Abstract
OBJECTIVE: To determine the prevalence and spectrum of mutations associated with long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) in a seemingly unexplained drowning cohort.
PATIENTS AND METHODS: From September 1, 1998, through October 31, 2010, 35 unexplained drowning victims (23 male and 12 female; mean ± SD age, 17±12 years [range, 4-69 years]) were referred for a cardiac channel molecular autopsy. Of these, 28 (20 male and 8 female) drowned while swimming, and 7 (3 male and 4 female) were bathtub submersions. Polymerase chain reaction, denaturing high-performance liquid chromatography, and DNA sequencing were used for a comprehensive mutational analysis of the 3 major LQTS-susceptibility genes (KCNQ1, KCNH2, and SCN5A), and a targeted analysis of the CPVT1-associated, RYR2-encoded cardiac ryanodine receptor was conducted.
RESULTS: Of the 28 victims of swimming-related drowning, 8 (28.6%) were mutation positive, including 2 with KCNQ1 mutations (L273F, AAPdel71-73 plus V524G) and 6 with RYR2 mutations (R414C, I419F, R1013Q, V2321A, R2401H, and V2475F). None of the bathtub victims were mutation positive. Of the 28 victims who drowned while swimming, women were more likely to be mutation positive than men (5/8 [62.5%] vs 3/20 [15%]; P=.02). Although none of the mutation-positive, swimming-related drowning victims had a premortem diagnosis of LQTS or CPVT, a family history of cardiac arrest, family history of prior drowning, or QT prolongation was present in 50%.
CONCLUSION: Nearly 30% of the victims of swimming-related drowning hosted a cardiac channel mutation. Genetic testing should be considered in the postmortem evaluation of an unexplained drowning, especially if a positive personal or family history is elicited.
Drownings claim the life of approximately 150,000 persons worldwide annually and are a leading cause of injury-related deaths in the young.1 From 1999 to 2007, more than 10,000 young people (<20 years) drowned in the United States; most of these drownings occurred inadvertently and could not be attributed to boating.2 Nearly half of all drownings occur in those younger than 20 years, and one-third of those victims are known to be accomplished swimmers.1 For every child who dies by drowning, it is estimated that another 4 children experience a serious non-fatal near-drowning episode, leaving many with permanent disabilities, including long-term neurologic deficits.3 Poor swimming ability, lack of adult supervision, alcohol or drug abuse, or risky behavior may be to blame for many drownings and near drownings.1 However, a significant number of drownings remain unexplained and may actually be secondary to a heritable and potentially lethal cardiac channelopathy, such as congenital long QT syndrome (LQTS) or catecholaminergic polymorphic ventricular tachycardia (CPVT).4-6
Long QT syndrome consists of a diverse group of cardiac channelopathies that are characterized by delayed repolarization of the myocardium; QT prolongation despite a structurally normal heart; and an increased clinical risk of syncope, seizures, and sudden cardiac death, usually after a precipitating event, such as extreme emotion, exertion, or an auditory trigger.7 About 75% of LQTS may be elucidated genetically with disease-causing mutations identifiable in the 3 major LQTS-causative genes encoding ion channel subunits that orchestrate the action potential of the heart8: KCNQ1(LQT1),9 KCNH2(LQT2),10 and SCN5A (LQT3).11 Like those with LQTS, persons with CPVT have a structurally normal heart, making a diagnosis difficult to establish by a traditional medicolegal autopsy. Typically precipitated by exertion, CPVT is an arrhythmogenic channelopathy that can manifest unpredictably with syncope or sudden death and is caused by mutations in the cardiac ryanodine receptor encoding gene, RYR2.12,13 Compared with other exertional activities, swimming is a well-established arrhythmogenic trigger among people with either LQTS (particularly LQT1) or CPVT.14-16
In 1999, we reported the first postmortem molecular diagnosis of an arrhythmia disorder, culminating with the diagnosis of inherited LQTS in a 19-year-old woman who died after nearly drowning.5 In 2003, Lunetta et al17 performed a postmortem genetic analysis involving 2 specific LQTS Finnish founder mutations and identified a single LQT2 mutation in 1 of 165 consecutive bodies found in water in Finland. In 2005, we provided proof of principle that some cases of unexplained drowning harbor CPVT-associated mutations in RYR2 through molecular autopsy of 2 young drowning victims referred by medical examiners.6
The extent to which such potentially lethal disorders may underlie an otherwise unexplained drowning is unknown. Using a comprehensive, open reading frame mutational analysis of KCNQ1, KCNH2, and SCN5A and a 64-exon targeted analysis of RYR2, we sought to determine the spectrum and prevalence of cardiac channel mutations associated with LQTS or CPVT in a large cohort of autopsy-negative, unexplained drowning victims referred by coroners, medical examiners, or forensic pathologists for further postmortem investigations, namely a cardiac channel molecular autopsy.
