24-well plates in advanced DMEM-F12 (Invitrogen) supplemented with 2 mM glutamine, 5% FCS, 50 µg/ml gentamicin, 100 U/ml penicillin, 100 µg/ml streptomycin and 12.5 µM 2-ME, and 6 ng/ml GM-CSF and 3 ng/ml IL-4 (both from PeproTech). On day 6, live stationary phase L. major promastigotes were added to BMDC cultures at 35 × 106 parasites per well. One day later, infected BMDC cultures were either used for cytospin preparations or for epitope display (see below). BMDC, cultured as above, and stained with anti-CD11b (clone M1/70), anti-CD11c (clone N418), and anti-MHC class II (clone M5/114) Abs and 7AAD (7-aminoactinomycin D) were 90–95% CD11c+ MHC class II-high cells. Immunofluorescence data were acquired on a BD FACSort cytometer and analyzed using Cell Quest v3.3 (BD Immunocytometry Systems). All Abs used in this study were from BD Biosciences, unless stated otherwise.
Cytospins of uninfected and infected BMDC (infected as above) were stained with Giemsa (Histoserv) and digitally photographed using a Zeiss Axiophot microscope at ×100 magnification to analyze their degree of L. major infection. Images were processed with IPLab 3.5 and Photoshop 3.0 software.
Epitope display by L. major-infected BMDC from BALB/c wild-type and mutant mice
Replicate cultures of 2-fold serial dilutions (375–192,000) or indicated numbers of WT, Ii−/−, DM−/−, and DM−/−Ii−/− BMDC of washed infected (as above) and uninfected BMDC were either not pulsed or pulsed with 1 µM LACK peptide 158–173 and cultured with 100,000 cells per well of LMR 16.2 hybridoma cells (specific for LACK peptide 158–173; a gift from Dr. N. Glaichenhaus, Institut National de la Santé et de la Recherche Médicale, Nice, France). After overnight culture, supernatants were analyzed for IL-2 by ELISA performed according to the manufacturer’s instructions (R&D Systems).
In vivo anti-IFN-γ treatment
Anti-IFN-γ (clone XMG1.2) and isotype control Ab (clone HRPN) were purchased from Bio X Cell. Mice were injected with 500 µg of Ab in 200 µl i.p. weekly for 5 wk.
Results
BALB/c DM−/− and DM−/−Ii−/− mice show protection against L. major infections
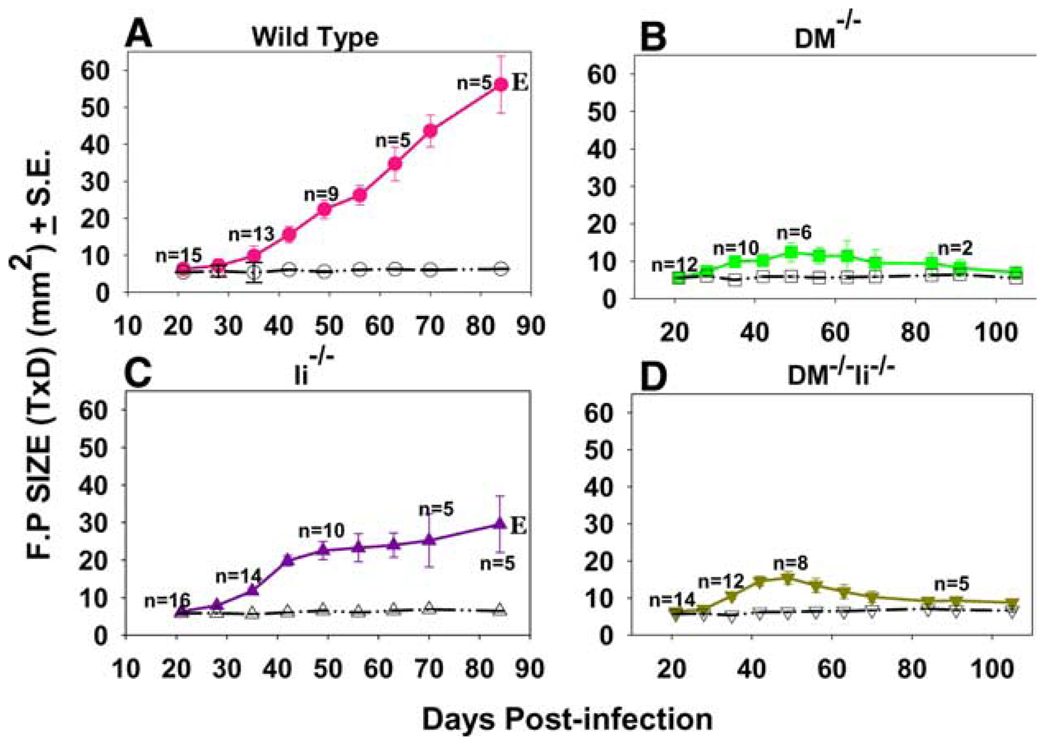
To assess whether Ag processing in the absence of DM could alter the course of L. major infection in BALB/c mice, we infected groups of wild-type, DM−/−, Ii−/−, and DM−/−Ii−/− BALB/c mice with 0.5 × 106 metacyclic parasites in the foot pad (FP) and measured FP size to determine disease progression. Fig. 1 shows the size of L. major-infected FP in mice during 15 wk after infection for each group from one representative of five experiments. As expected, the FP size in susceptible wild-type (DM+/+) BALB/c mice (Fig. 1A) progressively increases with time until it was necessary to sacrifice them. Strikingly, however, the infected DM−/− mice showed only a minimal increase in FP size (Fig. 1B). FP swelling in the infected feet of Ii−/− mice nearly paralleled those of the wild-type mice in that they exhibited progressive swelling at most time points (Fig. 1C). Remarkably, however, the DM−/− Ii−/− mice showed a FP swelling pattern that was similar to that of the resistant DM-deficient mice during the entire 15 wk postinfection period (Fig. 1D). Thus, while the infected wild-type (5) and Ii−/− (29) BALB/c mice were susceptible as expected, the DM−/− and DM−/−Ii−/− BALB/c mutant mice resisted the development of any noteworthy or sustainable FP lesions.
FIGURE 1.
DM−/− and DM−/−Ii−/− BALB/c mice are resistant to FP infection with L. major. BALB/c/J, DM−/−, Ii−/−, and DM−/−Ii−/− were infected in one hind FP with 0.5 × 106 metacyclic promastigote L. major parasites. Twelve to 18 age-matched female (two experiments) or male and female (three experiments) mice were used for each group. The thickness and diameter of the FP was measured each week postinfection. Closed symbols represent the sizes of the infected FP and the open symbols represent sizes of the uninfected foot pads. Note that the lesion size in the wild-type and Ii−/− mice continued to grow and these mice had to be euthanized (E) by day 84. In contrast, the DM−/− and DM−/−Ii−/− mice developed minimal lesions and the FP size of these mice was measured until day 105 postinfection. Note that in our experience the time point at which the wild-type mice reached maximal FP lesions varied between week 6 and 12 in different experiments. Mice infected with 2.0 × 106 parasites showed the same results.
