Abstract
Ubiquitin thioester is a key intermediate in the ubiquitylation of proteins and is formed enzymatically through the activation of α-COOH of ubiquitin in an ATP dependent manner using the E1 enzyme. The current methods used for the preparation of ubiquitin thioester rely on either the enzymatic machinery or on expressed protein ligation technology. In this article, we report a new chemical strategy, combining native chemical ligation and N-methylcysteine containing peptides, to chemically prepare ubiquitin thioester for the first time. The N-methylcysteine is utilized as an N→S acyl transfer device, and in its protected form serves as a latent thioester functionality. This enabled us to trigger the formation of ubiquitin thioester subsequent to the assembly of the ubiquitin polypeptide via native chemical ligation. The synthetic ubiquitin thioester showed a similar behavior in peptide ubiquitylation to the one obtained via expression. This approach should allow for higher flexibility in the chemical manipulation of ubiquitin thioester in a wide variety of ubiquitylated peptides and proteins for structural and biochemical analysis and for the synthesis of ubiquitin chains.
Introduction
Ubiquitin thioester is a key intermediate in the ubiquitylation of proteins. Ubiquitylation serves as a recognition marker for degradation in the case of polyubiquitylation and to regulate different biochemical processes in monoubiquitylation.1 Three distinct enzymes, known as the E1-E3 system, collaborate to achieve a site-specific ubiquitylation of the lysine residue(s) in the target protein.2 The activation of of α-COOH of ubiquitin is achieved in an ATP dependent manner using the E1 enzyme, which forms a thioester with the carboxyl group of Gly76. This step activates ubiquitin and triggers a nucleophilic attack by the conjugating enzyme E2. The latter transiently carries the activated ubiquitin, also as a thioester intermediate, and with the assistance of the E3 ligase transfers ubiquitin to a specific lysine residue of the protein substrate.2
The current methods for preparing ubiquitin thioester rely on either the use of the enzymatic machinery E1-E2 or on expressed protein technology.3 The latter approach was recently utilized to access ubiquitin with C-terminal electrophiles as activity-based probes to recover and identify members of each enzyme class in the ubiquitin-proteasome system.4 In another example, ubiquitin thioester was used in peptide and protein ubiquitylation using a chemical auxiliary5 or as we6 and Liu and coworkers7 have shown recently using mercaptolysine residue. Despite these successes, this approach is limited mainly to natural amino acid mutations in ubiquitin, thus inhibiting the chemical manipulation of this protein. Our interest in introducing unnatural functionalities into ubiquitin thioester, (e.g. mercaptolysine)6 to allow for the preparation of naturally occurring ubiquitin chains with different lengths and connectivities8 prompted us to explore chemical ways to synthesize ubiquitin thioester. Here we report a new chemical strategy combining native chemical ligation9 and N-methylcysteine containing peptides to prepare ubiquitin thioester in a highly efficient manner.
The chemical synthesis of protein thioester, wherein unnatural amino acids could be incorporated into the sequence, remains a synthetic challenge. Kent and coworkers reported an elegent “kinetically controlled ligation” strategy for preparing protein thioester.10 This approach exploits the different reactivities of aryl and alkyl thioesters as well as the differences in the bulkiness of the C-terminal residue of the thioester peptides for a convergent protein synthesis. Using this strategy, the group was able to assemble the covalent homo-dimer HIV protease by using HIV protease monomer with thioester functionality.10 However, “kinetically controlled ligation” could lead to an undesirable outcome when the C-terminal residue of the thioester peptides is intrinsically reactive in peptide ligation (e.g. Gly and His)11 as is the case in ubiquitin where the C-terminal residue is Gly. A sterically hindered amino acid at the C-terminal peptide bearing alkyl thioester has a very low reactivity compared to an unhindered amino acid at the C-terminal peptide bearing aryl thioester. On the other hand, the differences in the reactivities of the alkyl thioester peptide with C-terminal Gly compared to aryl thioester peptide bearing any C-terminal amino acid is not sufficient enough to inhibit ligation with the Gly thioester peptide. Thinking about the challenges while preparing ubiquitin thioester, it caught our attention that the methods which were reported to prepare thioester peptides according to Fmoc-SPPS could be useful in preparing ubiquitin thioester. Specifically, the uses of N→S acyl transfer as the key step in triggering thioester formation upon completion of peptide synthesis, on or off the resin.12 Of particular interest to us is the use of N-alkylated Cys at the C-terminal peptide, which upon treatment with 3-mercaptopropionic acid generates the desired peptide thioester.13 This method was found to be useful for the synthesis of a variety of thioester peptides and glycopeptides and for the sequential segment coupling to prepare glycopeptide dendrimer.14
Results and discussion
Our designed strategy for the chemical synthesis of ubiquitin thioester is described in Scheme 1. We envisioned the synthesis of the ubiquitin from two fragments, which would include peptide 1, Ub(46-76), and peptide 2, Ub(1-45), wherein Ala46 is mutated temporarily to Cys to facilitate native chemical ligation, bearing in mind that this Cys could be converted to Ala using the desulfurization reaction.15 To achieve the thioester C-terminal functionality, peptide 1 will be equipped with N-methylcysteine, as N→S acyl transfer device, and the thiol side chain is protected with the photolabile-protecting group (2-nitrobenzyl)16 to avoid an intramolecular N→S acyl transfer during the TFA-cleavage step. This would enable us to trigger the reaction providing a latent thioester functionality. Upon completion of the ligation step, the thiol-protecting group will be removed followed by treatment of the fully unprotected polypeptide with 3-mercaptopropionic acid to afford the ubiquitin thioester.
