Molecular imaging is defined as the in vivo diagnosis of complex pathological processes by detection of unique biological signatures at the sub-cellular level. To accomplish this, specialized imaging agents are required that accumulate at the site of interest and bring enough “payload” with them to be detected via magnetic resonance imaging.
Molecular imaging with perfluorocarbon nanoparticles has matured significantly during recent years. Experiments in a rabbit model have shown its potential to image angiogenesis, which is an early marker of disease, quantifying the extent and distribution of biomarkers to characterize tumors and predict and monitor the response to therapy.
As we continue to broaden our attention to the complete cycle of care for patients, the concept of individualized care becomes increasingly important, driving a paradigm shift leading toward personalized medicine [1]. Some newly-developed therapies are extremely potent and highly specific at treating disease, e.g. cancer; but they require detailed and precise patient stratification.
Given the vast diversity of pathology, a particular targeted therapy can help only a selected sub-group of patients whose disease expresses a specific molecular target. As the therapy side effects may be severe and targeted therapies are expensive, molecular imaging (MI) based stratification opens the way to reduce unnecessary risk to patients, while also helping to manage the associated healthcare costs. Furthermore, direct combination of MI techniques and novel therapeutic nanoparticles (i.e. “theranostics”) may provide a means to characterize disease, confirm and quantify therapy delivery, and monitor the response (or lack thereof) to therapy serially and noninvasively.
In 2003, a special issue of Medicamundi was devoted to “Molecular Imaging: The Road Ahead” [2] covering a large spectrum of molecular imaging modalities (from Nuclear Medicine to High Intensity Focused Ultrasound), in which we contributed results and an outlook on applications of magnetic resonance (MR) imaging using targeted imaging agents [3]. In the present article, we review where this road has led in the past years, in particular in the field of molecular imaging and targeted therapy based on site-targeted, paramagnetic, perfluorocarbon (PFC) nanoparticles in combination with water- and fluorine-based MR imaging. After a brief recapitulation of the original article, we cover state-of-the art applications in imaging and quantification of angiogenesis by means of pre-clinical examples as well as insights into the role of MR in therapy solutions.
MR molecular imaging
Written shortly after the completion of the Human Genome Project [4], the previous article heralded that one promise offered by genomics and proteomics is that the multitude of unique biomarkers uncovered could be applied to medical imaging and therapeutics at the molecular scale. Defined as “the in vivo diagnosis of complex pathological processes by detection of unique biological signatures at the sub-cellular level,” molecular imaging was predicted to be useful to improve the specificity, sensitivity and accuracy of diagnosis by visualizing and quantifying the molecular components and processes of disease.
To accomplish this, specialized imaging agents are required: agents that accumulate at the site of interest through targeting and bring enough “payload” with them to amplify the signal sufficiently to be detected via MRI. The specific targeting of these biomarkers can be via passive or active means.
Passive targeting is achieved based on the agents’ size, charge, or other attribute in combination with site-specific tissue properties (e.g. hyperpermeability, macrophage response) causing them to accumulate at the site of interest. An example is the uptake of iron oxide particles in macrophages, which accumulate within lymph nodes [5, 6].
Active targeting is achieved using ligand molecules that specifically bind to receptors of interest (proteins, cell surface markers, etc.). Ligands under investigation include antibodies, peptides, polysaccharides, aptamers and drugs [7–10].
In the case of biomarkers that are richly presented (as with fibrin in blood clots and ruptured atherosclerotic plaque), numerous copies of the molecular imaging agent can bind, thereby rising above the detection limit for measuring with MRI.
In the case of sparse biomarkers, other amplification schemes are required. One such scheme [3, 11, 12] utilizes nanometer-sized particles as targeted carriers (Figure 1). Each nanoparticle carries thousands of contrast molecules (i.e. paramagnetic gadolinium chelates) such that for every binding event, tens of thousands of gadolinium molecules are delivered to the imaging voxel. In this fashion, the longitudinal relaxivity (i.e. effectiveness of the agent in shortening T1 and enhancing signal intensity on T1-weighted images) can be many orders of magnitude stronger than typical gadolinium-based contrast agents used clinically. The PFC nanoparticles invented in our laboratories [11–14] and discussed in the prior review employ this technique, but also bring within their core high concentrations of fluorinated molecules, which are visible by MRI based on the fluorine nucleus as described by Morawski et al. [15], and elsewhere in this issue of Medicamundi by Makowski et al. [16].
Figure 1.

Paradigm for targeted molecular imaging agents based on nanoparticle (NP) technology. The NP core consists of perfluorocarbon compounds, containing a large number of fluorine nuclei. This example has a payload of gadolinium- chelates and antibodies. While gadolinium or fluorine serve as imaging labels for T1-weighted water MRI or 19F) MRI, respectively, therapeutic payloads like drugs or genes transform the particle to a ‘theranostic’ nano-device (Figure reprinted with permission from Lanza et al. [3]).
