Abstract
Objectives
To assess differences in cardiovascular risk profiles among rural-to-urban migrants and non-migrant groups.
Design
Cross-sectional study.
Setting
Ayacucho and Lima, Peru
Participants
rural (n=201); rural-urban migrants (n=589) and urban (n=199).
Main outcome measures
Cardiovascular risk factors were assessed according to migrant status (migrants vs. non-migrants), age at first migration, length of residency in an urban area and lifetime exposure to an urban area.
Results
For most risk factors, the migrant group had intermediate levels of risk between those observed for the rural and urban groups. Prevalences, for rural, migrant and urban groups, was 3%, 20% and 33% for obesity and 0.8%, 3% and 6% for type-2 diabetes. This gradient of risk was not observed uniformly across all risk factors. Blood pressure did not show a clear gradient of difference between groups. The migrant group had similar systolic blood pressure (SBP) but lower diastolic blood pressure (DBP) than the rural group. The urban group had higher SBP but similar DBP than rural group. Hypertension was more prevalent among the urban (29%) compared to both rural and migrant groups (11% and 16% respectively). For HbA1c, although the urban group had higher levels, the migrant and rural groups were similar to each other. No differences were observed in triglycerides between the three groups. Within migrants, those who migrated when aged older than 12 years had higher odds of diabetes, impaired fasting glucose and metabolic syndrome compared to people who migrated at younger ages. Adjustment for age, sex and socioeconomic indicators had little impact on the patterns observed.
Conclusions
The impact of rural to urban migration on cardiovascular risk profile is not uniform across different risk factors, and is further influenced by the age at which migration occurs. A gradient in levels was observed for some risk factors across study groups. This observation indicates that urbanization is indeed detrimental to cardiovascular health.
Introduction
Chronic non-communicable diseases dominate burden of disease statistics1 and are of growing concern in low and middle-income countries2 3. A recent consensus statement highlighted the need for data on the impact of urbanization on chronic diseases4. Urban areas of developing countries are growing much faster than rural areas5-7, partly through migration from rural to urban areas. Thus, it is imperative to more fully understand the impact of urban migration on health.
Previous research on the health effects of migration has largely focused on movement between countries8. Our knowledge and understanding of the health effects of migration from rural to urban areas within countries is less extensive. Because migration is often driven by economic and other factors that are likely to be related to health, migrants are often not representative of the rural area they come from making valid comparisons between migrants and non-migrants difficult. During the period 1970 through to the 1990’s mass migration from rural to urban areas occurred in Peru, triggered by political violence targeting rural dwellers9 10. This meant the usual selection effects that lead to migrants being an atypical group were reduced for two reasons. Firstly, in affected areas, large proportions of the population migrated. Secondly, the key factor leading to migration was to escape from violence rather than economic forces. Particularly given the marked contrast between urban and rural lifestyles, Peru therefore offers a unique opportunity to assess the health effects of migration to urban areas. This study aimed to assess differences in cardiovascular risk profiles among migrants and non-migrant groups.
Methods
Study design
Cross-sectional survey conducted in 2007-2008 of three population-based groups: rural, people born in Ayacucho who had always lived in a rural environment; rural-to-urban migrants, people born in Ayacucho who migrated from rural to urban areas and currently living in Lima; and, urban, people born and currently living in Lima. Details of the study design have been reported elsewhere11.
Setting
The village of San Jose de Secce (Santillana district, Huanta province) in Ayacucho was selected as the rural study site. This area located in the Andes was one of the most severely affected areas during this period of violence and was thus an area where a large proportion of the population migrated to urban areas9 12. According to Peru’s 2007 census, this district had a population of 7,215 people, 91% of them live in a rural area, 97% learnt a native language during childhood, and only 3.4% of its population were classified as migrants defined by place of birth13.
The area called “Las Pampas de San Juan de Miraflores” in Lima, a periurban shantytown in the south of Lima, was selected as the urban area for the study. This area was chosen because it is a typical shantytown where migrants from rural areas have settled over the years and migrants from the Southern part of Peru, including Ayacucho, are more likely to settle in San Juan de Miraflores. Both urban and rural-to-urban migrant participants were selected from this area. In 2007, the total population of the district of San Juan de Miraflores were 362,643 people, 100% urban, 11% learnt a native language during childhood and, based on place of birth, 50% were classified as migrants13.
The distance between these two settings chosen for the study, rural and urban, is about 310Km. However, due to the mountainous-type of geography due the Andes, the travelling distance to connect these two sites is 12 to 15 hours by land transportation, varying due to rainy season.
Participants
A single-stage random sampling method was used in all groups. In the case of San Jose de Secce in Ayacucho, a census was conducted in mid 2007. The sampling frame for the urban group was derived from the local census, conducted in year 2000, which was updated in 2006 to identify all those who referred to have been born in the department of Ayacucho and were currently living in Lima. From these updated censuses, the sampling frame of adults ≥30years-old was 398, 1,785, and 4,621 for the rural, rural-to-urban migrants and urban groups, respectively11.
For all study groups, individuals from both sexes aged 30 years-old and over, permanently living in their residence were considered eligible. Pregnant women, because of their transient physiological state, and those with mental disorders judged likely to impair survey completion were excluded. Language was not considered an exclusion criterion to take part in the study and some of our fieldwork personnel in Lima and all of them in Ayacucho were fluent in Quechua. Participants’ selection was stratified by 5-year age-groups and sex. The study target was to recruit a total of 1000 people, 200 people in each of the rural and urban groups and 600 migrants.
Study variables
The primary exposure was migration from a rural to an urban environment, defined by study group, i.e. rural, rural-to-urban migrant and urban groups. The migrant group was subsequently divided to explore if the pattern of cardiovascular risk factors in the migrant population vary by age at first migration (aged ≤ 12 years old when first migrated vs. >12yo), length of residency in an urban area (<20, 20-29 30-39 or ≥40 years in urban area) or lifetime exposure to an urban area (number of years lived in an urban area divided over current age, in quartiles).
CVD risk factors explored included systolic (SBP) and diastolic (DBP) blood pressure (mean of last two of three measures), hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg, or self report of physician diagnosis and currently receiving antihypertensive medication14 15), body mass index (BMI), obesity (BMI ≥ 30 Kg/m2), overweight or obesity (BMI ≥ 25 Kg/m2), skinfolds and waist-to-hip ratio, fasting glucose, diabetes (fasting glucose ≥ 126 mg/dL [≥ 7 mmol/L]16 or self report of physician diagnosis and currently receiving antidiabetic medication), impaired fasting glycaemia (IFG) or diabetes (fasting glucose ≥ 110 mg/dL [≥ 6.1 mmol/L]), lipoprotein profile, hypercholesterolemia (total cholesterol ≥ 200 mg/dL [≥ 5.2 mmol/L]), inflammatory markers (C-reactive protein [CRP], fibrinogen), insulin resistance and metabolic syndrome. Current smoker was defined as having smoked within the last six months and a lifetime total of more than 100 cigarettes.
Socioeconomic factors —educational level, household income, number of people per room and asset possession17— were assessed and the number of adverse factors combined into a deprivation index, as a marker of adulthood socioeconomic position18. Paternal and maternal education levels were combined into highest parental education as a proxy for childhood socioeconomic position. These markers were considered a priori to be potential confounder variables.
Skinfolds were measured at four sites (biceps, triceps, subscapular and suprailiac) to the nearest 0.2 mm using a Holtain Tanner/Whitehouse Skinfold Caliper. For skinfold, waist and hip circumference, three measurements were taken and the average used.
Fasting glucose, fasting insulin and glycosylated haemoglobin (HbA1c) were measured in plasma, serum and whole blood, respectively. All blood samples were analyzed in a single facility. For quality assurance, the quality of assays was checked with regular external standards and internal duplicate assays and monitored by BioRad (www.biorad.com). Insulin resistance was calculated using the HOMA calculator19, excluding those with diabetes. Metabolic syndrome was defined according to the recent 2009 unified definition adopted by several major organizations20.