DM−/− and DM−/−Ii−/− mice control local parasite load and are protected against systemic parasite spread
We next explored whether L. major resistance of DM−/− and DM−/−Ii−/− mice was due to clearance of parasites from the site of infection (FP) and/or from spleen and liver, the typical sites of systemic spread in the wild-type mice (30), by evaluating the parasite loads in these tissues of infected mice. Fig. 2 (bottom panels) shows that the parasite loads in the FP of the susceptible wild-type and Ii−/− mice increased exponentially during the course of infection. In contrast, the parasite numbers in FP of DM−/− and DM−/−Ii−/− mice were significantly lower and continued to consistently decline until the average parasite number was only 800 and 100, respectively, by day 90. These data demonstrate a control of infection in FP of both the DM−/− and DM−/−Ii−/− mice with time.
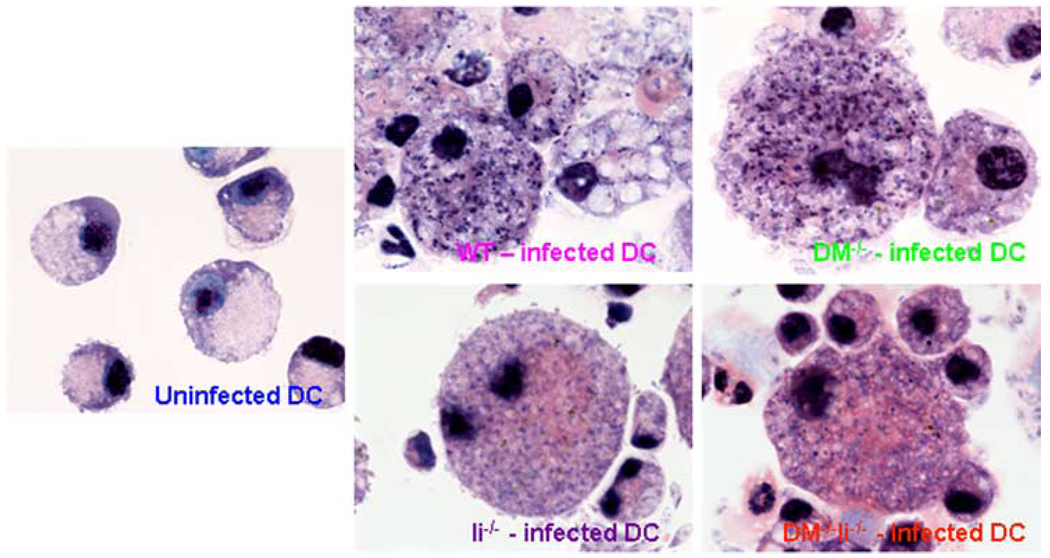
Systemic spread of parasites to visceral sites such as spleen and liver is one of the hallmarks of FP L. major infection in BALB/c mice (30). Accordingly, the spleens and livers of infected wild-type and Ii−/− mice showed sustained parasite presence and an exponential increase in parasite numbers until the last day before being sacrificed (Fig. 2, top and middle panels). Contrastingly, however, not only did the spleens and livers of infected DM−/− and DM−/−Ii−/− mice generally show remarkably lower numbers of parasites at all time points examined (an average of 300 and 9 in spleens and of fewer than 10 parasites in livers of DM−/− and DM−/−Ii−/− mice, respectively, on day 54 postinfection), they had completely cleared the infection by day 90 (Fig. 2, top and middle panels). These data in DM-deficient mice are strikingly similar to those obtained for the systemic parasite loads in the healer strain C57BL/6 (see supplemental Fig. 1). Our results strongly indicate that L. major is not able to establish and/or sustain systemic infection in DM−/− and DM−/−Ii−/− mice. Additionally, to ensure that resistance of DM−/− and DM−/−Ii−/− mice was not a result of an unknown intrinsic defect caused by deficiency of DM preventing L. major from infecting the mutant APC, we infected BMDC from wild-type and mutant (DM−/−, Ii−/−, and DM−/−Ii−/−) BALB/c mice in vitro with L. major. Giemsa staining of infected dendritic cells (DC) (indicates that L. major small intracellular black dots in Fig. 3) is able to infect wild-type and mutant DC in a comparable manner. Furthermore, the data shown in Fig. 2 indicate that the parasite load at the site of infection (FP) is not very different in the wild-type and the mutant strains of mice at early time points postinfection, providing additional evidence that infectivity of L. major for the different mutants is comparable to that of the wild-type mice.
FIGURE 3.
BALB/c wild type, DM−/−, Ii−/−, and DM−/−Ii−/− DC are infected by L. major in a comparable manner. BMDC, prepared as described before (28) and in Materials and Methods, were infected with 35 × 106 L. major promastigotes (at a DC/parasite ratio of 1:35) for 24 h. Cytospin slides prepared from infected and uninfected BMDC were stained with Giemsa (as described in Materials and Methods) and photographed (shown at ×100 magnification). Note that the small black (darkly stained) intracellular dots represent L. major nuclei (and the large black organelle is the DC nucleus).
L. major-infected APC from resistant BALB/c DM-deficient mice display a different set of epitopes as compared with infected wild-type and Ii−/− APC
Two different strategies were used to explore the effects of DM-mediated epitope editing on the display of Leishmania epitopes by L. major-infected APC in wild-type vs DM-deficient strains. It is well established that most T cells elicited in L. major infected wild-type BALB/c mice only recognize a single immunodominant epitope, LACK 158–173/Ad. Thus, in the first approach, we used the wild-type BALB/c-derived (LACK 158–173-specific) T cell hybridoma to probe the expression of this epitope on infected DM-deficient APC. Contrastingly, in the second approach, we developed a panel of L. major-specific T cell hybridomas from infected DM−/− mice and used them as probes to explore whether identical epitopes were expressed by the infected wild-type APC.
