Abstract
OBJECTIVE: To determine whether ethnic-specific differences in the prevalence of cardiovascular risk factors and outcomes exist worldwide among individuals with stable arterial disease.
PATIENTS AND METHODS: From December 1, 2003, to June 30, 2004, the prospective, observational REduction of Atherothrombosis for Continued Health (REACH) Registry enrolled 49,602 out-patients with coronary artery disease, cerebrovascular disease, and/or peripheral arterial disease from 7 predefined ethnic/racial groups: white, Hispanic, East Asian, South Asian, Other Asian, black, and Other (comprising any race distinct from those specified). The baseline demographic and risk factor profiles, medication use, and 2-year cardiovascular outcomes were assessed among these groups.
RESULTS: The prevalence of traditional atherothrombotic risk factors varied significantly among the ethnic/racial groups. The use of medical therapies to reduce risk was comparable among all groups. At 2-year follow-up, the rate of cardiovascular death was significantly higher in blacks (6.1%) compared with all other ethnic/racial groups (3.9%; P=.01). Cardiovascular death rates were significantly lower in all 3 Asian ethnic/racial groups (overall, 2.1%) compared with the other groups (4.5%; P<.001).
CONCLUSION: The REACH Registry, a large international study of individuals with atherothrombotic disease, documents the important ethnic-specific differences in cardiovascular risk factors and variations in cardiovascular mortality that currently exist worldwide.
BMI = body mass index; CAD = coronary artery disease; CVD = cerebrovascular disease; MI = myocardial infarction; PAD = peripheral arterial disease; REACH = REduction of Atherothrombosis for Continued Health
Atherothrombotic disease, which clinically manifests as coronary artery disease (CAD), peripheral arterial disease (PAD), and cerebrovascular disease (CVD), is a leading cause of morbidity and mortality worldwide. Despite established management guidelines, contemporary data indicate that undertreatment of risk factors and underuse of proven medical therapies in the treatment of atherothrombotic disease continue to be significant globally.1 Although the use of evidence-based therapies, including lifestyle interventions and pharmacotherapies, has been shown to improve patient outcomes, individuals with CAD, PAD, and CVD continue to have considerably high rates of adverse ischemic events.2 The clinical and socioeconomic implications of this disabling disease will only intensify as the prevalence of atherothrombotic disease continues to increase worldwide.
The natural history, prevalence, and individual risks for the disease subsets encompassed by atherothrombosis vary by ethnicity.3-5 Ethnicity has been shown to be an independent predictor of various adverse cardiovascular outcomes in patients with atherothrombotic disease, including cardiovascular and all-cause mortality.6 Ethnic-specific variations in the treatment of atherothrombotic disease have been demonstrated despite adjustments accounting for risk factor variations, differences in access to care, adherence to medical therapies, and other socioeconomic factors.7 However, the majority of previous data examining ethnicity and its impact in atherothrombotic disease have come from single regional studies limited in their geographic scope and their extent of ethnic diversity.
The international, prospective, observational REduction of Atherothrombosis for Continued Health (REACH) Registry is designed to evaluate those at risk for or with established atherothrombotic disease from highly diverse ethnic populations worldwide. The registry assessed the cardiovascular risk profiles, treatment regimens, and outcomes of nearly 68,000 outpatients enrolled in 44 countries. In the current study, we evaluate those individuals with symptomatic atherothrombotic disease (CAD, PAD, and/or CVD) and assess baseline demographic risk profiles, medication profiles, and 2-year cardiovascular event rates according to ethnicity.
PATIENTS AND METHODS
A detailed description of the study design, methodology, and baseline characteristics of individuals enrolled in the international, prospective REACH Registry has been previously reported.1,8 Briefly, the registry included outpatients aged 45 years or older with either established atherothrombotic disease (CAD, PAD, or CVD) or 3 or more cardiovascular risk factors from 5587 practices in 44 countries. Study participants were enrolled from December 1, 2003, to June 30, 2004 (except in Japan, where an enrollment delay resulted from regulatory requirements), and had an anticipated clinical follow-up of 2 years. Investigators submitted this protocol to the institutional review boards in each country according to local requirements, and all participants were required to sign informed consent.