Scheme 1.
Proposed synthetic strategy for the synthesis of ubiquitin thioester, also showing the amino acid sequence of ubiquitin and the ligation site (R = -CH2-CH2-COOH).
With this strategy in mind, we started with the preparation of peptide 1 according to the sequence of reactions shown in Scheme 2. Initially, the Rink amide resin was loaded with Fmoc-cys(2-nitrobenzyl)-OH using HBTU/DIEA coupling conditions. Subsequently, the Fmoc-protecting group was removed with 20% piperidine followed by coupling of the free amine with o-nitrobenzenesulfonyl chloride (o-NBS) to facilitate N-methylation.17 Selective deprotonation of the sulfonamide with DBU and alkylation with methyl p-nitrobenzenesulfonate in DMF led to the formation of the methylated sulfonamide resin 6. We found that TBAF/MeI could also serve as an excellent choice for the methylation step.18 Selective removal of the o-NBS was achieved by using mercaptoethanol and DBU in DMF allowing for the remaining assembly of the target peptide. Side chain deprotection and release from the solid support using TFA/TIS/H2O (95:2.5:2.5) afforded the desired peptide, after RP-HPLC purification, in 25–30% isolated yield (Supporting Information).
Scheme 2.
Synthetic strategy for peptide 1.
Next, we focused our efforts on synthesizing the Ub(1-45)-SR, (R: -CH2CH2-COOMe), using Fmoc-SPPS. For this target, we chose to apply the the N-acylurea chemistry developed by Dawson and coworkers (Supporting Information).19 The peptide with the N-acyl benzimidazolinone functionality was deprotected and cleaved from the resin by treatment with a mixture of TFA/H2O/TIS (95:2.5:2.5). After a lyophilization step, the crude peptide was treated with methyl 3-mercaptopropionate in 6M Gn.HCl, pH 7 to afford the Ub(1-45)-SR 2, after RP-HPLC purification step, in 20% yield (Supporting Information).
The ligation between peptide 1 and 2 was carried out under native chemical ligation conditions i.e. 6 M Gn.HCl, 200 mM phosphate buffer, pH ~7 in the presence of 2% (v/v) thiophenol/benzyl mercaptan. The reaction was followed by HPLC and mass spectrometry analysis, which indicated nearly a complete ligation after 8 h (Figure 1A). Following purification and lyophilization steps, the product was exposed to UV light (365 nm) for 2 h. This step was followed by the addition of 20% (v/v) 3-mercaptopropionic acid (pH ~1) and the reaction mixture was left at 37 °C. Gratifyingly, after 12 h a full conversion to the desired thioester product was achieved (Figure 1B). Preparative RP-HPLC purification and lyophilization steps afforded the ubiquitin thioester in 30% isolated yield (two steps). More recently, Macmillan and coworkers found that 3-mercaptopropionic acid induces peptide and protein fragmentation at Cys junction in a sequence dependent manner to give the corresponding peptide thioester.20 Importantly, under the 3-mercaptopropionic acid conditions we used in our study, the Phe-Cys junction was completely stable and had no by-product due to 3-mercaptopropionic acid cleavage at this site.
