Abstract
OBJECTIVE: To evaluate the efficacy of MAP0004, an orally inhaled dihydroergotamine, for acute treatment of migraine when administered at various time points from within 1 hour to more than 8 hours after migraine onset.
PATIENTS AND METHODS: This post hoc subanalysis was conducted using data from 902 patients enrolled in a randomized, double-blind, placebo-controlled, 2-arm, phase 3, multicenter study conducted from July 14, 2008, through March 23, 2009. End points were 2-hour pain relief and pain-free rates in patients who treated a migraine in ≤1 hour, from >1 hour to ≤4 hours, from >4 to ≤8 hours, or in >8 hours after onset of migraine, given that patients may be unwilling or unable to initiate treatment at headache inception.
RESULTS: Treatment with MAP0004 was significantly more effective than placebo in relieving pain at all treatment points (≤1 hour after start of migraine: 66% [74/112] for MAP0004 vs 41% [48/118] for placebo, P<.001; >1 to ≤4 hours: 60% [91/153] vs 35% [58/168], P<.001; >4 to ≤8 hours: 53% [36/68] vs 30% [16/54], P=.008; and >8 hours: 48% [25/52] vs 24% [11/46], P=.007). Pain-free rates were also significantly higher with MAP0004 than placebo for treatment within 8 hours after migraine onset (≤1 hour: 38% [43/112] for MAP0004 vs 13% [15/118] for placebo, P<.001; >1 to ≤4 hours: 28% [43/153] vs 10% [17/168], P<.001; >4 to ≤8 hours: 22% [15/68] vs 7% [4/54], P<.025) but not at >8 hours (19% [10/52] vs 9% [4/46], P=.106).
CONCLUSION: This post hoc subanalysis shows that MAP0004 was effective in treating migraine irrespective of the time of treatment, even more than 8 hours after onset of migraine pain.
Trial Registration: clinicaltrials.gov identifier: NCT00623636
AE = adverse event; Cmax = maximum concentration; DHE = dihydroergotamine mesylate; FEV1 = forced expiratory volume in 1 second; ICHD-II = International Classification of Headache Disorders, 2nd edition; mITT = modified intent-to-treat
Headache is one of the most prevalent neurologic disorders worldwide; 46% of the adult population report an active headache disorder, 11% of whom have migraine.1 The International Classification of Headache Disorders, 2nd edition (ICHD-II) provides clear diagnostic criteria to distinguish headache subtypes.2 The classification criteria for migraine include duration of headache from 4 to 72 hours and at least 2 of the 4 following features either untreated or unsuccessfully treated: unilateral location, pulsation, moderate or severe pain intensity, and aggravation by or causing avoidance of routine physical activity.2 The diagnosis of migraine also requires either (1) nausea and/or vomiting or (2) photophobia and phonophobia. Intuitively, one would expect patients to take their medication as soon as a headache starts. In reality, however, many patients delay treatment. Factors that may cause a delay in treatment initiation include severity of pain, presence of nonpain symptoms, and/or availability of or willingness to take migraine-specific medications.
Many studies show that delaying treatment after migraine onset may decrease the efficacy of some migraine-specific medications, including the selective 5-HT1B/1D agonists, commonly referred to as “triptans.”3 Specifically, several well-controlled studies have shown that rates of relief with triptans are highest when these drugs are used early in an attack.4-7
One proposed explanation for this drop in triptan efficacy associated with delayed treatment is the development of central sensitization over time8 that renders the patient less responsive to triptan therapy.9 Indeed, once central sensitization develops, it may not be reversible with triptan use.10,11 Animal model studies further suggest that sumatriptan does not inhibit either peripheral or central trigeminovascular neurons. Sumatriptan, which acts on presynaptic 5-HT1B/1D receptors, does not reverse developed sensitization.11 However, at least 3 randomized placebo-controlled studies have failed to link allodynia and treatment outcomes with triptan use.7,12,13 These data suggest that later treatment may be less successful simply because pain tends to be more severe as the migraine progresses unmanaged. In at least some patients, the efficacy of triptans may depend largely on prompt dosing after onset and before development of sensitization or onset of severe pain. Moreover, for patients at risk of developing central sensitization, treatment with an agent that does reverse this process may confer additional therapeutic benefits.7,12,13
Although the need for early treatment in patients with migraine is important for successful treatment with triptan drugs, patients frequently do not take their medication within 2 hours after onset of pain. The reasons for these delays vary, but delays may be exacerbated by drug hoarding for future use for more severe migraines or by concerns about side effects. The presence of nausea, the most specific and sensitive single-variable migraine-associated symptom,14 may deter patients from taking an oral medication. Delayed treatment is also associated with awakening with migraine pain, suggesting that the migraine started hours earlier, during sleep. Finally, gastric stasis, which has been reported to occur in migraineurs during or even outside of a migraine event, may slow oral drug absorption and contribute to or exacerbate nausea associated with migraine.15
The need exists for a migraine-specific medication that bypasses the gastrointestinal system and shows consistent efficacy, whether taken early or late after onset of a migraine and regardless of pain severity. Nasal triptan formulations (zolmitriptan and sumatriptan) are available for acute treatment of migraine; however, these formulations require early treatment to be most effective and are similarly associated with pharmacokinetic variations in time to reach maximal serum concentration (Tmax),16,17 which may be related to interindividual variations in the vascularity of the nasal mucosa, the amount of drug deposited in the nasal passage compared with what is lost, and the amount of dose swallowed and subsequently absorbed from the gastrointestinal tract.