The current study included only those individuals with symptomatic atherothrombotic disease (CAD, PAD, and/or CVD). Ethnicity was self-reported, and participants were instructed to choose one of the following 7 predefined ethnic/racial groups: white, Hispanic, East Asian, South Asian, Other Asian, black, or Other. Mixed-race individuals were asked to identify themselves by the racial subgroup that they thought had the greatest personal influence. Entry criteria for the study definitions of CAD, PAD, and CVD have been previously published.8 Established CAD was defined as one or more of the following: history of myocardial infarction (MI), history of coronary revascularization (percutaneous or surgical), or history of stable or unstable angina with documented CAD. Documented PAD included one or both of the following criteria: intermittent claudication with a documented ankle-brachial index less than 0.9 or a prior related peripheral revascularization procedure (angioplasty, bypass grafting surgery, or amputation). To fulfill the criteria for documented CVD, individuals had to have a history of transient ischemic attack and/or ischemic stroke.
On initial study enrollment, baseline data including atherosclerotic risk factors and pharmacologic therapies were obtained from the individual medical records at each physician practice. Smoking status was subclassified into either current, former, or never. Current smoking was defined as a mean of at least 5 cigarettes per day within the past month before enrollment, whereas former smoking was defined as having smoked an average of at least 5 cigarettes per day but stopping more than a month before enrollment. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2). Employment status was subdivided into full-time, part-time, unemployed, retired, and incapacitated. The proportion of individuals within the target level baseline blood pressure (<130/80 mm Hg), fasting blood glucose (<110 mg/dL; to convert to mmol/L, multiply by 0.0555), and fasting cholesterol (<175 mg/dL; to convert to mmol/L, multiply by 0.0259) measurements was used as an assessment of treatment efficacy.
Follow-up
Data regarding patient clinical outcomes were obtained from participating physicians at 2 years. Cardiovascular death included fatal MI, fatal stroke, and other cardiovascular death. Other cardiovascular death included other deaths of cardiac origin, pulmonary embolism, death due to heart failure, death after a vascular intervention or operation, and any observed or unobserved sudden death (unless identified as noncardiovascular in origin by autopsy). A death within 28 days of an MI or stroke was considered a fatal MI or fatal stroke, respectively.
Statistical Analyses
Continuous variables and categorical variables are expressed as mean ± SD and frequencies or percentages, respectively. Event rates are reported as 2-year event rates after adjustments for age, sex, enrolling country, type of enrolling physician, PAD, CAD, and CVD. Cumulative event rates were generated using the corrected group prognosis method.9 This method provides an alternative form for estimating survival rates from the Cox proportional hazards model. Event rates are reported only for those individuals with complete outcome and covariate information for the particular end point. Two-sided P values were considered significant when probability is <.05. Statistical analysis was performed using SAS version 8 (SAS Institute, Cary, NC).
RESULTS
Overall, 49,602 outpatients in the REACH Registry with established atherothrombotic disease at baseline were included in this analysis. At 2 years, 45,191 individuals (91%) were available for follow-up. A total of 747 participants (1.5%) died within the first 2 years, and 3664 (7%) were lost to follow-up at 2 years. The rate of lost to follow-up varied among the ethnic/racial groups: from 3% in East Asians to 16% in blacks. Baseline demographics, including atherosclerotic disease, risk factors, and employment status, are listed in Table 1. Overall, 44% (14,709/33,588) of whites in the registry were enrolled in the United States, whereas 95% (1655/1748) of blacks were from the United States. Most Other Asians were enrolled from the Philippines (30%; 796/2655), Thailand (15%; 411/2655), and Indonesia (15%; 400/2655). Hispanic enrollment was predominantly from the United States (36%; 819/2258), Mexico (31%; 690/2258), and Spain (11%; 239/2258).
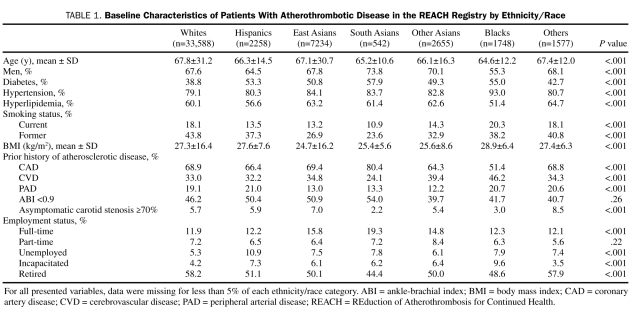
TABLE 1.