PATIENTS AND METHODS
Cases of Unexplained Drowning Referred by Medical Examiners
From September 1, 1998, through October 31, 2010, 35 cases of a seemingly unexplained drowning (23 male and 12 female drowning victims; mean ± SD age, 17±12 years [range, 4-69 years]) were referred by medical examiners for a cardiac channel molecular autopsy to Mayo Clinic’s Windland Smith Rice Sudden Death Genomics Laboratory. After receipt of written consent from the decedents’ parents, spouse, or appropriate next of kin for this Mayo Clinic Institutional Review Board–approved protocol, a postmortem genetic mutational analysis (ie, cardiac channel molecular autopsy) was performed.
Postmortem Mutational Analysis (Cardiac Channel Molecular Autopsy)
Genomic DNA was extracted from frozen necropsy tissue or autopsy blood using the Puregene DNA Isolation Kit (Qiagen, Valencia, CA). Comprehensive open reading frame mutation analysis of the 58 translated exons of the 3 major LQTS-susceptibility genes: KCNQ1, KCNH2, and SCN5A, and a targeted analysis of the 64 exons (3-28, 36-50, and 83-105) previously reported to encompass the 3 major mutation clustering domains of the 105 exon CPVT1-susceptibility gene, RYR2,18 were performed using polymerase chain reaction (PCR), denaturing high-performance liquid chromatography, and direct DNA sequencing as previously described.19
To be considered a putative pathogenic mutation that may have been responsible for a decedent’s otherwise unexplained drowning, the genetic variant had to be a nonsynonymous variant that involved a highly conserved residue and was absent among at least 400 reference alleles derived from healthy controls (100 white and 100 black). Control genomic DNA was acquired from the Human Genetic Cell Repository sponsored by the National Institute of General Medical Sciences and the Coriell Institute for Medical Research (Camden, NJ). The controls were used to distinguish common polymorphisms (heterozygote frequency, ≥1%) from rare genetic variants that may represent putative pathogenic mutations and were not specifically age- or sex-matched to the patient cohort.
Mutations were annotated using single-letter nomenclature: ie, L273F specifies a missense mutation whereby leucine (L) is mutated to phenylalanine (F) at amino acid 273. To assess whether mutations were sporadic or familial, direct DNA sequencing was used to verify the presence or absence of the putative mutations in DNA extracted from the parents of the deceased drowning victims when possible. Sequences for PCR primers and conditions for PCR/denaturing high-performance liquid chromatography can be obtained on request.
Statistical Analyses
Continuous data are summarized as mean ± SD, and categorical data are presented as number (percentage). Categorical variables were compared using the Fisher exact test. P=.05 was considered statistically significant.
RESULTS
Of the 35 unexplained drowning cases, 7 involved bathtub submersions (3 male and 4 female victims; mean ± SD age at death, 23±24 years [range, 3.5-69.0 years]), and 28 were swimming-related drownings (20 male and 8 female victims; mean ± SD age at death, 15.7±6.9 years [range, 4-39 years]). Table 1 summarizes the demographics and clinical phenotype of the 35 drowning victims. Importantly, no drowning victim or relative had been given a clinical diagnosis of a suspected heritable arrhythmia syndrome such as LQTS or CPVT before the unexplained drowning. However, either a positive personal or family history of syncope, seizures, cardiac arrest, near drownings or unexplained drowning (in a family member), or prolonged QT interval was documented explicitly by the medical examiner in 11 of the 35 cases (3 [42.9%] of the 7 bathtub submersions; 8 [28.6%] of the 28 swimming-related drownings; Table 2). Although 3 of those who drowned by bathtub submersion had a previous personal history of events (2 with seizures, 1 with syncope), none of the 7 cases had a positive family history suggestive of a familial cardiac arrhythmia syndrome. Of the 28 swimming-related drowning victims, 4 had a personal history (1 with near-drowning episodes, 1 with syncope, 1 with seizures, and 1 with exercise-induced cardiac arrest), and 4 had a family history of events (2 with unexplained drowning, 1 with palpitations while swimming, 1 with documented QT prolongation) suggestive of a familial cardiac arrhythmia syndrome.