Dihydroergotamine mesylate (DHE) is a 5-HT1B/1D receptor agonist that has been used effectively to treat migraine since 1946.18 Although originally proposed to exert its therapeutic effect solely via vasoconstrictive action on intracranial vessels,19 DHE is now thought to have central activity within the brain.20,21 Recent studies further support a central modulatory effect of DHE in animal models of pain and sensitization.22
Unlike triptans, DHE may reverse established central sensitization. In an animal model, early administration of DHE, as with sumatriptan, was shown to effectively block induction of central sensitization.23 However, when DHE and zolmitriptan were administered after central sensitization had been established, DHE reversed the changes in sensory thresholds but zolmitriptan did not. These results are consistent with earlier work showing that sumatriptan was also ineffective in reversing established central sensitization.8 Furthermore, in a small open-label study (N=9), intramuscular DHE was used to treat 2 migraines in patients whose episodic migraines were associated with cutaneous allodynia. Response was compared between patients treating early (≤2 hours after onset of throbbing pain) and patients treating late (≥4 hours after onset of throbbing pain). Results were similar in both groups, with 2-hour pain relief seen in greater than 55% of patients in each group.24 Although intravenous DHE and nasal DHE formulations are commercially available in addition to intramuscular DHE, neither of these choices offers the preferred combination of consistent delivery, ease of use, patient convenience, limited side effects, and effective migraine therapy.
MAP0004 (Levadex; MAP Pharmaceuticals, Mountain View, CA) is an orally inhaled formulation of DHE that delivers 0.6-mg emitted dose (1.0-mg nominal dose) to the lungs using the TEMPO inhaler. MAP0004 is quickly absorbed from the pleural mucosa into the systemic circulation as demonstrated by pharmacokinetic studies showing that maximum concentration (Cmax) is achieved in approximately 10 minutes.25 One of the benefits of inhaled delivery is that the Cmax is at least an order of magnitude lower than with intravenous DHE.25 Clinically, this may translate into improved tolerability and fewer adverse events (AEs) compared with intravenous DHE.25 The development of MAP0004 offers a potentially effective and consistently delivered non-oral migraine medication that may exert its antimigraine therapeutic effects both peripherally and centrally.
In a dose-finding study of MAP0004 (1.0-mg nominal dose, 2.0-mg nominal dose, and placebo), a significantly better 2-hour migraine response was reported for MAP0004 at 1.0 mg (72%) than for placebo (33%; P=.02).26 Pain relief with MAP0004 at 10, 15, and 30 minutes was 32%, 46%, and 55%, respectively, compared with 0%, 7%, and 14%, respectively, for placebo, which was significantly better (P<.05 for each). However, the clinical efficacy of MAP0004 when taken early (before sensitization) or late (after sensitization) had not been studied. This report presents the results of a post hoc subanalysis of data from the MAP0004 phase 3 study.27 This subanalysis evaluated the efficacy of MAP0004 when given at various time points (≤1 hour, between >1 hour and ≤4 hours, between >4 and ≤8 hours, and >8 hours) after onset of migraine.