Baseline Characteristics of Patients With Atherothrombotic Disease in the REACH Registry by Ethnicity/Race
There were significant differences among the ethnic/racial groups in each of the characteristics analyzed. The largest category of atherothrombotic disease within each group was CAD, followed by CVD and lastly PAD. The rates of CAD varied significantly from a low of 51.4% in blacks to a high of 80.4% in South Asians (P<.001). Rates of CVD ranged from as low as 24.1% in South Asians to a high of 46.2% in blacks (P<.001). The Asian populations had the lowest rates of PAD, with rates of 12.2% in Other Asians, 13.0% in East Asians, and 13.3% in South Asians. Hispanics had the highest rate of PAD at 21.0% (P<.001).
A large proportion of individuals within each ethnic/racial group had traditional risk factors for atherothrombosis, including diabetes mellitus, hypertension, and hyperlipidemia. Rates for each of these risk factors varied significantly across the groups (P<.001 for all 3 risk factors), which is similar to the results seen in the full REACH Registry cohort of individuals with asymptomatic and symptomatic disease.1 Blacks had the highest rate of hypertension (93.0%), followed by East Asians (84.1%) and South Asians (83.7%). The highest rates of diabetes mellitus were seen in South Asians, blacks, and Hispanics, with rates of 57.9%, 55.0%, and 53.3%, respectively. Whites had the lowest rates of diabetes mellitus (38.8%) and hypertension (79.1%), whereas blacks had the lowest rate of hyperlipidemia (51.4%). Most individuals were overweight and current or former smokers. Overall, the average BMI within each ethnic/racial group fulfilled the criterion for a diagnosis of being overweight (BMI ≥25 kg/m2), with the exception of East Asians, whose average BMI was 24.7 kg/m2. Despite having the second lowest mean BMI (25.4 kg/m2), South Asians had the highest rate of diabetes mellitus. Significant proportions of registry participants in each ethnic/racial group were not working full-time but rather were retired, unemployed, working part-time, or incapacitated. Full-time employment rates ranged from a low of 11.9% in whites to a high of 19.3% in South Asians (P<.001).
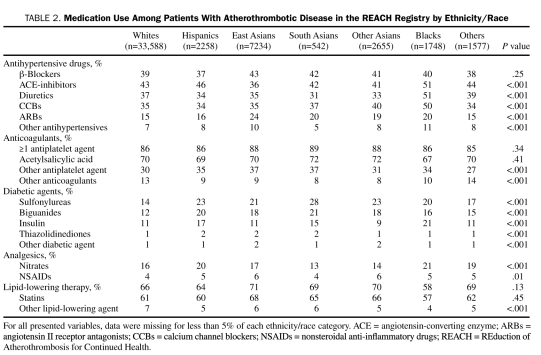
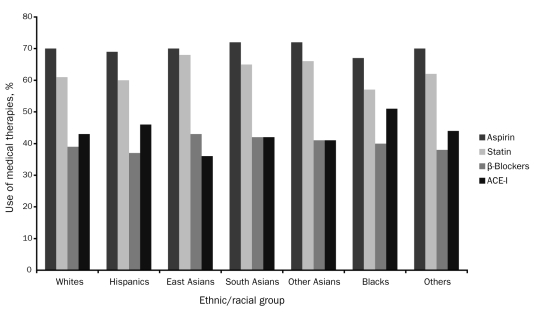
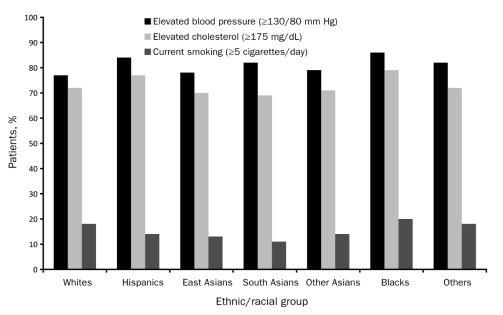
Medication use at the time of study enrollment is listed in Table 2. The use of antiplatelet therapies, aspirin, and statins was similar among all ethnic/racial groups (Figure 1). Nearly 70% of registry participants in each group were taking aspirin at baseline. The percentage of individuals within each group taking statins ranged from 57% to 68%. Overall use of statins in this population of individuals with established atherothrombotic disease was less than optimal, given that 50% or more of all individuals in each ethnic/racial group had a total cholesterol level of 175 mg/dL or higher at baseline (Figure 2). The proportion of individuals with inadequately controlled blood pressure at study enrollment, which varied significantly among the groups, was highest in blacks (86%) (Figure 2). However, a large number of participants among the other ethnic/racial groups also had poorly controlled blood pressure at baseline, with rates ranging from 77% in whites to 84% in Hispanics (P<.001).