TABLE 1.
Clinical Characteristics and Demographics of the Unexplained Drowning Cohorta
TABLE 2.
Nonsynonymous Mutations/Polymorphisms in 35 Victims of Unexplained Drowninga
Postmortem genetic testing for LQT1-3– and CPVT1-susceptibility mutations revealed 9 (2 novel) putative, pathogenic channel mutations in 8 (22.9%) of the 35 cases overall (8 [28.6%] of 28 swimming-related, 0 of 7 bathtub submersions; P=.17; Table 2 and Figures 1 and 2). All mutations (6 RYR2 and 3 KCNQ1) were absent in controls. Of the 8 swimming-related drowning victims hosting mutations, 75% (6 cases, 21.4% overall) had a mutation in the CPVT1-associated gene RYR2, and 25% (2 cases, 7.1% overall) had a mutation in the LQT1-associated gene KCNQ1. One individual hosted 2 KCNQ1 mutations (AAPdel71-73 and V524G). No mutations were identified in the LQT2-associated KCNH2 or LQT3-associated SC-N5A genes.
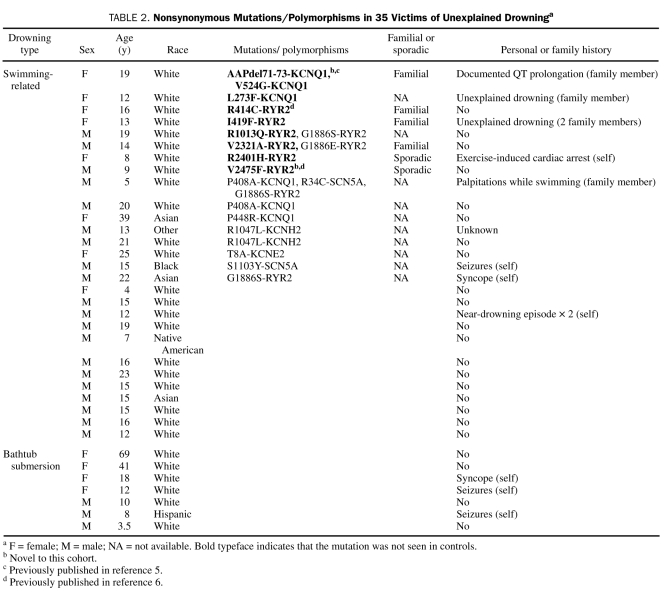
FIGURE 1.
Summary of cardiac channel mutations identified in a molecular autopsy series of otherwise unexplained drownings. The putative pathogenic drowning-associated mutations identified in this study (black circles) are depicted with their proximate location on the linear topologies (not drawn to scale) of the LQT1-associated KCNQ1-encoded cardiac channel Kv7.1 alpha subunit (left) and the CPVT1-associated RYR2-encoded calcium release channel (cardiac ryanodine receptor) alpha subunit (right). CPVT = catecholaminergic polymorphic ventricular tachycardia; LQT = long QT; SR = sarcoplasmic reticulum.
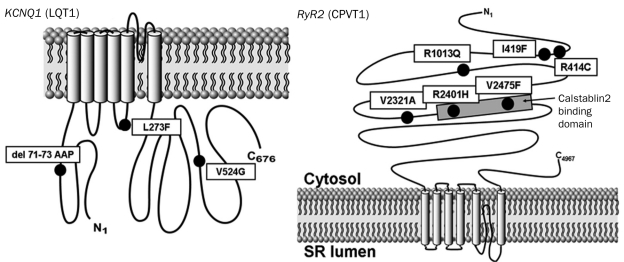
FIGURE 2.