In accordance with the International Physical Activity Questionnaire (IPAQ) protocol, the categorical physical activity levels (low, moderate, high) were coded based on total days of physical activity and metabolic equivalents (MET) minutes/week21. Moderate physical activity was coded as 5 or more days of any combination of walking, moderate-intensity or vigorous-intensity activities achieving at least 600 MET minutes per week. High physical activity was coded as 7 or more days of any combination of walking, moderate-intensity or vigorous-intensity activities achieving a minimum total physical activity of at least 3000 MET minutes/week. Sedentary physical activity was defined as less than 150 MET-min in one week21.
Statistical methods
Continuous non-normally distributed variables were log-transformed leading to normal or near normal distributions and age- and sex-adjusted arithmetic means (± standard deviations [SD]) or geometric means (ratios)22 23 were calculated. Direct standardization to the World Health Organization (WHO) standard population24 was used to calculate age-standardized prevalences by specific age groups (five yearly age groups: 30-34, 35-39, 40-44, 45-49, 50-54. 55-59, ≥60 years-old).
Multivariable logistic regression and linear regression were used for categorical and continuous outcomes respectively. Three core models were as follows: Model 1 was adjusted for age, sex, individual socioeconomic deprivation and highest parental education. Model 2 added BMI and Model 3 added physical activity. For the analysis of coefficients, adjustment for treatment effects, e.g. antihypertensive therapy on blood pressure outcomes, was undertaken using censored normal regression25. For continuous outcomes, to enable comparisons between risk factors, standardized mean differences (SMD) were calculated using linear regression using the standard deviation (SD) for each group. Interpretation of SMDs followed the convention of 0.2 SMD representing a small effect, 0.5 a moderate effect, and 0.8 a large effect26 27.
Results
Response rate at enrolment was 73.2% (1176/1606) and overall response rate at completion of the study was 61.6% (989/1606)11. The final sample size of 989 comprised 52.8% females. A higher proportion of refusals was observed among males in the urban group and among older people (>60 years old) in all study groups. In the urban group, more urban non-responders had completed secondary level education (70.3% compared to 56.6% in urban responders). No differences in self reported diagnosis of diabetes or hypertension were seen between response groups. In relation to migration indicators, non-responders migrant’s median age at first migration was similar compared to responders. Both, individual socioeconomic reasons —studies or working reasons— and terrorism were listed amongst the two main reasons for migration in both responders and non-responders11.
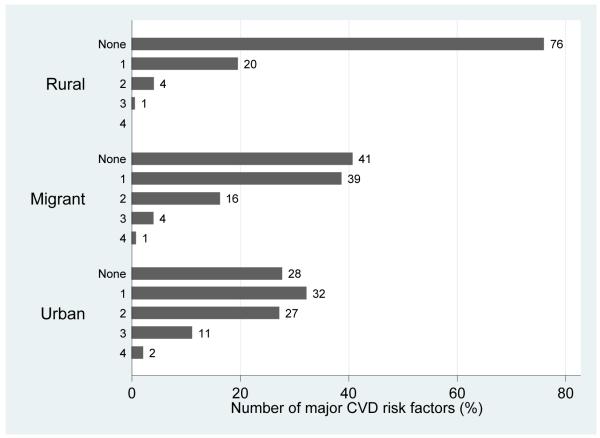
The distribution of sociodemographic indicators, profile of migrants and distribution of cardiovascular risk factors by migration status are shown in Table 1, Table 2 and Table 3, respectively. The rural group was the most socioeconomically disadvantaged, followed by the migrants. As shown in Figure 1, the majority of the rural population had no risk factors and the majority of migrants had none or at least one risk factor. A gradient of doubling prevalence were observed for current smoking status but this was not necessarily accompanied by larger cigarette consumption (Table 1).
Table 1.
Demographic and socioeconomic variables by migration status.
| Rural | Migrant | Urban | Missing data | |
|---|---|---|---|---|
| n = 201 | n = 589 | n = 199 | n/989 | |
| Demographic variables | ||||
| Age, mean (SD) | 48.3 (13.1) | 47.8 (11.7) | 48.1 (11.9) | 0 |
| Female, n(%) | 106 (52.7%) | 309 (52.5%) | 107 (53.8%) | 0 |
| Individual’s education level, n(%) | 2 (0.2%) | |||
| None | 68 (33.8 %) | 59 (10 %) | 2 (1 %) | |
| Primary incomplete | 64 (31.8 %) | 124 (21.1 %) | 11 (5.6 %) | |
| Primary complete | 30 (14.9 %) | 99 (16.8 %) | 23 (11.6 %) | |
| Secondary incomplete | 16 (8 %) | 126 (21.4 %) | 50 (25.3 %) | |
| Secondary complete or more | 23 (11.4 %) | 180 (30.6 %) | 112 (56.6 %) | |
| Household income, n(%) | 83 (8.4%) | |||
| ≤$50 US dollars | 109 (69 %) | 8 (1.4 %) | 2 (1 %) | |
| $51-150 US dollars | 32 (20.3 %) | 143 (25.8 %) | 36 (18.7 %) | |
| $151-250 US dollars | 10 (6.3 %) | 292 (52.6 %) | 104 (53.9 %) | |
| $251-350 US dollars | 4 (2.5 %) | 82 (14.8 %) | 40 (20.7 %) | |
| $351-450 US dollars | 2 (1.3 %) | 26 (4.7 %) | 8 (4.2 %) | |
| ≥$450 US dollars | 1 (0.6 %) | 4 (0.7 %) | 3 (1.6 %) | |
| Number of people per room, n(%) | 6 (0.6%) | |||
| <2 people per room | 34 (17 %) | 217 (37.1 %) | 68 (34.3 %) | |
| 2-3 people per room | 72 (36 %) | 240 (41 %) | 75 (37.9 %) | |
| 3-4 people per room | 43 (21.5 %) | 78 (13.3 %) | 37 (18.7 %) | |
| 4 or more people per room | 51 (25.5 %) | 50 (8.6 %) | 18 (9.1 %) | |
| Possessions weighted asset index, n(%) | 0 | |||
| Lowest tertile | 196 (97.5%) | 110 (18.7%) | 24 (12.1%) | |
| Middle | 5 (2.5%) | 259 (44%) | 72 (36.2%) | |
| Highest tertile | 0 | 220 (37.4%) | 103 (51.8%) | |
| Number of deprivations per individual, n(%)* | 0 | |||
| None | 3 (1.5%) | 265 (45%) | 131 (65.8%) | |
| One deprivation | 18 (9%) | 217 (36.8%) | 55 (27.6%) | |
| Two deprivations** | 89 (44.23%) | 85 (14.4%) | 11 (5.5%) | |
| Three deprivations** | 91 (45.3%) | 22 (3.7%) | 2 (1%) | |
| Highest parental education level, n(%) | 0 | |||
| None | 122 (60.7%) | 298 (50.6%) | 30 (15.1%) | |
| Some primary | 52 (25.9%) | 147 (25%) | 26 (13.1%) | |
| Primary complete or more | 27 (13.4%) | 144 (24.5%) | 143 (71.9%) | |
| Cigarettes consumption in the last 30 days*** | ||||
| Individuals, n (%) | 6 (3%) | 37 (6.3%) | 32 (16.1%) | |
| Cigarettes, median (IQR range) | 10 (1 – 20) | 5 (3 – 20) | 5.5 (1 – 26.5) | |
| Current smokers**** Individuals, n (%) | 11 (5.5 %) | 59 (10 %) | 40 (20.1 %) |
Notes: Number of deprivations based on the sum of deprivations in education (none or incomplete primary education), income (household income < US $150 dollars per month) and assets (lowest tertile of possessions weighted asset index) in the same individual;
Those with two or more deprivations were considered socioeconomically deprived;
All individuals that smoked in the last 30 days qualified as current smokers;
The difference between current smokers and those who reported cigarette consumption in the last 30 days is accounted for those who reported having smoked in the last 6 months but not in the last 30 days.
Table 2.