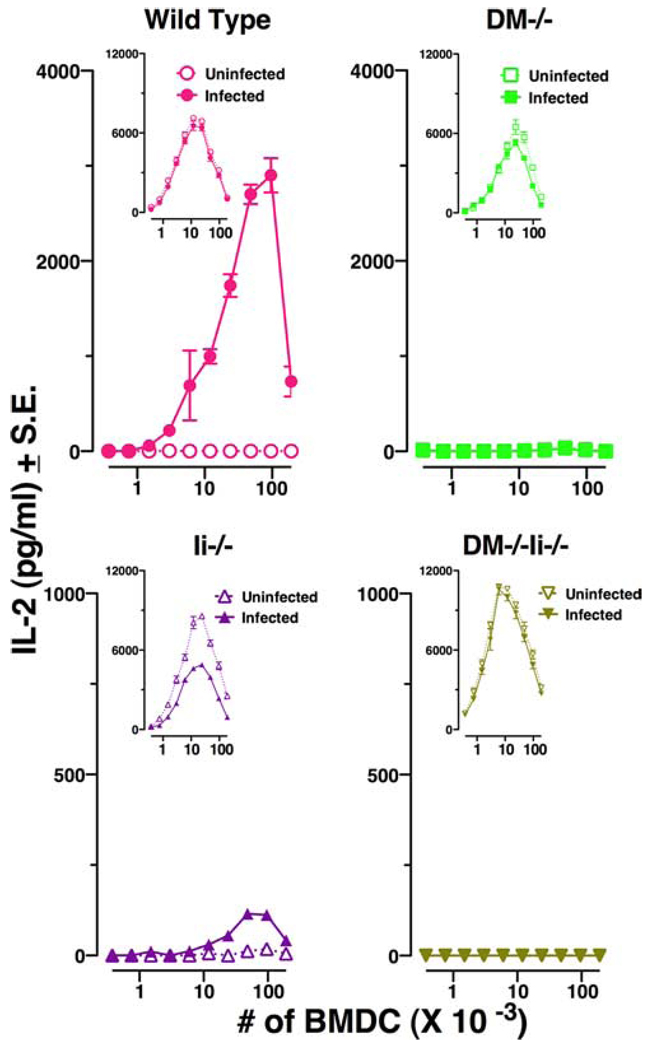
L. major infected DM−/− APC fail to display the immunodominant epitope LACK 158–173
Wild-type BALB/c-derived LACK 158–173-speciifc T cell hybridoma LMR 16.2 was used as a probe to examine the display of the immunodominant epitope LACK 158–173 by infected DC from the wild-type, DM−/−, Ii−/−, and DM−/− Ii−/− mice. As expected, L. major-infected DC from wild-type BALB/c mice efficiently displayed the immunodominant LACK epitope, and rather low numbers (n = 375) of infected APC were able to stimulate IL-2 production from the T cell hybridoma (Fig. 4, top left panel). In sharp contrast, infected DM−/− DC did not stimulate the LACK 158–173-specific hybridoma even at the highest numbers (n = 192,000) of infected DC per well (Fig. 4, right upper panel). As a consequence of abnormal trafficking of MHC class II in the absence of Ii, it can be expected that infected Ii−/− and DM−/−Ii−/− APC (19) will display quantitatively low levels of parasite-derived epitopes (19, 24). Accordingly, APC from Ii−/− DC were able to stimulate LACK 158–173-specific hybridoma but only when 48,000–192,000 infected DC were used in the culture well (Fig. 4, bottom left panel). These data reflect a definite though quantitatively scarce display of epitope 158–173 by infected Ii−/− APC (19). Interestingly, however, DM−/−Ii−/− APC failed to stimulate LACK 158–173-specific T hybridoma even at the highest numbers of infected DC (Fig. 4, right lower panel). This result suggests that the expression of low amount of LACK 158–173 in Ii−/− APC was dependent upon presence of DM. Note that the infected or uninfected DC from all mice including DM−/−Ii−/− mice were able to show activation of LMR 16.2 when exogenous peptide 158–173 was added to the culture wells (Fig. 4, insets), indicating that levels of expression of MHC class II and costimulatory molecules in all DC were sufficient to stimulate T cell hybridomas. Data in Fig. 4 thus indicate that display of MHC class II-bound LACK 158–173 is completely abrogated in DM-deficient mice.
FIGURE 4.
L. major-infected DM−/− APC fail to display the immunodominant epitope LACK 158–173. Bone marrow DC, prepared from 1 × 106 BM cells as described before (28) and in Materials and Methods, were infected with 35 × 106 L. major promastigotes for 24 h. Infected and uninfected DC were washed and used at various concentrations with the T cell hybridoma (LMR 16.2) specific for LACK 158–173. The data are depicted as IL-2 release, a measure of activation of the T cell hybridoma, and are representative of three to five experiments. Peptide 158–173 (1 µM) was exogenously added in the data shown in the insets. Open symbols represent uninfected and closed symbols represent infected BMDC: Wild-type (●, ○), DM−/− (■, □), Ii−/− (▲, △), and DM−/−Ii−/− (▼, ▽).
L. major-infected DM−/− APC present newer epitopes not displayed by infected wild-type APC
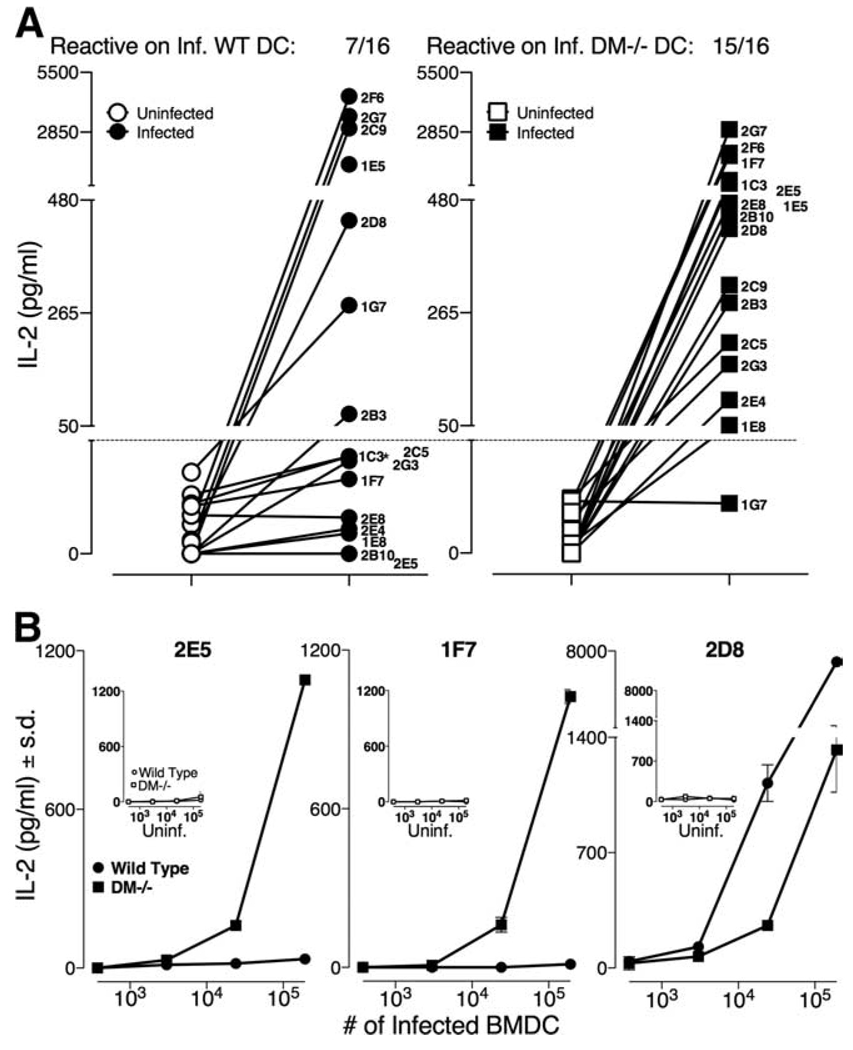
As mentioned above, a panel of 16 L. major-specific T cell hybridomas was derived from infected BALB/c DM−/− mice 4 wk postinfection (see Materials and Methods). All 16 DM−/−-derived hybridomas showed IL-2 secretion only when L. major-infected DC were used as APC but no IL-2 production when uninfected DC were used as APC (Fig. 5A). The peptide specificity of these 16 hybridomas is unknown (preliminary analyses (not shown) indicate that these DM−/−-derived hybridomas may not be reactive to LACK-derived peptides; however, our data do not entirely rule out this possibility).