Summary of the yield of postmortem cardiac channel genetic testing (molecular autopsy) in cases of unexplained drowning. Depicted is a bar graph comparing the percent yield in mutation identification between victims of a bathtub submersion (n=7) and victims of a swimming-related unexplained drowning (n=28; P=.3). Of the 8 mutation-positive victims of a swimming-related drowning, 75% (n=6) had mutations in RYR2, and 25% (n=2) had mutations in KCNQ1.
Among the 8 decedents with a positive cardiac channel molecular autopsy, surviving family members for 6 of the cases chose to participate with confirmatory, mutation-specific genetic testing. For these 6 participating families, 4 were shown to host familial mutations, and 2 victims were established to have sporadic de novo mutations (R2401H-RYR2 and V2475F-RYR2; Table 2). We were unable to assess whether the mutation was familial or sporadic in the 2 nonparticipating families. However, the drowning victim hosting the L273F-KCNQ1 mutation was said to have had a family history of an unexplained drowning. Presumably, this mutation residing in a critical region of the Kv7.1/IKs potassium channel (S5 transmembrane spanning domain) represents a familial LQT1-causing mutation.
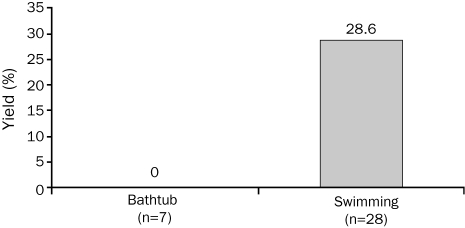
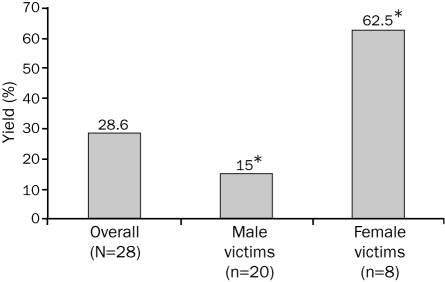
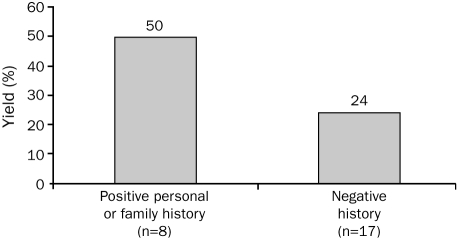
Interestingly, the yield of mutation detection in the swimming-related drowning cohort was significantly higher in female victims (5 [62.5%] of 8) than in male victims (3 [15%] of 20; P=.02; Figure 3). The yield of mutation detection among those swimming-related drowning victims with either a positive personal or family history was 50% (4/8) compared with 24% (4/17) for decedents with a negative history, but this difference was not statistically significant (P=.35; Figure 4). Although 8 (34.8%) of the 23 drowning victims aged 20 years or younger were found to be mutation positive, no mutations were identified in the 5 drowning victims older than 20 years. However, this finding was not statistically significant, most likely because of the small sample size (P=.28).
FIGURE 3.
Sex-specific yield of postmortem cardiac channel genetic testing in unexplained drowning. Depicted is a bar graph showing the significant difference in mutation detection yield between male (3 [15%] of 20) and female (5 [63%] of 8; P=.02) drowning victims. *P<.05.
FIGURE 4.
The effect of a personal or family history of cardiac events on the yield of postmortem cardiac channel genetic testing in unexplained drowning. Depicted is a bar graph showing the difference in mutation detection yield between drowning victims with either a negative (n=17) or positive (n=8) documented personal or family history of cardiac events or other “warning” signs, including syncope, cardiac arrest, seizures, sudden cardiac death, drowning, or documented QT prolongation. Importantly, despite a personal or family history of such “warning” signs, neither the decedent nor any family member carried an arrhythmia syndrome diagnosis (ie, long QT syndrome or catecholaminergic polymorphic ventricular tachycardia) before the drowning event.