Distribution of migrants by patterns of migration.
| Group | n (%) | Mean (SD)* | Median (IQR)* |
Range (min – max) |
|---|---|---|---|---|
| By age at first migration** | 585 (100%) | 14.7 (9) | 14 (7) | |
| ≤ 12 years old when first migrated | 225 (38.4%) | 8.2 (3.2) | 8 (5) | |
| > 12 years old when first migrated | 360 (61.6%) | 18.7 (9) | 16 (4) | |
|
By length of residence in urban area
(in years)*** |
559 (100%) | 32 (10.5) | 31 (14) | |
| Migrant <20 years in urban area**** | 53 (9.5%) | 15 (4.1) | 16 (4) | |
| Migrant 20-29 years in urban area | 203 (36.3%) | 25.1 (2.6) | 25 (4) | |
| Migrant 30-39 years in urban area | 169 (30.2%) | 34.4 (2.9) | 34 (5) | |
| Migrant ≥40 years in urban area | 134 (24%) | 46.1 (6.3) | 44 (6) | |
|
By lifetime exposure to urban
area***** |
559 (100%) | 67.7 (15.1) | 69.4 (18.3) | 0 – 100 |
| Quartile 1, lowest | 141 (25.2%) | 47.8 (11.6) | 51.8 (14.6) | 0 – 59.5 |
| Quartile 2 | 139 (24.9%) | 64.8 (2.9) | 65 (4.9) | 59.5 – 69.4 |
| Quartile 3 | 142 (25.4%) | 73.7 (2.4) | 73.5 (4) | 69.6 – 77.8 |
| Quartile 4, highest | 137 (24.5%) | 85 (5.7) | 83.6 (7.7) | 78 – 100 |
Summary statistics, mean (SD) and median (IQR), are provided for this variable in relation to each group. All units for descriptive statistics correspond to absolute number of years or percentages of total age in the case of lifetime exposure to urban area.
Sub-classification based on 585/589 (99.3%) observations in the migrant group.
Sub-classification based on 559/589 (95%) observations in the migrant group.
In order to ensure sufficient numbers in each strata, the first two groups, “<10 years in urban area” (n = 6) and “10-19 years in urban area” (n = 47), were merged into a single stratum “<20 years in urban area”.
Quartiles were created based on proportion (percentage) of lifetime exposure to urban environment, defined as number of years lived in an urban area divided over age. Sub-classification based on 559/589 (95%) observations in the migrant group.
Table 3.
Distribution* of cardiovascular risk factors by migration status.
| Rural | Migrant | Urban | Missing data | |
|---|---|---|---|---|
| n = 201 | n = 589 | n = 199 | ||
| Anthropometric and adiposity markers | ||||
| Weight (Kg), mean (SD) | 53.9 (8.2) | 64.1 (10.6) | 69.4 (14.5) | 2 (0.2%) |
| Height (m), mean (SD) | 1.5 (0.1) | 1.5 (0.1) | 1.6 (0.1) | 2 (0.2%) |
| BMI (Kg/m2), mean (SD) | 23.2 (2.7) | 27 (4.3) | 28.3 (5.4) | 2 (0.2%) |
| Waist circumference (cm), mean (SD) | 76.1 (8.4) | 88.1 (9.9) | 91.4 (12.1) | 6 (0.6%) |
| Hip circumference (cm), mean (SD) | 87.4 (5.3) | 94.4 (7.5) | 98.9 (10.6) | 8 (0.8%) |
| Waist-to-hip ratio, mean (SD) | 0.87 (0.07) | 0.93 (0.07) | 0.92 (0.07) | 8 (0.8%) |
| Skinfolds, all (mm), mean (SD) | 42 (20.1) | 81.4 (30.6) | 96 (35.1) | 13 (1.3%) |
| Biceps (mm), median (IQR) | 4.2 (3.1) | 8.5 (8.4) | 13.1 (13.6) | 9 (0.9%) |
| Triceps (mm), median (IQR) | 10.2 (8.3) | 21.1 (19.5) | 30 (21.4) | 9 (0.9%) |
| Subscapular (mm), median (IQR) | 11.3 (6.9) | 19.7 (11.2) | 23.3 (12) | 9 (0.9%) |
| Suprailiac (mm), median (IQR) | 9.7 (10.3) | 27.1 (11.3) | 29.1 (14.4) | 13 (1.3%) |
| Blood pressure and Lipids | ||||
| Systolic blood pressure (mmHg), mean (SD) | 120.9 (18.7) | 119.9 (16.4) | 128.2 (22.9) | 1 (0.1%) |
| Diastolic blood pressure (mmHg), mean (SD) | 74.2 (9.2) | 71.3 (9.3) | 76.2 (11.5) | 1 (0.1%) |
| Total cholesterol (mg/dL), mean (SD) | 155.7 (33.3) | 190.8 (39.5) | 194.9 (40) | 1 (0.1%) |
| Triglycerides (mg/dL), median (IQR) | 113 (71) | 133 (95.5) | 135 (109) | 1 (0.1%) |
| HDL (mg/dL), mean (SD) | 44.1 (13.1) | 44 (11.2) | 44.4 (11) | 1 (0.1%) |
| LDL (mg/dL), mean (SD) | 85.6 (27.1) | 115.9 (33) | 119.8 (34.2) | 1 (0.1%) |
| TC/HDL ratio, mean (SD) | 3.8 (1.2) | 4.6 (1.4) | 4.7 (1.6) | 1 (0.1%) |
| Metabolic-related markers | ||||
| Glucose (mg/dL), median (IQR) | 80 (12) | 86 (12) | 88 (12) | 1 (0.1%) |
| HbA1c (%), median (IQR) | 5.7 (0.5) | 5.5 (0.5) | 5.7 (0.6) | 0 |
| Insulin (µIU/mL), median (IQR) | 2.5 (3.9) | 6.7 (5.9) | 8.5 (7.8) | 11 (1.1%) |
| HOMA-IR, median (IQR) | 0.31 (0.46) | 0.86 (0.77) | 1.1 (1.13) | 12 (1.2%) |
| Inflammatory variables | ||||
| CRP (mg/L), median (IQR) | 0.7 (1.6) | 1.6 (2.7) | 1.6 (2.7) | 1 (0.1%) |
| Fibrinogen (mg/dL), median (IQR) | 351.4 (115.7) | 386.9 (96.5) | 383 (77.5) | 1 (0.1%) |
Values represent crude means (SD) or crude medians (IQR)
Figure 1.
Number of major cardiovascular risk factors by migration status.
Note: Major risk factors considered were smoking, hypertension, diabetes, obesity and hypercholesterolaemia (defined as total cholesterol ≥200 mg/dL or ≥5.2 mmol/L). The aggregations shown correspond to the sum of “Yes” of each individual risk factors and it ranges from zero, no risk factors, up to 4, presence of four risk factors concomitantly.
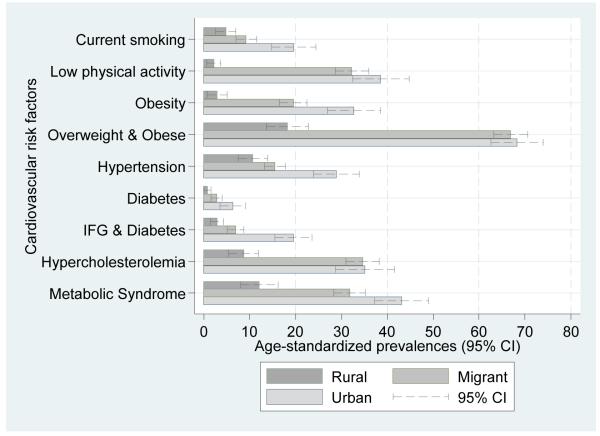
Obesity was markedly low in the rural group. Overweight and obesity prevalences were over 65% in both migrant and urban groups, as shown in Figure 2 (see also Supplementary Table 1). In multivariable regression, after adjustment for age, sex and socioeconomic factors, all anthropometric measurements were consistently higher in migrant and urban groups compared to the rural group (see Supplementary Table 1). In the case of the sum of all skinfolds, migrants and urban people were on average 34.8 mm (95% CI 29.5; 40.1) and 45.8 mm (95% CI 39.3; 52.3) units higher compared to the rural group (see Table 4). As shown in Figure 3, the magnitude of the differences in anthropometric risk factors were substantial, ranging between 0.5 to 2 SD units higher in both migrant and urban people when compared to the rural group. Further adjustments for BMI and physical activity only attenuated mildly most of the outcome estimates but two exceptions were noted. First, further adjustment decreased the effect sizes observed in insulin and HOMA-IR. Second, HDL changed from zero to a range of difference of 0.2 to 0.3 SD units (Figure 3 and Supplementary Table 2)
Figure 2.