FIGURE 5.
L. major infected DM−/− DC present newer epitopes not displayed by infected wild-type DC. A, Sixteen L. major-specific hybridomas derived from infected DM−/− mice were examined for activation using L. major-infected or uninfected wild-type or DM−/− BMDC (104 DC per well) as described in Fig. 4. The data show that while 15 of the 16 DM−/−-derived hybridomas responded to infected DM−/− APC, only 7 of 16 recognized infected wild-type APC, thus indicating that L. major-infected wild-type DC failed to express epitopes recognized by 8 DM−/− T cell hybridoms. The horizontal dotted line represents the cutoff point for the T hybridomas not recognizing L. major infected APC (with an exception of 1C3, which shows a minor response to wild-type-infected APC). Symbols are: wild-type uninfected (○), infected (●); BALB/c DM−/− uninfected (□), infected (■). B, Three DM−/−-derived T hybridomas were examined for stimulation with varying doses of uninfected or infected BMDC as described in A. The data show that 2E5 and 1F7 hybridomas (representing the eight T cell hybridomas that fail to be stimulated by infected wild-type APC) are not stimulated despite the presence of 192,000 infected wild-type DC per well. The hybridoma 2D8 (representing seven T hybridomas that are stimulated by both the infected wild-type and DM−/− DC) shows an enhanced response to wild-type DC as compared with infected DM−/− DC. Symbols used are as in A.
Nevertheless, we used these hybridomas to explore whether identical epitopes were being displayed by L. major-infected DM−/− and wild-type APC. As shown in Fig. 5A, while 15 of 16 hybridomas were stimulated by epitopes displayed by infected DM−/− APC (right panel), only 7 of 16 hybridomas were stimulated by L. major-infected wild-type APC (left panel). Thus, eight different L. major-specific hybridomas were unable to recognize any epitope presented on infected wild-type DC. Dose response of infected wild-type DC shown in Fig. 5B confirms that infected wild-type APC completely lack presentation of these epitopes for hybridomas 2E5 and 1F7, as there is no secretion of IL-2 even when they are stimulated by 192,000 infected DC. These data affirm that relative to the wild-type-infected APC, 1) at least one (and very likely more than one) newer pathogen-derived epitope is displayed by L. major-infected DM−/− APC, and 2) the display of these newer pathogen epitopes is quantitatively sufficient for activation of a distinct repertoire of T cells in DM−/− mice in response to L. major infections. To precisely state the diversity of newer epitopes displayed by infected DM−/− APC but not by the similarly infected wild-type APC, we await identification of peptides recognized by these eight distinct hybridomas.
Furthermore, 7 of 15 hybridomas recognize epitopes displayed on L. major-infected wild-type and DM−/− DC (Fig. 5A). A dose response of one of these T hybridomas, 2D8, is shown in Fig. 5B. Since infected DM-deficient APC lack a display of LACK 158–173 (Fig. 4), LACK 158–173 epitope can be precluded as an epitope recognized by this set of seven hybridomas. Our preliminary data (not shown) affirm this prediction. The data, however, suggest that 2D8 hybridoma responds to an epitope that can be presented in the absence or in the presence of DM.
DM−/− and DM−/−Ii−/− mice produce IFN-γ and IL-17 but not IL-4/IL-10
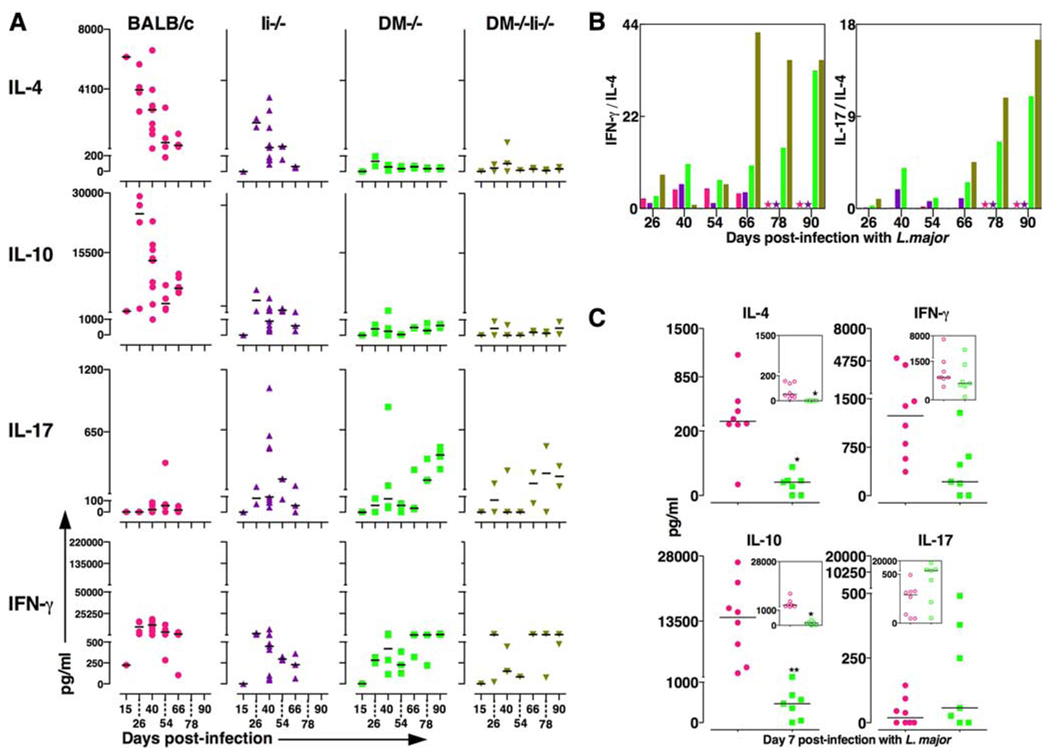
To explore whether the effector cytokine response of the resistant DM−/− and DM−/−Ii−/− mice matched that of the healing strain C57BL/6, we examined the cytokines produced by LN cells from infected DM−/− and DM−/−Ii−/− (as well as by the wild -type and Ii−/− mice) at various time points postinfection (Fig. 6A). As expected, the wild-type and Ii−/− mice produced high levels of IL-4 and IL-10 (accompanied by modest amounts of IFN-γ) (2–5, 31). Strikingly, however, BALB/c DM−/− and DM−/−Ii−/− mice showed no production of IL-4 or IL-10 in response to L. major infections at any time point, yet they produced IFN-γ throughout the course of disease (see Fig. 6A, right panels). Lack of IL-4 and IL-10 production in protected DM-deficient mice matched the absence of IL-4 and IL-10 production in the healer C57BL/6 mice (32–34) (supplemental Fig. 2).