TABLE 2.
Medication Use Among Patients With Atherothrombotic Disease in the REACH Registry by Ethnicity/Race
FIGURE 1.
Medication use among patients with atherothrombotic disease in the REduction of Atherothrombosis for Continued Health (REACH) Registry by ethnicity. ACE-I = angiotensin-converting enzyme inhibitor.
FIGURE 2.
Undertreatment of risk factors among patients with atherothrombotic disease in the REduction of Atherothrombosis for Continued Health (REACH) Registry by ethnicity. SI conversion factor: To convert cholesterol value to mmol/L, multiply by 0.0259.
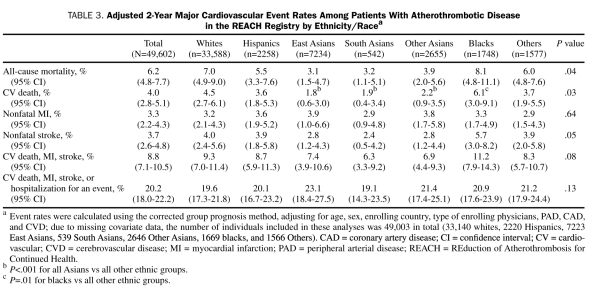
The adjusted 2-year cardiovascular event rates are listed in Table 3. The all-cause mortality rate for the overall population was 6.2%, and the cardiovascular death rate was 4.0%. There were significant differences in both all-cause and cardiovascular mortality among the ethnic/racial groups. Mortality rates were consistently lower in all 3 Asian populations (East, South, and Other Asians), whereas rates were uniformly higher in blacks. Blacks had a significantly higher cardiovascular death rate compared with all other ethnic/racial groups (6.1% vs 3.9%, respectively; P=.01). East Asians, South Asians, and Other Asians had significantly lower cardiovascular death rates of 1.8%, 1.9%, and 2.2%, respectively (all Asians combined, 2.1% vs 4.5%; P<.001). There were no significant differences among the groups in the rates of nonfatal MI, or the composite end point of cardiovascular death, MI, stroke, or hospitalization for an atherothrombotic event. Nonfatal stroke rates were lowest in Asians and highest in blacks, although this difference was not significant (P=.05).
TABLE 3.
