Abstract
Most viral diseases, with the exception of those caused by human immunodeficiency virus, are self-limited illnesses that do not require specific antiviral therapy. The currently available antiviral drugs target 3 main groups of viruses: herpes, hepatitis, and influenza viruses. With the exception of the antisense molecule fomivirsen, all antiherpes drugs inhibit viral replication by serving as competitive substrates for viral DNA polymerase. Drugs for the treatment of influenza inhibit the ion channel M2 protein or the enzyme neuraminidase. Combination therapy with Interferon-α and ribavirin remains the backbone treatment for chronic hepatitis C; the addition of serine protease inhibitors improves the treatment outcome of patients infected with hepatitis C virus genotype 1. Chronic hepatitis B can be treated with interferon or a combination of nucleos(t)ide analogues. Notably, almost all the nucleos(t) ide analogues for the treatment of chronic hepatitis B possess anti–human immunodeficiency virus properties, and they inhibit replication of hepatitis B virus by serving as competitive substrates for its DNA polymerase. Some antiviral drugs possess multiple potential clinical applications, such as ribavirin for the treatment of chronic hepatitis C and respiratory syncytial virus and cidofovir for the treatment of cytomegalovirus and other DNA viruses. Drug resistance is an emerging threat to the clinical utility of antiviral drugs. The major mechanisms for drug resistance are mutations in the viral DNA polymerase gene or in genes that encode for the viral kinases required for the activation of certain drugs such as acyclovir and ganciclovir. Widespread antiviral resistance has limited the clinical utility of M2 inhibitors for the prevention and treatment of influenza infections. This article provides an overview of clinically available antiviral drugs for the primary care physician, with a special focus on pharmacology, clinical uses, and adverse effects.
ALT = alanine aminotransferase; CHB = chronic hepatitis B; CHC = chronic hepatitis C; CMV = cytomegalovirus; CSF = cerebrospinal fluid; EBV = Epstein-Barr virus; FDA = US Food and Drug Administration; HBeAg = hepatitis B e antigen; HBV = hepatitis B virus; HCV = hepatitis C virus; HHV = human herpesvirus; HIV = human immunodeficiency virus; HSV = herpes simplex virus; HSV-1 = HSV type 1; HSV-2 = HSV type 2; IFN = interferon; IV = intravenous; mRNA= messenger RNA; RSV = respiratory syncytial virus; SC = subcutaneous; SVR = sustained virologic response; TK = thymidine kinase; VZV = varicella zoster virus
Most diseases caused by viral pathogens are self-limited and do not require specific antiviral therapy. Other than therapies targeting the human immunodeficiency virus (HIV), currently available antiviral drugs in the clinical setting target 3 principal groups of viruses—the herpes, hepatitis, and influenza viruses. This review article is structured to discuss antiviral therapeutics on the basis of these 3 major antiviral categories, with the caveat that some drugs discussed in these sections possess other potential applications, such as ribavirin for the treatment of respiratory syncytial virus (RSV) and cidofovir for the treatment of cytomegalovirus (CMV) and other DNA viral infections. Nucleos(t)ide analogues for the treatment of chronic hepatitis B (CHB) may also possess anti-HIV properties, but their clinical utility for the treatment of HIV will be discussed in a separate article in this symposium. Experimental and novel therapies that have not reached clinical application will not be reviewed.
ANTIHERPES DRUGS
Acyclovir
Acyclovir is a synthetic guanosine analogue used for treating herpes simplex virus (HSV) and varicella zoster virus (VZV) infections.1-3 Intravenous (IV) acyclovir provides excellent tissue and fluid penetration, including the cerebrospinal fluid (CSF), whereas oral acyclovir provides modest bioavailability of 15% to 30%. Bioavailability is improved with the use of valacyclovir, the valyl ester formulation of acyclovir. Acyclovir is excreted by glomerular filtration and tubular secretion.
Herpesviruses have varying degrees of susceptibility to acyclovir, with HSV type 1 (HSV-1) being most susceptible, followed by HSV type 2 (HSV-2) and VZV, and to a lesser extent Epstein-Barr virus (EBV).1-3 High acyclovir concentrations may also inhibit CMV in vitro, but acyclovir is not recommended clinically for CMV treatment. Acyclovir is not active against human herpesvirus (HHV) 6, 7, and 8.
To exert antiviral activity, acyclovir must be converted to acyclovir-triphosphate; this process is initially catalyzed by viral thymidine kinase (TK) and subsequently by human enzymes. Acyclovir-triphosphate serves as a competitive substrate for viral DNA polymerase, and its incorporation into the DNA chain results in termination of viral replication.
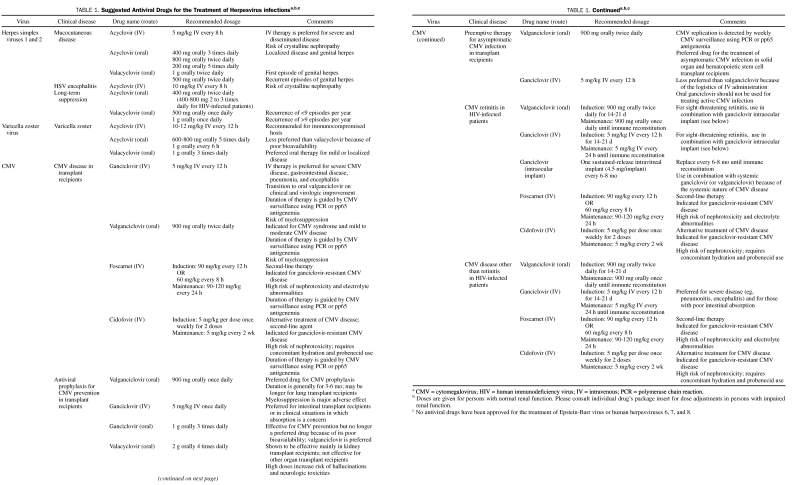
Acyclovir is approved for the treatment of primary and recurrent genital HSV infection (Table 1).2,4,5 Topical acyclovir may be used to treat genital herpes, but the oral formulation is generally recommended6; IV acyclovir is used for severe cases.1,4 Suppressive therapy with oral acyclovir is also indicated to reduce the incidence of recurrent genital herpes.4,7
TABLE 1.