One of the first demonstrated applications of ligand-directed molecular imaging was in the context of atherosclerosis [12]. A defining characteristic of ruptured atherosclerotic plaque is the formation of micro-thrombi within the fractures. This type of plaque is vulnerable to rupture again in the future, which may result in life-threatening thrombi. The fibrin associated with the micro-thrombi can be exploited as a target. MR imaging of a thrombus situated in the external jugular vein of a dog was highlighted earlier (Figure 3a in Lanza et al. [3]) where the fibrin-bound paramagnetic nanoparticles caused conspicuous enhancement on T1-weighted gradient-echo imaging.
Advancements in molecular imaging of angiogenesis
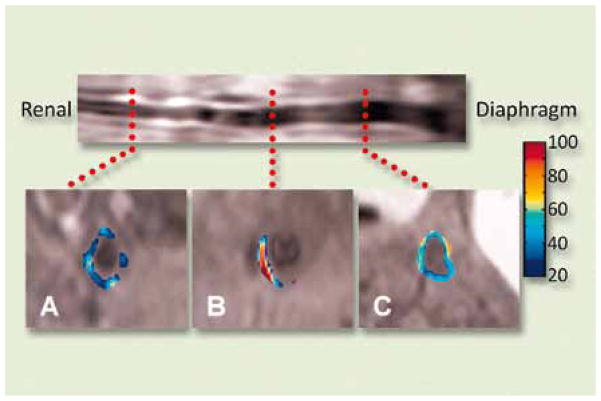
Angiogenesis plays a key role in many diseases, including atherosclerosis [17, 18] and cancer [19]. As atherosclerosis progresses, an increase in the vasa vasorum is required to support it. This neo-vasculature, as with all new vessel angiogenesis, can be targeted specifically through biomarkers such as the αvβ3-integrin, which is exposed to the vessel lumen on activated endothelial cells, but is not expressed in mature, quiescent vasculature [20, 21]. By targeting paramagnetic nanoparticles to the αvβ3-integrin [22], this early marker of atherosclerosis can be detected via MR molecular imaging as was demonstrated in hyperlipidemic rabbit models [23]. The extent and distribution of atherosclerosis was measured through pre- and post-contrast T1-weighted MI (Figure 2) and verified by histology. Similarly, αvβ3-integrin has been targeted to provide 3D visualization of tumor-related angiogenesis in cancer models uch as the Vx-2 tumor in rabbits [24, 25].
Figure 2.
In vivo spin-echo image reformatted to display the long axis of the aorta from renal arteries to diaphragm of a cholesterol-fed rabbit (top) and at three transverse levels - renal (A), central (B) and diaphragm (C) - with color-coded signal enhancement above baseline (in percent) upon binding of αvβ3-targeted nanoparticles in the neo-vasculature of atherosclerotic plaque (Figure reprinted with permission from Winter et al. [23]).
The potential to image biomarkers early in the disease process is certainly a central benefit of MI. However, in addition to early diagnostic information, MI promises better characterization of disease. For example, it has been shown by Wu et al. that high levels of angiogenesis and are linked to the aggressiveness of a tumor αvβ3 [19]. Using αvβ3-integrin-targeted PFC nanoparticles, one can measure the extent and distribution of angiogenesis around a growing tumor to characterize its aggressiveness. Schmieder et al. [26] did this in a serial fashion for the Vx-2 rabbit model illuminating the “angiogenic switch” which occurred between 8 and 14 days when the tumor, with the oxygen demand exceeding the limit supplied via diffusion, began instigating rapid new vessel growth. Similarly, they used MR molecular imaging to characterize smaller, less aggressive MDA435 tumors in mice which, indeed, exhibited much less αvβ3-integrin and angiogenesis. The 3D maps of neo-vasculature created from T1-weighted MI (cf. Figure 7) are a valuable tool in characterizing tumors. Nevertheless, the absolute quantification of the degree of angiogenesis can be difficult. Exploiting the fluorine signal from these targeted PFC nanoparticles, however, brings many benefits to quantitative imaging of angiogenesis.
Figure 7.

Three-dimensional neo-vascular maps of Vx-2 tumors in rabbits showing the peritumor angiogenesis as detected with αvβ3-targeted paramagnetic nanoparticles, derived from T1-weighted fat-suppressed, 3-D gradient echo MR images (250 × 250 × 500 μm3, TR/TE = 40/5.6 ms, 65° excitation angle, 1.5T). The tumor surface (gray mesh) and T1-enhanced angiogenesis (blue) are displayed at day 16 following therapy. Treatment with αvβ3-targeted fumagillin nanoparticles (a) is compared with the effect of αvβ3-targeted nanoparticles without drug (b). The tumor receiving therapy has much less neo-vasculature and remains significantly smaller than the control tumor which exhibits much more neo-vasculature and has grown unabated. Similar 3D neovascular maps can be used to predict response to anti-angiogenic therapy [46]. (Figure reprinted with permission from Winter et al. [25]).