In addition to the 8 cases hosting putative pathogenic mutations, 7 (25%) swimming-related drowning victims hosted nonsynonymous channel polymorphisms other than the extremely common channel polymorphisms of K897T-KCNH2, H558R-SCN5A, and Q2958R-RyR2; Table 2). For example, a 15-year-old black male victim of a swimming-related drowning who had a personal history of seizures was identified as having the common proarrhythmic and sudden death–associated SCN5A polymorphism S1103Y.20 Two white male drowning victims (7% [2/28]; one with a family history: his mother had palpitations while swimming) were identified as having P408A-KCNQ1, a 1% to 2% heterozygote polymorphism21 observed in ostensibly healthy blacks and Hispanics but not in white controls. Two cases (7% [2/28]) hosted R1047L-KCNH2, a 3% to 4% heterozygote polymorphism21 in healthy whites that is associated with drug (dofetilide)-induced torsades de pointes,22 a hallmark arrhythmia in LQTS. It is unknown whether these 2 decedents were being treated with dofetilide (Kv11.1 channel blocker, also called HERG [Human Ether-a-go-go Related Gene] channel blocker) or any other drug known to induce torsades de pointes or prolong the QT interval.
DISCUSSION
In a cohort of autopsy-negative drowning victims who were referred by medical examiners or coroners and whose cause of death was otherwise unexplained, we provide molecular evidence suggesting that nearly 30% of swimming-related drowning cases may host putative pathogenic mutations in critical ion channel genes associated with the potentially lethal arrhythmia syndromes known as LQTS and CPVT. Additionally, another 25% of this cohort host nonsynonymous channel polymorphisms that may or may not confer risk for an arrhythmogenic event during swimming as a result of functional perturbations in these cardiac ion channels.
Although most drownings occur in swimming pools or open waters like lakes or rivers, 15% of drownings occur in bathtubs.23 In our study, none of the 7 bathtub submersion victims were mutation positive for LQTS or CPVT. Although drowning in the bathtub is not a common occurrence among persons diagnosed as having LQTS or CPVT, it is a common cause of accidental death in persons with epilepsy.24 In one study, 60% of epileptic patients who drowned did so in the bathtub at home.25 Moreover, a study on submersion injury in epileptic children placed a relative risk of submersion in the bathtub at 47.0 vs 18.7 in a swimming pool and a relative risk of drowning at 96.0 in the bathtub vs 23.4 in the pool.26 Given the higher relative risk for bathtub drowning vs swimming-related drowning, one might speculate that our cohort of bathtub drowning victims may represent a higher proportion of persons with a neurologic seizure disorder rather than a primary cardiac channelopathy. Notably, 3 (43%) of the 7 bathtub drowning cases had a history of seizures or syncope, despite not carrying any premortem diagnosis.
The genetic predilection for KCNQ1 and RYR2 mutations in this drowning cohort is remarkably congruent with the relative gene-specific arrhythmogenic trigger of swimming in LQT1 compared with other LQTS genotypes. Swimming is thought to trigger events in up to 15% of children and young adults with symptomatic LQTS and, compared with LQT2 and LQT3, is a relatively gene-specific trigger for LQT1. In 1999, 2 small case series (Ackerman et al,14 n=6; Moss et al,15 n=19) suggested swimming-triggered events as pathognomonic for LQT1 with the observation that all 25 LQTS patients with a personal or family history of swimming-triggered events had LQT1-causing mutations in KCNQ1. In a larger cohort of 43 LQTS referral cases with a personal or family history of swimming-triggered events, Choi et al27 demonstrated that 91% had a putative arrhythmia syndrome–causing mutation. Among the 33 cases with clinically definite LQTS and a swimming-triggered event, 85% had LQT1, 6% had LQT2, and 9% were genotype-negative. Near drowning or drowning was the sentinel event for all 16 LQT1-positive index cases with a personal symptomatic history.
Swimming is also a common trigger observed in CPVT. Among LQTS referral patients with a swimming-triggered event but low clinical probability for their referral diagnosis of LQTS, 90% had mutations in RYR2, the gene causative for CPVT1.27
Taken together, these reports anticipated a strong predilection for KCNQ1 and RYR2 mutations in mutation-positive “cardiac channelopathic” unexplained drowning cases. In this necropsy cohort of 28 swimming-related drowning cases, nearly 30% had either CPVT1- or LQT1-associated mutations, with most (75%) being CPVT1.