PATIENTS AND METHODS
Study Design
This evaluation was a post hoc subanalysis of a randomized, double-blind, placebo-controlled parallel group phase 3 study27 that assessed the efficacy of MAP0004, a novel, orally inhaled formulation of DHE (0.6-mg emitted dose or 1.0-mg nominal dose) for acute treatment of migraine with or without aura. This study was conducted at 102 sites in the United States between July 14, 2008, and March 23, 2009, and was performed in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and US Food and Drug Administration regulations for informed consent and protection of patients’ rights. The study was reviewed and approved by the respective institutional review boards at each of the participating study centers. The FREEDOM-301 study was registered at ClinicalTrials.gov (NCT00623636).
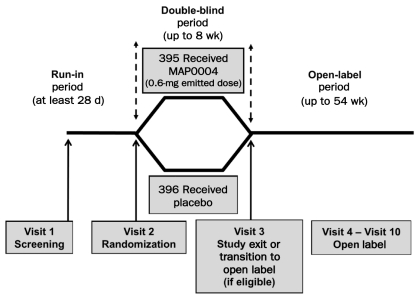
Study participants were required to visit the clinic at least 3 separate times for screening (visit 1), randomization (visit 2), and study exit (visit 3) (Figure 1). Although results are not reported herein, this study also included an open-label extension phase in which eligible patients could enroll in a 54-week open-label drug safety phase and were evaluated for long-term drug safety during clinic visits 4 to 10. During the screening visit, patients meeting inclusion and exclusion criteria were assigned a study identification number, entered into a run-in period of at least 28 days’ duration, and given an electronic diary. During the run-in period, patients were required to experience a minimum of 2 migraines but no more than 8 migraines and to record their migraine information in the diary. Patients were also required to have had at least 20 migraine-free days within the last 28 days of the run-in period. After completion of the run-in period, patients returned to the clinic for randomization in visit 2.
FIGURE 1.
This randomized, double-blind, placebo-controlled, single-attack, parallel group study assessed the effects of MAP0004 for acute treatment of migraine. Patients attended the clinic for 3 visits during the blinded treatment phase. Primary efficacy and safety results have been published previously.25 Qualifying patients were invited to participate in a 54-week open-label safety phase.
Patient Participation
Inclusion criteria. Participating patients were men or women aged 18 to 65 years with a documented history of migraine with or without aura, as defined by the ICHD-II classification criteria,2 diagnosed before age 50 years. Eligible patients had a diagnosis of migraine for a minimum of 1 year before the study and, in the 6 months before the screening visit, experienced an average of 2 to 8 migraines per month. Patients were required to have a normal or clinically insignificantly abnormal 12-lead electrocardiogram and rhythm strip. Patients also were required to be able to satisfactorily use an electronic diary, enter data into the diary, and hear the alarms set in the diary to capture data at specific time points after treatment. In addition, patients were screened for pulmonary function status at baseline and were required to have a forced expiratory volume in 1 second (FEV1) ≥50% of predicted and an FEV1/forced vital capacity ratio ≥70% of predicted. Patients had to satisfactorily demonstrate the use of a trainer inhaler. Moreover, patients were required to use adequate contraception or were sterile. Women were required to have a negative pregnancy test result or, if postmenopausal, to have had absence of menses for at least 12 months.
Exclusion criteria. Study exclusion criteria included having a history of suspected or diagnosed coronary artery disease, coronary vasospasm (including Prinzmetal angina), or peripheral vascular disease; a history of a risk of or diagnosed ischemic disease (such as ischemic bowel syndrome or Raynaud syndrome) or cardiac disorder (such as any clinically significant dysrhythmia); or any history of heart attack or stroke. A patient history of diabetes mellitus, liver or kidney disease, aortic aneurysm, or chronic pulmonary disease; recent (within 3 months) sepsis or vascular surgery; or recent (within 12 months) pulmonary disease (such as chronic obstructive pulmonary disease or uncontrolled asthma) was also cause for exclusion. A diagnosis of cancer (other than noninvasive skin cancer) within the past 5 years was reason for exclusion. Also excluded were patients with intermediate or significant risk factors for cardiac disease (such as having ≥2 cardiac risk factors, including cigarette smoking, hypertension, hyperlipidemia, or family history of premature coronary artery disease). Any hospitalization within the prior 30 days or use of prohibited or illegal drugs within 2 weeks of screening or during the run-in period was an additional reason for exclusion. The use of other DHE products, triptans, or ergot-based drugs was prohibited during the run-in period and during the treatment phase. Patients with a known allergy or hypersensitivity to DHE or users of any concomitant prohibited medications were also excluded. Finally, patients who had participated in another clinical trial in the preceding 30 days, or in any other trial that included MAP0004, or who had a history of hemiplegic or basilar migraine were excluded.