Suggested Antiviral Drugs for the Treatment of Herpesvirus infectionsa,b,c
Oral acyclovir is modestly efficacious against orolabial herpes. In immunocompetent individuals, orolabial herpes is often self-limited, and antiviral treatment is generally not recommended.7 However, oral acyclovir may be indicated for severe cases, for those with recurrent orolabial herpes, and in those who are immunocompromised.7,8
Intravenous acyclovir is the first-line treatment for HSV encephalitis9 and should be started as soon as the disease is suspected clinically. Magnetic resonance imaging of the brain typically demonstrates temporal lobe involvement, and diagnosis is confirmed by detection of HSV DNA in the CSF. Major studies have evaluated the efficacy of 10 days of acyclovir treatment for HSV encephalitis; however, the recommended duration of treatment in the clinical setting is 2 to 3 weeks because shorter durations have been associated with relapse.10 The treatment duration may be further prolonged in immunocompromised patients.8
Acyclovir is also approved by the US Food and Drug Administration (FDA) for the treatment of VZV11,12; however, young immunocompetent patients with zoster may not require treatment if the lesions are localized and have been present for more than 72 hours. Intravenous acyclovir is recommended for patients with disseminated zoster disease or visceral involvement. Acyclovir treatment of zoster reduces duration of viral shedding, formation of new lesions, and short- and long-term neuralgia.13 Therapy should be started early, but even delayed initiation of acyclovir may still be beneficial in immunocompromised patients. Short-course prednisone may be added as an adjunct to acyclovir treatment of zoster to improve quality of life, especially in elderly patients.
Acyclovir has been used in the treatment of acute retinal necrosis (which is associated with HSV or VZV), eczema herpeticum, and oral hairy leukoplakia due to EBV. Oral acyclovir is used to prevent HSV during the early period after transplant in patients not receiving ganciclovir or valganciclovir prophylaxis.14
Acyclovir is generally well tolerated. However, IV acyclovir may cause reversible nephrotoxicity in 5% to 10% of patients because of intratubular precipitation of acyclovir crystals. Acyclovir crystalline nephropathy is more common when acyclovir is given as a rapid infusion (reaching serum concentrations >25 μg/mL)15 and in patients with dehydration and preexisting renal impairment.16 Adequate hydration, a slower rate of infusion, and dosing based on renal function may reduce this risk. Reversible neurologic symptoms such as delirium and seizures may occur rarely in elderly people and those with renal impairment; this toxicity has been associated with high serum acyclovir concentrations15 and high CSF levels of its metabolite 9-carboxymethoxymethylguanine.17-19 Other adverse effects are gastrointestinal symptoms, myelosuppression, and rash.3,20,21
Acyclovir-resistant HSV has been reported, especially in immunocompromised patients.22-24 Resistance occurs by selection of viral mutants that are deficient in TK (which results in an inability to activate acyclovir) or that have altered DNA polymerase with reduced affinity to acyclovirtriphosphate.23
Brivudin
Brivudin is a 5′-halogenated thymidine nucleoside analogue that is highly active against HSV-1 and VZV.25,26 Brivudin is phosphorylated by viral TK and cellular kinases to brivudin-triphosphate, which serves as a competitive inhibitor of viral DNA polymerase, thereby terminating viral DNA synthesis. It is available in some countries for the treatment of herpes zoster and herpes simplex. However, concerns about its toxicity halted its clinical development in the United States. Its metabolite, bromovinyluracil, irreversibly inhibits dihydropyridine dehydrogenase, which regulates nucleoside metabolism. Coadministration with 5-fluorouracil has resulted in lethal bone marrow toxicity and severe gastrointestinal toxicity.25,26
Cidofovir
Cidofovir is a nucleoside analogue used for the treatment of CMV, other herpesviruses, and other DNA viral infections.27 It is available as an IV formulation, and an oral prodrug of cidofovir (known as CMX-001) is under clinical development.28 This investigational lipid ester formulation of cidofovir has enhanced bioavailability, resulting in improved 50% inhibitory concentrations.28 Direct intraocular injection of cidofovir is contraindicated due to ocular hypotony.27 Serum cidofovir concentrations decline rapidly after IV infusion, with a half-life of 2 hours; however, the intracellular half-life of active cidofovir-diphosphate is as long as 65 hours. Cidofovir is eliminated by glomerular filtration and tubular secretion; probenecid reduces its excretion by blocking tubular secretion.29,30
Cidofovir is phosphorylated by cellular kinases into cidofovir-diphosphate, a competitive substrate for viral DNA polymerase, thereby halting viral DNA synthesis.27 The major clinical indication for cidofovir is the treatment of CMV retinitis in HIV-infected patients (Table 1).31 Cidofovir is also used as rescue therapy for immunocompromised patients with CMV disease resistant or unresponsive to ganciclovir.32 Because activation of cidofovir does not rely on viral kinases, it retains activity against CMV with the UL97 mutation and HSV with the TK mutation.33 Resistance to cidofovir occurs when the virus develops mutations in the DNA polymerase gene (ie, CMV-UL54 gene mutations).33 Cidofovir has also been used off-label for various illnesses, such as acyclovir-resistant HSV disease, condyloma acuminatum, BK virus–associated hemorrhagic cystitis, JC virus–associated progressive multifocal leukoencephalopathy, and other infections due to double-stranded DNA viruses.34-47
Nephrotoxicity is the most common serious adverse effect of cidofovir.27 The incidence and severity of nephrotoxicity may be reduced by hydration and probenecid.48 Blood cell counts should be monitored to assess myelosuppression, and ophthalmological surveillance is recommended because of the risk of ocular hypotony, uveitis, and iritis.49,50
Famciclovir
Famciclovir is a diacetyl 6-deoxy analogue of penciclovir. Oral famciclovir is rapidly absorbed and achieves a bioavailability of 77%.51 Famciclovir is metabolized into penciclovir, reaching peak plasma penciclovir concentrations within 1 hour. Because of extensive hepatic metabolism, virtually no famciclovir is detectable in plasma.51 Famciclovir is excreted renally as penciclovir and its 6-deoxy precursor.9
Famciclovir is active against HSV-1, HSV-2, and VZV, and, to a lesser extent, against EBV. Its mechanism of action is through penciclovir; penciclovir triphosphate inhibits herpes DNA synthesis by acting as a substrate for viral DNA polymerase. The major clinical indications for famciclovir use are treatment of herpes zoster, recurrent genital herpes,52 and recurrent herpes labialis.53 Famciclovir can also be used as suppression therapy to reduce the risk of recurrent genital herpes as well as oral treatment of uncomplicated varicella in HIV-infected patients.