FIGURE 6.
A, DM−/− and DM−/−Ii−/− mice do not produce IL-4 and IL-10 in response to L. major infections. The supernatants derived from LN cells (shown) and spleen (not shown) cultured in vitro with SLA for 7 days were analyzed for a panel of cytokines using ELISA technique as described in Ref. 11. Time kinetics of cytokines expressed in supernatants from LN cells cultured for 24, 48, 72, 96, and 168 h are shown in supplemental Fig. 4. The data in supplemental Fig. 4 validate our choice of a 7-day period of in vitro culture. The data from individual mice from three to nine mice per strain per time point are shown. The horizontal lines represent median values of each group. The pattern of cytokine secretion from spleen cells (not shown) was similar to that from lymph node cells. Wild-type (●), DM−/− (■), Ii−/− (▲), and DM−/−Ii−/− (▼). B, DM−/− and DM−/−Ii−/− mice have significantly higher ratios of IFN-γ vs IL-4 and IL-17 vs IL-4. Data from A are shown as ratio of average value of IFN-γ vs IL-4 (left panel) and of IL-17 vs IL-4 (right panel) at a given time point postinfection to indicate that the resistance phenotype of BALB/c DM−/− and DM−/−Ii−/− mice is correlated with markedly higher ratios of inflammatory cytokines IFN-γ (left panel) and IL-17 (right panel). Color of bars are as in A. C, DM−/− mice do not make IL-4 and IL-10 even 7 days postinfection. The supernatants derived from LN cells derived from mice infected 7 days prior were cultured in vitro with SLA and were analyzed for a panel of cytokines as described in A. Data from seven to nine mice are shown as in A. GraphPad Prism 5 for MAC OS X was used for unpaired t test analysis in C. *, p < 0.05; **, p < 0.005. Symbol colors are as in A. Open symbols indicate no Ag (insets); closed symbols, with SLA.
The ratio of IFN-γ/IL-4 depicted in Fig. 6B suggests that the strikingly higher ratio of IFN-γ to IL-4 in DM−/− and DM−/− Ii−/− mice parallels resistance, and the lower ratio corresponds to susceptibility for both the wild-type and Ii−/− mice. These results support the previously held conviction that in the absence of the inhibitory cytokines IL-4 and IL-10, even low amounts of IFN-γ are sufficient to activate macrophages to produce inducible NO synthase and other effector molecules necessary for parasite clearance (33). The absence of IL-4/IL-10 in DM−/− and DM−/−Ii−/− strains should therefore allow relatively potent activation of DC and parasite clearance (33, 35).
We also measured levels of IL-17 (another proinflammatory cytokine) in infected mice and show that the resistant DM−/−, DM−/−Ii−/−, and C57BL/6 mice produced IL-17, especially at the late stage of disease (Fig. 6A and supplemental Fig. 2). In contrast, IL-17 was not produced by wild-type mice at any time point postinfection. Nevertheless, a markedly high ratio of IL-17 to IL-4 symbolizes protection in DM−/− and DM−/−Ii−/− (Fig. 6B, right panel) and C57BL/6 mice (not shown), and a low ratio correlates with susceptibility of DM+/+ mice. Note that production of IL-17 by infected DM-deficient mice was neither just exclusive to L. major infections nor was it produced in an Ag-nonspecific manner. LN cells from hen egg lysozyme-immunized DM−/− and the wild-type mice showed equivalent production of IL-17 in response to the immunodominant HEL 106–116 but none when cultured without Ag (data not shown).
To determine exactly how early after infection with L. major divergence of cytokine in DM-deficient mice vs the wild-type mice could be observed, we examined cytokine secretion in infected mice on day 7 postinfection (Fig. 6C). It is clear that the hallmark cytokine response of no IL-4/IL-10 but secretion of IFN-γ and IL-17 in resistant DM−/−, DM−/−Ii−/− (Fig. 6C), and B6 mice (supplemental Fig. 2, bottom panels) was already in existence as early as day 7 postinfection. The pattern of high IL-4/IL-10 response observed in the susceptible strains of mice was also already existent at day 7 (Fig. 6C). Furthermore, our preliminary data indicate that this characteristic cytokine response of DM- deficient mice vs the susceptible DM-sufficient mice is detectable on day 2 postinfection (not shown). These results suggest that pathogen processing in DM-deficient mice triggers events (such as an activation of a newer group of T cells in place of LACK 158–173-reactive T cells) that can steer the host toward protective immune responses as early as day 2 postinfection. We propose that this early differential cytokine response between the susceptible wild-type and the resistant DM-deficient mice underlies the presence and absence, respectively, of FP swelling in these strains shown in Fig. 1.
