Abstract
Rhabdomyolysis is a common condition with potentially devastating complications, including acute renal failure, arrhythmias, and death. The standard of care is to use supportive measures such as aggressive fluid repletion to prevent kidney injury and attenuate clinical symptoms. Besides fluid management, few therapeutic options are available for the treatment of acute rhabdomyolysis. As a result, acute and refractory cases remain difficult to manage. We report a case of alcohol-induced rhabdomyolysis that responded dramatically to high-dose corticosteroids. A 55-year-old man presented to the emergency department for evaluation of diffuse muscle pain, weakness, and darkening urine. On admission, his creatine kinase (CK) level was 50,022 U/L. Despite aggressive fluid repletion, his CK level continued to increase, peaking at 401,280 U/L with a concomitant increase in muscle pain and urine darkening. On administration of high-dose corticosteroids, clinical symptoms and CK levels improved dramatically, and the patient was discharged 36 hours later with complete resolution of muscle pain and weakness. Given their low toxicity profile, short-term high-dose corticosteroids may be a valid treatment option for recurrent rhabdomyolysis unresponsive to fluid repletion.
CK = creatine kinase; IL = interleukin
Rhabdomyolysis is the rapid breakdown of striated muscle caused by a wide variety of conditions, including trauma, drugs, viruses, and metabolic disorders. Rhabdomyolysis is a potentially fatal condition with a mortality rate of approximately 8% and a long-term survival rate of approximately 80%.1 The most common complication is renal failure, and rhabdomyolysis accounts for 7% to 10% of all cases of acute kidney injury.2 First-line treatment for rhabdomyolysis is aggressive fluid repletion, which reduces the accumulation of toxic intracellular contents caused by the rapid breakdown of muscle and subsequent renal damage. Unfortunately, few treatments are available for rhabdomyolysis besides those that address the underlying insult, making cases refractory to hydration difficult to manage.3
REPORT OF A CASE
A 55-year-old man presented to the emergency department for evaluation of 2 days of diffuse muscle pain and muscle weakness. The pain occurred at rest and increased on exertion. Associated symptoms included darkening of the urine during the same period without dysuria. The patient denied any illicit drug use, trauma, or recent illness. His medical history was remarkable for hypertension. He had no family history of muscle disease or metabolic disorders. The patient had 1 episode of similar symptoms 2 months previously that was induced by alcohol, and the patient consumed alcohol before the onset of his current symptoms.
On presentation, his vital signs were as follows: temperature, 36.3°C; heart rate, 114 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 161/98 mm Hg. Physical examination revealed tenderness to palpation and 5/5 strength in the upper and the lower extremities with no edema or skin changes. Findings on physical examination were otherwise unremarkable. Laboratory studies on admission revealed a normal complete blood cell count. A complete metabolic profile yielded the following results: sodium, 128 mmol/L; potassium, 3.5 mmol/L; chloride, 96 mmol/L; bicarbonate, 24 mmol/L; blood urea nitrogen, 8 mg/dL; creatinine, 0.46 mg/dL; glucose, 128 mg/dL; calcium, 8.0 mg/dL; total protein, 8.5 g/dL; albumin, 2.9 g/dL; total bilirubin, 1.8 g/dL; aspartate aminotransferase, 1057 U/L; alanine aminotransferase, 288 U/L; and alkaline phosphatase, 95 U/L. The levels of creatine kinase (CK) and C-reactive protein were 50,022 U/L and 4.6 mg/dL, respectively. Urinalysis showed brown urine, a specific gravity greater than 1.030, a pH of 7.0, a protein value of 150 mg/dL, a blood value of 50 mg/dL, and an urobilinogen level of 4 mg/dL, without glucose, nitrites, or leukocyte esterase. Microscopic examination revealed 3 to 5 red blood cells, 0 to 2 white blood cells, 2 to 20 squamous epithelial cells, few bacteria, and 0 to 2 casts. Urine toxicology was unremarkable. Laboratory investigation 3 months previously during a similar episode revealed significant myoglobinuria (myoglobin, 0.85 μg/mL [reference ranges provided parenthetically] [0-0.13 μg/mL]) and an aldolase level of 124.4 U/L (<7.7 U/L).
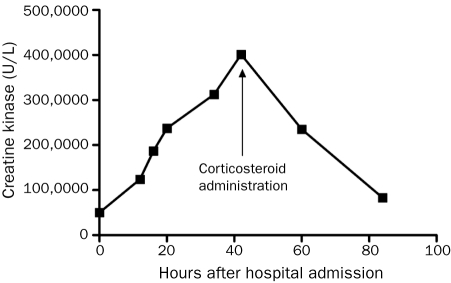
The patient received 2 L of normal saline in the emergency department followed by a continuous infusion of 250 mL/h of normal saline while in the hospital. Despite fluid repletion, the patient’s symptoms worsened. The following day, he was unable to ambulate or sit up in bed secondary to muscle pain and weakness. The patient’s CK level steadily increased to 401,280 U/L, and his urine darkened to a coffee color; however, creatinine and blood urea nitrogen levels remained within normal limits throughout hospitalization. At this point, the patient received 1 g of methylprednisolone. After corticosteroid treatment, the patient’s symptoms improved; within 18 hours, his CK level had decreased to 235,100 U/L (Figure). Within 36 hours, the patient’s CK level decreased to 83,240 U/L, his urine color returned to normal, and his muscle pain and weakness resolved. The patient was discharged 96 hours after admission with a diagnosis of acute alcohol-induced rhabdomyolysis.