In 2003, Lunetta et al17 performed a postmortem genetic analysis identifying only a single LQT2 mutation in 1 of 165 consecutive bodies found in water in Finland, a bathtub death of a 44-year-old woman initially ruled as a suicidal drowning. This study concluded that the minimum prevalence of LQTS in drownings was only 0.61% and provided a point estimate of 1% to 2% overall. However, this very limited mutational analysis sought to identify only 2 specific LQTS Finish founder mutations in this cohort: G589D-KCNQ1 (LQT1) and L522S-KCNH2 (LQT2), which encompass about 35% of familial LQTS in Finland. This retrospective 165-person study consisted of mostly accidental (n=107 [64.8%]) or suicidal (n=37 [22.4%]) deaths. Only 9% of the cohort represented “undetermined intent.” Further, the cohort was much older than our cohort of mostly swimming-related drowning victims, with a mean age of 49.1 years (range, 1.0-89.0 years) vs 15.7 years (range, 4-39 years) in our study. Given the substantial differences between the phenotypes of these 2 cohorts and the limited mutational analysis conducted in the Finnish study, the large discordance in mutation detection yield (Finnish, 0.6%, vs Mayo Clinic, 28%) is not surprising.
Interestingly, although 1 of every 7 male victims of a swimming-related drowning hosted a channel mutation, nearly two-thirds of the female victims were mutation positive. According to Howland et al,28 the drowning rate among males is 10-fold higher than among females during late adolescence and early adulthood. Possible reasons for this male predominance include men’s greater exposure to aquatic activity, their probable overestimation of their swimming ability, and their greater likelihood of displaying risky behavior than women.28 The fact that the unexplained drowning for most male victims in our cohort appears unrelated to either LQTS or CPVT may simply reflect this inherent behavior in young men rather than a direct or indirect cause of an underlying pathology. However, for young women, who are less likely to undertake the same risky behaviors that leave young men more vulnerable, it stands to reason that there may be a higher percentage of otherwise unexplained drownings due to an underlying pathology such as an arrhythmia syndrome.
Our data also suggest that some unexplained drownings may be preventable. Although the unexplained drowning was the first event in most cases, half of the 8 drowning victims with a cardiac channel mutation exhibited potential cautionary signs, either in the family or personally, yet no premortem diagnosis of LQTS or CPVT was established. It is essential that such warning signs be noticed and critically interrogated. Given the effectiveness of LQTS- and CPVT-related therapies, a premortem diagnosis of these conditions may well have prevented the sudden death.
A molecular autopsy can greatly affect surviving relatives and ought to be considered in all unexplained drowning cases. In fact, our pedigree expansion for most of the genotype-positive decedents revealed that two-thirds of the mutations were familial. The postmortem elucidation of a channelopathy-susceptibility mutation may provide molecular confirmation as to the underlying basis of death and a potential life-saving indication for the clinical evaluation and management of those relatives still living.
CONCLUSION
A cardiac channel molecular autopsy revealed that nearly 30% of the victims of swimming-related drowning hosted a cardiac channel mutation consistent with LQTS or CPVT. Cardiac channel genetic testing should be considered in the postmortem evaluation of an otherwise unexplained drowning. Our data suggest the viability of performing a tiered approach to genetic testing beginning with RYR2 followed by KCNQ1, especially among young female victims of otherwise unexplained drownings and among drowning victims of either sex with a positive personal or family history consistent with the presence of a heritable arrhythmia syndrome.