Randomization. Patients were randomly assigned to receive either MAP0004 or placebo in a 1:1 ratio with no stratification. Randomization was performed by an automated interactive voice recognition system. Neither patients nor investigators were informed of treatment assignment. Unblinding occurred only after formal database lock after completion of the treatment phase.
Randomized patients were instructed to use the study drug to treat a single qualifying migraine as instructed by the electronic diary after responding to qualifying assessment questions. Qualifying migraines were defined as migraines characterized by moderate or severe pain that was either unilateral, throbbing (pulsating), or worsened with activity and that occurred in the presence of either nausea or vomiting or both phonophobia and photophobia.
Treatment
Qualifying migraines were treated with 2 actuations of either MAP0004 or placebo using matching TEMPO inhalers. The MAP0004 treatment delivered a 0.6-mg emitted dose of DHE (1.0-mg nominal dose) suspended in a blend of hydrofluoroalkane propellants. The placebo treatment delivered only the blend of hydrofluoroalkane propellants. Patients then recorded specific data at selected time points as prompted by the electronic diary, including the time of treatment onset, severity of migraine symptoms, and rescue medication use. The first patient was enrolled in July 2008, and the last patient completed this phase of the study in March 2009.
Patients were to refrain from taking any type of rescue medication for at least 2 hours after administration of study medication. If patients failed to respond at 2 hours, nonergot and nontriptan rescue medications could be taken. If there was no response at 24 hours, patients were allowed to take ergot or triptan medications.
Efficacy Assessments and Study End Points
Efficacy assessments were based on the treatment of a single moderate to severe migraine using MAP0004 or matching placebo. The 4 co-primary study end points were pain relief and freedom from photophobia, phonophobia, and nausea, all at 2 hours posttreatment. Pain relief was defined as a reduction in moderate to severe pain to mild or no pain. Freedom from photophobia and phonophobia and from nausea was defined as a reduction of a mild, moderate, or severe symptom to the absence of the symptom with no rescue medication use.
The pain relief at 2 hours posttreatment was further evaluated in this post hoc subanalysis to assess the response at 4 time intervals at which treatment occurred after onset of the migraine: ≤1 hour, from >1 to ≤4 hours, from >4 to ≤8 hours, and >8 hours after onset. A similar post hoc subanalysis was conducted for the pain-free assessment (moderate or severe pain to the absence of pain), also at 2 hours posttreatment.
Safety and Tolerability
Safety was assessed from spontaneous reports of AEs and events recorded by patients in their electronic diaries. All reported AEs were mapped using the Medical Dictionary for Regulatory Activities and were grouped by system organ class and preferred term and tabulated by treatment group. The incidence of AEs in each treatment group was also tabulated by seriousness, severity, and relationship to study drug.
Statistical Analyses
Investigators planned for approximately 850 randomized patients to obtain 766 treated patients after accounting for a 10% dropout rate. For the primary analysis, the study power was at least 86% for a 2-sided test with a 5% type I error rate based on assumed pain relief rates of 60% for MAP0004 and 40% for placebo; photophobia-free rates of 55% for MAP0004 and 40% for placebo; phonophobia-free rates of 55% for MAP0004 and 40% for placebo; and nausea-free rates of 71.5% for MAP0004 and 60% for placebo. A gatekeeping strategy was used to control for the family-wise error rate of .05.
The study intent-to-treat population included all randomized patients. The predefined modified intent-to-treat (mITT) population (defined for primary, secondary, and post hoc end points) was defined as all randomized patients who experienced a qualifying migraine, received at least 1 dose of study drug, and had at least 1 posttreatment efficacy evaluation. The mITT population was further refined in this subanalysis to include only patients who reported migraine onset. The safety population was defined as all patients who received at least 1 dose of study drug. The post hoc subanalysis for the end points relative to time of treatment included all patients who also reported the time of migraine onset.
The Cochran-Mantel-Haenszel test, stratified by baseline pain severity, was applied to all study end points to assess differences between treatment and placebo groups and to follow-up pair-wise comparisons. For the post hoc assessments, this test was applied for the overall test of differences between treatment groups; however, these analyses were not adjusted for multiple comparisons. All P values were 2-tailed and significant if less than the 5% significance level.