FIGURE.
Effect of corticosteroids on creatine kinase. Administration of methylprednisolone (1 g) was followed by a steady decrease in creatine kinase in this patient.
DISCUSSION
This case presented a challenge in that our patient did not respond to first-line therapy. No standardized second-line treatment is available for rhabdomyolysis, and this patient was at substantial risk of acute renal injury.1,4,5 The risk of renal failure in these cases is directly proportional to CK levels.6,7 This patient’s CK level increased to more than 400,000 U/L, an abnormally high value even in acute cases of rhabdomyolysis.8 Thus, our patient was at high risk of end-organ damage with limited treatment options. Our patient’s clinical symptoms and CK level dramatically improved after a single administration of corticosteroids.
Data on the effectiveness of corticosteroids in the treatment of rhabdomyolysis are limited. The primary pharmacologic effects of corticosteroids are both immunosuppressive and anti-inflammatory. Corticosteroids block the response of neutrophils to damage tissues and inhibit the chemotaxis of monocytes and neutrophils to sites of inflammation. Furthermore, these drugs block T-cell activation through inhibition of cytokine releases, thus decreasing levels of interleukin (IL) 1, IL-2, IL-6, and tumor necrosis factor α.9 The anti-inflammatory and immunosuppressive effects of corticosteroids are both dose-dependent and time-dependent. Short-term exposure to a high “lympholytic” dose, such as 500 to 1000 mg of methylprednisolone, induces apoptosis of both immature and mature T cells.10-12 Corticosteroids are commonly used to prevent recurrent rhabdomyolysis in those with chronic myositis. However, chronic myositis cases are generally immune-mediated, whereas acute rhabdomyolysis cases have direct causes, such as toxins, drugs, endocrine disorders, and traumatic muscle damage. Corticosteroids can cause rhabdomyolysis in rare cases, and few studies have been conducted involving this class of drugs. In fact, to our knowledge, only 3 cases of methylprednisolone administration to treat rhabdomyolysis have been reported. Hirohama et al13 and Yasumoto et al14 each reported a case of cytomegalovirus-induced rhabdomyolysis responsive to corticosteroids in the setting of respiratory failure and renal failure, respectively. Brown and Mitchell15 reported a case of rhabdomyolysis caused by heat stroke and strenuous exercise that responded to corticosteroid treatment.
A previously negative autoimmune work-up, including an extended nuclear antigen-6 panel, suggests that our patient’s rhabdomyolysis was likely induced by ethanol. A follow-up muscle biopsy after discharge confirmed a diagnosis of polymyositis, suggesting alcohol-induced rhabdomyolysis in our patient with underlying poly myositis. Ethanol causes rhabdomyolysis through disruption of aden osine triphosphatase pump function, breakdown of the muscle membrane, and alteration of the sarcoplasmic reticulum.16 The muscle damage caused by rhabdomyolysis is thought to be exacerbated by activated neutrophils that infiltrate damaged muscle tissue and release proteases in response to liberated toxic cellular contents.17 Furthermore, the inflammation increases rhabdomyolysis-induced hypoalbuminemia, resulting in increased renal injury.18 Thus, the inflammatory response to rhabdomyolysis amplifies muscle damage, as well as secondary renal insult. The first-line therapy is fluid repletion, which helps prevent kidney injury by correcting hypovolemia, maintaining the glomerular filtration rate, and attenuating myoglobin accumulation.1 However, fluid management does not address the underlying causes of myotoxicity or minimize muscle damage.
In the current case, we hypothesize that corticosteroid administration helped diminish the inflammatory exacerbation of muscle damage. High, lympholytic dosing of corticosteroids is likely justified in acute cases of rhabdomyolysis given the additional benefit of short-term T-cell suppression compared with low-dose administration. We cannot exclude this patient’s natural disease progression as a cause of his recovery. However, the time course of corticosteroid administration and the dramatic decreases in CK level and symptom improvement suggest a pharmacologic effect. As seen in the Figure, the patient’s CK level decreased abruptly after methylprednisolone dosing.