Fluorine MR molecular imaging
There are two principal shortcomings of molecular MRI using imaging agents that rely on the water signal relaxation for detection:
The necessity to acquire pre- and post-injection images
The indirect method for agent quantification.
The MR water signal is ubiquitous and shows a large range of native relaxation values - which form the basis for the rich spectrum of possible MR soft tissue contrasts. Hence, the influence of exogenous agents on the water signal can only be assessed via comparison of images taken pre- and post-contrast injection. For most targeted molecular imaging agents, a significant delay on the order of a few hours is necessary between these two image acquisitions, because the target mechanism accumulates agent concentration locally over time and the blood-pool concentration has to return to near pre-contrast levels for a good specificity. In clinical workflow, this would result in a double examination per patient, adding preparation time and introducing error sources due to re-positioning – a potential hurdle for the clinical introduction.
MR physics offers a complementary label for specific agent detection and direct quantification: the nucleus of fluorine atoms. While the MR-active isotope (19F, spin ½) is 100% abundant, there is virtually no native signal from biological tissue. Thus, 19F-nuclei from exogenous agents are visible as “hot spots” on a dark background with no anatomical information, comparable to nuclear imaging techniques (e.g. PET). The MR signal from fluorine is well distinguished from the water (proton) signal because of a 6% difference in resonance frequency. Furthermore, the signal strength of individual 19F-nuclei is comparable to that of protons (83%) - significantly higher than other MR-active nuclei (e.g. 23Na, 31P, C).
The nanoparticle-based MR molecular imaging approach, as described thus far, directly benefits from fluorine MRI because of the PFC core. The core contains a large number of 19F-nuclei - about 108 per particle - which provides an enormous amplification factor per pathophysiological target site. Furthermore, fluorine signal intensity, measured in a single time point examination, can directly be related to the local agent concentration and, thus, supersedes the need for pre- and post-injection imaging. Multi-nuclear (i.e. non-proton) MR spectroscopy and imaging capability, including support of fluorine MRI and basic methodology, is commercially available on most clinical systems. Advanced imaging methods, e.g. for motion compensated and calibrated fluorine quantification, are active areas of research, many of which are ripe for clinical translation [27–31].
First results in fluorine-based pre-clinical MR imaging of angiogenesis in cardiovascular diseases (atherosclerosis [14–16]) and oncology (tumor angiogenesis [30]) demonstrate the potential for targeted diagnosis, stratification for anti-angiogenic therapy, and subsequent therapy monitoring.
Figure 3. shows an example of combined fluorine and water imaging based on αvβ3-integrin targeted nanoparticles for quantitative assessment of angiogenesis in a rabbit tumor model (Vx-2 adenocarcinoma). This study was performed on a 3.0T clinical MR scanner (Philips Achieva) using a small animal coil which is simultaneously operable on both the fluorine and the water MR frequency [30].
Figure 3.
Quantification of αvβ3-targeted nanoparticle (NP) concentrations via fluorine MRI on a Vx-2 tumor in the hind leg of a New Zealand White Rabbit, measured on a 3.0 T Achieva MR scanner (Philips Healthcare). The milli-molar fluorine concentration from the NP at the target site (color scale 10–70 mM, right b,d,f) is superimposed with T1-weighted gradient echo images (left a,c,e) for anatomical co-localization in three selected image slices (out of 15). For an in-plane resolution of 2.2 mm and a slice thickness of 4 mm, the data acquisition was completed in 35 minutes. NP density can be derived from the fluorine concentrations and correlated with the degree of angiogenesis, which shows a heterogeneous pattern at the tumor rim as expected from tumor pathophysiology.
Within a measurement time of 35 minutes, the sensitivity of the fluorine image acquisition is high enough to monitor angiogenesis in the tumor rim in three dimensions at a spatial resolution of 2.2 × 2.2 × 4 mm as visualized as color overlay in Figure 3b,d,e. A 3-D gradient echo sequence was used for fluorine and a 3-D T1-weighted gradient-echo sequence in water image acquisition to depict morphology (specifically, the popliteal fossa of the hind leg, Figure 3a,c,e). The water image was acquired without changing the examination setup and was merged with the fluorine image for anatomical co-localization (Fig. 3b,d,e). The color scale corresponds quantitatively to the milli-molar (mM) concentration of fluorine nuclei at the molecular target site and can be correlated to the degree of angiogenesis.
As expected from the pathophysiology, angiogenesis is a heterogeneous process around the tumor, which is well reflected in the present fluorine MR molecular imaging example. The concentration of the occupied integrin targets amounts to about 100 pM, while the accumulated fluorine concentration, via nanoparticle based amplification, ranges up to 60 mM. The quantitative assessment of the angiogenic state is particularly important for therapy monitoring, because the effectiveness of anti-angiogenic therapy can be noninvasively confirmed at a very early time point.