RESULTS
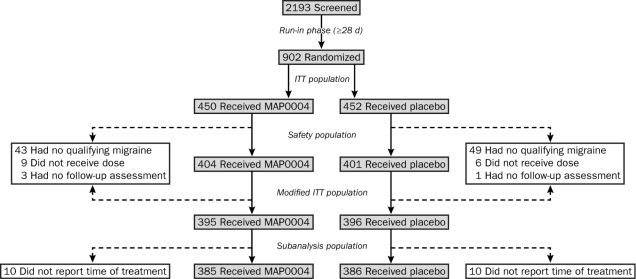
The study population included 902 patients with confirmed history of ICHD-II–defined migraine who were randomly assigned to receive active treatment (n=450) or matching placebo (n=452). The safety population comprised 814 patients who self-treated with the study medication (Figure 2). The 794 mITT patients who treated a migraine of moderate to severe pain were included in the primary efficacy analysis of the study. Of these 794 patients, 771 reported the time of migraine onset. The patient baseline demographics showed no differences between treatment groups for general demographic features or baseline pulmonary function tests (Table 1). The primary end points for the study were statistically significant for MAP0004 relative to placebo, including pain relief (58% vs 34%; P<.001), phonophobia-free (52% vs 34%; P<.001), photophobia-free (46% vs 27%; P<.001), and nausea-free (67% vs 59%; P=.02), all at 2 hours after treatment; these data have been published previously.27
FIGURE 2.
Study population included 902 patients randomized to the treatment phase, with 55 patients in the MAP0004 treatment group and 56 patients in the placebo group discontinuing or withdrawing from the study because of the lack of a qualifying migraine, not receiving a dose of study medication, or lacking a follow-up assessment. In all, 10 patients in the MAP0004 treatment group and 10 patients in the placebo group were excluded from the subanalysis population because they did not report the time of their treatment. ITT = intent-to-treat.
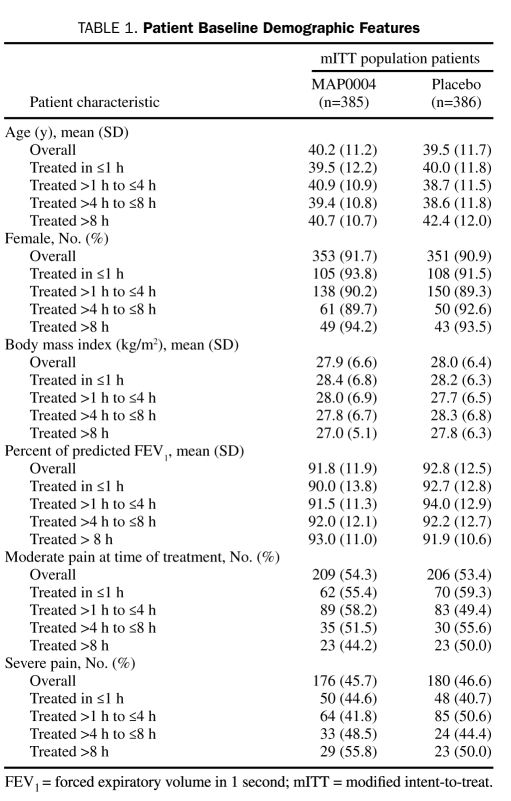
Table 1.
Patient Baseline Demographic Features
This post hoc subanalysis of the exploratory end point of pain relief and pain-free relative to the time of treatment after the onset of migraine included 771 patients who reported the time of migraine onset.
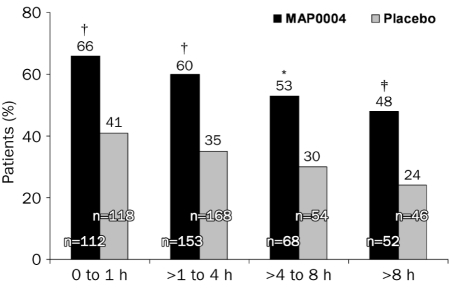
For pain relief, results for MAP0004 for all times to treatment were statistically significant relative to placebo (Figure 3). Specifically, 66% of patients (74/112) who treated their migraine ≤1 hour after onset with MAP0004 achieved pain relief at 2 hours posttreatment compared with 41% (48/118) who treated with placebo (P<.001). Results at 2 hours were similar for patients who treated their migraine with MAP0004 vs placebo between >1 hour and ≤4 hours after onset (60% [91/153] vs 35% [58/168]; P<.001), between >4 and ≤8 hours after onset (53% [36/68] vs 30% [16/54]; P=.008), and >8 hours after onset (48% [25/52] vs 24% [11/46]; P=.007).