Some have suggested the use of mannitol or bicarbonate in conjunction with fluid repletion in the treatment of rhabdomyolysis.4 However, the efficacy of alkalinization therapy has yet to be thoroughly established and, to date, this strategy is a topic of much debate in the literature.1 Urine alkalinization may trap myoglobin in its reduced state, thereby preventing toxic myoglobin precipitation that causes direct kidney injury.19 Alkalinization also decreases lipid peroxidation, reactive oxygen species formation, and myoglobin-induced vasoconstriction, all of which contribute to acute kidney injury.20,21 Studies have shown a mild benefit with urine alkalinization in reducing kidney injury, particularly when the alkalinizing agent is administered early in the course of rhabdomyolysis.1,3,4,22,23 Because CK levels correlate with the development of renal damage, and given that this patient’s presentation CK value was greater than 50,000 U/L, damage likely had already occurred, limiting the effectiveness of alkalinization.3 Given the lack of evidence of alkalinization utility, as well as the advanced stage in which our patient presented, we did not use this treatment strategy.
CONCLUSION
Rhabdomyolysis is a common condition that, if left untreated, can have devastating complications, including renal failure and death. Few treatment options are available for cases of acute rhabdomyolysis unresponsive to first-line fluid management. Corticosteroids, such as methylprednisolone, can diminish the inflammatory response to muscle damage and potentially decrease secondary immune-mediated muscle damage. Administration of high-dose corticosteroids may break the cycle of primary muscle toxicity followed by inflammation-induced muscle damage. Given the lack of second-line treatment options, corticosteroids may be a viable treatment option in cases of acute or refractory rhabdomyolysis. Priorities for further research include identifying risk factors for refractory cases in which corticosteroid therapy may be a viable option, as well as determining the optimal dose of corticosteroids and the timing of their administration.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review from the editorial board of this journal.
REFERENCES
- 1. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62-72 [DOI] [PubMed] [Google Scholar]
- 2. Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27(5):803-811 [DOI] [PubMed] [Google Scholar]
- 3. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010;48(6):749-756 [DOI] [PubMed] [Google Scholar]
- 4. Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196 [DOI] [PubMed] [Google Scholar]
- 5. Szpirt WM. Plasmapheresis is not justified in treatment of rhabdomyolysis and acute renal failure [letter]. J Cardiovasc Surg (Torino). 1997;38(5):557 [PubMed] [Google Scholar]
- 6. Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore). 2005;84(6):377-385 [DOI] [PubMed] [Google Scholar]
- 7. Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988;148(7):1553-1557 [PubMed] [Google Scholar]
- 8. de Meijer AR, Fikkers BG, de Keijzer MH, van Engelen BG, Drenth JP. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29(7):1121-1125 [DOI] [PubMed] [Google Scholar]
- 9. Sayer HG, Longton G, Bowden R, Pepe M, Storb R. Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood. 1994;84(4):1328-1332 [PubMed] [Google Scholar]
- 10. Leussink VI, Jung S, Merschdorf U, Toyka KV, Gold R. High-dose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leukocytes. Arch Neurol. 2001;58(1):91-97 [DOI] [PubMed] [Google Scholar]
- 11. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55-89 [DOI] [PubMed] [Google Scholar]
- 12. Barnes PJ. Corticosteroids: the drugs to beat. Eur J Pharmacol. 2006;533(1-3):2-14 [DOI] [PubMed] [Google Scholar]
- 13. Hirohama D, Shimizu T, Hashimura K, et al. Reversible respiratory failure due to rhabdomyolysis associated with cytomegalovirus infection. Intern Med. 2008;47(19):1743-1746 [DOI] [PubMed] [Google Scholar]
- 14. Yasumoto N, Hara M, Kitamoto Y, Nakayama M, Sato T. Cytomegalovirus infection associated with acute pancreatitis, rhabdomyolysis and renal failure. Intern Med. 1992;31(3):426-430 [DOI] [PubMed] [Google Scholar]
- 15. Brown J, Mitchell S. A complicated case of exertional heat stroke in a military setting with persistent elevation of creatine phosphokinase. Mil Med. 1992;157(2):101-103 [PubMed] [Google Scholar]
- 16. Song SK, Rubin E. Ethanol produces muscle damage in human volunteers. Science. 1972;175(19):327-328 [DOI] [PubMed] [Google Scholar]
- 17. Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11(8):1553-1561 [DOI] [PubMed] [Google Scholar]
- 18. Slater MS, Mullins RJ. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. J Am Coll Surg. 1998;186(6):693-716 [DOI] [PubMed] [Google Scholar]
- 19. Zager RA. Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab Invest. 1989;60(5):619-629 [PubMed] [Google Scholar]
- 20. Moore KP, Holt SG, Patel RP, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273(48):31731-31737 [DOI] [PubMed] [Google Scholar]
- 21. Heyman SN, Greenbaum R, Shina A, Rosen S, Brezis M. Myoglobinuric acute renal failure in the rat: a role for acidosis? Exp Nephrol. 1997;5(3):210-216 [PubMed] [Google Scholar]
- 22. Gunal AI, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15(7):1862-1867 [DOI] [PubMed] [Google Scholar]
- 23. Shimazu T, Yoshioka T, Nakata Y, et al. Fluid resuscitation and systemic complications in crush syndrome: 14 Hanshin-Awaji earthquake patients. J Trauma. 1997;42(4):641-646 [DOI] [PubMed] [Google Scholar]