Abstract
Estrogens play a pivotal role in the control of female reproductive function. Recent studies using primate GnRH neurons derived from embryonic nasal placode indicate that 17β-estradiol (E2) causes a rapid stimulatory action. E2 (1 nM) stimulates firing activity and intracellular calcium ([Ca2+]i) oscillations of primate GnRH neurons within a few min. E2 also stimulates GnRH release within 10 min. However, the classical estrogen receptors, ERα and ERβ, do not appear to play a role in E2-induced [Ca2+]i oscillations or GnRH release, as the estrogen receptor antagonist, ICI 182,780, failed to block these responses. Rather, this rapid E2 action is, at least in part, mediated by a G-protein coupled receptor GPR30. In the present study we further investigate the role of ERα and ERβ in the rapid action of E2 by knocking down cellular ERα and ERβ by transfection of GnRH neurons with specific siRNA for rhesus monkey ERα and ERβ. Results indicate that cellular knockdown of ERα and ERβ failed to block the E2-induced changes in [Ca2+]i oscillations. It is concluded that neither ERα nor ERβ is involved in the rapid action of E2 in primate GnRH neurons.
Keywords: GnRH neurons, ER alpha, ER beta, GPR30, primates, rapid estradiol action
Introduction
17β-Estradiol (E2) modulates a wide variety of neuronal functions. It is a key regulator of the stimulatory and inhibitory release of GnRH during the reproductive cycle [1], it is necessary for induction of reproductive behavior in most mammalian species [2], it is important for memory [3], and neuroprotection against brain insult or neurodegenerative diseases [4]. Importantly, estrogen action in those neuronal functions are a result of “nuclear-initiated steroid signaling” pathways involving the estrogen receptors, ERα and ERβ [5], through which E2 activates gene transcription.
More recently, a rapid action of E2 in several neuronal cell types through “membrane-initiated steroid signaling” pathways [5] has been reported. For example, E2 hyperpolarizes guinea pig GnRH neurons [6], depolarizes guinea pig β-endorphin neurons [7], modifies cAMP production in GT1-7 cells [8], stimulates intracellular calcium, [Ca2+]i, oscillations in mouse GnRH neurons [9], and modulates metabotropic glutamate receptor function in rat hippocampal as well as striatal neurons [10-12]. “Membrane-initiated E2 signaling” occurs within one minute to 10-15 min, whereas “nuclear-initiated E2 signaling” takes hours to days.
We have also shown that E2 induces a rapid action in primate GnRH neurons in the presence or absence of tetrodotoxin (TTX). Using a patch clamp recording method both E2 and E2-BSA, a plasma membrane impermeable form of E2, increased firing activity within a few minutes [13]. Similarly, with a calcium imaging method both E2 and estrogen dendrimer conjugate (EDC) [14], a nuclear membrane impermeable form of E2, resulted in an increase in the pulse frequency and synchronization of [Ca2+]i oscillations with a short latency in GnRH neurons [15]. Moreover, E2 stimulates GnRH release in vitro [16]. However, the classical estrogen receptors, ERα and ERβ, do not appear to play a role in E2-mediated [Ca2+]i oscillations or GnRH release, as the estrogen receptor antagonist, ICI 182,780, failed to block these responses [16]. Rather, this rapid E2 action appears to be mediated by a G-protein coupled receptor GPR30 as: 1) PTX treatment completely blocked the E2-induced [Ca2+]i oscillations and GnRH release, 2) a GPR30 agonist, G1, mimicked E2 action and 3) human GPR30 specific siRNA blocked E2-induced [Ca2+]i oscillations [16].
Even though ICI 182,780 failed to block the rapid E2 action in primate GnRH neurons in our previous study, one can argue that the involvement of ERα and ERβ in the rapid E2 action is not ruled out unless ERα and ERβ are eliminated from our preparation. Therefore, in the present study, we conducted experiments to examine whether cellular knockdown of ERα and ERβ influences E2 action in primate GnRH neurons.