FIGURE 3.
Pain relief at 2 hours as a function of time of initiating treatment after start of migraine. Pain relief rates at 2 hours were significantly higher for patients treated with MAP0004 than with placebo when assessed at ≤1 hour after onset of the migraine (†P<.001), between >1 and ≤4 hours after onset (†P<.001), between >4 and ≤8 hours after onset (*P=.008), and >8 hours after onset (‡P=.007). P values were calculated using the Cochran-Mantel-Haenszel test controlling for baseline pain score; exploratory end points were not adjusted for multiplicity. Therapeutic gain (active minus placebo) was similar at all time points.
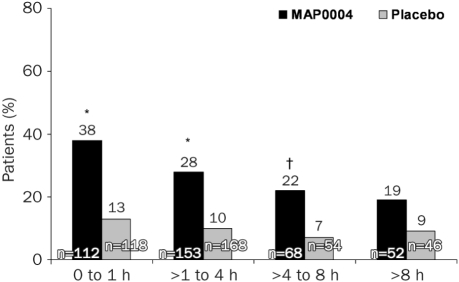
The pain-free assessments also consistently favored MAP0004 compared with placebo when patients were treated ≤1 hour (38% [43/112] vs 13% [15/118]; P<.001), between >1 and ≤4 hours (28% [43/153] vs 10% [17/168]; P<.001); and >4 and ≤8 hours (22% [15/68] vs 7% [4/54]; P<.025) after migraine onset. When treatment was taken >8 hours after migraine onset, pain-free rates at 2 hours did not differ significantly between MAP0004 and placebo (19% [10/52] vs 9% [4/46]; P=.106) (Figure 4).
FIGURE 4.
Pain freedom at 2 hours as a function of time of initiating treatment after start of migraine. Pain-free rates at 2 hours were significantly higher for patients treated with MAP0004 than with placebo regardless of the time to treatment after onset of the migraine. Specifically, early treatment, as defined as treatment within 1 hour of migraine, was effective in achieving a pain-free response in significantly more patients taking MAP0004 than placebo. Similarly, MAP0004 treatments between >1 and ≤4 hours and between >4 and ≤8 hours were also significantly more effective than placebo in achieving pain freedom (*P<.001 and †P<.025, respectively). P values were calculated using the Cochran-Mantel-Haenszel test controlling for baseline pain score; exploratory end points were not adjusted for multiplicity.
Treatment with MAP0004 was well tolerated, and no drug-related serious AEs were reported. One or more AEs were reported in 119 patients (29%) in the MAP0004 treatment group compared with 95 patients (23.5%) in the placebo group. Five patients in the MAP0004 group and 2 in the placebo group discontinued the study because of AEs. (Full safety and tolerability results are reported elsewhere.27)
DISCUSSION
The current study showed that MAP0004 was effective for acute treatment of migraine with or without aura, as noted by the significantly greater improvement relative to placebo for all 4 co-primary end points, including 2-hour pain relief and freedom from phonophobia, photophobia, and nausea. Similarly, this post hoc subanalysis of the efficacy of MAP0004 when taken up to many hours after the onset of the migraine also showed statistical significance relative to placebo. When MAP0004 was taken within the first hour after the onset of migraine pain, 66% of patients achieved migraine pain relief, compared with 41% who took placebo, and 38% achieved complete pain freedom, compared with 13% who took placebo. Although pain relief rates declined with increasing delay for time to treatment up to more than 8 hours, the associated therapeutic gains of approximately 25% were comparable when treatment was taken ≤1 hour, between >1 and ≤4 hours, between >4 and ≤8 hours, and even after >8 hours after migraine pain onset. Similarly, statistically significant pain freedom was achieved when MAP0004 was taken at time points within 8 hours after the onset of migraine pain.
This post hoc analysis supports the current migraine treatment recommendations that patients treat early after the onset of migraine pain; however, because early treatment is not possible for some patients, these results also suggest that MAP0004 may offer a viable treatment option with similar efficacy and therapeutic gains irrespective of the time of treatment relative to migraine onset.