The most common adverse effects of famciclovir are headache and nausea.54 Rare adverse events include jaundice, rash, pruritus, somnolence, and confusion.54 Acute renal failure has occurred in patients taking inappropriately high doses of famciclovir.
Fomivirsen
Fomivirsen is a 21-nucleotide phosphorothionate oligonucleotide complementary to messenger RNA (mRNA) of the immediate-early region of CMV.55-57 Its antiviral property is exerted by antisense inhibition of target gene expression. Other potential mechanisms of antiviral activity include its nonspecific interactions with viral particles that may prevent adsorption or lead to inhibition of enzymes required for viral DNA synthesis.
Fomivirsen is given intravitreally. Its main indication is the treatment of CMV retinitis in patients with AIDS who have not benefited from or are intolerant of standard CMV therapies, or whose virus is resistant to ganciclovir and foscarnet.55-57 The main adverse effect of fomivirsen is increased intraocular pressure and inflammation. Blurred vision, conjunctival hemorrhage, retinal detachment, and retinal edema are other adverse effects.55-57
Foscarnet
Foscarnet is a nonnucleoside pyrophosphate analogue that is given intravenously for the treatment of herpesviruses.58 Its pharmacokinetic profile is complicated by a high incidence of nephrotoxicity and by its deposition and subsequent gradual release from bone.58 Its half-life depends on the duration of therapy; in patients with normal renal function, the plasma half-life is about 2 to 4 hours, but terminal half-lives up to about 8 days may occur when it has accumulated in bones. Foscarnet is excreted through glomerular filtration.
Foscarnet selectively inhibits pyrophosphate binding on viral DNA polymerases, thus suppressing HSV-1, HSV-2, and CMV replication. It is also active against VZV, HHV-6, and EBV.59 Unlike ganciclovir, foscarnet does not require intracellular conversion to active triphosphate, thus maintaining activity against herpesviruses with TK or UL97 kinase mutations.33
Foscarnet is approved for the treatment of CMV retinitis in patients with AIDS.58 It has been used to treat other CMV diseases in immunocompromised patients, especially those unable to tolerate ganciclovir and those infected with ganciclovir-resistant virus.60,61 Foscarnet is also used for treating acyclovir-resistant mucocutaneous HSV and VZV in immunocompromised patients.58 On rare occasion, it has been used for prevention of CMV in transplant recipients; however, its toxicity limits this clinical indication.60,61
Nephrotoxicity is the most common serious adverse effect of foscarnet, affecting 30% of patients. It is caused by deposition of foscarnet crystals in the glomerular capillary lumen.62,63 Foscarnet may cause myelosuppression, with anemia as the most common effect. It can chelate bivalent metal ions and may lead to reductions in ionized calcium. Other electrolyte disturbances are hypokalemia, hypomagnesemia, and hypophosphatasemia, which could manifest as paresthesias, cardiac dysrhythmias, and neurologic symptoms, including seizures.64 Patients should be hydrated to prevent nephrotoxicity, and electrolyte abnormalities should be corrected to avoid cardiac and neurologic complications.
Ganciclovir
Ganciclovir is an acyclic 2′-deoxyguanosine analogue for the management of CMV.65 It is available in oral and parenteral formulations. Oral ganciclovir is poorly absorbed, with a bioavailability of only 5%.65 Management of active CMV disease is therefore with IV ganciclovir or its oral valyl prodrug valganciclovir. Intravitreal ganciclovir implants are also available, with minimal systemic absorption. Ganciclovir is excreted renally.
Ganciclovir undergoes triphosphorylation to become active, with the initial monophosphorylation catalyzed by UL97-encoded kinase and subsequently by cellular kinases. Ganciclovir triphosphate inhibits viral DNA synthesis through competitive incorporation during viral DNA synthesis, thereby leading to DNA chain termination. In vitro, it is 10 times more potent than acyclovir against CMV and EBV and is just as effective as acyclovir against HSV-1, HSV-2, and VZV.66 Ganciclovir is active against HHV-6 and HHV-8 but not against HHV-7.67
Ganciclovir is approved for the treatment of CMV retinitis in patients with AIDS, the treatment of herpes simplex keratitis, and CMV prophylaxis in transplant recipients. Intravenous ganciclovir may also be used to treat other forms of CMV disease, such as colitis or esophagitis. Induction therapy with IV ganciclovir for CMV retinitis in patients with AIDS has an efficacy of 85% to 95% in stabilizing disease.68 Because it is poorly absorbed, oral ganciclovir should not be used for induction treatment of CMV disease.69 Because CMV disease often recurs or progresses in patients with advanced AIDS, oral or IV ganciclovir (or valganciclovir) is given as maintenance therapy until immune reconstitution is achieved.69 Intravitreal ganciclovir may also be surgically implanted for the treatment of CMV retinitis, although this treatment should be used together with systemic therapy with IV ganciclovir or oral valganciclovir therapy.
Oral ganciclovir may be used to prevent CMV in patients with AIDS; however, the benefit of this strategy is not as pronounced in the era of highly active antiretroviral therapy. Although IV and oral ganciclovir have also been used to prevent CMV disease in transplant recipients, valganciclovir is currently the preferred drug for this indication.60,61,70 Intravenous ganciclovir is also used as a first-line treatment of CMV disease in bone marrow and solid organ transplant recipients.61
Reversible bone marrow suppression is the most common adverse effect of ganciclovir. Other adverse effects of the drug are rash, pruritus, diarrhea, nausea, vomiting, and increased levels of serum creatinine and liver enzymes. Neurotoxicity may occur occasionally.
Resistance to ganciclovir occurs most commonly in severely immunocompromised patients with prolonged exposure to the drug. The most common mechanism for ganciclovir resistance is UL97 gene mutation71; this mutation leads to deficiency in the viral kinase that is necessary for the initial phosphorylation of ganciclovir into its active form. A less common mechanism is a mutation in the UL54 gene, which encodes for the CMV DNA polymerase.71
Penciclovir
Penciclovir is an acyclic guanine analogue that is chemically similar to acyclovir. Because it is poorly absorbed from the gastrointestinal tract, it is only available as topical therapy for mucocutaneous herpes. For systemic use, penciclovir has been reformulated into the oral prodrug famciclovir.