Fig. 1.

Representative analytical HPLC traces/(ESMS) of ligation reaction between 1 and 2 (A) followed by thioester formation (B). Reported mass is for total protein. A) Ligation after 8 h: Peak a, unreacted peptide 1 with the observed mass of 3752.1 Da. Peak b, thioester hydrolysis by product (Ub1-45-COOH) with the observed mass 5096.1 Da. Peak c, ligation product with the observed mass of 8832 Da (calcd m/z 8830.9 Da). Peak d, unreacting benzyl thioester of peptide 2 with the observed mass of 5201.2 Da (1.1 eq of peptide thioester was used in the ligation reaction). B) Photolysis (2 h) of the ligation product (365 nm) followed by treatment with 20% 3-mercaptopropionic acid (12 h): Peak a, photolysis mixture. Peak b, ubiquitin thioester with the observed mass of 8668 Da (calcd m/z 8668.8 Da).
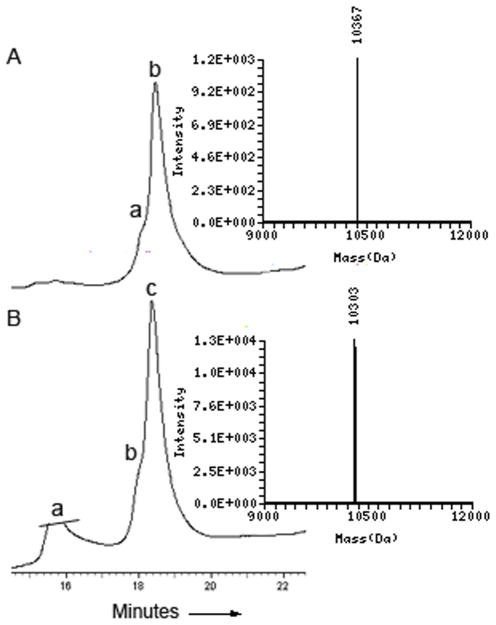
To further support the integrity of the C-terminal thioester functionality, the synthetic ubiquitin thioester was tested in peptide ubiquitylation using α-synuclein(1-17) model peptide bearing the mercaptolysine residue (Figure 2).6 Our results show that ubiquitin thioester is indeed an excellent substrate in the ligation reaction and within 4 h a complete reaction was observed to afford the ubiquitylated peptide in 60% isolated yield (Figure 2A). Subsequently the ligation product was desulfurized using metal free desulfurization conditions21 to convert Cys46 to Ala along with a full removal of the thiol handle from the mercaptolysine to furnish the ubiquitylated peptide 8 (Figure 2B). The desulfurized product was isolated in 75% yield and was treated with ubiquitin C-terminal hydrolase, UCH-L3 for 12 h. Our results show that the desulfurized product is indeed a UCH-L3 susbstrate affording both the hydrolyzed ubiquitin and the α-syn(1-17) peptide (Supporting Information). Our synthetic strategy of ubiquitin thioester is in analogy to the E1-E2 activation steps and when combined with the ubiquitylation step using mercaptolysine, which resemble the E3 ligase activity, shows that the entire ubiquitylation process could be mimicked using chemical tools only.
Fig. 2.
Representative analytical HPLC traces/(ESMS) of ubiquitylation reaction between 5 and 7 (A) followed by desulfurization (B). Reported mass is for total protein. A) Ubiquitylation after 4 h: Peak a, hydrolyzed thioester that was not fully seperated from the previous step with the observed mass of 5096.1 Da. Peak b, the desired ubiquitylation product with the observed mass 10367 Da (calcd m/z 10367.9 Da). B) Desulfurization after 3 h: Peak a, desulfurization mixture. Peak b, byproduct carried from previous step. Peak c, the desired desulfurized product with the observed mass 10303 Da (calcd m/z 10303.8 Da).
Conclusion
Several groups, including our laboratory, have recently reported new approaches for the synthesis and semisynthesis of ubiquitylated peptides and proteins.5–7 In all of these studies, the preparation of ubiquitin thioester, as a key precursor, was achieved using the intien technology, which limits the introduction of unnatural functionalities for the chemical manipulation of ubiquitin. To overcome these limitations we have developed a novel route for the first total chemical synthesis of ubiquitin thioester. The key step in the synthesis relies on the use of the N→S acyl transfer reaction after the chain assembly of the ubiquitin polypeptide via native chemical ligation. The synthetic ubiquitin thioester showed a similar behavior in peptide ubiquitylation to the expressed ubiquitin thioester. This approach should allow a higher flexibility in the chemical manipulation of ubiquitin thioester in a wide variety of ubiquitylated peptides and proteins for structural and biochemical analysis and for the synthesis of ubiquitin chains.8 We are currently working towards achieving these goals.