Adjusted 2-Year Major Cardiovascular Event Rates Among Patients With Atherothrombotic Disease in the REACH Registry by Ethnicity/Racea
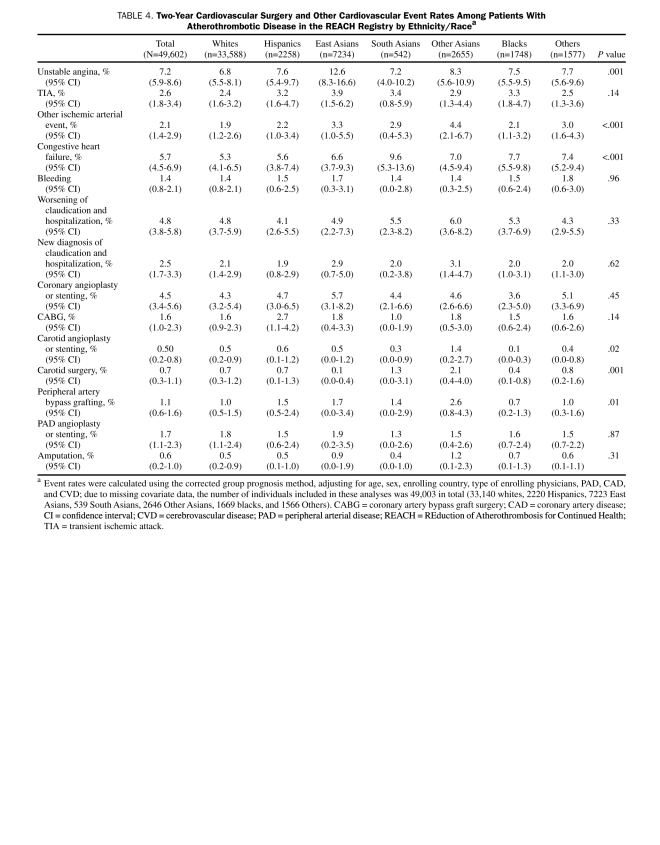
Data on other secondary cardiovascular outcomes resulting in hospitalizations are provided in Table 4. Although the overall rates of cardiovascular revascularization procedures were similar among the ethnic/racial groups, there were significant differences in the rates of carotid and peripheral revascularization procedures. The rates of carotid angioplasty or stenting and carotid surgery were nearly twice as high in Other Asians (1.4% and 2.1%, respectively) compared with the other ethnic/racial groups. The rate of peripheral artery bypass graft surgery was also highest in the Other Asian population (2.6%; P=.01); however, PAD angioplasty or stenting and amputation rates were similar among the groups. Despite having higher rates of hospitalization due to carotid and peripheral revascularization procedures, Other Asians were among the groups with the lowest cardiovascular death rates and nonfatal stroke rates. In contrast, blacks, who tended to have the highest rates of hard cardiovascular outcomes (cardiovascular death, nonfatal stroke, and nonfatal MI), had similar or slightly lower rates of ischemic events resulting in hospitalization and cardiovascular, cerebrovascular, or peripheral revascularization procedures.
TABLE 4.
Two-Year Cardiovascular Surgery and Other Cardiovascular Event Rates Among Patients With Atherothrombotic Disease in the REACH Registry by Ethnicity/Racea
DISCUSSION
The results of this wide-reaching, contemporary analysis composed of a markedly diverse population with stable CAD, CVD, and/or PAD demonstrate important ethnic-specific variations in the risks, management, and outcomes associated with atherothrombotic disease. Blacks with atherothrombotic disease, mainly comprising African Americans from the United States (95%), had the highest rate of cardiovascular death among the ethnic/racial groups worldwide. In contrast, Asians had significantly lower rates of both all-cause mortality and cardiovascular death. Despite being the ethnic/racial group with the largest proportion of CAD, South Asians had one of the lowest rates of MI seen at 2 years, with no apparent explanation. Nonfatal stroke rates were uniformly lower in Asians compared with the other groups. Overall, all hard cardiovascular events tended to be higher in blacks and lower in South Asians, East Asians, and Other Asians (largely composed of Asians from the Philippines, Thailand, and Indonesia).
Traditional atherosclerotic risk factors varied with ethnicity/race, as did the rates of obesity, which have now risen to epidemic proportions on a global scale. Although obesity rates were uniformly lower in the Asian populations, this did not translate into lower rates of comorbid conditions such as diabetes mellitus—South Asians had the highest burden of diabetes mellitus. These findings are consistent with previous studies demonstrating variability in the amount of body fat per BMI and the subsequent propensity to develop cardiovascular risk factors, particularly diabetes mellitus, for a given BMI measurement among the various ethnic/racial groups, especially Asians.10-12
The distribution of atherosclerotic disease also varied among the ethnic/racial groups. South Asians had a much higher rate of CAD with lower rates of CVD or PAD in comparison with the other groups. These findings confirm previously published data illustrating the increasingly high burden of CAD in the developing countries of South Asia.13 Although blacks tended to have the greatest burden of traditional atherosclerotic risk factors, they had the smallest proportion of individuals with documented CAD; yet their rate of CVD was highest among the groups. Whether this denotes a difference in atherothrombotic disease predilection for certain vascular beds (ie, coronary, cerebrovascular, or peripheral) among individuals within various ethnic/racial groups is unknown. It is conceivable that certain atherosclerotic risk factors confer increased susceptibility toward a particular disease subset. For example, the increased prevalence of hypertension, particularly uncontrolled and/or undertreated hypertension as seen in the current study, within the black population may predispose them to having a cerebrovascular event (eg, stroke, transient ischemic attack) as their initial clinical manifestation of atherothrombotic disease.