The underlying mechanisms that may contribute to the consistent efficacy of MAP0004 when it is taken are not clearly understood, but several factors may play a contributing role. MAP0004 bypasses gastrointestinal absorption associated with oral deliveries, as noted in pharmacokinetic studies demonstrating MAP0004 plasma levels within 5 minutes, Cmax of 10 minutes, and clinical efficacy as early as 10 minutes.25,26,28 Inconsistent gastrointestinal absorption, which may be further exacerbated in migraineurs because of gastric stasis, is avoided with MAP0004. DHE, unlike oral triptans, is also thought to reverse central sensitization. Additional studies are warranted to prospectively assess the efficacy of MAP0004 when taken anytime after the onset of migraine.
CONCLUSION
In this study, MAP0004 was effective for acute treatment of migraine with or without aura in adults. In this post hoc subanalysis, significant pain relief was achieved when MAP0004 was taken anytime after migraine onset. Although patients should optimally treat as soon as possible after the start of a migraine, when early treatment is not possible, MAP0004 may be an effective treatment option for those seeking pain relief even in excess of 8 hours after the onset of the migraine.
Acknowledgments
We thank Starr Pearlman, PhD, for her assistance in the preparation of the initial manuscript draft.
Footnotes
Dr Tepper reports having received grants and research support from ATI, GlaxoSmithKline, MAP, and Merck; serving as a consultant and on the Advisory Board for GSK, MAP, Merck, NuPathe, and Zogenix; and serving on the Speaker’s Bureau for GSK, Valeant, and Merck. Dr Kori and Dr Wang are employees of MAP and own stock or stock options in MAP. Dr Goadsby reports having received research grants from Boston Scientific, Medtronic, GSK, MSD, MAP, Johnson & Johnson, and Neuralieve and having received consulting fees or honoraria from Allergan, Almirall, ATI, BMS, Boehringer, Boston Scientific, Coherex, Colucid, Lilly, Medtronic, Minster, MSD, MAP, Neuralieve, NeurAxon, NTP, and Pfizer. Dr Winner reports having received research grants from Novartis, GSK, Merck, Pfizer, Allergan, and Eli Lilly and having received consulting fees or honoraria from GSK, Merck, and MAP. Dr Silberstein reports having received grants or honoraria from Advanced Neuromodulation Systems, AGA, Allergan, Boston Scientific, Capnia, Coherex, Endo, GSK, Lilly, MAP, Medtronic, Merck, NuPathe, and Valeant. Dr Cutrer reports serving as a consultant on Advisory Boards for MAP and Allergan and serving on the Scientific Review Committee for the MIgraine Research Foundation.
This research was funded by MAP Pharmaceuticals, Inc. MAP Pharmaceuticals provided financial and material support, monitoring, data collection and management, and data analysis to the authors and study investigators.
REFERENCES
- 1. Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193-210 [DOI] [PubMed] [Google Scholar]
- 2. Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition Cephalalgia. 2004;24(suppl 1):9-160 [DOI] [PubMed] [Google Scholar]
- 3. Valade D. Early treatment of acute migraine: new evidence of benefits. Cephalalgia. 2009;29(suppl 3):15-21 [DOI] [PubMed] [Google Scholar]
- 4. Cady R, Elkind A, Goldstein J, Keywood C. Randomized, placebo-controlled comparison of early use of frovatriptan in a migraine attack versus dosing after the headache has become moderate or severe. Curr Med Res Opin. 2004;20(9):1465-1472 [DOI] [PubMed] [Google Scholar]
- 5. Cady R, Martin V, Mauskop A, et al. Efficacy of rizatriptan 10 mg administered early in a migraine attack. Headache. 2006;46(6):914-924 [DOI] [PubMed] [Google Scholar]
- 6. Brandes JL, Kudrow D, Cady R, Tiseo PJ, Sun W, Sikes CR. Eletriptan in the early treatment of acute migraine: influence of pain intensity and time of dosing. Cephalalgia. 2005;25(9):735-742 [DOI] [PubMed] [Google Scholar]