Materials and Methods
Animals
Rhesus monkey embryos (Macaca mulatta) from time-mated pregnancies were delivered by cesarean section under isoflurane anesthesia. A total of 7 fetuses between embryonic day 36 (E36) and E39 were used in this study. All experimental procedures were conducted in accordance with the standards outlined in the Principles for the Use of Animals and Guide for the Care and Use of Laboratory Animals. The protocol used in these studies was approved by the Animal Care and Use Committee of the University of Wisconsin-Madison.
Tissue Culture from the olfactory placode
Culture methods for GnRH neurons derived from the fetal nasal placode region have previously been described [17,18]. Briefly, the nasal placode and the ventral GnRH neuronal migratory pathway (terminal nerve region) were dissected out and cut into small (<0.5 mm3) pieces. Two to three pieces were plated on each collagen coated 25-mm round glass coverslip. On the fourth day of culture, cells were exposed to an antimitotic agent, 5-fluoro-5-deoxyuridine (40 μM), for 2 days in order to eliminate non-neuronal cells and to better visualize GnRH neurons. Cultures were incubated at 37°C, 1.5% CO2 in culture media (Medium 199 + l-glutamine, Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Inc. Logan, UT), 0.6% glucose, and 50 μg/ml gentamycin (Sigma) for at least two weeks before experiments. Medium was replaced every 1-3 days as needed. All experiments began after two weeks in culture and finished by the end of the fourth week in culture. Transfections: As previously described [16], 48-72 h prior to initiation of experiments GnRH neurons were transferred to serum free media and transiently transfected with human specific ERα, ERβ, or control siRNA using Fugene HD Transfection reagent (Roche Diagnostics, Indianapolis, IN) at 37°C following the manufacturer’s instructions. On-target plus siRNA for human ERα provided by Dharmacon (Lafayette, CO) was targeted at the sequence: GAAUGUGCCUGGCUAGAGA, Human ERβ On-target plus siRNA was targeted at the sequence: GGAAAUGCGUAGAAGGAAU, and the On-target plus scramble control (catalog number D-001810-01-20) was a nonspecific sequence control. ERα and ERβ siRNA sequences were 100% identical to macaca mulatta ESR1 (accession # XM_001097228.1) and ESR2 (accession # XM_001101433.1), respectively.
Quantitative Real time PCR
RNA was isolated from neuronal cultures using RNA STAT-60 (Tel-test, Friendswood, TX) and the RT-reaction was performed using GeneAmp RNA PCR core kit (Applied biosystems). Quantitative RT-PCR was performed using SYBR GREEN Jump Start Taq ready Mix for Quantitative PCR (Sigma), primers were designed based on macaca mulatta ESR1 (accession # XM_001097228.1), ESR2 (accession # XM_001101433.1), and β-actin (accession # NM_001033084.1) genes using sequences that cross an intron/exon junction to eliminate genomic DNA amplification in the reaction. Primer sequences were as follows:
ESR1: Sense: 5′- CCTGATGATTGGTCTCGTCTG -3′
Antisense: 5′- GGCACACAAACTCCTCTCC -3′
ESR2: Sense: 5′- AGTATCTCTGTGTCAAGGC -3′
Antisense: 5′- GAGCATCAGGAGGTTAGC -3′
β-actin: Sense: 5′- CTCTTCCAGCCTTCCTTCCT -3′
Antisense: 5′- AGCACTGTGTTGGCGTACAG -3′
Relative amounts of mRNA were determined using the ΔΔ CT method with β-actin as the internal control and relative to control siRNA samples. Quantitative RT PCR reactions were run in triplicate on a DNA Engine Opticon System PTC-200 DNA engine Cycler with CFD-3200 Opticon Detector (MJ Research, San Francisco, CA) using the following protocol, 94°C for 2 min (initial denaturing), followed by 45 cycles of amplification (plate read after each cycle): 94°C for 15 sec (denaturing); 58°C ERα primers (59.3°C for ERβ, 60°C for β–actin) for 30 sec (annealing); 72°C for 45 sec, and completed with a dissociation step for melting point analysis from 50°C-94°C with a plate read every 1°C. Melt curves yielded single peaks, which were distinct for each product. PCR products were run on a 5% agarose gel, cut out, isolated using Quantum Prep Freeze ‘N Squeeze DNA Gel Extraction spin Columns (Bio Rad), and sequenced at the University of Wisconsin-Madison biotechnology sequencing center. Reactions yielded single bands with sequences matching macaca mulatta ESR1 for ERα primers and ESR2 for ERβ primers.