Another advantage of fluorine-labeled imaging agents is the rich content of the magnetic resonance spectrum. The chemical shift, known from the water-fat shift in water based MRI, is particularly strong in fluorine compounds and ranges over more than 100 ppm of the fluorine resonance frequency (as compared to 3.5 ppm for the frequency shift between water and fat).
Figure 4 shows the spectra of two typical PFCs, perfluoro-octyl-bromide (PFOB) and perfluoro-crown-ether (PFCE). While PFOB exhibits seven individual resonance frequencies, PFCE is represented by a single central resonance. Imaging methodology can be specialized to separate and individually quantify different label substances, relying on these “fingerprint” spectral features. This is not possible for imaging agents acting over the water relaxivity (although an alternative approach in water MRI is achieved via chemical exchange saturation transfer (CEST) agents [32, 33]).
Figure 4.
Two typical fluorine (19F) spectra from perfluoro-carbon compounds: perfluoro-octyl-bromide (PFOB) shows 7 individual MR resonances (blue), well resolved in this spectrum acquired on a 3.0T clinical MRI scanner, over a large frequency range (about 60 ppm). Perfluoro-Crown-Ether is characterized by a single characteristic spectral features (“fingerprint”), different fluorine labels can be identified and quantified simultaneously.
Many pathophysiologies express diverse proteins the (dark red). Given these molecular level, like the large variety of different integrins and growth-factors in angiogenesis, and a single molecular target might not be sufficient for comprehensive and specific diagnosis. A cocktail of agents with different targeting moieties and discernible “multi-color” fluorine labels would be particularly suitable for diagnosis and therapy stratification within a large class of tumor phenotypes.
An example for “multi-color” fluorine MRI is shown in Figure 5 [34]. Fibrin targeting nanoparticles with PFOB and PFCE were supplied in variable mixtures to fibrin clots in vitro. The two nanoparticle types could be separately imaged by selecting different fluorine MR resonances (Fig. 5c,d, green for PFCE, cyan for PFOB) and the composition of the mixture was quantified via the signal intensity levels.
Figure 5.
Fluorine (19F) MRI of a mixture of differently labeled PFC nanoparticles bound to fibrin clots in vitro, acquired with steady-state gradient echo MR sequences within 3 minutes (at 1.5T). The clots are positioned at the bottom of water- filled vials, as visible in a water T1-weighted MR image (a). In the non-selective image acquisition (b), all fibrin clots are enhancing brightly irrespective the nanoparticle type or mixture. Variable RF excitation schemes were used for ‘multi-color’ detection the twofluorine image labels, (c) perfluoro-crown-ether (PFCE) in green and (d) perfluoro-octyl-bromide (PFOB) in cyan. The mixtures (PFCE:PFOB = 1:0 …0:1) are quantitatively reproduced in the signal intensity levels. (Figure reprinted in adapted form with permission from Caruthers et al. [34]).
Image-guided targeted therapeutics
Early diagnosis with quantitative characterization of disease could have considerable clinical relevance. However, in the context of the complete care cycle, this information is far more valuable if it can help to guide and monitor earlier and more effective therapies. Nanoparticles designed for MI can also be designed with multi-functionality as vehicles for targeted drug delivery.
Typically, particulate drug delivery vehicles are designed to carry the therapeutic agent within the particle itself. Such a design requires cellular uptake of the vehicle or particle destruction to accomplish drug release [35–37]. PFC nanoparticles, however, can be manufactured with lipophilic drugs embedded in the outer lipid monolayer of the particle. Since the drugs are lipophilic, they do not readily diffuse into the surrounding aqueous environment and they also do not dissolve in the PFC particle core. Therefore, the therapeutics are sequestered at the particle surface, presenting a high drug concentration to the target cell upon binding, but otherwise are not released and have no effect.
The drug is released primarily through interactions between phospholipid surface of the particle and the opposing targeted cell membrane [38]. This process is highly dependent on ligand-directed binding of the nanoparticles to cell surface receptors. The increased frequency and duration of interactions between the particle monolayer and the cellular bilayer substantially enhance the net transfer of drug into the target cell. As a result, these therapeutic nanoparticles do not require cellular uptake or particle disruption in order to release the drug at the target site [39]. Conversely, non-targeted nanoparticles do not induce any significant therapeutic effects either in cell culture experiments [38, 39] or in vivo animal models [40]. In addition, increasing the interactions between the nanoparticle and cellular surfaces, such as exposure to ultrasound energy, can largely augment the lipid exchange [40] (in this case, a tenfold increase was observed). These findings suggest that drug delivery is dependent upon specific targeting to the cellular membrane and lipid exchange.