Footnotes
This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program and the National Institutes of Health HD42569. Dr Ackerman is a consultant for Biotronik, Boston Scientific, Medtronic, St. Jude Medical, Inc, and Transgenomic. Intellectual property derived from Dr Ackerman’s research program resulted in license agreements in 2004 between Mayo Clinic Health Solutions (formerly Mayo Medical Ventures) and PGxHealth (formerly Genaissance Pharmaceuticals, recently acquired by Transgenomic). Dr Ackerman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
REFERENCES
- 1. Layon AJ, Modell JH. Drowning: update 2009. Anesthesiology. 2009;110:1390-1401 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) Injury prevention and control: data & statistics (WISQARS). Web site. www.cdc.gov/ncipc/wisqars Accessed August 16, 2011
- 3. Kemp A, Sibert JR. Drowning and near drowning in children in the United Kingdom: lessons for prevention. BMJ. 1992;304:1143-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackerman MJ. Cardiac channelopathies: it’s in the genes. Nat Med. 2004;10:463-464 [DOI] [PubMed] [Google Scholar]
- 5. Ackerman MJ, Tester DJ, Porter CJ, Edwards WD. Molecular diagnosis of the inherited long-QT syndrome in a woman who died after near-drowning. N Engl J Med. 1999;341:1121-1125 [DOI] [PubMed] [Google Scholar]
- 6. Tester DJ, Kopplin LJ, Creighton W, Burke AP, Ackerman MJ. Pathogenesis of unexplained drowning: new insights from a molecular autopsy. Mayo Clin Proc. 2005;80:596-600 [DOI] [PubMed] [Google Scholar]
- 7. Roden DM. Long-QT syndrome. N Engl J Med. 2008;358:169-176 [DOI] [PubMed] [Google Scholar]
- 8. Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569-580 [DOI] [PubMed] [Google Scholar]
- 9. Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17-23 [DOI] [PubMed] [Google Scholar]
- 10. Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795-803 [DOI] [PubMed] [Google Scholar]
- 11. Wang Q, Shen J, Splawski I, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805-811 [DOI] [PubMed] [Google Scholar]
- 12. Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196-200 [DOI] [PubMed] [Google Scholar]
- 13. Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485-490 [DOI] [PubMed] [Google Scholar]
- 14. Ackerman MJ, Tester DJ, Porter CJ. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clin Proc. 1999;74:1088-1094 [DOI] [PubMed] [Google Scholar]
- 15. Moss AJ, Robinson JL, Gessman L, et al. Comparison of clinical and genetic variables of cardiac events associated with loud noise versus swimming among subjects with the long QT syndrome. Am J Cardiol. 1999;84:876-879 [DOI] [PubMed] [Google Scholar]
- 16. Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89-95 [DOI] [PubMed] [Google Scholar]
- 17. Lunetta P, Levo A, Laitinen P, Fodstad H, Kontula K, Sajantila A. Molecular screening of selected long QT syndrome (LQTS) mutations in 165 consecutive bodies found in water. Int J Legal Med. 2003;117:115-117 [DOI] [PubMed] [Google Scholar]
- 18. Medeiros-Domingo A, Bhuiyan Z, Tester D, et al. Comprehensive open reading frame mutational analysis of the RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome. J Am Coll Cardiol. 2009;54:2065-2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507-517 [DOI] [PubMed] [Google Scholar]
- 20. Splawski I, Timothy KW, Tateyama M, et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333-1336 [DOI] [PubMed] [Google Scholar]
- 21. Ackerman MJ, Tester DJ, Jones G, Will MK, Burrow CR, Curran M. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78:1479-1487 [DOI] [PubMed] [Google Scholar]
- 22. Sun Z, Milos PM, Thompson JF, et al. Role of KCNH2 polymorphism (R1047L) in dofetilide-induced Torsades de Pointes. J Mol Cell Cardiol. 2004;37:1031-1039 [DOI] [PubMed] [Google Scholar]
- 23. Wintemute GJ, Kraus JF, Teret SP, Wright M. Drowning in childhood and adolescence: a population-based study. Am J Public Health. 1987;77:830-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breningstall GN. Mortality in pediatric epilepsy. Pediatr Neurol. 2001;25:9-16 [DOI] [PubMed] [Google Scholar]
- 25. Ryan CA, Dowling G. Drowning deaths in people with epilepsy. CMAJ. 1993;148:781-784 [PMC free article] [PubMed] [Google Scholar]
- 26. Diekema DS, Quan L, Holt VL. Epilepsy as a risk factor for submersion injury in children. Pediatrics. 1993;91:612-616 [PubMed] [Google Scholar]
- 27. Choi G, Kopplin LJ, Tester DJ, Will ML, Haglund CM, Ackerman MJ. Spectrum and frequency of cardiac channel defects implicated in swimming-triggered arrhythmia syndromes. Circulation. 2004;110:2119-2124 [DOI] [PubMed] [Google Scholar]
- 28. Howland J, Hingson R, Mangione TW, Bell N, Bak S. Why are most drowning victims men? sex differences in aquatic skills and behaviors. Am J Public Health. 1996;86:93-96 [DOI] [PMC free article] [PubMed] [Google Scholar]