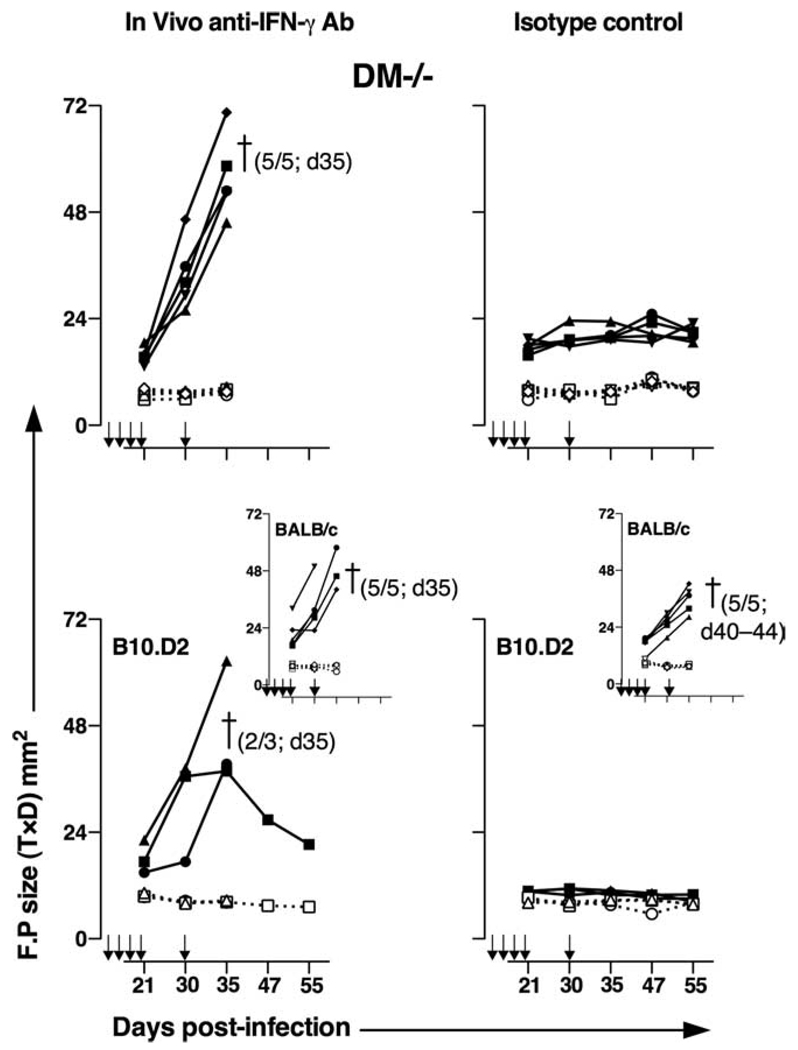
Anti-IFN-γ Ab treatment abrogates protection of BALB/c DM−/− mice against L. major infections
To explore whether resistance of DM-deficient mice was indeed due to IFN-γ production and a high IFN-γ/IL-4 ratio, we treated DM−/− and wild-type BALB/c mice weekly with 500 µg of anti-IFN-γ or an isotype control Ab for 5 wk. Mice were infected with L. major a day after the first injection with the Abs. B10.D2 and B6 mice, treated with the same regimen of Abs and infection, were included as controls. As shown in Fig. 7, all DM−/− mice treated with anti-IFN-γ Ab showed remarkable susceptibility to infections (measured by the FP swelling) and had to be euthanized by day 35 postinfection (top panels). Contrastingly, as expected (Fig. 1), DM−/− mice that received isotype control Ab were protected. Wild-type mice treated with IFN-γ Ab developed a more severe disease (larger FP swelling) relative to those treated with the isotype control (Fig. 7, see insets in bottom panels). All of the normally resistant B10.D2 (Fig. 7, bottom panels) and B6 mice (supplemental Fig. 3) treated with anti-IFN-γ also became susceptible to infections, whereas those treated with isotype control Ab remained resistant to infections. These results demonstrate that the resistance to L. major in DM-deficient mice is dependent on IFN-γ produced in response to infection.
FIGURE 7.
IFN-γ is essential for controlling disease in DM−/− mice. Groups of five BALB/c wild type and DM−/− mice each and three B10.D2 mice each were injected i.p with 500 µg of anti-IFN-γ Ab or with isotype control Ab weekly for 5 wk starting 1 day before FP infection with 0.5 × 106 L.major as described in Fig. 1. FP swelling was measured over the course of 55 days and is depicted as shown in Fig. 1. Closed symbols represent the sizes of the infected FP and open symbols represent sizes of the uninfected FP. All of the IFN-γ-treated DM−/−, wild-type BALB/c mice and two of three IFN-γ-treated B10.D2 mice and all of the isotype Ab-treated wild-type BALB/c mice had to be sacrificed between day 35 and 44 due to animal protocol stipulations. Due to strikingly similar results in all individual mice in each group, this particular experiment was done once.
Discussion
We identify DM as a host susceptibility gene for L. major infections in BALB/c mice since the presence of DM leads to susceptibility and its absence leads to host protection against this parasite. Paradoxically, the opposite is true in the case in C57BL/6 mice. As mentioned before, unlike Ad and Ed, Ab MHC encodes a very tight CLIP-MHC association, and in the absence of DM, all Ab molecules expressed by APC exclusively display CLIP (19, 20, 21). This attribute of Ab makes DM a host resistance gene in B6 mice since DM-deficient B6 mice, unable to make any pathogen-specific CD4 T cell response, become susceptible to L. major infections (22). These data highlight how molecular events in pathogen processing can profoundly alter the course of susceptibility to parasite infections.
BALB/c response to L. major infection is characterized by very high levels of IL-4 and IL-10 production (5), immunodominance of the LACK 158–173 epitope (9), and systemic spread of the parasites from the skin (the site of infection) to visceral sites such as spleen and liver (30). We show herein that ablation of the DM molecule in BALB/c mice also ablates presentation of the immunodominant LACK 158–173 epitope and in its place allows display of newer epitopes (one or more) not presented by DM-sufficient, wild-type infected APC (Fig. 5). Although the identity of the newer peptides displayed by DM deficient APC is unknown, our preliminary data (not shown) indicate that they may not be derived from LACK. The presentation of the newer epitopes, not displayed by the wild-type APC, is quantitatively sufficient to have activated CD4 T cells leading to isolation of 16 L. major-specific T cell hybridomas from infected DM−/− mice. We show that these events, including an ablation of presentation of LACK158–173 and activation of a newer set of T cells specific for epitopes excluded from presentation in wild-type mice, have profound immunological consequences in that the DM−/− mice are now resistant to L. major infection and are capable of controlling systemic spread of parasites, and the immune response consists of protective levels of IFN-γ and IL-17 and an absence of IL-4 or IL-10.