Specific targeting of lipophilic therapeutics, such as the potent anti-angiogenic compound fumagillin [41], can be achieved by formulating the drug in the surface of αvβ3-integrin targeted nanoparticles. The nanoparticles also carry gadolinium such that MRI can be performed at treatment to assess the level of plaque angiogenesis, while simultaneously confirming and quantifying the specific binding of drug-loaded nanoparticles. As a model of atherosclerosis, cholesterol-fed rabbits were injected with αvβ3-targeted paramagnetic nanoparticles with or without fumagillin [40] (Figure 6).
Figure 6.

images of the abdominal aorta showing outline of segmented vessel wall ROI (top a,b), false-colored overlay of percent signal enhancement (middle c,d) at time of treatment with αvβ3-targeted paramagnetic nanoparticles either with (left c,e) or without (right d,f) fumagillin drug, and one week post-treatment (bottom e,f). The color overlays are thresholded at 10% enhancement to show some anatomical detail within the ROI, and were calculated using a semi-automated method by analyzing pre- and 3 hr post-injection T1-weighted black-blood images (spin echo, fat saturation, TR/TE = 380/11 ms, 0.25 × 0.25 × 5 mm, NSA = 8) (Figure reprinted with permission from Winter et al. [40]).
MRI signal enhancement averaged over the abdominal aorta was identical for both groups of animals four hours after injection, 16.7 ± 1.1% and 16.7 ± 1.6% (see Figure 6c,d). One week later, the anti-angiogenic effect was assessed by injecting diagnostic αvβ3-targeted nanoparticles (i.e. without drug). Aortic enhancement was dramatically reduced in fumagillin treated rabbits, 2.9 ± 1.6% (p < 0.05, Figure 6e), whereas control animals showed no change, 18.1 ± 2.1% (Figure 6f). A second group of control animals received non-targeted fumagillin nanoparticles, which did not produce any significant therapeutic response. These results suggest that effective drug delivery is only possible when the particles specifically bind to the target cell membrane in order to facilitate lipid exchange.
Histological sections showed a significant decrease in neo-vascular density in fumagillin treated animals compared to the controls (24 ± 5 vs. 73 ± 28, respectively, p = 0.05), corroborating the anti-angiogenic effect of targeted fumagillin nanoparticles observed by MRI. Furthermore, the T1-weighted signal enhancement measured on the day of initial treatment predicted the therapeutic response. Targeted fumagillin nanoparticles provided effective inhibition of angiogenesis at a dose 50,000 times lower than achieved with traditional oral delivery [41]. Coupled with the site-specific delivery, this extreme reduction in dose could greatly diminish the adverse side effects associated with fumagillin treatment, including temporary neurotoxic effects [43], while maintaining therapeutic efficacy.
Serial molecular imaging of angiogenesis was also used to monitor fumagillin treatment in conjunction with oral statin therapy [44] and demonstrated that two fumagillin doses combined with statin treatment provided a sustained anti-angiogenic effect (Figure 3 in Winter et al. [44]). Furthermore, the density of vessels co-staining for PECAM and αvβ3-integrin was directly related to the level of MRI enhancement measured in the aortas of untreated animals (Figure 6 in Winter et al. [44]). These results show that molecular imaging of angiogenesis with targeted nanoparticles correlates with microvascular density and can noninvasively monitor the effects of anti-angiogenic therapies.
Targeted PFC nanoparticles can also serve as an anti-angiogenic drug delivery platform against tumors [25], using a much lower dose than is required for systemic administration. Tumor bearing rabbits were treated with αvβ3-targeted nanoparticles carrying 30 μg/kg of fumagillin. Four days later, MRI was performed with αvβ3- targeted paramagnetic nanoparticles to quantify tumor size and assess neo-vascularity. Tumor volume was reduced among treated rabbits compared with controls (Figure 7).
Molecular imaging of control rabbits revealed angiogenesis predominantly located in the tumor rim (Figure 7b), which decreased significantly with -targeted nanoparticle treatment (Figure αvβ3 7a). Microscopically, the tumor parenchyma tended to show T-cell infiltration after targeted drug treatment, which was not appreciated in control animals. These results suggest that -targeted fumagillin nanoparticles can elicit αvβ3a marked anti-angiogenic effect and diminish tumor growth with drug levels 1000-fold lower than those used in systemic delivery applications (30 μg/kg versus 30 mg/kg).
In a similar study, Schmieder et al. [46] delivered anti-angiogenic therapy to MDA435 tumors in mice. Though the amount of angiogenesis in these tumors was small, there was a measurable, albeit much lower, effect of therapy. Comparing these two studies, one can begin to appreciate the power MI offers in determining the amount of angiogenesis prior to commencing therapy, thereby predicting response and guiding choices [46]. Ultimately, this can lead to reduced costs and, more importantly, prevent the unnecessary exposure of the patient to extreme side effects of a chemotherapy which is not going to be effective in fighting the cancer.