Measurement of [Ca2+]i
As previously detailed [15,16] [Ca2+]i levels were assessed by loading cultured cells on glass coverslips with 18 μM fura-2 AM (Teflab, Austin, TX) and 6 μl of a mixture of pluronic F-127 and DMSO (BASF Pharma/Knoll AG, Parsippany, NY) for 30 min at 37°C and 1.5% CO2. The coverslip was then placed in a Dvorak-Stotler chamber. Flourescence imaging of the dye loaded cells was achieved with an inverted microscope. GnRH neurons were identified and viewed through a 20X objective lens with a 750×750 μm recording field. Cultures were perifused with serum free M199 media (pH 7.4, 95% O2, 5% CO2) at a speed of 50 μl/min at room temperature under low light conditions.
[Ca2+]i was determined from the function of the ratio of the 510-nm fura-2 emission excited by illuminations at 340 and 380 nm with a Lambda DG-4 light source and filter exchanger (Sutter Instruments, Novato, CA). Fura-2 flourescence was recorded at 10 sec intervals with a charge-coupled device camera (Photometrics, Tucson, AZ) and using Metafluor imaging software (Molecular Devices Corp., Downingtown, PA). The ratio of the fluorescence intensities (ΔF/F0) from the 340- and 380-nm excitation was used to calculate free [Ca2+]i levels and saved as a text file in Microsoft Excel for analysis. Viability of GnRH neurons were assessed with a 56 mM KCl challenge at the end of each experiment.
GnRH neurons for [Ca2+]i imaging were identifiable by their unique morphology, size, and migratory pattern: large oval shaped soma (>10 μM) with large bundled somatic processes. Because these cultures contain many other cell types including epithelial cells, fibroblasts, and other unidentified cells as well as a small proportion of non-GnRH neurons [17,19], the identity of each GnRH neuron was confirmed by immunocytochemistry. The immunocytochemistry results were compared with a fluorescent image of the view area and matched with the location on the reference grid of the coverslip [19].
Immunocytochemistry
Culture dishes used for [Ca2+]i imaging experiments were stained using standard immunocytochemical procedures with an antisera cocktail of GF-6 and LR-1 [gifts from Dr. N.M. Sherwood (University of British Columbia, Victoria, Canada; 1:9000 dilution) and Dr. R.A. Benoit (University of Montreal, Montreal, Canada; 1:15,000)] for 40-42 h, the Vectastain ABC peroxidase system (Vector laboratories, Burlingame, CA), and 3,3′-diaminobenzidine (Sigma) as the chromogen. GnRH-positive cells were matched up with a digitized fluorescent image from the [Ca2+]i imaging experiments. Fibroblasts, epithelial cells and other GnRH negative neurons were excluded from all analyses.
Data Analysis
Peaks in [Ca2+]i oscillations were determined by Pulsar algorithm [20] as described previously [15]. After [Ca2+]i peaks were established, the average amplitude, interpeak interval, and number of peaks were counted in each neuron in 20 min blocks: −20-0, 0-20, 20-40, and 40-60 with time 0 corresponding to the initiation of treatment. Mean values for all neurons in a culture dish (n = 10-60) were calculated from these parameters. For statistical comparison of each treatment, overall mean values were obtained by averaging the mean values of each culture. For the purpose of graphical representation the frequency was presented as a percentage relative to 100 % at −20-0 min. All statistical analyses were conducted using raw data.
Synchronization of [Ca2+]i peaks in each culture was determined as previously described [15]. First, the average number of peaks was determined for the entire experimental time period. Then the mean number of peaks was calculated in consecutive 50 sec periods for the entire experiment. A synchronization was considered to occur if the mean rate in two consecutive 50 sec periods was greater than the total mean + 3 SD, based on previous observations [18,19]. The total number of synchronizations in the 60 min period during and after treatments was expressed as the synchronization frequency. The effects of treatments were analyzed against their respective controls and statistics were performed using student’s t-test (unpaired, two tailed). All groups consisted of six cultures per group. Data are presented as means ±SEM.