How does Ag processing in the absence of DM lead to a divergence in effector cytokine response in BALB/c mice in response to L. major infections? Effector cytokines are secreted by L. major specific host CD4 T cells. As mentioned above, most CD4 T cells isolated from wild-type BALB/c mice recognize LACK 158–173. In contrast, CD4 T cell response in L. major-infected DM−/− mice is represented by 1) an absence of LACK 158–173-specific CD4 T cells, and 2) the specificity of eight L. major-specific DM−/−-derived CD4 T cell hybridomas for epitopes exclusively displayed by DM−/− APC and precluded from epitopes presented by infected wild-type APC (Fig. 5). We propose that a switch to a protective response (secretion of both IFN-γ and IL-17) in DM−/− mice is a consequence of the absent LACK 158–173-specific T cell response coupled with elicitation of T cells specific for newer epitopes that were precluded from presentation in DM-sufficient APC. It is expected that T cells specific for different epitopes would be nonidentical in their ability to elicit different effector type response (e.g., TH1 or TH2 or TH17 type) based on their avidity interactions and strength of signaling induced in the target T cell (17, 18). Thus, it can be speculated that avidity interactions encoded within the newer epitopes presented in absence of DM lead to IFN-γ response and lack of IL-4/IL-10 response, while the opposite may be the case for LACK 158–173 response of a high IL-4/IL-10 production. However, note that in the case of the immunodominant LACK 158–173, elicitation of high IL-4/IL-10 response has also been postulated to be the result of a preexistent memory response thought to be due to a cross-reactive epitope expressed by a previously encountered common pathogen (36). Such a memory response might be absent from DM−/− mice as a consequence of the absent presentation of the putative LACK 158–173 cross-reactive epitope.
How does one view the epitopes displayed by both the wild-type and DM-deficient APC in the context of the well-established immunodominant response LACK 158–173 in the wild-type mice in response to L. major infections? We anticipate that the immunodominant presentation of LACK 158–173 might mask the presentation of other minor epitopes or/and that T cells specific for epitopes other than LACK 158–173 might have been deleted as a result of a differential negative selection in the wild-type BALB/c mice. Although both possibilities could occur, evidence for one of these possibilities was reported in our earlier work in which it was shown that LACK 33–48-specific T cells could not be elicited in the wild-type BALB/c mice despite immunization with the peptide 33–48, which requires no Ag processing (10). In contrast, the DM-deficient mice immunized with the same peptide (or LACK protein) were able to show activation of these T cells. From the perspective of a differential T cell repertoire potentially underlying a differential outcome of disease, it is pertinent to note the differences in TCR repertoires of the susceptible wild-type BALB/c (self-superantigens Mls-2a, Mls-3a) and the normally resistant B10.D2 (self-superantigens Mls-2b, Mls-3b) mice due to expression of different self-superantigens. We found that although TCR Vβ5, Vβ11, and Vβ12 are completely deleted in BALB/c mice, the same TCR gene segments are only partly deleted in B10.D2 mice (data not shown). Furthermore, unlike their BALB/c counterparts, the B10.D2 mice have an intact repertoire of TCR Vβ3 gene segments (37). It can thus be speculated that the different TCR repertoire available in B10.D2 mice could in part play a role in the resistance of these mice to L. major infections as a consequence of activation of T cells that recognize other epitopes (besides LACK 158–173) displayed by (DM-sufficient) B10.D2 APC and that produce protective cytokines.
Due to the deleterious nature of the immunodominant response in the wild-type BALB/c mice thought to underlie the strong IL-4/IL-10 and varying levels of IFN-γ (2, 6, 36, 38) seen in response to L. major infections, a number of studies have attempted to obliterate the typical LACK 158–173-specific T cell response of infected wild-type BALB/c mice by either inducing tolerance to this epitope or by engineering the parasite to deliver an altered version of LACK 158–173 peptide ligand to prevent activation of LACK 158–173-specific T cells (9, 36, 38). The results of these studies have been ambiguous in terms of achieving resistance to L. major. Our results suggest that the ambiguity of these studies might be due to a lack of elicitation of the protective T cells in the wild-type mice since DM-sufficient APC predominantly express the immunodominant Leishmania epitopes, and presentation of other epitopes is either reduced or precluded. Similarly, an obliteration of the nonprotective LACK 158–173 response in some of these cases could have been incomplete while in others it might have allowed some elicitation of a protective response. Note that BALB/c Ii-deficient hosts display scarce levels of LACK 158–173 on APC and yet produce detrimental levels of IL-4/IL-10 and are susceptible (Fig. 1–Fig. 6). The present study shows that a requirement for protection of BALB/c hosts is dependent on T cells specific for the nonimmunodominant epitopes to produce the modest amount of IFN-γ required for protection. This is further affirmed by our preliminary data (not shown) showing production of signature cytokines IFN-γ and IL-17 in short-term (4–6 wk) cell lines derived from infected DM−/− mice in response to L. major-infected DC. The role of IL-17 in protection is less clear. However, a high ratio of IFN-γ to IL-4 and IL-17 to IL-4 production symbolizes protective responses in DM-deficient mice (Fig. 6B). Interestingly, a robust innate immune response activation is seen in L. major-infected DM−/− mice as indicated by significantly higher production of IL-12p40 (supplemental Fig. 5). How ablation of DM leads to greater activation of innate responses is unclear.
A recent study in MHC class I pathway showed ERAAP (the endoplasmic reticulum aminopeptidase associated with Ag processing) to be associated with resistance to Toxoplasma gondii (39). Unlike our study, protection against the parasite was dependent on expression of an immunodominant response to the parasite. Taken together, these studies highlight that events in Ag processing pathway are consequential for host protection against pathogens. We propose that strategies to down-regulate and/or antagonize DM function will be successful in protecting wild-type BALB/c hosts against L. major. Development of agents that act as antagonists for HLA-DM function could also be potentially used to treat susceptibility to human infectious disease when the MHC class II alleles dissociate from CLIP with relative ease.
Supplementary Material
Acknowledgments
We thank Drs. Ronald Schwartz and Polly Matzinger (National Institutes of Health) for the generous and crucial support that made this work possible. We are grateful to Dr. Elizabeth K. Bikoff for supplying us with breeder pairs for all of the BALB/c mutant mice, and May Awkal, Andrew Heitman, Abhi Bhirud, Love Wade, and Dave Mallon for their excellent technical assistance. We extend our special thanks to Dr. David Usharauli for his suggestions. We are thankful to Drs. Ronald Schwartz, Polly Matzinger, David Usharauli, David Ucker, David Sacks, Elizabeth K. Bikoff, and Nevil Singh for a critical reading of this manuscript.