Experimental
SPPS of N-methylcysteine Peptide 1 Ub(46-76)
Cys(2-nitrobenzyl)-OH16 was coupled to Rink amide resin (0.56 mmol/g; 0.1 mmol scale, 178 mg) using HBTU/DIEA in 5/10 fold excess of the initial loading of the resin. The coupling was performed for 30 min. Fmoc deprotection was achieved by treatment of the resin with 20% piperidine.
Sulfonylation
Collidine (264 μL, 20 eq) was dissolved in 1.5 mL of CH2Cl2 and was added to the resin, followed by the addition of solution of o-nitrobenzenesulfonyl chloride (442 mg, 20 eq) in 1.5 mL of CH2Cl2. The resin was shaken for 2 h at RT and was washed using CH2Cl2 (3 × 5 mL) and DMF (3 × 5 mL).
Alkylation
To the washed resin, DBU (74 μL, 5 eq) in 1.5 mL of DMF was added followed by the addition of methyl 4-nitrobenzenesulfonate (108 mg, 5eq) in 1.5 mL of DMF. The resin was shaken for 1 h at RT and was washed with DMF (3 × 5 mL). Alternatively, MeI (124 μL, 20 eq) in 1 ml TBAF was added to the resin and was shaken for 1 h.
Removal of NBS
To the suspension of the previously treated resin, DBU (38 μL, 5 eq) and mercaptoethanol (35μL, 10 eq) were added in DMF and shaken well for 30 min at RT followed by DMF wash (3 × 5 mL).
SPPS
The first amino acid (Gly) was coupled using HATU (4 eq) and DIEA (10 eq) for 45 min (2x). The remaining amino acids were coupled using peptide synthesizer. The peptide synthesis using the peptide synthesizer was caried out in presence of 4 eq of AA, 2 eq of DIEA and 4 eq of HBTU/HOBT to the initial loading of the resin. The coupling was kept for 1 h and Fmoc-deprotection was achieved using 20% piperidine with 5/10/5 min cycles.
Cleavage from the resin
A mixture of TFA, triisopropylsilane and water (95:2.5:2.5) was added to the dried peptide-resin and the reaction mixture was shaken for 2 h at RT. The resin was removed by filtration and was washed with TFA (2 × 2 mL). To precipitate the peptide, the combined filtrate was added drop-wise to 10 fold volume of cold ether, centrifugation, decanting of ether, followed by dissolution of residue in acetonitrile-water and HPLC purification afforded the corresponding peptide in 25–30 % yield (90–100 mg).
Peptide analysis and purification
Analytical RP-HPLC was performed on a Thermo instrument (Spectra System p4000) using an analytical column (Jupiter 5 micron, C18, 300A 150 × 4.6 mm) and a flow rate of 1.2 ml/min. Preparative RP-HPLC was performed on an ECOM instrument using a preparative column (Jupiter 5 micron, C18, 300A, 250 × 10 mm).
Synthesis of Peptide 2
Rink amide resin (0.2 mmol/g, 0.1mmol scale, 500 mg ) was used for the synthesis of peptide 2. The first two amino acids, (i.e. 3-Fmoc-4-damino benzoic acid (Fmoc-Dbz), and Phe), were each double coupled manually for 1 h using HBTU/HOBT in 4 fold excess of the initial loading of the resin. DIEA was used in 10 fold excess. Fmoc deprotection was achieved by treatment of the resin with 20% piperidine. The remaining amino acids were coupled using the peptide synthesizer as previously described. The last amino acid was coupled in its Boc protected form.
On resin activation
After peptide elongation, the resin was washed with CH2Cl2 and a solution of p-nitrophenylchloroformate (100 mg, 5 eq) in 10 mL of CH2Cl2 was added and shaken for 1 h at RT. The resin was washed with CH2Cl2 (3 × 5 mL), and DMF (3 × 5 mL). To the washed resin, a solution of 0.5 M DIEA in DMF (5 mL) was added and shaken for additional 30 min. The resin was washed using DMF (3 × 5 mL).