Effects of treatment (E2 vs. vehicle, E2/vehicle in the presence of ERα/ERβ/control siRNA) for [Ca2+]i imaging, were examined using two-way ANOVA repeated measure followed by Bonferroni analysis. Statistical analysis of qPCR was conducted with one-way ANOVA. Significance was established at P<0.05.
Results
1. Effects of E2 on [Ca2+]i oscillations
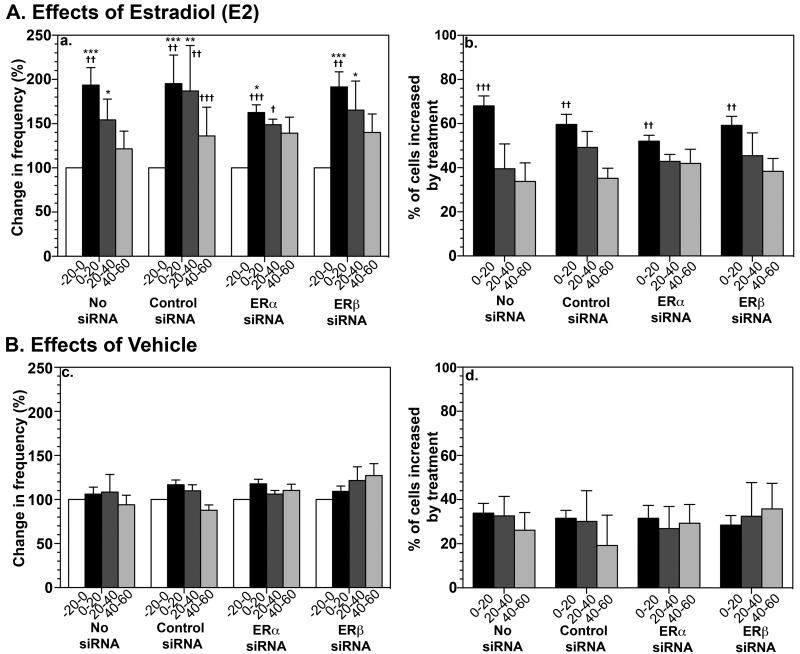
As we have shown previously, E2 (1 nM) exposure of GnRH neurons resulted in an increase in the frequency of [Ca2+]i oscillations and number of activated cells (Fig. 1A). E2 also stimulated the synchronization frequency of [Ca2+]i oscillations (Table 1). In contrast, vehicle infusion did not cause any significant effect (Fig. 1B, Table 1).
Figure 1.
Effects of siRNA transfection with ERα or ERβ specific siRNA on [Ca2+]i changes in GnRH neurons in the absence and presence of E2. E2 treatment resulted in a significant increase in the frequency of [Ca2+]i oscillations (Aa), calculated as frequency as a % of control, and the percentage of activated cells (Ab) regardless of siRNA transfection with control, ERα specific, or ERβ specific siRNA. These results were not significantly different from untransfected cultures (no siRNA). Vehicle treated cultures showed no significant changes in the frequency of [Ca2+]i oscillations (Bc), nor percentage of cells increased by treatment (Bd). *, P<0.05, **, P<0.01, ***, P<0.001 vs. before treatment; =, P<0.05, =, P<0.01, =, P<0.001 vs. respective vehicle control.
Table 1.
Effects of treatments on the synchronization of calcium oscillations in primate GnRH neurons
| Treatments | Synchronizations in the 60-min period after initiation of treatment (n) |
|---|---|
| No siRNA + Vehicle | 1.00± 0.18 |
| No siRNA+ E2 | 2.33± 0.47** |
| Control siRNA + Vehicle | 1.00± 0.26 |
| Control siRNA + E2 | 2.50± 0.62** |
| ERα siRNA + Vehicle | 0.57± 0.30 |
| ERα siRNA + E2 | 1.83± 0.40** |
| ERβ siRNA + Vehicle | 1.17± 0.54 |
| ERβ siRNA + E2 | 2.14± 0.80** |
N=6 in all treatment groups except for vehicle treatment ( N=12).
p< 0.01 vs. respective vehicle control.