The antiviral activity of penciclovir is similar to that of acyclovir, with efficacy against HSV-1, HSV-2, and VZV, and, to a lesser extent, against EBV.72 Penciclovir is monophosphorylated by TK and subsequently by cellular kinases into active penciclovir-triphosphate, which inhibits herpes DNA polymerase activity by serving as a competitive inhibitor of deoxyguanosine triphosphate.73 Penciclovir is approved as topical therapy for recurrent herpes labialis, resulting in a faster healing rate and reduction in pain and viral shedding.74
Valacyclovir
Valacyclovir, an L-valyl ester prodrug of acyclovir,75 provides a higher bioavailability (55%) than oral acyclovir. After absorption, valacyclovir is hydrolyzed almost completely to acyclovir by first-pass intestinal and hepatic metabolism. It achieves peak serum concentration in 1 to 3 hours. Serum acyclovir levels are much higher with valacyclovir than with oral acyclovir.75
The mechanism of action and spectrum of activity of valacyclovir is identical to those of acyclovir. It is approved for the treatment of initial or recurrent episodes of genital herpes76 and for the treatment of recurrent herpes labialis.4 Treatment is most efficacious when initiated at the earliest onset of symptoms.4 Suppressive therapy with valacyclovir is recommended to prevent recurrent genital herpes76 and has the potential to reduce transmission to sexual partners.4
Valacyclovir is approved for treatment of VZV. Varicella often resolves without antiviral therapy in those who are immunocompetent. However, in immunocompromised patients, such as HIV-infected patients and transplant recipients, valacyclovir may be used to treat varicella, even if it is uncomplicated.77 Valacyclovir is the most commonly used drug for the treatment of zoster.75,77 In a randomized double-blind trial of older immunocompetent patients, valacyclovir was as effective as acyclovir, with similar resolution rates of cutaneous zoster but accelerated resolution of herpetic pain and a lower risk of postherpetic neuralgia.75 The typical course of zoster treatment is 7 days, and the first dose should be started within 48 hours of rash onset. Treatment can be prolonged, continuing until all lesions have crusted, in immunocompromised patients.77
Valacyclovir has also been used to treat acute retinal necrosis and for prevention of CMV disease in kidney transplant recipients.78 Although ganciclovir is the backbone for CMV prevention in transplant recipients, the efficacy of valacyclovir prophylaxis for CMV prevention was demonstrated in kidney transplant recipients.78 However, valacyclovir has not been proven effective for preventing CMV in heart, liver, lung, pancreas, and small bowel transplant recipients.
The adverse effects of valacyclovir are similar to those of acyclovir. At very high doses, neurotoxicity characterized by confusion, hallucinations, and seizures may occur,78 especially in elderly patients and in those with dehydration and renal disease. The mechanism of resistance for valacyclovir is identical to that of acyclovir (TK mutation); however, achieving higher serum acyclovir levels with valacyclovir could reduce the risk of resistance compared with oral acyclovir.
Valganciclovir
Valganciclovir is the L-valyl ester prodrug of ganciclovir. Oral valganciclovir is well absorbed and converted to ganciclovir by first-pass intestinal or hepatic metabolism.79 The bioavailability of ganciclovir after valganciclovir administration is about 60%, and peak plasma concentrations are achieved in 1 to 3 hours.80,81 Valganciclovir is eliminated renally as ganciclovir.80,82
Valganciclovir exerts its antiviral activity in the form of ganciclovir-triphosphate, which inhibits viral replication by serving as a competitive substrate for CMV DNA polymerase. Valganciclovir was first approved by the FDA for treatment of CMV retinitis in patients with AIDS.83 For immediate sight-threatening lesions, valganciclovir is used in combination with an intravitreal ganciclovir implant. Valganciclovir is also used for preventing CMV disease in high-risk CMV donor-positive/recipient-negative recipients of kidney, heart, or kidney-pancreas transplants.61,84 In the United States, valganciclovir is not approved for preventing CMV disease in liver recipients because of a higher incidence of tissue-invasive CMV disease in patients who received valganciclovir vs oral ganciclovir prophylaxis. In other countries, valganciclovir is used for preventing CMV disease in all solid organ transplant recipients. It has recently gained approval for the prevention of CMV disease in pediatric heart and kidney transplant recipients. It can also be used to preemptively treat asymptomatic CMV infection in transplant recipients.85-88 Valganciclovir was recently demonstrated to be as effective as IV ganciclovir for treating mild to moderate CMV disease in transplant recipients.86,87,89
Bone marrow suppression is the most common adverse effect of valganciclovir. Gastrointestinal manifestations, such as diarrhea, nausea, and vomiting, may be observed. Resistance to valganciclovir occurs through mechanisms identical to those underlying ganciclovir resistance, ie, through mutations in the UL97 gene, which encodes for CMV kinase, and the UL54 gene, which encodes for CMV DNA polymerase.71
Vidarabine
Vidarabine, a purine nucleoside obtained from Streptomyces antibioticus, was historically used for treating HSV and VZV. Acyclovir has since become the preferred drug for these conditions.90 Vidarabine is currently available only as an ophthalmic solution for treating recurrent epithelial keratitis and acute keratoconjunctivitis.90 Once phosphorylated into its active form, vidarabine inhibits viral DNA polymerase. Adverse effects of ophthalmic vidarabine include irritation, pain, photophobia, lacrimation, and occlusion of the lacrimal duct.90
ANTIVIRAL DRUGS FOR INFLUENZA
M2 Inhibitors
Amantadine. Amantadine is a symmetric tricyclic amine that inhibits replication of influenza A virus by impairing the function of the membrane protein M2.91 Present only in influenza A virus, M2 is an acid-activated ion channel required for nucleocapsid release.91 Amantadine is well absorbed after oral administration. It has an elimination half-life of 11 to 15 hours and is excreted by glomerular filtration and tubular secretion.