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study of approximately 15,000 patients with atherothrombosis found that blacks had significantly higher all-cause and cardiovascular mortality compared with Asian patients.6 Several prior studies have also demonstrated ethnic disparities in the use and outcomes associated with coronary, cerebrovascular, and peripheral revascularization procedures.14-20 There were significant differences in the rates of specific cerebrovascular and peripheral revascularization procedures among the ethnic/racial groups in this cohort. Despite having one of the lowest rates of stroke, the Other Asian group was twice as likely to have undergone carotid angioplasty or stenting or carotid surgery within 2 years compared with the other groups. Rates of peripheral artery bypass graft surgery were also highest in Other Asians. Although blacks had consistently higher rates of hard cardiovascular events, the rates of carotid angioplasty, carotid surgery, and peripheral artery bypass grafting were among the lowest seen. There were no ethnic-specific variations in rates of coronary revascularization procedures (angioplasty or stenting or coronary artery-bypass grafting).
The current analysis provides a contemporary “real-world” reflection of specific ethnic data from a large-scale, extremely diverse population group with atherothrombotic disease with excellent 2-year follow-up rates given its geographic reach. Despite these facts, the article has limitations. Ethnicity was self-reported and required mixed-race individuals to choose only one identity. Furthermore, despite best attempts to adequately adjust for confounding variables associated with ethnicity/race, such as the impact of geographic locale, access and delivery of primary and secondary medical care, physician practice preferences, and physician and patient beliefs, delineating their impact on our findings is difficult. Although the REACH Registry enrolled an extensively heterogeneous population, many important ethnic/racial groups are underrepresented. Likewise, REACH is an observational, non–population based registry and, therefore, our findings are vulnerable to criticism regarding their external validity. Medical compliance, including contraindications and financial hardship, was not recorded, and their effect on physician prescribing practices is unknown.
CONCLUSION
Our findings from this large global registry of individuals with atherothrombotic disease illustrate several important ethnic differences in treatment patterns and outcomes from the current era. In general, blacks (consisting mainly of African Americans) had higher rates of adverse cardiovascular outcomes, with the highest rate of cardiovascular death among the groups. However, Asians had significantly lower rates of all-cause and cardiovascular death. The underlying etiologies for these findings have yet to be defined and should be the focus of continued research endeavors in this area. Continued efforts are needed to establish the impact of specific social, cultural, and environmental factors among various ethnicities on health outcomes. Cooperative efforts from both physicians and the patients they treat to adhere to evidence-based risk factor and lifestyle modifications are paramount in efforts to eliminate any ethnic disparities in outcomes from this increasingly burdensome disease.
Footnotes
A complete list of the REACH Registry investigators is published in JAMA. 2006;295(2):180-189.
Dr Bhatt has received institutional research support from AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, sanofi-aventis, and The Medicines Company. Dr Gersh is a member (<3 years) of the advisory boards of Bristol-Myers Squibb, Boston Scientific, Amorcyte Inc, Merck, Cardiovascular Therapeutics, and AstraZeneca. Dr Röther has received honoraria and consulting fees from sanofi-aventis, Bristol-Myers Squibb, Lundbeck, and Boehringer Ingelheim. Dr Goto has received honoraria and consulting fees from Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Kowa, Novartis, Otsuka, sanofi-aventis, Schering-Plough, and Takeda, and he has received research grants from Eisai, Ono, sanofi-aventis, AstraZeneca, Kowa, and Pfizer. Dr Wilson has received grant support from sanofi-aventis. Dr Salette is an employee of sanofi-aventis. Dr Steg has received research grants from sanofi-aventis and has been either a speaker or a consultant for Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Endotis, GlaxoSmithKline, Medtronic, MSD, Nycomed, sanofi-aventis, Servier, and The Medicines Company.