Prevalence (age-standardized to WHO standard population) of cardiovascular disease risk factors by migration status.
Note: p for trend in all cases <0.01.
Table 4.
β coefficients (95% CI) of continuous normally distributed cardiovascular risk factors by migration status.
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Rural | Migrant | Urban | Migrant | Urban | Migrant | Urban | |
| β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
||
| BMI | Reference | 3.2 (2.4; 4.1) | 4.3 (3.2; 5.3) | -- | -- | 3.4 (2.4; 4.3) | 4.3 (3.2; 5.5) |
| Waist-to-hip ratio | Reference | 0.06 (0.04; 0.07) | 0.04 (0.03; 0.06) | 0.03 (0.02; 0.04) | 0.01 (0; 0.02) | 0.03 (0.02; 0.05) | 0.01 (0; 0.03) |
| All skinfolds | Reference | 34.8 (29.5; 40.1) | 45.8 (39.3; 52.4) | 20.8 (16.9; 24.7) | 27.7 (22.9; 32.5) | 19.6 (15.4; 23.8) | 26.1 (21; 31.2) |
| Systolic blood pressure* | Reference | 0.7 (−2.7; 4.2) | 9.1 (4.8; 13.3) | −2.1 (−5.6; 1.3) | 5.5 (1.2; 9.7) | −2.1 (−5.9; 1.7) | 5.5 (1; 10.1) |
| Diastolic blood pressure* | Reference | −3.4 (−5.4; −1.4) | 1.3 (−1.2; 3.8) | −5.9 (−7.8; −3.9) | −1.9 (−4.4; 0.5) | −5.8 (−8; −3.7) | −1.9 (−4.5; 0.7) |
| Total cholesterol | Reference | 34.9 (27; 42.8) | 36.2 (26.4; 46) | 29.4 (21.4; 37.4) | 28.8 (18.9; 38.7) | 26.2 (17.6; 34.9) | 25.5 (15; 36.1) |
| HDL | Reference | −0.5 (−2.9; 1.9) | −0.1 (−3.1; 2.9) | 2 (−0.4; 4.3) | 3.1 (0.1; 6.1) | 2 (−0.6; 4.6) | 3.2 (0.1; 6.4) |
| LDL | Reference | 31.1 (24.5; 37.8) | 32.9 (24.7; 41.2) | 26.9 (20.2; 33.6) | 27.3 (18.9; 35.6) | 24.1 (16.8; 31.4) | 24.4 (15.5; 33.3) |
| TC/HDL ratio | Reference | 0.9 (0.6; 1.2) | 0.9 (0.6; 1.3) | 0.5 (0.2; 0.8) | 0.4 (0.1; 0.8) | 0.4 (0.1; 0.7) | 0.3 (−0.1; 0.7) |
Notes: Model 1: adjusted for age, sex, individual’s socioeconomic deprivation and parental education; Model 2: as Model 1 plus BMI; Model 3: as Model 2 plus physical activity in 3 MET score categories (except only for BMI estimates where BMI was not included).
SBP and DBP estimates derived from censored regression analyses.
Figure 3.
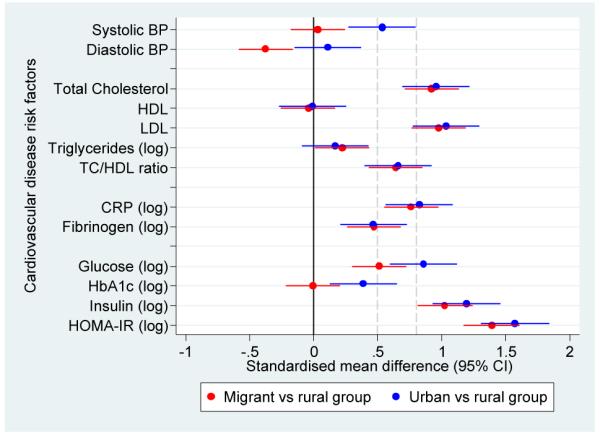
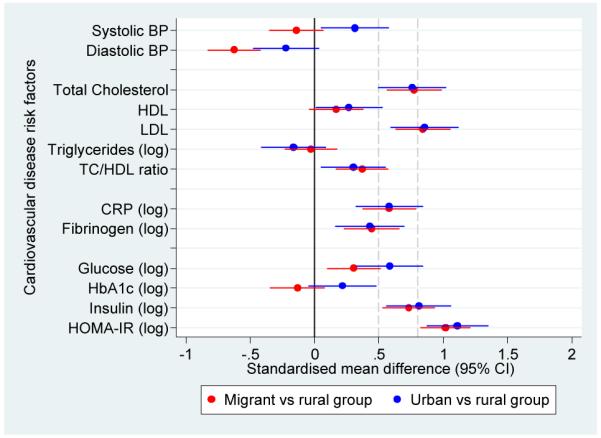
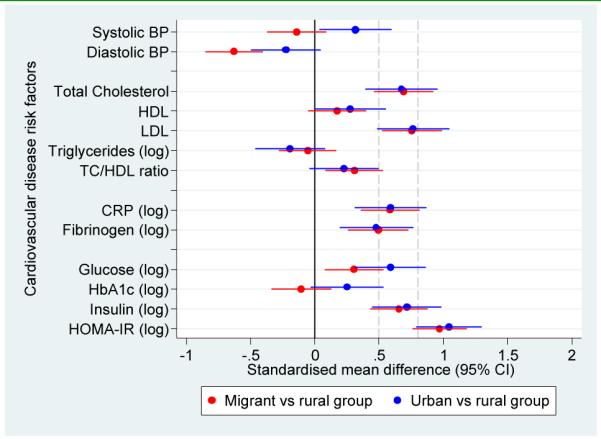
Adjusted standardised mean differences in cardiovascular disease risk factors in migrant and urban population compared to rural population.
a. Standardised mean differences adjusted for age, sex, socioeconomic position and parental education
b. Standardised mean differences adjusted for age, sex, socioeconomic position, parental education and body mass index
c. Standardised mean differences adjusted for age, sex, socioeconomic position, parental education, body mass index and levels of physical activity
Notes: The solid line at zero indicate no difference compared to rural group. Additional dashed lines at 0.5 and 0.8 correspond to thresholds for moderate and large differences, respectively.
For most risk factors migrant and urban groups were similar and had significantly higher levels than the rural group. One of the exceptions was blood pressure, which despite showing a gradient from rural to migrant to urban in terms of age-standardized prevalences (Figure 2), no difference was observed between rural and migrants in SBP (β coefficient 0.7 mmHg). However, migrants had a DBP on average −3.4 mmHg (95% CI −5.4 to −1.4) lower than the rural group. Compared to the rural group, the urban group had higher SBP but similar DBP and further adjustment for BMI and physical activity did not change this observation (Table 4 and Figure 3).
For lipid measures and inflammatory markers, migrants were generally similar to the urban group. However, differences between groups in triglycerides were small (Table 4 and Figure 3). After multivariable adjustment and compared to the rural group, both migrant and urban populations had significantly higher geometric means of CRP (Table 5). The CRP geometric means of migrant and urban groups were 196% and 224% greater than the geometric mean of the rural group, respectively. In the case of fibrinogen, the geometric mean of the migrant and urban groups was 11% greater than the geometric mean of rural group.
Table 5.
Geometric mean (95% CI) and ratios (95% CI) of non-normally distributed cardiovascular risk factors by migration status.