Amantadine is effective for treating susceptible influenza A virus infection.91 It results in a more rapid functional recovery and reduces the duration of fever and other symptoms by about 1 day, if given within 48 hours of disease onset.91 Amantadine is effective as prophylaxis for preventing symptomatic influenza A infection in exposed persons.92,93 It is usually given for 14 days or for at least 7 days after the last confirmed illness. Seasonal influenza vaccination, however, remains the preferred method for prevention.
Amantadine is generally well tolerated. Among its adverse effects are mild neurologic symptoms such as anxiety, disorientation, and headache, especially in elderly patients and those taking neuroaffective drugs. Emergence of amantadine resistance has limited its use in the clinical setting.94,95 Amantadine resistance, characterized by amino acid substitutions in the M2 protein, emerges within 2 to 4 days of treatment. Because of widespread resistance, amantadine is no longer recommended for empiric treatment of influenza.94,95 M2 mutation confers cross-resistance with rimantadine.
Rimantadine. Rimantadine is a symmetric tricyclic amine that inhibits influenza virus.93 It is well absorbed after oral administration, reaching peak plasma concentration in 3 to 5 hours. Rimantadine undergoes extensive hepatic metabolism before it is excreted in the urine.
The mechanism of action of rimantadine is similar to that of amantadine; it inhibits the ion channel function of M2, thereby inhibiting viral uncoating. Rimantadine is indicated for prevention and treatment of influenza A virus93; however, its clinical utility is currently limited by drug resistance.96,97 A few trials that compared amantadine and rimantadine suggested similar efficacy; however, neurologic adverse events are less severe and frequent with rimantadine.
Neuraminidase Inhibitors
Oseltamivir. Oseltamivir phosphate is a prodrug of oseltamivir carboxylate, which is an inhibitor of neuraminidase that is essential in the replication of influenza A and B viruses.98 Oral oseltamivir is well absorbed and reaches peak serum concentrations in 1 hour. Bioavailability of oseltamivir phosphate is at least 75%. The prodrug oseltamivir phosphate undergoes extensive hepatic metabolism via ester hydrolysis. More than 99% of active oseltamivir carboxylate is excreted renally.
Oseltamivir carboxylate, the active drug metabolite, selectively blocks viral neuraminidase, thereby preventing the release of virus from infected cells.98 Oseltamivir is approved for the treatment of children (≥1 year) and adults with influenza A or B viral infections.98 Treatment should start within 48 hours of disease onset and continue for 5 days. Oseltamivir is as effective as the other neuraminidase inhibitor, zanamivir, in reducing the febrile period during infection with influenza A (H1N1), influenza A (H3N2), and influenza B virus.99
Oseltamivir is also used for postexposure prophylaxis against influenza A and B, including pandemic strains. For this indication, oseltamivir should be started within 48 hours of exposure and continued daily for at least 10 days or for up to 6 weeks during an outbreak. A systematic review reported no statistically significant difference between oseltamivir and zanamivir prophylaxis for preventing symptomatic influenza among immunocompetent adults.100
The most common adverse effects of oseltamivir are nausea, vomiting, diarrhea, abdominal pain, insomnia, and vertigo. Neuropsychiatric adverse effects, including delirium, abnormal behavior, and hallucinations, have been reported. Oseltamivir-resistant influenza A virus has been reported.101-103 Mutations in the neuraminidase gene, such as R292K101 and H274Y,98 account for oseltamivir resistance. Surveillance conducted during the 2009 H1N1 influenza pandemic detected sporadic and infrequent incidence of oseltamivir-resistant pandemic (H1N1) 2009 influenza virus. All resistant viruses had neuraminidase mutations (most commonly H275Y mutation) that conferred resistance to oseltamivir, but not to zanamivir.104 Oseltamivir resistance among influenza B viruses occurs less frequently.105
Zanamivir. Zanamivir is an inhaled neuraminidase inhibitor that is used for the treatment and prophylaxis of influenza A and B viruses.106 Zanamivir is not available orally since it is poorly absorbed.106 Inhaled zanamivir produces high concentrations in the respiratory tract where influenza virus infection occurs. About 4% to 20% of inhaled zanamivir is absorbed systemically, producing peak serum concentrations at 1 to 2 hours. The absorbed drug is not metabolized and is excreted unchanged in the urine, while the unabsorbed drug is excreted in the feces.106
The mechanism of action of zanamivir is similar to oseltamivir, by inhibiting neuraminidase, which is essential for release of newly formed viral particles from infected cells.106 For treatment, zanamivir is given by inhalation twice daily for 5 days, with the therapy begun within 48 hours after symptom onset. Zanamivir can be given once daily for 10 days as postexposure prophylaxis of influenza A and B in household or close contacts. Zanamivir prophylaxis during community outbreaks may be given for 28 days. Zanamivir has occasionally been given IV to treat critically ill patients with influenza.107,108
Inhaled zanamivir is well tolerated.106 Acute bronchospasm with decline in respiratory function has been reported; a bronchodilator should be available if given as treatment for patients with underlying pulmonary disease. Other adverse effects include headache and gastrointestinal symptoms. Hypersensitivity reactions and neuropsychiatric adverse effects occur rarely.109
ANTIVIRAL DRUGS FOR HEPATITIS AND OTHER VIRUSES
Interferons
Interferons (IFNs) are naturally occurring proteins produced in response to viral infection.110 The 3 major classes of IFNs are α, β, and γ; IFN-α and IFN-β are further classified as type I, whereas IFN-γ is type II.110 Available only in parenteral formulation, more than 80% of a subcutaneous (SC) or intramuscular dose of IFN-α is absorbed.110 After an intramuscular injection, peak IFN concentrations occur within 4 to 8 hours and return to baseline levels in 16 to 24 hours.110 Pegylation, which is the process of attachment of IFN to a large inert polyethylene glycol, markedly reduces the rate of absorption and excretion of IFN and therefore increases its plasma concentration.111 For example, after an SC dose of peginterferon α-2b, the peak serum concentration occurs in 15 to 44 hours, high concentrations are maintained for 48 to 72 hours, and the mean terminal half-life is about 40 hours.110 In contrast, the peak serum concentration of peginterferon α-2a is reached in 72 to 96 hours after an SC dose, and the mean terminal half-life is 160 hours.110 Interferon-α undergoes extensive renal catabolism, and negligible amounts of IFN are excreted in the urine.