2. Transfection of GnRH neurons with specific siRNA for human ERα and ERβ
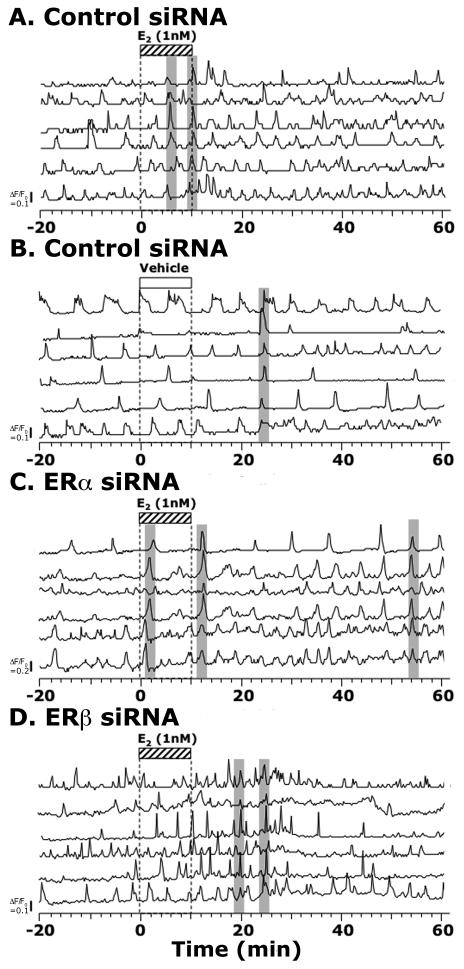
E2 (1 nM) exposure of GnRH neurons, which had been transfected with control siRNA, resulted in an increase in the number of activated cells and frequency of [Ca2+]i oscillations (Fig. 1A and 2A) as well as the synchronization frequency of [Ca2+]i oscillations (Table 1). E2 exposure of GnRH neurons, which had been transfected with specific siRNA for human ERα, failed to block the E2-induced changes in [Ca2+]i oscillations: E2 increased the number of activated cells and frequency of [Ca2+]i oscillations (Fig. 1A and 2C) and the synchronization frequency of [Ca2+]i oscillations (Table 1). Similarly, E2 exposure of ERβ siRNA transfected GnRH neurons failed to block the E2-induced changes in [Ca2+]i oscillations (Fig. 1A and 2D and Table 1). In contrast, vehicle exposure of GnRH neurons transfected with ERα siRNA, ERβ siRNA, or control siRNA did not cause any significant effect on [Ca2+]i oscillations (Fig. 1B and 2B, and Table 1).
Figure 2.
The effects of E2 on [Ca2+]i oscillations in siRNA transfected cultures. Examples of E2 effects on a control siRNA transfected culture (A), an ERα siRNA transfected culture (C), and an ERβ siRNA transfected culture (D) are shown. Effects of vehicle (as a control for E2) in a control siRNA transfected culture are also shown (B) for comparison. Each example shows 6 cells from a single culture.
3. Effects of siRNA transfection on expression of ERα mRNA and ERβ mRNA
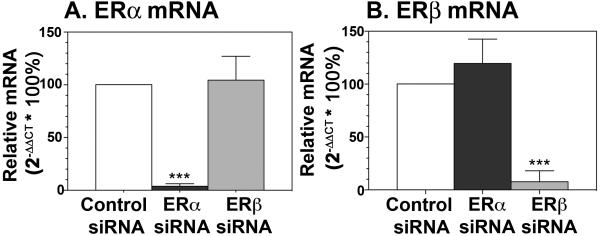
To determine the efficacy of transfection with ERα siRNA and ERβ siRNA, we transfected neurons and assessed ERα mRNA and ERβ mRNA levels using qPCR. Neurons from the fetal brain were dissected out after the olfactory placode was removed and cultured for at least 2 weeks. ER mRNA levels were standardized relative to β-actin mRNA for each sample, and comparisons were made using the ΔΔ CT method. Transfection with ERα siRNA significantly (p<0.001) reduced ERα mRNA levels, but not ERβ mRNA levels, when compared to those of control siRNA transfected cells (Fig. 3A). Similarly, transfection with ERβ siRNA significantly reduced ERβ mRNA but not ERα mRNA (Fig. 3B).