Cleavage and purification
The procedure used for peptide 1 was followed.
Thioesterification
The pure peptide was dissolved in 0.2 M phosphate buffer (pH ~7) containing 6 M guanidine.HCl to a final concentration of ~1 mM, followed by the addition of 2% (v/v) methyl-mercaptopropionate. The solution was kept at RT for 1 h and purified by preparative reverse-phase HPLC using a linear gradient of 10–60% B over 30 min (buffer A: 0.1% TFA in water; buffer B: 0.1% TFA in acetonitrile) to afford the corresponding thioester in ~20% yield (~100 mg).
Procedure for NCL
Peptide 1 (3.2 mg) and peptide 2 (5 mg, 1.1 eq) were dissolved in 440 μL of 0.2 M phosphate buffer (pH ~7) containing 6 M guanidine.HCl to a final concentration of 2 mM. Thiophenol and benzylmercaptan (2% v/v, 8.7 μL) were added and the ligation reaction was performed in a heating block at 37 °C. The reaction was monitored using RP-HPLC analysis on a C4 column using a linear gradient (10–60% B) over 30 min and purified on a preparative HPLC (~ 3 mg, 36 % yield).
Synthesis of ubiquitin thioester
Photolysis
Peptide 3 (3 mg) was dissolved in the photolysis buffer containing 10 mM ascorbic acid; 10 mM semicarbazide and 10 mM 3-mercaptopropionic acid in 0.2 M phosphate buffer (pH ~7)/6 M guanidine.HCl for a final concentration of ~1 mM. The mixture was irradiated with a UV lamp at 365 nm, 28 °C for 2 h. Subsequently, 20% of 3-mercaptopropionic acid was added (pH ~1 )and the reaction was left at 37 °C for 12 h. After the completion of thioester formation of the ubiquitin thioester was purified using preparative RP- HPLC on C4 column and a linear gradient of 10–60% B over 30 min. The fractions were analyzed by ESI-MS and the desired fractions were collected, lyophilized to afford ubiquitin thioester in ~30% yield (1 mg).
Procedure for the ligation of peptide (7) with ubiquitin thioester(5)
Peptide 5, (1.60 mg, 1 eq) and 7 (1 mg, 3 eq), were dissolved in 100 μL of 6 M guanidine.HCl, 200 mM phosphate buffer pH ~7.0. To this solution 2 μL each of benzylmercaptan and thiophenol were added and incubated for 5 h at 37 °C. The reaction was followed using an analytical column and a gradient of 10–60% B over 30 min. For preparative HPLC a similar gradient was used to afford the ligation product in ~60% yield (1.0 mg).
Desulfurization
The ubiquitylated peptide was dissolved in argon purged 6 M guanidine.HCl 0.2 M Phosphate buffer pH ~7.0 to a concentration of 2 mM. To this solution, a solution of TCEP (0.5 M ) in argon purged guanidine.HCl/phosphate buffer pH 7, 10% (v/v) of t-BuSH and 0.1 M radical initiatior VA-044 were added, sequentially. The mixture was left at 37 °C for 3 h. The extent of the reaction was analyzed using C-4 analytical RP-HPLC employing a gradient of 10–60% B over 30 min to afford pure desulfurized peptide 8 in 75% yield.
Enzymatic cleavage of isopeptide
Peptide 8 was dissolved in 482 μL of Tris buffer (50 mM Tris, 150 mM NaCl, 1 mM DTT, pH 7.5) to a final concentration of ~100 μM and reacted with recombinant human ubiquitin C-terminal hydrolase L3 (UCH-L3, Aldrich). 10 μg of UCH-L3 in 15.5 μL of assay buffer containing 50 mM Tris, 150 mM NaCl, 12 mM DTT, pH 8.0 was incubated for 20 min at 25 °C. To the reduced UCH-L3 peptide 8 in 187 μL in Tris buffer was added. The mixture was incubated for 12 h at 37 °C, at which a complete hydrolysis was acheived. The reaction was analyzed using C-4 analytical RP-HPLC, employing a gradient of 10–60% B for 30 min.
Supplementary Material
Acknowledgments
We are grateful to the Edmond J. Safra Foundation, the Israel Science Foundation (A.B.) and NIH (P.E.D., GM059380) for financial support.