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Rural* | Migrant | Urban | Migrant | Urban | Migrant | Urban | |
| Geometric Mean (95% CI) |
Ratio (95% CI) | Ratio (95% CI) | Ratio (95% CI) | Ratio (95% CI) | Ratio (95% CI) | Ratio (95% CI) | ||
| Triglycerides (mg/dL) | 988 | 116.2 (102.2; 132.2) | 1.12 (1.01; 1.25) | 1.09 (0.95; 1.25) | 0.99 (0.89; 1.09) | 0.92 (0.81; 1.05) | 0.97 (0.87; 1.09) | 0.9 (0.79; 1.04) |
| Glucose (mg/dL) | 988 | 80 (76.6; 83.6) | 1.09 (1.05; 1.13) | 1.16 (1.11; 1.22) | 1.05 (1.02; 1.09) | 1.11 (1.06; 1.16) | 1.05 (1.01; 1.1) | 1.11 (1.06; 1.16) |
| HbA1c (%) | 989 | 5.7 (5.5; 5.9) | 1 (0.97; 1.03) | 1.05 (1.02; 1.08) | 0.98 (0.96; 1.01) | 1.03 (0.99; 1.06) | 0.99 (0.96; 1.02) | 1.03 (1; 1.07) |
| Insulin (µIU/mL) | 978 | 2.6 (2; 3.3) | 2.93 (2.35; 3.66) | 3.51 (2.67; 4.62) | 2.16 (1.75; 2.67) | 2.34 (1.8; 3.04) | 1.99 (1.58; 2.5) | 2.12 (1.6; 2.8) |
| HOMA-IR | 953 | 0.3 (0.3; 0.4) | 2.96 (2.5; 3.49) | 3.41 (2.77; 4.19) | 2.21 (1.91; 2.57) | 2.38 (1.98; 2.86) | 2.13 (1.81; 2.51) | 2.26 (1.85; 2.75) |
| CRP (mg/L) | 988 | 0.6 (0.4; 0.8) | 2.96 (2.2; 3.97) | 3.23 (2.24; 4.67) | 2.28 (1.7; 3.07) | 2.29 (1.58; 3.3) | 2.3 (1.67; 3.18) | 2.31 (1.56; 3.42) |
| Fibrinogen (mg/dL) | 988 | 349.9 (331.7; 369.1) | 1.11 (1.06; 1.16) | 1.11 (1.05; 1.17) | 1.10 (1.05; 1.15) | 1.10 (1.04; 1.16) | 1.11 (1.06; 1.17) | 1.11 (1.04; 1.18) |
Notes: Model 1: adjusted for age, sex, individual’s socioeconomic deprivation and parental education; Model 2: as Model 1 plus BMI; Model 3: as Model 2 plus physical activity in 3 MET score categories.
Rural geometric mean values were derived from Model 1 adjusting for age, sex, individual socioeconomic deprivation and highest parental education.
In terms of fasting glucose and compared to the rural group, both migrant and urban groups had higher geometric mean ratios of 9% and 16% higher, respectively. In the case of HbA1c there was no difference between migrant and rural populations, and the urban group had a slightly higher average value (Table 5). Compared to the rural group, fasting insulin and insulin resistance were markedly higher in migrant (in the range of 200% greater) and urban group (around 250% greater) (Table 5), which were also reflected as substantial effect sizes between 1 and 1.6 SD units with some attenuation following adjustment for BMI and physical activity (Figure 3 and Supplementary Table 2).
All cases of diabetes were type-2 as none of the participants reported type-1 diabetes mellitus. A gradient was observed in age-standardized prevalence of diabetes (0.8% to 2.8% to 6.3%) and IFG and diabetes (2.9% to 6.9% to 19.5%). Metabolic syndrome was consistently higher in migrants and in urban groups (Figure 2 and Supplementary Table 1). OR for diabetes, IFG and diabetes and metabolic syndrome were substantially high but their confidence intervals were wide. Further adjustment for BMI and physical activity showed different directions on these estimates in migrant and urban groups: it gradually increased the odds of diabetes but attenuated the odds of metabolic syndrome (Figure 4 and Supplementary Table 3).
Figure 4.
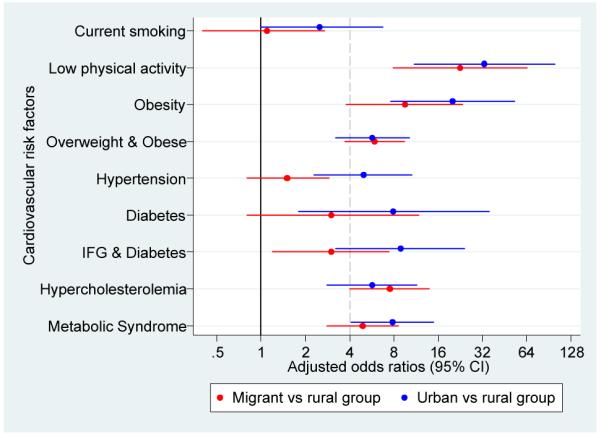
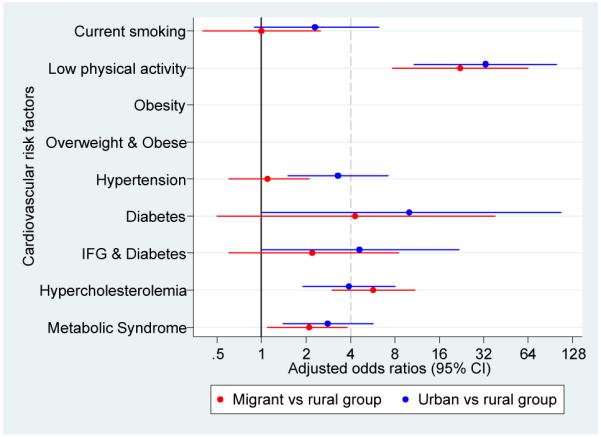
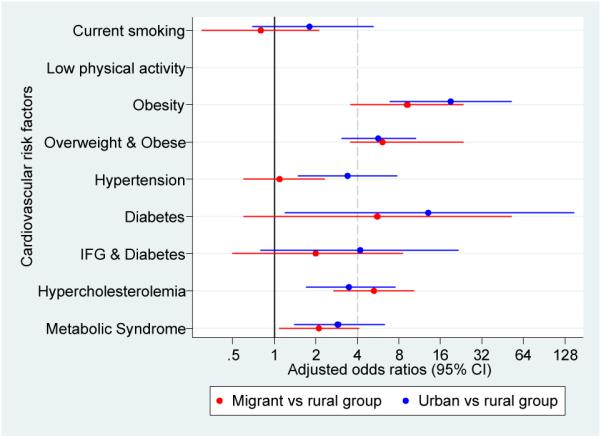
Adjusted odds ratios for cardiovascular disease risk factors in migrant and urban population compared to rural population.
a. Odds ratios adjusted for age, sex, socioeconomic position and parental education
b. Odds ratios adjusted for age, sex, socioeconomic position, parental ducation and body mass index
c. Odds ratios adjusted for age, sex, socioeconomic position, parental education, body mass index and levels of physical activity
Pattern of migration
Separate analyzes, adjusting for multiple confounders, explored whether the pattern of CVD risk factors in the migrant group varied by length of residence in urban environment, lifetime exposure to urban environment or age at first migration (see Table 2). No consistent pattern of variation in cardiovascular risk factors was observed using migrants’ sub-classifications (Supplementary Table 4 to 6), apart from glucose- and obesity-related variables.
Compared to those who migrated younger than 12 years-old, those who migrated aged 12 years or older had 3% and 2% higher geometric mean ratio of blood glucose and HbA1c, respectively. In the adjusted models, they had higher odds of: diabetes, OR 7.05 (95% CI 0.9; 55.48); IFG or diabetes, OR 6.07 (95% CI 1.36; 27.06); metabolic syndrome, OR 1.66 (95% CI 1.08; 2.57).