While the reported studies on serial monitoring of therapy successfully relied on water relaxivity measurements in pre-/post-injection, we would like to point out the high potential of fluorine MRI for nanoparticle based therapy control. It is possible to define thresholds of paramagnetic enhancement (pre- and post-injection) for the assessment of the level of angiogenesis, when monitoring therapy with gadolinium-labeled nanoparticles, as reported above. However, the fluorine-based quantification yields a direct readout of local nanoparticle concentrations and may result in a reproducible measure of the degree of angiogenesis. Thus, the degree of angiogenesis can be reliably monitored over time and accurately compared to standards, which would be particularly helpful in comparative effectiveness studies. The two MI techniques, water- and fluorine-based MR of targeted nanoparticles, will have to be compared in terms of sensitivity, specificity and reproducibility for future clinical applications.
Conclusion and future directions
Magnetic Resonance Molecular Imaging with PFC nanoparticles has matured significantly during the last few years. It was applied to image early markers of disease, quantifying the extent and distribution of biomarkers to characterize tumors and predict the response to therapy. MI has been proven to be useful in serial monitoring of targeted therapy delivery, with the potential of guiding therapeutic choices toward personalized medicine. As these and other imaging agents are initiating translation into human clinical trials, the clinical use of targeted MI agents seems to be well within sight on “the road ahead.”
Contributor Information
S.D. Caruthers, C-TRAIN, Washington University, St. Louis, MO, USA
P.M Winter, C-TRAIN, Washington University, St. Louis, MO, USA.
S.A. Wickline, C-TRAIN, Washington University, St. Louis, MO, USA
G.M. Lanza, C-TRAIN, Washington University, St. Louis, MO, USA
J. Keupp, Philips Research Europe, Hamburg, Germany
References
- 1.Pettigrew RI, Fee CA, Li KC. Changes in the World of Biomedical Research are Moving the Field of “Personalized Medicine” From Concept To Reality. J Nucl Med. 2004;45(9):1427. [PubMed] [Google Scholar]
- 2.Molecular Imaging: The Road Ahead. Medicamundi. 2003;47(1) http://www.philips.com/medicamundi.
- 3.Lanza GM, Lamerichs R, Caruthers S, Wickline SA. Molecular Imaging in MR with Targeted Paramagnetic Nanoparticles. Medicamundi. 2003;47(1):34 – 39. [Google Scholar]
- 4.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial Sequencing and Analysis of the Human Genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall Superparamagnetic Iron Oxide: An Intravenous Contrast Agent for Assessing Lymph Nodes with MR Imaging. Radiology. 1990;175(2):494–8. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 6.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, Van De Kaa CH, et al. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 7.Gaffney PJ, Gascoine PS, Creighton LJ, Tymkewycz PM. Monoclonal Antibodies for the Detection of Thrombosis. Adv Exp Med Biol. 1990;281:419–27. doi: 10.1007/978-1-4615-3806-6_45. [DOI] [PubMed] [Google Scholar]
- 8.Britz-Cunningham SH, Adelstein SJ. Molecular Targeting with Radionuclides: State of the Science. J Nucl Med. 2003;44(12):1945–61. [PubMed] [Google Scholar]
- 9.Lipinski MJ, Fuster V, Fisher EA, Fayad ZA. Technology Insight: Targeting of Biological Molecules for Evaluation of High-Risk Atherosclerotic Plaques with Magnetic Resonance Imaging. Nat Clin Pract Cardiovasc Med. 2004;1(1):48–55. doi: 10.1038/ncpcardio0013. [DOI] [PubMed] [Google Scholar]
- 10.Shukla R, Thomas TP, Peters JL, Desai AM, Kukowska-Latallo J, Patri AK, et al. HER2 Specific Tumor Targeting with Dendrimer Conjugated Anti-HER2 mAb. Bioconjug Chem. 2006;17(5):1109–1115. doi: 10.1021/bc050348p. [DOI] [PubMed] [Google Scholar]