Footnotes
Supported in part by Department of Defense (award W81XWH-04-0013; to N.K.N.) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this paper: LACK, Leishmania homolog of activated receptor for c-kinase; BMDC, bone marrow-derived dendritic cell; CLIP, class II invariant chain peptide; DC, dendritic cell; FP, foot pad; Ii, invariant chain; LN, lymph node; 7AAD, 7-aminoactinomycin D; SLA, soluble Leishmania Ag.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chakkalath HR, Theodos CM, Markowitz JS, Grusby MJ, Glimcher LH, Titus RG. Class II major histocompatibility complex-deficient mice initially control an infection with Leishmania major but succumb to the disease. J. Infect. Dis. 1995;171:1302–1308. doi: 10.1093/infdis/171.5.1302. [DOI] [PubMed] [Google Scholar]
- 2.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 3.Scott P, Artis D, Uzonna J, Zaph C. The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol. Rev. 2004;201:318–338. doi: 10.1111/j.0105-2896.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 4.Muller I, Pedrazzini T, Farrell JP, Louis J. T-cell responses and immunity to experimental infection with leishmania major. Annu. Rev. Immunol. 1989;7:561–578. doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- 5.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowell DJ, Locksley RM. Leishmania major infection of inbred mice: unmasking genetic determinants of infectious diseases. Bioessays. 1999;21:510–518. doi: 10.1002/(SICI)1521-1878(199906)21:6<510::AID-BIES7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Lauvau G, Glaichenhaus N. Mini-review: presentation of pathogenderived antigens in vivo. Eur. J. Immunol. 2004;34:913–920. doi: 10.1002/eji.200424944. [DOI] [PubMed] [Google Scholar]
- 8.Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang ZE, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 9.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 10.Nanda NK, Bikoff EK. DM peptide-editing function leads to immunodominance in CD4 T cell responses in vivo. J. Immunol. 2005;175:6473–6480. doi: 10.4049/jimmunol.175.10.6473. [DOI] [PubMed] [Google Scholar]
- 11.Nanda NK, Sant AJ. DM determines the cryptic and immunodominant fate of T cell epitopes. J. Exp. Med. 2000;192:781–788. doi: 10.1084/jem.192.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Ham SM, Gruneberg U, Malcherek G, Broker I, Melms A, Trowsdale J. Human histocompatibility leukocyte antigen (HLA)-DM edits peptides presented by HLA-DR according to their ligand binding motifs. J. Exp. Med. 1996;184:2019–2024. doi: 10.1084/jem.184.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ. Editing of the HLA-DR-peptide repertoire by HLADM. EMBO J. 1996;15:6144–6154. [PMC free article] [PubMed] [Google Scholar]
- 14.Weber DA, Evavold BD, Jensen PE. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618–620. doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]
- 15.Lich JD, Jayne JA, Zhou D, Elliott JF, Blum JS. Editing of an immunodominant epitope of glutamate decarboxylase by HLA-DM. J. Immunol. 2003;171:853–859. doi: 10.4049/jimmunol.171.2.853. [DOI] [PubMed] [Google Scholar]
- 16.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 17.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 18.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 19.Bikoff EK, Wutz G, Kenty GA, Koonce CH, Robertson EJ. Relaxed DM requirements during class II peptide loading and CD4+ T cell maturation in BALB/c mice. J. Immunol. 2001;166:5087–5098. doi: 10.4049/jimmunol.166.8.5087. [DOI] [PubMed] [Google Scholar]
- 20.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 21.Koonce CH, Wutz G, Robertson EJ, Vogt AB, Kropshofer H, Bikoff EK. DM loss in k haplotype mice reveals isotype-specific chaperone requirements. J. Immunol. 2003;170:3751–3761. doi: 10.4049/jimmunol.170.7.3751. [DOI] [PubMed] [Google Scholar]
- 22.Swier K, Brown DR, Bird JJ, Martin WD, Van Kaer L, Reiner SL. A critical, invariant chain-independent role for H2-M in antigen presentation. J. Immunol. 1998;160:540–544. [PubMed] [Google Scholar]
- 23.Bikoff EK, Germain RN, Robertson EJ. Allelic differences affecting invariant chain dependency of MHC class II subunit assembly. Immunity. 1995;2:301–310. doi: 10.1016/1074-7613(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 24.Kenty G, Bikoff EK. BALB/c invariant chain mutant mice display relatively efficient maturation of CD4+ T cells in the periphery and secondary proliferative responses elicited upon peptide challenge. J. Immunol. 1999;163:232–241. [PubMed] [Google Scholar]
- 25.Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- 26.Dozmorov I, Eisenbraun MD, Lefkovits I. Limiting dilution analysis: from frequencies to cellular interactions. Immunol. Today. 2000;21:15–18. doi: 10.1016/s0167-5699(99)01561-3. [DOI] [PubMed] [Google Scholar]
- 27.Nanda NK, Apple R, Sercarz E. Limitations in plasticity of the T-cell receptor repertoire. Proc. Natl. Acad. Sci. USA. 1991;88:9503–9507. doi: 10.1073/pnas.88.21.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 29.Brown DR, Swier K, Moskowitz NH, Naujokas MF, Locksley RM, Reiner SL. T helper subset differentiation in the absence of invariant chain. J. Exp. Med. 1997;185:31–41. doi: 10.1084/jem.185.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill JO. Pathophysiology of experimental leishmaniasis: pattern of development of metastatic disease in the susceptible host. Infect. Immun. 1986;52:364–369. doi: 10.1128/iai.52.2.364-369.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiner SL, Wang ZE, Hatam F, Scott P, Locksley RM. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 32.Morris L, Troutt AB, McLeod KS, Kelso A, Handman E, Aebischer T. Interleukin-4 but not gamma interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect. Immun. 1993;61:3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J. Immunol. 2004;172:3793–3797. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 34.Padigel UM, Farrell JP. Control of infection with Leishmania major in susceptible BALB/c mice lacking the common γ-chain for FcR is associated with reduced production of IL-10 and TGF-β by parasitized cells. J. Immunol. 2005;174:6340–6345. doi: 10.4049/jimmunol.174.10.6340. [DOI] [PubMed] [Google Scholar]
- 35.Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J. Dermatol. Sci. 2007;46:159–167. doi: 10.1016/j.jdermsci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Julia V, McSorley SS, Malherbe L, Breittmayer JP, Girard-Pipau F, Beck A, Glaichenhaus N. Priming by microbial antigens from the intestinal flora determines the ability of CD4+ T cells to rapidly secrete IL-4 in BALB/c mice infected with Leishmania major. J. Immunol. 2000;165:5637–5645. doi: 10.4049/jimmunol.165.10.5637. [DOI] [PubMed] [Google Scholar]
- 37.Schirrmacher V, Beutner U, Bucur M, Umansky V, Rocha M, von Hoegen P. Loss of endogenous mouse mammary tumor virus superantigen increases tumor resistance. J. Immunol. 1998;161:563–570. [PubMed] [Google Scholar]
- 38.Kelly BL, Locksley RM. The Leishmania major LACK antigen with an immunodominant epitope at amino acids 156 to 173 is not required for early Th2 development in BALB/c mice. Infect. Immun. 2004;72:6924–6931. doi: 10.1128/IAI.72.12.6924-6931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 2008;9:937–944. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.