Those with a longer period living in an urban area had higher odds of obesity, three to four times more. There was weak evidence that longer periods of residence in an urban area (20+ years vs. <20 years) was associated with increased levels of HOMA insulin resistance but these were attenuated with further adjustment by BMI and physical activity. This pattern was not observed in those migrants by length of exposure to urban environment, where the higher odds of obesity and overweight were only present for the second quartile.
Discussion
Main findings
This study assessed three groups in Peru: migrants from a rural to an urban area; people who had remained in the rural area, and people who had always lived in the urban setting. A wide range of cardiovascular risk factors were assessed including anthropometry, blood pressure, lipids, glycaemia, metabolic and inflammatory markers. For most risk factors, the profile of migrants is close to that observed among an urban group or is intermediate between the levels seen among rural and urban groups. However, there were notable exceptions to this pattern. For blood pressure and HbA1c, values for migrants were similar to those seen in the rural group, while the urban group had substantially higher levels. For triglycerides and HDL cholesterol, there was little difference between any of the groups. The findings challenge a simple view that following migration, risk factors uniformly converge to that of the host urban population.
Strengths and limitations of the study
Our results complements, and expand to other regions, the recent observations arising from India on the impact of rural-to-urban migration28. In most studies economic factors determine migration resulting in selection bias which is an important concern29. Due to the unique circumstances of the Peruvian context, where a forced migration process occurred, the whole population had strong pressures to migrate. Rural and urban control groups were defined a priori to match the rural area of origin of most migrants as well as their urban destination. A separate analysis30 of this sample addressing the issue of selection bias using both the instrumental variable method and propensity score matching showed no differences between the migrant and the rural groups. Numbers of death by political violence, which strongly affected the study area, was used as an instrument. In addition, based on observable covariates, propensity score matching helped to mimic an experimental setting. The results from both methods suggested that selection bias did not influence our study findings, and thus the observations reported contributes to expand our knowledge of the impacts of rural to urban migration in low and middle income settings30. Ideally, longitudinal measures would be made starting prior to migration and repeated for several years to examine the evolution of risk factor changes with migration. Such a design is seldom feasible and although a cross-sectional design was used in this study, it is likely that a large part of the differences observed is attributable to the effects of migration. Of note, migrants in this study moved to and remained in a low socioeconomic area, so its findings may not generalize to the overall effect of migration considering the minority group within migrants who migrate to better areas.
Comparison with previous research
Relatively few studies have addressed the impact of rural-to-urban migration on cardiovascular disease outcomes in low- and middle-income countries. For blood pressure, while results have been somewhat inconsistent, in general studies have observed higher blood pressures —both systolic and diastolic— among people who have migrated to urban areas compared to their rural counterparts31-38.
The observation from this study that migrants from a wide age range, after a sustained process of migration and establishment into an urban environment for a number of years —a median of 32 years (IQR 25–39) in an urban environment11— maintain similar SBP to their rural counterparts, to the best of our knowledge, has not been previously reported. However, findings of surprising decrease in BP following migration in a much younger cohort and within 6 months of migration have recently been reported in Tanzania39. As suggested by Unwin et al. these findings “suggest that the pattern of change on rural to urban migration may be more complex than commonly thought and is worthy of further study”39.
In our study, the prevalence of type-2 diabetes was 0.8%, 2.8% and 6.3% in the rural, migrant and urban populations respectively. These findings of gradients are broadly similar to those seen in previous studies38 40-43. Similarly, higher HbA1c levels have been reported in urban compared to rural settings in China and Fiji44 45. Given our findings on diabetes however, the lack of difference in HbA1c between rural and migrant groups is surprising and to our knowledge has not previously been observed. These findings pose additional challenges to and signals to potential shortcomings of current recommendations seeking to consider HbA1c as diagnostic criteria for diabetes46.
Our finding that age at migration accounts for some of the variability in cardiovascular risk among migrants suggests that urban living has a heterogeneous impact on physiology. The effects of change in dietary patterns and stress may occur quite rapidly following migration, and this may account for the similar blood lipid and CRP profiles of migrant and urban populations. Other components of risk may more strongly reflect developmental experience, with a longer period during childhood spent in rural conditions conferring a degree of protection against adult risk, as is plausible for blood pressure. These findings indicate that migration may be particularly detrimental later in life, perhaps because stronger long-term physiological effects may be generated during the more plastic earlier period of growth47. Although requiring confirmation in other settings, these differential findings according to the age-profile of migration raise further question on the role and long-term impact of migration on the development of chronic conditions, particularly in low- and middle-income countries. The study by Colon-Lopez on elder Mexican migrants to the US found that those who migrated before age 20 had greater rates of cardiovascular mortality48. Although different outcomes were studied, the directionality of these observations differ from what it is suggested by our results. The observations arising from this Mexican migration study are not necessarily comparable to ours as the migration was not necessarily a rural-to-urban process, and, as clearly noted by the authors, those who migrated before the age of 20 years had higher income and education, were more likely to speak English, were culturally more Anglo48.
Importance of the study findings
The PERU MIGRANT study highlights a few observations relevant for the assessment of chronic non-communicable diseases in Peru and other LMICs. First, the profile of cardiovascular risk factors is different in the groups studied, thus demonstrating the negative impact of migration and urbanization on cardiovascular health as demonstrated by the similar metabolic and anthropometric profiles between migrant and urban populations. Second, the magnitude of difference between risk factors is not uniform across risk factors. The latter observation is demonstrated by the fact that some metabolic outcomes in migrants behave pretty much in the same way as in urban population but blood pressure does not. Third, the finding that age at migration accounts for some of the variability in cardiovascular risk in the migrants suggests that urban living has a heterogeneous impact on physiology. The impact of migration appears less if it occurs before 12 years, suggesting that adaptation is more successful if it is initiated during childhood rather than adolescence. This may be because of greater physiological plasticity during childhood may aid the alignment of homeostatic physiology with the development of body size and metabolic load47.
Studies like PERU MIGRANT describing such unexpected heterogeneity in the profile of cardiovascular risk between groups are key to the understanding of the epidemic of non-communicable diseases in LMICs. This heterogeneity adds to views proposed by Geoffrey Rose, in his classic “Sick individuals and sick populations” paper49 that prevention may be feasible at the population level and that, at least in Peru, genetic factors may not be the only factor in explaining the totality of differential cardiovascular risk.
Together with PREVENCION study —conducted solely in a middle-class urban city in another Andean area50 51— this is one of largest comprehensive CVD studies conducted in Peru to date. Unlike PREVENCION study, this study took advantage of rural and urban residents. Findings from this study contribute to fill the massive knowledge gap on NCD and CVD in LMIC as currently advocated52 53. As Yusuf et al. pointed out, there is urgent need to better document current rates —incidence and prevalence— of CVD mortality and morbidity in LMIC in order to properly assess burdens and future projections53. In the same vein, Unwin et al. argue for improved surveillance of all diseases in order to place non-communicable diseases properly within the context of the overall burden of disease54.
These findings can inform other similar LMIC but also, notoriously, challenges the adoption and incorporation of research findings from developed countries, particularly in CVD epidemiology55, to other LMIC settings without prior knowledge of the risk profile of such populations.
Conclusions
The reduction of risk of chronic disease among rural to urban migrants in low and middle income countries presents one of the major challenges to public health in the 21st century. An important finding of this study is that for some risk factors there seems to be a gradient in levels across the three groups which do not appear to be explained by age, sex, socioeconomic position, BMI and physical activity differences. This observation indicates that urbanization is indeed detrimental to cardiovascular health. Following on this, an additional observation derived from this study is that the impact of migration on cardiovascular risk profile is not uniform across risk factors, and is further influenced by the age at which migration occurs. In low- and middle-income countries, prevention strategies delivered in urban areas —presumably designed for urban dwellers— may need to be tailored to the needs of rural-to-urban migrants.
Supplementary Material
“What this paper adds” box.
What is already known on this subject?
Previous research on the health effects of migration has largely focused on movement between countries. Our knowledge and understanding of the health effects of migration from rural to urban areas within countries is less extensive.
What this study adds?
This study suggests that the impact of migration on cardiovascular risk profile is not uniform across risk factors, and is further influenced by the age at which migration occurs.