Figure 3.
Transfection of fetal brain cells with siRNA specific to ERα significantly reduced ERα mRNA levels (A) but not ERβ levels (B). Transfection with siRNA specific to ERβ significantly reduced ERβ mRNA (B) but not ERα mRNA (A) as determined by qPCR by the ΔΔ CT method using β-actin as an internal control. Data is representative of triplicate samples from 3 independent experiments. ***, P<0.001 vs. control siRNA.
Discussion
The results of the present study indicate that cellular knockdown of ERα and ERβ does not interfere with the rapid action of E2, as transfection of fetal neurons with siRNA for human ERα and ERβ reduced mRNA by 80-90 %, yet E2 still induced an increase in [Ca2+]i oscillations. Because there is a possible compensatory mechanism between ERα and ERβ, we further conducted experiments with double knockdown of ERα and ERβ. Preliminary data indicate that ERα and ERβ double knockdown does not block the E2-induced increase in [Ca2+]i oscillations.
It has been proposed in Chinese hamster ovarian cells that membrane ERs mediating E2 action are products of the same nuclear ERα and ERβ gene transcripts [21]. Indeed, the importance of ERα and ERβ for membrane initiated signaling has been reported in some neuronal cells. For example, rapid stimulatory action of E2 resulting in mitogen-activated protein kinase (MAPK)-dependent activation of cAMP-response element binding (CREB) protein is mediated by ERα with the glutamate receptor mGluR1, whereas E2’s attenuation of L-type calcium channel-mediated CREB phosphorylation is mediated by ERβ with mGluR2/3 phosphorylation in rat hippocampal pyramidal neurons [10,11]. Similarly, E2 via coupling of ERα with mGluR5 activates MAPK-dependent CREB phosphorylation, whereas E2 through ERβ with mGluR3 attenuates L-type calcium channel-mediated CREB signaling in striatal neurons [12]. In mouse GnRH neurons, an E2-induced increase in [Ca2+]i oscillations is blocked by ICI 182,780 [9], and presumably mediated by ERβ, as mouse GnRH neurons express ERβ, but not ERα [22,23]. Likewise, E2 stimulates firing activity of mouse GnRH neuronsthrough ERβ [24], modifying L-type voltage gated calcium currents (VGCCs), as this E2 action is blocked by an ERβ antagonist [25].
There are also several examples that E2 signaling causing rapid action is not mediated by ERα and ERβ in the brain. First, Toran-Allerand et al. [26] show that E2 elicits rapid and sustained phosphorylation and MAPK-dependent activation, specifically extracellular signal-related kinases (ERK1 and ERK2), in neocortical neurons obtained from neonatal mice. Because this action of E2 is not blocked by ICI 182,780 and because a similar action of E2 can be induced in cortical neurons from ERα knockout mice, the authors have proposed the presence of ER-X, a membrane estrogen receptor, yet to be identified. Second, Kelly and his colleague show that the diphenyl acrylamide compound, STX, induces E2-like signaling in mouse hypothalamic neurons. Although this STX effect is blocked by ICI 182,780 [27], STX also causes E2-like action in ERα or ERβ knockout and ERα/ERβ double knockout mice [28], suggesting that STX sensitive receptors are independent of both ERα and ERβ. Third, GPR30, which was originally identified as a membrane estrogen receptor in cancer cells [29,30], appears to be involved in rapid E2 action in neuronal cells. In mouse GnRH neurons, E2 rapidly increases high voltage activated (HVA) currents primarily through L- and R-type VGCCs and this E2 action is blocked by ICI 182,780 in a subset of cells and appears to be mediated by ERβ, but it is also mediated by GPR30 in another subset of GnRH neurons, as they are stimulated by the GPR30 agonist G1 [25]. E2 also enhances excitatory postsynaptic potentials (EPSPs) in rat CA1 hippocampal neurons by stimulating Schaffer collateral fibers [31]. Similarly, E2 causes enhancement of EPSPs in CA1 hippocampal neurons from gonadectomized male and female ERα knockout mice. ICI 182,780 does not block the E2-induced potentiation of EPSPs in ERα knockout mice, and ICI 182,780 alone increased EPSPs in 5 of 12 ERα knockout mice [32], indicating membrane receptors, such as GPR30, not ERα or ERβ, mediate E2 actions. Indeed, in rat hippocampal neurons it has been shown that GPR30 is responsible for the E2-induced increase in EPSPs [33].