We thank sanofi-aventis and Bristol-Myers Squibb for their support of the REACH Registry. The REACH Registry is endorsed by the World Heart Federation. The sponsor was able to review the manuscript before submission but did not have the authority to change any aspect of the manuscript.
REFERENCES
- 1. Bhatt DL, Steg PG, Ohman EM, et al. ; REACH Registry Investigators International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180-189 [DOI] [PubMed] [Google Scholar]
- 2. Steg PG, Bhatt DL, Wilson PW, et al. ; REACH Registry Investigators One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197-1206 [DOI] [PubMed] [Google Scholar]
- 3. Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112(17):2703-2707 [DOI] [PubMed] [Google Scholar]
- 4. Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163(12):1469-1474 [DOI] [PubMed] [Google Scholar]
- 5. Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371(9616):915-922 [DOI] [PubMed] [Google Scholar]
- 6. Mak KH, Bhatt DL, Shao M, et al. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J. 2009;157(4):658-665 [DOI] [PubMed] [Google Scholar]
- 7. Lillie-Blanton M, Maddox TM, Rushing O, Mensah GA. Disparities in cardiac care: rising to the challenge of Healthy People 2010. J Am Coll Cardiol. 2004;44(3):503-508 [DOI] [PubMed] [Google Scholar]
- 8. Ohman EM, Bhatt DL, Steg PG, et al. ; REACH Registry Investigators The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J. 2006;151(4):786.e1-786.e10 [DOI] [PubMed] [Google Scholar]
- 9. Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35(8):669-674 [DOI] [PubMed] [Google Scholar]
- 10. Wang J, Thornton JC, Burastero S, et al. Comparisons for body mass index and body fat percent among Puerto Ricans, blacks, whites and Asians living in the New York City area. Obes Res. 1996;4(4):377-384 [DOI] [PubMed] [Google Scholar]
- 11. Razak F, Anand S, Vuksan V, et al. ; SHARE Investigators Ethnic differences in the relationships between obesity and glucose-metabolic abnormalities: a cross-sectional population-based study. Int J Obes (Lond). 2005;29(6):656-667 [DOI] [PubMed] [Google Scholar]
- 12. Yang JJ, Shiwaku K, Nabika T, Masuda J, Kobayashi S. High frequency of cardiovascular risk factors in overweight adult Japanese subjects. Arch Med Res. 2007;38(3):337-344 [DOI] [PubMed] [Google Scholar]
- 13. Ramaraj R, Chellappa P. Cardiovascular risk in South Asians. Postgrad Med J. 2008;84(996):518-523 [DOI] [PubMed] [Google Scholar]
- 14. Kennedy BS, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission. J Natl Med Assoc. 2007;99(5):480-488 [PMC free article] [PubMed] [Google Scholar]
- 15. Vaccarino V, Rathore SS, Wenger NK, et al. ; National Registry of Myocardial Infarction Investigators Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353(3):671-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen MS, Bhatt DL, Chew DP, Moliterno DJ, Ellis SG, Topol EJ. Outcomes in African Americans and whites after percutaneous coronary intervention. Am J Med. 2005;118(9):1019-1025 [DOI] [PubMed] [Google Scholar]
- 17. Halm EA, Tuhrim S, Wang JJ, et al. Racial and ethnic disparities in outcomes and appropriateness of carotid endarterectomy: impact of patient and provider factors. Stroke. 2009;40(7):2493-2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrissey NJ, Giacovelli J, Egorova N, et al. Disparities in the treatment and outcomes of vascular disease in Hispanic patients. J Vasc Surg. 2007;46(5):971-978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson CT, Fisher E, Welch HG. Racial disparities in abdominal aortic aneurysm repair among male Medicare beneficiaries. Arch Surg. 2008;143(5):506-510 [DOI] [PubMed] [Google Scholar]
- 20. Meadows TA, Bhatt DL, Hirsch AT, et al. ; REACH Registry Investigators Ethnic differences in the prevalence and treatment of cardiovascular risk factors in US outpatients with peripheral arterial disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Am Heart J. 2009;158(6):1038-1045 [DOI] [PubMed] [Google Scholar]