Footnotes
Electronic Supplementary Information (ESI) available: [synthesis of peptide HPLC and masspectrometry analysis of precourses and products]. See DOI: xxx
References
- 1.(a) Ciechanover A, Hod Y, Hershko A. Biochem Biophys Res Commun. 1978;81:1100. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]; (b) Fallon L, Belanger CML, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen en Henegouwen PM, Fon EA. Nat Cell Biol. 2006;8:834. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 2.Pickart CM. Annu Rev Biochem. 2001;70:503. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 3.Muir TW, Sondhi D, Cole PA. Proc Natl Acad Sci USA. 1998;95:6705. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM. Chem Biol. 2002;9:1149. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee C, McGinty RK, Pellois JP, Muir TW. Angew Chem Int Ed. 2007;46:2814. doi: 10.1002/anie.200605155. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KSA, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Angew Chem Int Ed. 2009;48:8090. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Pasunooti KK, Li F, Liu XW, Liu CF. J Am Chem Soc. 2009;131:13952. doi: 10.1021/ja905491p. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda F, Dikic I. EMBO Reports. 2008;9:546. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 10.(a) Bang D, Pentelute BL, Kent SBH. Angew Chem Int Ed. 2006;45:3985. doi: 10.1002/anie.200600702. [DOI] [PubMed] [Google Scholar]; (b) Torbeev VY, Kent SBH. Angew Chem Int Ed. 2007;119:1697. [Google Scholar]
- 11.Hackeng TM, Griffin JH, Dawson PE. Proc Natl Acad Sci USA. 1999;96:10068. doi: 10.1073/pnas.96.18.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Kawakami T, Sumida M, Nakamura K, Vorherr T, Aimoto S. Tetrahedron Lett. 2005;46:8805. [Google Scholar]; (b) Ollivier N, Behr JB, El-Mahdi Q, Blanpain A, Melnyk O. Org Lett. 2005;7:2647. doi: 10.1021/ol050776a. [DOI] [PubMed] [Google Scholar]; (c) Kawakami T, Aimoto S. Tetrahedron. 2009;65:3871. [Google Scholar]; (d) Tsuda S, Shigenaga A, Bando K, Otaka A. Org Lett. 2009;11:823. doi: 10.1021/ol8028093. [DOI] [PubMed] [Google Scholar]
- 13.(a) Nagaike F, Onuma Y, Kanazawa C, Hojo H, Ueki A, Nakahara Y, Nakahara Y. Org Lett. 2006;8:4465. doi: 10.1021/ol0616034. [DOI] [PubMed] [Google Scholar]; (b) Hojo H, Onuma Y, Akimoto Y, Nakahara Y, Nakahara Y. Tetrahedron Lett. 2007;48:25. [Google Scholar]
- 14.Ozawa C, Katayama H, Hojo H, Nakahara Y, Nakahara Y. Org Lett. 2008;10:3531. doi: 10.1021/ol801340m. [DOI] [PubMed] [Google Scholar]
- 15.(a) Yan LZ, Dawson PE. J Am Chem Soc. 2001;123:526. doi: 10.1021/ja003265m. [DOI] [PubMed] [Google Scholar]; (b) Bang D, Makhatadze GI, Tereshko V, Kossiakoff AA, Kent SBH. Angew Chem Int Ed. 2005;44:3852. doi: 10.1002/anie.200463040. [DOI] [PubMed] [Google Scholar]
- 16.Smith AB, Savinov SN, Manjappara UV, Chaiken IM. Org Lett. 2002;4:4041. doi: 10.1021/ol026736d. [DOI] [PubMed] [Google Scholar]
- 17.Miller SC, Scanlan TS. J Am Chem Soc. 1997;119:2301. [Google Scholar]
- 18.(a) Wu CY, Brik A, Wang SK, Chen YH, Wong CH. ChemBioChem. 2005;6:2176. doi: 10.1002/cbic.200500295. [DOI] [PubMed] [Google Scholar]; (b) Brik A, Wu CY, Best MD, Wong CH. Bioorg Med Chem. 2005;13:4622. doi: 10.1016/j.bmc.2005.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco-Canosa JB, Dawson PE. Angew Chem Int Ed. 2008;47:6851. doi: 10.1002/anie.200705471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J, Richardson JP, Macmillan D. Chem Commun. 2009;4:407. doi: 10.1039/b815888f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan Q, Danishefsky SJ. Angew Chem Int Ed. 2007;46:9248. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.