Interferons have multiple overlapping biological activities, including antiviral, antiproliferative, and immunoregulatory functions. After binding to receptors, IFNs initiate a cascade of events that lead to various cellular responses, such as inhibition of virus replication, suppression of cell proliferation, enhancement of the phagocytic activity of macrophages, and augmentation of the specific cytotoxicity of lymphocytes for target cells.
Interferons have been used in treating multiple viral infections and are most commonly used for treating chronic viral hepatitis.112 Interferon-α was the first drug approved for treatment of compensated liver disease due to CHB; it is not approved for treating acute hepatitis B. For CHB, IFN α-2a or α-2b is given parenterally, depending on dosing schedule, for 4 to 6 months or up to 48 weeks.112,113 Interferon was most effective in patients with recently acquired hepatitis B virus (HBV), high pretreatment levels of alanine aminotransferase (ALT), and low levels of HBV DNA. Subcutaneous peginterferon-α is as effective or slightly more effective than SC IFN-α.114 Likewise, SC peginterferon-α may be more effective than lamivudine in hepatitis B e antigen (HBeAg)–positive and HBeAg-negative patients with CHB,115-117 and the addition of lamivudine to peginterferon-α did not significantly enhance efficacy.118 Interferon-α is effective in patients with HBV and hepatitis D virus coinfection,119 although they are less responsive than patients infected with HBV alone.120 Guidelines for the management of CHB in HIV-infected patients were recently published121,122; for patients not requiring anti-HIV therapy, peginterferon-α for 12 months is considered a therapeutic option. Lamivudine and the other antiviral nucleos(t)ides for the treatment of HBV often have anti-HIV properties and may result in the development of HIV resistance if given as monotherapy in HBV-HIV coinfected patients.
Interferon-α-2a and -2b are approved for the treatment of chronic hepatitis C (CHC); however, they are not approved for acute hepatitis C. A meta-analysis found that IFN-α for at least 12 months had the best risk-benefit ratio for patients with CHC.123 Once-weekly peginterferon-α was more effective than IFN-α given 3 times weekly in patients with CHC.124-126 However, combination therapy with IFN-α and oral ribavirin is more effective than either drug used alone.127 Combining oral ribavirin with peginterferon-α may be more effective than combining it with IFN-α.128,129 Therefore, the British Society for Gastroenterology and the American Association for the Study of Liver Diseases130 recommends once-weekly SC peginterferon-α combined with oral ribavirin as the first line of treatment of CHC. The recommended duration of treatment of CHC in patients not infected with HIV is 24 weeks (for hepatitis C virus [HCV] genotype 2 or 3) or 48 weeks (for HCV genotype 1).
For patients coinfected with HIV and HCV, the rate of intolerance to a combination regimen of IFN-α and ribavirin is higher and the rate of sustained virologic response (SVR) lower than in patients infected with HCV alone.131-133 Use of peginterferon-α resulted in a higher SVR rate than use of IFN-α.131,133 The APRICOT study reported an SVR rate of 40% for patients treated with peginterferon-α plus ribavirin, compared with 20% for those treated with peginterferon-α monotherapy, and 12% for those treated with IFN-α plus ribavirin.132 A lower SVR rate to combination peginterferon-α plus ribavirin therapy was observed in patients coinfected with HCV genotype 1 (29%) than with HCV genotypes 2 and 3 (62%).132,133 Guidelines for the management of HIV and HCV coinfection have been published recently.130 In general, the guidelines recommend combination therapy with peginterferon-α and ribavirin for 48 weeks.
Interferons are generally not recommended in acute viral hepatitis, but treatment of acute HCV with IFN-α has resulted in a more rapid resolution of viremia and reduced progression to chronic hepatitis.134,135 The American Association for the Study of Liver Diseases recommends either IFN-α or peginterferon-α for at least 6 months for acute HCV if infection persists for 2 to 4 months after diagnosis.
Interferons are also approved as intralesional therapy for condyloma acuminatum of genital and perianal areas.136 Intralesional injection ensures relatively high concentrations of IFN at the local site of infection, but occurrence of systemic adverse effects suggests its absorption from this site. Currently, HSV is generally treated with acyclovir, but beneficial responses to topical IFN-α have been reported for genital herpes137 and HSV keratitis.90 Beneficial responses to IFN-α have been reported for HIV-associated progressive multifocal leukoencephalopathy138; however, these findings are debatable because IFN-α may not provide added benefits when used with highly active antiretroviral therapy.139
Most patients receiving IFN may develop flulike symptoms, which appear to be dose-related, are more likely to occur at the start of treatment, and typically respond to acetaminophen. Among the more serious adverse effects are neuropsychiatric disorders (eg, depression and homicidal and suicidal ideation), neurologic disturbances (eg, confusion and seizures), myelosuppression (neutropenia [most commonly] and aplastic anemia [rarely]), cardiovascular disorders (eg, arrhythmias), endocrine disorders (eg, thyroid disorders), and pulmonary disorders (eg, dyspnea and pneumonitis).140-142 Patients at risk for developing depression are those with preexisting mood and anxiety disorders, those with a history of major depression, and those receiving higher doses of IFN-α or undergoing long-term treatment regimens. Selective serotonin reuptake inhibitors have been used successfully to treat patients with IFN-associated depression, allowing therapy to be continued,143 and as a pretreatment to prevent its occurrence in high-risk patients.144 Other adverse effects are altered liver function,145 renal insufficiency,146 and gastrointestinal manifestations.147
Ribavirin
Ribavirin, a synthetic nucleoside analogue of guanine, is available in oral, aerosolized, and IV formulations. Oral ribavirin is absorbed extensively, but its bioavailability is only 65% because of first-pass metabolism. Peak plasma ribavirin concentrations occur within 1 to 2 hours after oral dose.148 Peak plasma concentrations increase over time and are 6 times higher after 4 weeks of treatment. Administration of aerosolized ribavirin leads to high concentrations in the respiratory tract, with some ribavirin absorbed systemically. Ribavirin is mainly excreted in the urine.149
The mechanism of action of ribavirin is known to be diverse but is not completely understood. It may be a competitive inhibitor of cellular enzymes because its antiviral activity is reversed by guanosine. Its triphosphorylated form, ribavirin triphosphate, is a potent competitive inhibitor of inosine monophosphate dehydrogenase, influenza virus RNA polymerase, and mRNA guanylyltransferase. As a result of this competitive inhibition, intracellular guanosine triphosphate pools are markedly reduced and viral nucleic acid and protein synthesis are inhibited. Ribavirin does not alter viral attachment, penetration, or uncoating, nor does it induce IFN production.