A gradient in levels, which do not appear to be explained by age, sex, socioeconomic position, body mass index, and physical activity differences, was observed for some risk factors across study groups. This observation indicates that urbanization is indeed detrimental to cardiovascular health.
In low- and middle-income countries, prevention strategies delivered in urban areas —presumably designed for urban dwellers— may need to be tailored to the needs of rural-to-urban migrants.
Acknowledgments
Professor David A. Leon and Professor Shah Ebrahim, both at the London School of Hygiene and Tropical Medicine, provided critical inputs at the design phase, throughout the study and carefully revised the manuscript. Professor Héctor H. García at UPCH provided direct advice for the various phases of the study in Peru. Valerie McCormack, Juan Pablo Casas and Pablo Perel (LSHTM) advised on data analysis. Jonathan C. K. Wells (Institute of Child Health, University College London) and Victor M. Montori (Mayo Clinic) provided critical comments to the interpretation of the data.
Our special gratitude to various colleagues at Universidad Peruana Cayetano Heredia and A.B. PRISMA in Lima, Peru and several others in the UK, as well as to the staff and the team of fieldworkers that contributed to different parts of this study. Most importantly, our sincere gratitude is extended to the people that agreed to take part in the study and to Juan Francisco Chiroque, Candice Romero and Lilia Cabrera who coordinated the fieldwork phase of this study.
Funding This work was funded in whole by the Wellcome Trust (GR074833MA). LS is supported by a Wellcome Trust Senior Research Fellowship in Clinical Science. The CRONICAS Center of Excellence in Chronic Diseases at UPCH is funded by the National Heart, Lung and Blood Institute (NHLBI), National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268200900033C. The funder had no role in study design; data collection, analysis, or interpretation; in writing the report, or in the decision to submit the article for publication. The researchers are all independent from the funding source.
Abbreviations
- AHA
American Heart Association
- BMI
Body mass index
- CI
Confidence intervals
- CRP
C-reactive protein
- CVD
Cardiovascular diseases
- DBP
Diastolic blood pressure
- HDL
High-density lipoprotein cholesterol
- HOMA
Homeostatic model assessment
- IFG
Impaired fasting glycaemia
- IQR
Interquartile range
- IR
Insulin resistance
- LDL
Low-density lipoprotein cholesterol
- MS
Metabolic syndrome
- NHLBI
National Heart, Lung, and Blood Institute
- PERU MIGRANT
PEru’s Rural to Urban MIGRANTs
- SBP
Systolic blood pressure
- SD
Standard deviations
- SMD
Standardized mean differences
- WHO
World Health Organization
- WHR
Waist-to-hip ratio
Footnotes
Contributiors JJM conceived the study, conducted the study and wrote the initial draft of this manuscript. RHG participated in the design of the study and actively supported the fieldwork phase of the study. LS contributed to the design of the study, the coordination of the study and provided critical input during data analysis and interpretation of results. All authors read and approved the final manuscript. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. JJM is the guarantor of the study.
Competing interests All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) no support from any organisation for the submitted work other than the funding grant; (2) no relationships with any organisation that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children do not have financial relationships that may be relevant to the submitted work; and (4) no non-financial interests that may be relevant to the submitted work.
Ethical approval Ethical approval for this protocol was obtained from ethics committees at Universidad Peruana Cayetano Heredia in Peru and London School of Hygiene and Tropical Medicine in the UK. The purpose of the study was explained to each of the study participants and written informed consent was obtained.
Data sharing Technical appendix, statistical code, and dataset available from the corresponding author at Jaime.Miranda@upch.pe or Jaime.Miranda@lshtm.ac.uk. Consent for data sharing was not obtained but the presented data are anonymised and risk of identification is low.
Exclusive licence statement The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in BMJ editions and any other BMJPGL products and sublicences to exploit all subsidiary rights, as set out in our licence (http://resources.bmj.com/bmj/authors/checklists-forms/licence-for-publication).
References
- 1.World Health Organization . Preventing chronic diseases: A vital investment. WHO Global Report. World Health Organization; Geneva: 2005. [Google Scholar]
- 2.Miranda JJ, Kinra S, Casas JP, Smith G Davey, Ebrahim S. Non-communicable diseases in low- and middle-income countries: context, determinants and health policy. Trop Med Int Health. 2008;13(10):1225–34. doi: 10.1111/j.1365-3156.2008.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GF, Chu E. Expanding Priorities -- Confronting Chronic Disease in Countries with Low Income. N Engl J Med. 2007;356(3):209–11. doi: 10.1056/NEJMp068182. [DOI] [PubMed] [Google Scholar]
- 4.Daar AS, Singer PA, Persad D Leah, Pramming SK, Matthews DR, Beaglehole R, et al. Grand challenges in chronic non-communicable diseases. Nature. 2007;450(7169):494–6. doi: 10.1038/450494a. [DOI] [PubMed] [Google Scholar]
- 5.van Ginkel H. Urban Future. Nature. 2008;456(n1s):32–33. [Google Scholar]
- 6.United Nations Population Fund . The State of World Population 2007: Unleashing the Potential of Urban Growth. UNFPA; New York: 2007. [Google Scholar]
- 7.Patel RB, Burke TF. Urbanization -- An Emerging Humanitarian Disaster. N Engl J Med. 2009;361(8):741–43. doi: 10.1056/NEJMp0810878. [DOI] [PubMed] [Google Scholar]
- 8.McKay L, Macintyre S, Ellaway A. Migration and health : a review of the international literature. Medical Research Council, Social & Public Health Sciences Unit, University of Glasgow; Glasgow: 2003. Occasional paper No. 12. [Google Scholar]
- 9.Comisión de la Verdad y Reconciliación . Informe Final de la Comisión de la Verdad y Reconciliación. [Peruvian Truth and Reconciliation Comision’s Final Report]. Comisión de la Verdad y Reconciliación; Lima: 2003. Available at: http://www.cverdad.org.pe/ifinal/index.php. [Google Scholar]
- 10.Coral I. Documentos de Trabajo, 58. Serie Politica, 6. Instituto de Estudios Peruanos; Lima: 1994. Desplazamiento por violencia politica en el Peru, 1980-1992; p. 35. [Google Scholar]
- 11.Miranda JJ, Gilman RH, Garcia HH, Smeeth L. The effect on cardiovascular risk factors of migration from rural to urban areas in Peru: PERU MIGRANT Study. BMC Cardiovascular Disorders. 2009;9(1):23. doi: 10.1186/1471-2261-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen D, Tremblay J, Errázuriz C, Gamarra J. The sequelae of political violence: Assessing trauma, suffering and dislocation in the Peruvian highlands. Soc Sci Med. 2008;67(2):205–17. doi: 10.1016/j.socscimed.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Instituto Nacional de Estadística e Informática . Censos Nacionales 2007: XI de Población y VI de Vivienda. INEI; Lima, Perú: 2007. Available from: http://censos.inei.gob.pe/Censos2007/IndDem// [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–536. doi: 10.1093/eurheartj/ehm236. 2007. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications. World Health Organization; Geneva: 1999. [Google Scholar]
- 17.Galobardes B, Lynch J, Smith G Davey. Measuring socioeconomic position in health research. Br Med Bull. 2007;81-82(1):21–37. doi: 10.1093/bmb/ldm001. [DOI] [PubMed] [Google Scholar]