- 11.Lanza G, Wallace K, Scott M, Cacheris W, Abendschein D, Christy D, et al. A Novel Site-Targeted Ultrasonic Contrast Agent with Broad Biomedical Application. Circulation. 1996;94:3334–3340. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- 12.Flacke S, Fischer S, Scott M, Fuhrhop R, Allen J, Mc Lean M, et al. A Novel MRI Contrast Agent for Molecular Imaging of Fibrin: Implications for Detecting Vulnerable Plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 13.Lanza G, Lorenz C, Fischer S, Scott M, Cacheris W, Kaufman R, et al. Enhanced Detection of Thrombi with a Novel Fibrin-Targeted Magnetic Resonance Imaging Agent. Acad Radiol. 1998;5(Suppl 1):S173–S176. doi: 10.1016/s1076-6332(98)80097-4. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Song SK, Chen J, Scott MJ, Fuhrhop RJ, Hall CS, et al. High-Resolution MRI Characterization of Human Thrombus Using a Novel Fibrin-Targeted Paramagnetic Nanoparticle Contrast Agent. Magn Reson Med. 2000;44(6):867–872. doi: 10.1002/1522-2594(200012)44:6<867::aid-mrm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Morawski AM, Winter PM, Yu X, Fuhrhop RW, Scott MJ, Hockett F, et al. Quantitative “Magnetic Resonance Immunohistochemistry” with Ligand-Targeted (19)F Nanoparticles. Magn Reson Med. 2004;52(6):1255–1262. doi: 10.1002/mrm.20287. [DOI] [PubMed] [Google Scholar]
- 16.Makowski MR, Indermeule A, Andia M, Jansen CHP, Botnar RM, Wiethoff AJ, et al. Molecular MRI of Atherosclerosis: From Mouse to Man. Medicamundi. 2010;54(2):xx–yy. [Google Scholar]
- 17.Moulton KS. Plaque Angiogenesis: Its Functions and Regulation. Cold Spring Harb Symp Quant Biol. 2002;67:471–482. doi: 10.1101/sqb.2002.67.471. [DOI] [PubMed] [Google Scholar]
- 18.Krupinski J, Font A, Luque A, Turu M, Slevin M. Angiogenesis and Inflammation in Carotid Atherosclerosis. Front Biosci. 2008;13:6472–6482. doi: 10.2741/3167. [DOI] [PubMed] [Google Scholar]
- 19.Wu I, Moses MA. Angiogenic Molecules and Mechanisms in Breast Cancer. Curr Oncol Rep. 2000;2(6):566–571. doi: 10.1007/s11912-000-0111-z. [DOI] [PubMed] [Google Scholar]
- 20.Eliceiri BP, Cheresh DA. Role of Alpha V Integrins During Angiogenesis. Cancer J. 2000;6 (Suppl 3):S245–249. [PubMed] [Google Scholar]
- 21.Kerr JS, Mousa SA, Slee AM. Alpha(V)Beta(3) Integrin in Angiogenesis and Restenosis. Drug News Perspect. 2001;14(3):143–150. [PubMed] [Google Scholar]
- 22.Anderson SA, Rader RK, Westlin WF, Null C, Jackson D, Lanza GM, et al. Magnetic Resonance Contrast Enhancement of Neovasculature with Alpha(V)Beta(3)-Targeted Nanoparticles. Magn Reson Med. 2000;44(3):433–9. doi: 10.1002/1522-2594(200009)44:3<433::aid-mrm14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, et al. Molecular Imaging of Angiogenesis in Early-Stage Atherosclerosis with Alpha(V)Beta3-Integrin-Targeted Nanoparticles. Circulation. 2003;108(18):2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 24.Winter PM, Caruthers SD, Kassner A, Harris TD, Chinen LK, Allen JS, et al. Molecular Imaging of Angiogenesis in Nascent Vx-2 Rabbit Tumors Using a Novel Alpha(Nu)Beta3-Targeted Nanoparticle and 1.5 Tesla Magnetic Resonance Imaging. Cancer Res. 2003;63(18):5838–5843. [PubMed] [Google Scholar]
- 25.Winter PM, Schmieder AH, Caruthers SD, Keene JL, Zhang H, Wickline SA, et al. Minute Dosages of Alpha(Nu)Beta3-Targeted Fumagillin Nanoparticles Impair Vx-2 Tumor Angiogenesis and Development in Rabbits. Faseb J. 2008;22(8):2758–2767. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmieder AH, Williams TA, Allen JS, Hu G, Zhang H, Caruthers SD, et al. Time-Resolved Molecular Imaging of the “Angiogenic Switch” in Animal Models of Cancer. Proc Intl Soc Mag Reson Med. 2008:14. [Google Scholar]
- 27.Keupp J, Mazurkewitz PC. Simultaneous 19F/1H Imaging for Quantification: Calibration and Sensitivity Assessment. Proc Intl Soc Mag Reson Med. 2007:1334. [Google Scholar]
- 28.Keupp J, Rahmer J, Waters EA, Caruthers SD, Lanza GM, Wickline SA. In Vivo Quantification of 19F Molecular Imaging Agents with Improved Accuracy and Sensitivity Using Motion Correcting, Simultaneous 19F/1H Radial MRI. Proc Intl Soc Mag Reson Med. 2008:17. [Google Scholar]
- 29.Rahmer J, Keupp J, Caruthers SD. Self-Navigated Motion Compensation in Simultaneous 19F/1H 3D Radial Imaging Using Golden Means Profile Interleaving. Proc Intl Soc Mag Reson Med. 2008:1471. [Google Scholar]
- 30.Keupp J, Caruthers SD, Rahmer J, Williams TA, Wickline SA, Lanza GM. Fluorine-19 MR Molecular Imaging of Angiogenesis On Vx-2 Tumors in Rabbits Using Targeted Nanoparticles. αvβ3 Proc Intl Soc Mag Reson Med. 2009:223. [Google Scholar]