Ribavirin inhibits multiple viruses in vitro. Among the susceptible DNA viruses are herpesviruses, adenoviruses, and poxviruses. Susceptible RNA viruses include HCV, Lassa virus, influenza, parainfluenza, measles, mumps, RSV, and HIV. However, no correlation has been found between ribavirin’s in vitro activity and its activity against human infections.
Oral ribavirin is approved for use, in combination with IFN-α or peginterferon-α, for the treatment of CHC. However, it is not effective when given as monotherapy.150,151 The duration of treatment, and sometimes its dose, may be dictated by HCV genotype. Treatment for infections with HCV genotype 1, and probably with genotype 4, should generally continue for 48 weeks, whereas those with genotype 2 or 3 may be treated for 24 weeks. Treatment of HCV in patients coinfected with HIV should be for 48 weeks.150,151
Ribavirin is approved for the treatment of RSV in children, including hematopoietic stem cell transplant recipients. When used for the treatment of RSV pneumonia, ribavirin is usually given by the aerosol route, which delivers high concentrations at the site of infection.152 Oral ribavirin has also been used with good outcomes.153 Ribavirin has been used, off-label, for the treatment of HSV, influenza, severe acute respiratory syndrome coronavirus,154,155 La Crosse encephalitis,156 Nipah encephalitis,157 Lassa fever,158 hemorrhagic fever with renal syndrome,159 Crimean-Congo hemorrhagic fever,160,161 Bolivian hemorrhagic fever,162 and hantavirus pulmonary syndrome.163
Aerosolized ribavirin can cause sudden deterioration of respiratory function and cardiovascular effects. Precipitation of inhaled ribavirin may occur in ventilatory tubings. Hemolytic anemia occurs commonly,154 and ribavirin should not be given to patients with preexisting medical conditions exacerbated by ribavirin-induced hemolysis, including significant cardiac disease or hemoglobinopathies. Severe depression, suicidal ideation, and relapse of drug abuse may occur, and ribavirin is contraindicated in patients with a history of, or existing, psychiatric disorders. Significant teratogenic and/or embryocidal effects have been observed in animals exposed to ribavirin. Ribavirin is therefore contraindicated in pregnant women and their male partners, and it is recommended that patients use 2 forms of contraception and avoid pregnancy during therapy and for 6 months thereafter.
Nucleos(t)ide Analogues for CHB
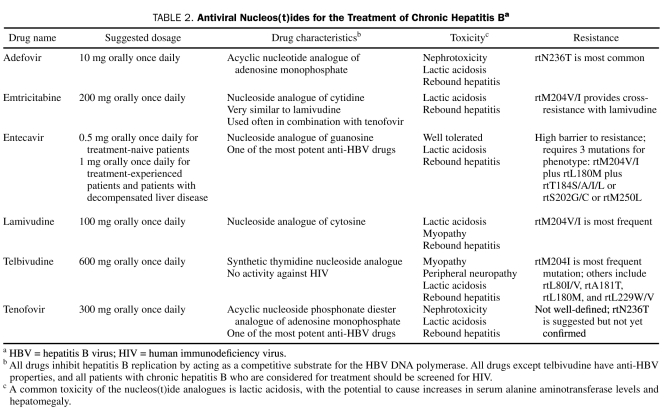
In addition to IFN, several nucleos(t)ide analogues are available for the treatment of CHB (Table 2). With the exception of telbivudine, these drugs possess anti-HIV properties, serving as inhibitors of the HIV reverse transcriptase inhibitors. The specific mechanism of their anti-HBV property is through competitive inhibition of HBV DNA polymerase. Because of their anti-HIV properties, it is highly recommended that CHB patients considered for treatment with these drugs be tested for HIV infection, and monotherapy with these drugs should be avoided for HIV-infected patients to reduce the risk of HIV resistance. Hepatitis B virus may also develop resistance to these drugs, usually after prolonged exposure, and this risk may be reduced by a strategy of combination antiviral therapies. The exact duration of anti-HBV treatment is not defined, and HBV relapse often occurs after discontinuation of treatment. Severe exacerbation of hepatitis may also occur on discontinuation of these drugs; hence, monitoring for hepatotoxicity should be performed after stopping treatment. Lactic acidosis may occur with nucleos(t)ide analogues, and the drugs should be withdrawn if there is a rapid increase in ALT levels, progressive hepatomegaly or steatosis, or acidosis.
TABLE 2.
Antiviral Nucleos(t)ides for the Treatment of Chronic Hepatitis Ba
Adefovir. Adefovir dipivoxil is an acyclic nucleotide analogue of adenosine monophosphate.164 Oral adefovir dipivoxil is rapidly absorbed and converted to adefovir. Its oral bioavailability is about 59%. Excretion of the drug is by glomerular filtration and active tubular secretion.
Adefovir is converted intracellularly by cellular kinases to its active metabolite, adefovir diphosphate, which competitively inhibits HBV DNA polymerase.165 Although adefovir has the distinction of being the least potent among currently available anti-HBV drugs, it has been used in adults with decompensated liver disease, or with compensated liver disease with evidence of active viral replication, persistently elevated ALT levels, and histologic evidence of active inflammation and fibrosis.164
The major adverse effect of adefovir is nephrotoxicity, including proximal renal tubular dysfunction and Fanconi syndrome. Gastrointestinal symptoms, such as nausea, diarrhea, and abdominal pain, may be observed. Adefovir resistance, characterized by the rtN236T mutation, gradually increases over time to 11%, 18%, and 29% at year 3, 4, and 5, respectively.166-169 To minimize the risk of resistance, adefovir is used in combination with other drugs such as lamivudine.165 However, concomitant use with the related drug tenofovir disoproxil fumarate is not recommended because of the augmented risk of nephrotoxicity.