- 18.Gordon D. Census based deprivation indices: their weighting and validation. J Epidemiol Community Health. 1995;49(Suppl 2):S39–44. doi: 10.1136/jech.49.suppl_2.s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR. Use and Abuse of HOMA Modeling. Diabetes Care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–45. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health and Human Services . Physical Activity Guidelines for Americans. Department of Health and Human Services; 2008. 2008. [Google Scholar]
- 22.Newson R. Stata tip 1: The eform() option of regress. The Stata Journal. 2003;3(4):445. [Google Scholar]
- 23.Statistical Consulting Group . FAQ: How do I interpret a regression model when some variables are log transformed? UCLA, Academic Technology Services; Los Angeles, CA: Available at: http://www.ats.ucla.edu/stat/mult_pkg/faq/general/log_transformed_regression.htm. [Google Scholar]
- 24.World Health Organization . The WHO Standard Population. World Health Organization; Geneva: Available at: https://apps.who.int/infobase/help.aspx?helpid=293. [Google Scholar]
- 25.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd ed Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ: 1988. [Google Scholar]
- 27.Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. The Cochrane Collaboration; [updated February 2008]. 2008. Available at www.cochrane-handbook.org. [Google Scholar]
- 28.Ebrahim S, Kinra S, Bowen L, Andersen E, Ben-Shlomo Y, Lyngdoh T, et al. The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study. PLoS Med. 2010;7(4):e1000268. doi: 10.1371/journal.pmed.1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razum O. Commentary: Of salmon and time travellers—musing on the mystery of migrant mortality. Int. J. Epidemiol. 2006;35(4):919–21. doi: 10.1093/ije/dyl143. [DOI] [PubMed] [Google Scholar]
- 30.Miranda JJ. Working Paper. Georgia State University; Atlanta, GA: 2009. Migration from Rural to Urban Areas in Peru: Impact on Health Outcomes. Available at: http://aysps.gsu.edu/ECON_MA_MonteroJJ.pdf. [Google Scholar]
- 31.Poulter NR, Khaw KT, Hopwood BE, Mugambi M, Peart WS, Rose G, et al. The Kenyan Luo migration study: observations on the initiation of a rise in blood pressure. BMJ. 1990;300(6730):967–72. doi: 10.1136/bmj.300.6730.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Klag MJ, Whelton PK, Chen JY, Mo JP, Qian MC, et al. Migration, blood pressure pattern, and hypertension: the Yi Migrant Study. Am J Epidemiol. 1991;134(10):1085–101. doi: 10.1093/oxfordjournals.aje.a116012. [DOI] [PubMed] [Google Scholar]
- 33.He J, Tell GS, Tang YC, Mo PS, He GQ. Effect of migration on blood pressure: the Yi People Study. Epidemiology. 1991;2(2):88–97. doi: 10.1097/00001648-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Carvalho JJ Mancilha, Baruzzi RG, Howard PF, Poulter N, Alpers MP, Franco LJ, et al. Blood pressure in four remote populations in the INTERSALT Study. Hypertension. 1989;14(3):238–46. doi: 10.1161/01.hyp.14.3.238. [DOI] [PubMed] [Google Scholar]
- 35.Intersalt Cooperative Research Group INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297(6644):319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadim A, Amini H, Malek-Afzali H. Blood Pressure and Rural-Urban Migration in Iran. Int. J. Epidemiol. 1978;7(2):131–38. doi: 10.1093/ije/7.2.131. [DOI] [PubMed] [Google Scholar]
- 37.Poulter NR, Khaw KT, Mugambi M, Peart WS, Sever PS. Migration-induced changes in blood pressure: a controlled longitudinal study. Clin Exp Pharmacol Physiol. 1985;12(3):211–6. doi: 10.1111/j.1440-1681.1985.tb02633.x. [DOI] [PubMed] [Google Scholar]
- 38.Sobngwi E, Mbanya JC, Unwin NC, Porcher R, Kengne AP, Fezeu L, et al. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int J Epidemiol. 2004;33(4):769–76. doi: 10.1093/ije/dyh044. [DOI] [PubMed] [Google Scholar]
- 39.Unwin N, McLarty D, Machibya H, Aspray T, Tamin B, Carlin L, et al. Changes in blood pressure and lipids associated with rural to urban migration in Tanzania. J Hum Hypertens. 2006;20(9):704–6. doi: 10.1038/sj.jhh.1002056. [DOI] [PubMed] [Google Scholar]
- 40.Sadikot SM, Nigam A, Das S, Bajaj S, Zargar AH, Prasannakumar KM, et al. The burden of diabetes and impaired glucose tolerance in India using the WHO 1999 criteria: prevalence of diabetes in India study (PODIS) Diabetes Res Clin Pract. 2004;66(3):301–7. doi: 10.1016/j.diabres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Yajnik CS, Joglekar CV, Lubree HG, Rege SS, Naik SS, Bhat DS, et al. Adiposity, inflammation and hyperglycaemia in rural and urban Indian men: Coronary Risk of Insulin Sensitivity in Indian Subjects (CRISIS) Study. Diabetologia. 2008;51(1):39–46. doi: 10.1007/s00125-007-0847-1. [DOI] [PubMed] [Google Scholar]
- 42.Aspray TJ, Mugusi F, Rashid S, Whiting D, Edwards R, Alberti KG, et al. Rural and urban differences in diabetes prevalence in Tanzania: the role of obesity, physical inactivity and urban living. Trans R Soc Trop Med Hyg. 2000;94(6):637–44. doi: 10.1016/s0035-9203(00)90216-5. [DOI] [PubMed] [Google Scholar]
- 43.Alberts M, Urdal P, Steyn K, Stensvold I, Tverdal A, Nel JH, et al. Prevalence of cardiovascular diseases and associated risk factors in a rural black population of South Africa. Eur J Cardiovasc Prev Rehabil. 2005;12(4):347–54. doi: 10.1097/01.hjr.0000174792.24188.8e. [DOI] [PubMed] [Google Scholar]
- 44.Gao M, Ikeda K, Hattori H, Miura A, Nara Y, Yamori Y. Cardiovascular risk factors emerging in Chinese populations undergoing urbanization. Hypertens Res. 1999;22(3):209–15. doi: 10.1291/hypres.22.209. [DOI] [PubMed] [Google Scholar]
- 45.Russell-Jones DL, Hoskins P, Kearney E, Morris R, Katoaga S, Slavin B, et al. Rural/urban differences of diabetes--impaired glucose tolerance, hypertension, obesity, glycosolated haemoglobin, nutritional proteins, fasting cholesterol and apolipoproteins in Fijian Melanesians over 40. Q J Med. 1990;74(273):75–81. [PubMed] [Google Scholar]
- 46.International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells JC. Historical cohort studies and the early origins of disease hypothesis: making sense of the evidence. Proc Nutr Soc. 2009;68(2):179–88. doi: 10.1017/S0029665109001086. [DOI] [PubMed] [Google Scholar]
- 48.Colon-Lopez V, Haan MN, Aiello AE, Ghosh D. The effect of age at migration on cardiovascular mortality among elderly Mexican immigrants. Ann Epidemiol. 2009;19(1):8–14. doi: 10.1016/j.annepidem.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14(1):32–8. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 50.Medina-Lezama J, Chirinos JA, Diaz H Zea, Morey O, Bolanos JF, Munoz-Atahualpa E, et al. Design of PREVENCION: a population-based study of cardiovascular disease in Peru. Int J Cardiol. 2005;105(2):198–202. doi: 10.1016/j.ijcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 51.Medina-Lezama J, Zea-Diaz H, Morey-Vargas O, Bolanos-Salazar J, Postigo-Macdowall M, Paredes-Diaz S, et al. Prevalence and patterns of hypertension in Peruvian Andean Hispanics: The PREVENCION study. J Am Soc Hypertens. 2007;1(3):216–25. doi: 10.1016/j.jash.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease control priorities in developing countries. 2nd ed. Oxford University Press; New York: 2006. [PubMed] [Google Scholar]
- 53.Yusuf S, Vaz M, Pais P. Tackling the challenge of cardiovascular disease burden in developing countries. Am Heart J. 2004;148(1):1–4. doi: 10.1016/j.ahj.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 54.Unwin N, Setel P, Rashid S, Mugusi F, Mbanya JC, Kitange H, et al. Noncommunicable diseases in sub-Saharan Africa: where do they feature in the health research agenda? Bull World Health Organ. 2001;79(10):947–53. [PMC free article] [PubMed] [Google Scholar]
- 55.Ebrahim S, Smith G Davey. Exporting failure? Coronary heart disease and stroke in developing countries. Int J Epidemiol. 2001;30(2):201–5. doi: 10.1093/ije/30.2.201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.