- 31.Rahmer J, Keupp J, Caruthers SD, Lips O, Williams TA, Wickline SA, et al. 19F/1H Simultaneous 3D Radial Imaging of Atherosclerotic Rabbits Using Self-Navigated Respiratory Motion Compensation. Proc Intl Soc Mag Reson Med. 2009:4611. [Google Scholar]
- 32.Woods M, Woessner DE, Sherry AD. Paramagnetic Lanthanide Complexes As PARACEST Agents for Medical Imaging. Chem Soc Rev. 2006;35(6):500–511. doi: 10.1039/b509907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD. PARACEST Agents: Modulating MRI Contrast via Water Proton Exchange. Acc Chem Res. 2003;36(10):783–790. doi: 10.1021/ar020228m. [DOI] [PubMed] [Google Scholar]
- 34.Caruthers SD, Neubauer AM, Hockett FD, Lamerichs R, Winter PM, Scott MJ, et al. In Vitro Demonstration Using 19F Magnetic Resonance To Augment Molecular Imaging with Paramagnetic Perfluorocarbon Nanoparticles At 1.5 Tesla. Invest Radiol. 2006;41(3):305–312. doi: 10.1097/01.rli.0000199281.60135.6a. [DOI] [PubMed] [Google Scholar]
- 35.Guzman LA, Labhasetwar V, Song C, Jang Y, Lincoff AM, Levy R, et al. Local Intraluminal Infusion of Biodegradable Polymeric Nanoparticles. A Novel Approach for Prolonged Drug Delivery After Balloon Angioplasty. Circulation. 1996;94(6):1441–1448. doi: 10.1161/01.cir.94.6.1441. [DOI] [PubMed] [Google Scholar]
- 36.Kolodgie FD, John M, Khurana C, Farb A, Wilson PS, Acampado E, et al. Sustained Reduction of In-Stent Neointimal Growth with the Use of a Novel Systemic Nanoparticle Paclitaxel. Circulation. 2002;106(10):1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- 37.Muller RH, Radtke M, Wissing SA. Nanostructured Lipid Matrices for Improved Microencapsulation of Drugs. Int J Pharm. 2002;242(1–2):121–128. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 38.Lanza GM, Yu X, Winter PM, Abendschein DR, Karukstis KK, Scott MJ, et al. Targeted Antiproliferative Drug Delivery to Vascular Smooth Muscle Cells with a Magnetic Resonance Imaging Nanoparticle Contrast Agent: Implications for Rational Therapy of Restenosis. Circulation. 2002;106(22):2842–2847. doi: 10.1161/01.cir.0000044020.27990.32. [DOI] [PubMed] [Google Scholar]
- 39.Crowder KC, Hughes MS, Marsh JN, Barbieri AM, Fuhrhop RW, Lanza GM, et al. Sonic Activation of Molecularly-Targeted Nanoparticles Accelerates Transmembrane Lipid Delivery to Cancer Cells through Contact-Mediated Mechanisms: Implications for Enhanced Local Drug Delivery. Ultrasound Med Biol. 2005;31(12):1693–1700. doi: 10.1016/j.ultrasmedbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, et al. Endothelial Alpha(V)Beta3 Integrin-Targeted Fumagillin Nanoparticles Inhibit Angiogenesis in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 41.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis Inhibitors Endostatin or TNP-470 Reduce Intimal Neovascularization and Plaque Growth in Apolipoprotein E-Deficient Mice. Circulation. 1999;99(13):1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 42.Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, et al. Synthetic Analogues of Fumagillin that Inhibit Angiogenesis and Suppress Tumour Growth. Nature. 1990;348(6301):555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 43.Herbst RS, Madden TL, Tran HT, Blumenschein GR, Jr, Meyers CA, Seabrooke LF, et al. Safety and Pharmacokinetic Effects of TNP-470, An Angiogenesis Inhibitor, Combined with Paclitaxel in Patients with Solid Tumors: Evidence for Activity in Non-Small-Cell Lung Cancer. J Clin Oncol. 2002;20(22):4440–4447. doi: 10.1200/JCO.2002.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic Synergism of Integrin-Targeted Fumagillin Nanoparticles and Atorvastatin in Atherosclerosis. JACC: Cardiovascular Imaging. 2008;1(5):624. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmieder AH, Caruthers SD, Zhang H, Williams TA, Robertson JD, Wickline SA, et al. Three-Dimensional MR Mapping of Angiogenesis with α5β1(αvβ3)-Targeted Theranostic Nanoparticles in the MDA-MB-435 Xenograft Mouse Model. Faseb J. 2008;22:4179–4189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmieder AH, Winter PM, Williams TA, Allen JS, Hu G, Zhang H, et al. MR Molecular Imaging of Neovasculature may Predict Response to Antiangiogenic Therapy in Animal Cancer Models. Proc Intl Soc Mag Reson Med. 2008:799. [Google Scholar]