- 7. Goadsby PJ, Zanchin G, Geraud G, et al. Early versus non-early intervention in acute migraine-’Act when Mild (AwM)’: a double-blind, placebo-controlled trial of almotriptan. Cephalalgia. 2008;28(4):383-391 [DOI] [PubMed] [Google Scholar]
- 8. Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55(1):27-36 [DOI] [PubMed] [Google Scholar]
- 9. Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55(1):19-26 [DOI] [PubMed] [Google Scholar]
- 10. Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris). 2005;161(6-7):658-660 [DOI] [PubMed] [Google Scholar]
- 11. Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101(12):4274-4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cady R, Martin V, Mauskop A, et al. Symptoms of cutaneous sensitivity pre-treatment and post-treatment: results from the rizatriptan TAME studies. Cephalalgia. 2007;27(9):1055-1060 [DOI] [PubMed] [Google Scholar]
- 13. Schoenen J, De Klippel N, Giurgea S, et al. Almotriptan and its combination with aceclofenac for migraine attacks: a study of efficacy and the influence of auto-evaluated brush allodynia. Cephalalgia. 2008;28(10):1095-1105 [DOI] [PubMed] [Google Scholar]
- 14. Martin VT, Penzien DB, Houle TT, Andrew ME, Lofland KR. The predictive value of abbreviated migraine diagnostic criteria. Headache. 2005;45(9):1102-1112 [DOI] [PubMed] [Google Scholar]
- 15. Aurora SK, Kori SH, Barrodale P, McDonald SA, Haseley D. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46(1):57-63 [DOI] [PubMed] [Google Scholar]
- 16. Moore KH, Hussey EK, Shaw S, Fuseau E, Duquesnoy C, Pakes GE. Safety, tolerability, and pharmacokinetics of sumatriptan in healthy subjects following ascending single intranasal doses and multiple intranasal doses. Cephalalgia. 1997;17(4):541-550 [DOI] [PubMed] [Google Scholar]
- 17. Rapoport AM, Bigal ME, Tepper SJ, Sheftell FD. Zolmitriptan (Zomig). Expert Rev Neurother. 2004;4(1):33-41 [DOI] [PubMed] [Google Scholar]
- 18. Pollock LA. Dihydroergotamine (D.H.E. 45), a new and effective drug in the treatment of migraine. Rocky Mt Med J. 1946;43(11):895-897 [PubMed] [Google Scholar]
- 19. Carpi A, Virno M. The action of ergotamine on the intracranial venous pressure and on the cerebral venous outflow of the dog. Br J Pharmacol Chemother. 1957;12(2):232-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goadsby PJ, Gundlach AL. Localization of 3H-dihydroergotamine-binding sites in the cat central nervous system: relevance to migraine. Ann Neurol. 1991;29(1):91-94 [DOI] [PubMed] [Google Scholar]
- 21. Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine: a c-Fos and electrophysiological study. Brain. 1996;119:249-256 [DOI] [PubMed] [Google Scholar]
- 22. Masterson CG, Durham PL. DHE repression of ATP-mediated sensitization of trigeminal ganglion neurons. Headache. 2010;50(9):1424-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pozo-Rosich P, Oshinsky ML. Dihydroergotamine (DHE) blocks the induction of central sensitization in the trigeminal nucleus caudalis [abstract]. Neurology. 2005;64(suppl 1):A151 [Google Scholar]
- 24. Silberstein SD, Young WB, Hopkins MM, Gebeline-Myers C, Bradley KC. Dihydroergotamine for early and late treatment of migraine with cutaneous allodynia: an open-label pilot trial. Headache. 2007;47(6):878-885 [DOI] [PubMed] [Google Scholar]
- 25. Cook RO, Shrewsbury SB, Ramadan NM. Reduced adverse event profile of orally inhaled DHE (MAP0004) vs IV DHE: potential mechanism. Headache. 2009;49(10):1423-1434 [DOI] [PubMed] [Google Scholar]
- 26. Aurora SK, Rozen TD, Kori SH, Shrewsbury SB. A randomized, double blind, placebo-controlled study of MAP0004 in adult patients with migraine. Headache. 2009;49(6):826-837 [DOI] [PubMed] [Google Scholar]
- 27. Aurora SK, Silberstein SD, Kori SH, et al. MAP0004, orally inhaled DHE: a randomized, controlled study in the acute treatment of migraine. Headache. 2011;51(4):507-517 [DOI] [PubMed] [Google Scholar]
- 28. Shrewsbury SB, Cook RO, Taylor G, Edwards C, Ramadan NM. Safety and pharmacokinetics of dihydroergotamine mesylate administered via a novel (Tempo) inhaler. Headache. 2008;48(3):355-367 [DOI] [PubMed] [Google Scholar]