It has been proposed that GPR30 may not be a “stand-alone” receptor, but it may require a collaborator, forming a complex with ERα and ERβ [34]. For example, in some cancer cells the effects of both E2 and G1 are blocked by ICI 182,780 [35] and the formation of an E2-inducible ERα and GPR30 complex are also blocked by 10 μM ICI 182,780 [36]. In a subset of myelinated vagal nerves both E2 and G1 stimulate firing activity and these changes are sensitive to 10 μM ICI 182,780 [37]. Similarly, E2 and G1 cause protective action for the ischemia-induced global cell death in hippocampal CA1 neurons and this E2 action is blocked by 100 μM ICI 182,780 [33].
In nonhuman primate GnRH neurons, GPR30, rather than an ERα or ERβ-mediated signaling mechanism, appears to be involved in rapid E2 action: The E2-induced increase in the frequency of [Ca2+]i oscillations, the number of activated cells, and the frequency of synchronization in primate GnRH neurons [15] are not blocked by 100 nM ICI 182,780, but they are abrogated by cellular knockdown of GPR30 [16]. Results of the present study showing that both cellular knockdown of ERα and ERβ and ERα/ERβ double knockdown (Kenealy and Terasawa, unpublished observations) failed to modify the changes in the E2-induced changes in [Ca2+]i oscillations, support the notion that ERα and ERβ are not involved in the rapid E2 action characterized in primate GnRH neurons.
In ERα and ERβ negative breast cancer cells, the G protein coupled estrogen receptor, GPR30, is activated by 1 μM of ICI 182,780 [29,30]. Similarly, ICI 182,780 at a 1 μM dose alone elicits changes in the frequency of [Ca2+]i oscillations and the number of activated cells in primate GnRH neurons [16]. The mechanism of ICI 182,780 stimulation in GPR30 signaling is currently unknown. While E2 effects on changes in [Ca2+]i oscillations are not blocked by 100 nM ICI 182,780, E2 action does not occur in the absence of GPR30 [16]. Thus, it appears that GPR30, independent from ERα and ERβ, is responsible for E2 action in primate GnRH neurons. Nonetheless, in future studies we need to examine 1) whether GPR30 effects are blocked by a high dose of ICI 182,780 and 2) whether stimulatory effects of ICI 182,780 alone are blocked by the GPR30 antagonist G15.
The mechanism of rapid E2 action leading to an increase in [Ca2+]i oscillations and GnRH release is unknown at this time. It is quite possible that E2 may potentiate KATP channel activity through GPR30, as Zhang et al. (38) have shown in mouse GnRH neurons that both E2 and STX activate the protein kinase C (PKC)-protein kinase A (PKA) signaling pathway resulting in a rapid increase in KATP channel activity. We will investigate the role of the PKC-PKA signaling pathway in rapid E2 action in primate GnRH neurons in near future.
Rapid E2 action is mediated by multiple receptors and multiple signaling pathways in single neurons [39,40]. For example, G1, STX, and E2 all have neuroprotective effects against an ischemic insult to rat hippocampal CA1 neurons [41]. In mouse GnRH neurons ERβ and GPR30 both caused effects on different VGCCs [25]. We have also shown that in primate GnRH neurons, E2 action appears to be mediated by GPR30 and STX sensitive receptors [42]. Importantly, all membrane bound ERs are seven transmembrane (7TM) GPCRs or associated with 7TM GPCRs. In rat hippocampal neurons ERα and ERβ together with mGluR1 and mGluR2/3, respectively, activate Gαq and Gαi/o pathways [10,11], while STX sensitive receptors are thought to signal through Gαq/ Gβγ mechanisms [27,43]. Although the signal transduction mechanisms of E2 through GPR30 in primate GnRH neurons are yet to be clarified, it has been shown in cancer cells that GPR30 is associated with Gαs/Gβγ [29,30]. Considering the importance of E2 action with the complexity of neurocircuitry in the brain, we propose that E2 signals through different types of GPCRs in various neuronal cell types to provide flexibility for versatile neuronal function.
Acknowledgments
This research was supported by NIH grants: R01HD15433 and R01HD11355 and was possible to perform by NIH supports (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Footnotes
Disclosure summary: The authors have nothing to disclose.
References
- [1].Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–30. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- [2].Devidze N, Lee AW, Zhou J, Pfaff DW. CNS arousal mechanisms bearing on sex and other biologically regulated behaviors. Physiol Behav. 2006;88:283–93. doi: 10.1016/j.physbeh.2006.05.030. [DOI] [PubMed] [Google Scholar]
- [3].McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- [4].Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29:199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- [5].Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- [6].Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17b and m-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–4. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- [7].Kelly MJ, Ronnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002;67:447–56. doi: 10.1016/s0039-128x(01)00181-7. [DOI] [PubMed] [Google Scholar]
- [8].Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, et al. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neuroscretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- [9].Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–33. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neurosci. 2010;170:1045–55. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–20. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- [15].Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endocrinology. 2008;149:1155–62. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–59. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133:2379–90. doi: 10.1210/endo.133.5.8404690. [DOI] [PubMed] [Google Scholar]
- [18].Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone - releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140:1432–41. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- [19].Richter TA, Keen KL, Terasawa E. Synchronization of Ca2+ oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol. 2002;88:1559–67. doi: 10.1152/jn.2002.88.3.1559. [DOI] [PubMed] [Google Scholar]
- [20].Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- [21].Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear receptors (ERs originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- [22].Sharifi N, Reuss AE, Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology. 2002;143:2503–7. doi: 10.1210/endo.143.7.8897. [DOI] [PubMed] [Google Scholar]
- [23].Herbison, Pape New Evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- [24].Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–27. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–23. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy SJ, et al. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–55. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- [30].Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- [31].Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. PNAS. 2000;97:3602–7. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor alpha knockout mice. Neurosci Lett. 2001;309:207–9. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- [33].Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–61. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Levin ER. Minireview: Extranuclear steroid receptors: rolesin modulation of cell functions. Mol endocrinol. 2010 Sep 22; doi: 10.1210/me.2010-0284. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to β–estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–66. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- [36].Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, et al. G protein-couped receptor 30 expression is upregulated by EGF and TGF{alpha} in estrogen receptor{alpha}-positive cancer cells. Mol Endocrinol. 2009;23:1815–26. doi: 10.1210/me.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qiao G, Li B, Lu Y, Fu Y, Schild JH. 17b-estradiol restores excitability of a sexually dimorphic subset of myelinated vagal afferents in ovariectomized rats. Am J Physiol Cell Physiol. 2009;297:C654–C664. doi: 10.1152/ajpcell.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang C, Kelly MJ, Rønnekleiv OK. 17Beta-estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151:447784. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in the luteinising hormone-releasing hormone neurons: the role of GPR30. J Neuroendocrinol. 2009;21:316–21. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kenealy BP, Keen KL, Ronnekleiv OK, Terasawa E. STX, a novel non-steroidal estrogenic compound, induces rapid action, calcium signaling and decapeptide release in primate GnRH neurons; SRC summer Research Conferences FASEB; The physiology of integrated nuclear and extranuclear steroid signaling; Snowmass CO. Aug 8-13.2010. [Google Scholar]
- [43].Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Ronnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–24. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]