Emtricitabine. Emtricitabine is an analogue of cytidine. Although not currently approved for the treatment of CHB, emtricitabine has been used clinically in combination with tenofovir in HIV/HBV–coinfected patients. Emtricitabine is very similar to lamivudine, and cross-resistance between these drugs is common. Emtricitabine may be more potent than lamivudine; however, it should not be used as monotherapy because of high rates of resistance development.170 The rate of emtricitabine resistance among patients with HBV monoinfection is 18% at 96 weeks.170 Adverse effects are reportedly uncommon and include mild to moderate headache, nausea, diarrhea, and rash.170
Entecavir. Entecavir, a nucleoside guanosine analogue,171 is considered one of the most potent agents for the treatment of patients with CHB, including those resistant to lamivudine.172 Oral entecavir is extensively absorbed: peak plasma concentrations occur in 30 to 90 minutes, and oral bioavailability is almost 100%. Despite low plasma concentrations, entecavir maintains its potency by the long intracellular half-life of its active metabolite entecavir triphosphate. Entecavir is mainly excreted by glomerular filtration and active tubular secretion.
The mechanism of action of entecavir is somewhat unique because it inhibits 3 specific functions of the HBV DNA polymerase: priming of the HBV DNA polymerase, reverse transcription of the negative strand from the pregenomic mRNA, and synthesis of positive-strand HBV DNA.172 Entecavir is approved for the treatment of CHB, at a dose of 0.5 mg orally once daily for nucleoside treatment–naive patients, and a dose of 1 mg orally once daily for patients with a history of HBV viremia while receiving lamivudine, those with lamivudine- or telbivudine-resistant mutations, and those with decompensated liver disease.173 In randomized trials of HBeAg-positive and HBeAg-negative patients, entecavir demonstrated better outcomes than lamivudine, with improvement in histologic responses, higher percentages of HBV DNA suppression, and normalization or improvement of ALT levels.
Adverse effects of entecavir are generally mild and include headache, fatigue, nausea, diarrhea, and insomnia. Entecavir has a high barrier to resistance and requires at least 3 mutations for phenotypic resistance. Entecavir resistance requires a baseline rtM204V/I and rtL180M mutation plus either rtT184S/A/I/L, rtS202G/C, or rtM250L. Among nucleoside-naive patients, the rate of entecavir resistance is less than 1% after 5 years, but patients with preexisting rtM204V/I have a higher rate of entecavir resistance (51%) after 5 years.173
Lamivudine. Lamivudine is a nucleoside analogue of cytosine. Oral lamivudine provides bioavailability of about 85%, and peak serum concentrations occur in 1.0 to 1.5 hours. Hepatic metabolism is low, and up to 70% is excreted unchanged by the kidneys.174
Lamivudine is phosphorylated intracellularly into its active 5′-triphosphate metabolite, lamivudine triphosphate. When the active metabolite is incorporated into viral DNA by HBV polymerase, it results in DNA chain termination.
Lamivudine was the first drug to be used as an alter native to IFN-α for the treatment of CHB.175,176 In a double-blind study involving about 350 patients with CHB, lamivudine was associated with substantial histologic improvement, HBeAg antibody seroconversion, and ALT normalization.177 However, relapses are common once treatment is discontinued.178
The adverse effects of lamivudine are mild and include abdominal pain, nausea, and headache. The clinical utility of lamivudine is limited by the rapid development of antiviral resistance. Lamivudine shares with the L-nucleosides the primary resistance mutation, rtM204V/I, which occurs easily and confers cross-resistance. After 4 years of lamivudine monotherapy, rtM204V/I resistance develops in up to 70% and 90% of patients with HBV monoinfection and HIV/HBV coinfection, respectively.
Telbivudine. Telbivudine is a synthetic thymidine nucleoside analogue. Unlike other anti-HBV drugs, telbivudine has no activity against HIV. Oral telbivudine is well absorbed and achieves peak plasma concentrations after about 3 hours. It is mainly excreted by glomerular filtration, with a terminal elimination half-life of 30 to 50 hours.179
Telbivudine-triphosphate inhibits HBV by competitive inhibition of viral DNA polymerase. Oral telbivudine is approved for the treatment of CHB in patients with compensated liver disease and evidence of active viral replication, persistently increased serum ALT concentrations, and histologic evidence of active liver inflammation and fibrosis.180,181 It is considered more effective than lamivudine and adefovir.182,183 Compared with lamivudine, telbivudine was associated with a higher degree of reduction in HBV DNA levels; however, no significant differences were found in ALT level normalization, loss of HBeAg, or anti-HBe seroconversion.
The most common adverse effects reported for telbivudine are dizziness, fatigue, gastrointestinal symptoms, and rash. Unique adverse effects are peripheral neuropathy and myopathy with elevation in creatine kinase levels. Telbivudine treatment should be discontinued if either peripheral neuropathy or myopathy is diagnosed. The rate of resistance to telbivudine is 25% after 96 weeks of treatment.
Tenofovir. Tenofovir disoproxil fumarate, an acyclic nucleoside phosphonate diester analogue of adenosine monophosphate, is considered one of the most potent anti-HBV drugs. In oral form, it is rapidly absorbed and converted to tenofovir, reaching peak plasma concentrations in 1 to 2 hours.184 Oral bioavailability, which is only 25% in the fasting state, can be enhanced when taken with a high-fat meal. The terminal elimination half-life of tenofovir is 12 to 18 hours, and it is excreted mainly by active tubular secretion and glomerular filtration.184
Tenofovir disoproxil fumarate is a prodrug that requires diester hydrolysis for conversion to tenofovir. Subsequent phosphorylation by cellular enzymes forms tenofovir diphosphate, which competes with the natural substrate deoxyadenosine 5′-triphosphate for incorporation into the viral DNA strand.
Tenofovir is used for the treatment of CHB. In a randomized trial comparing tenofovir and adefovir, a higher percentage of patients receiving tenofovir achieved HBV DNA level suppression. In HBeAg-positive patients, the biochemical response was higher with tenofovir; however, the anti-HBe seroconversion rates and histologic responses were similar for adefovir and tenofovir.185-188
The adverse effects of tenofovir include gastrointestinal symptoms, dizziness, fatigue, and headache. Renal toxicities, including nephritis, proximal renal tubulopathy (including Fanconi syndrome), and renal failure, have been associated with tenofovir.189-193
Primary tenofovir resistance mutations have not been well defined. Although viruses with rtN236T are not resistant to tenofovir, they have a slower response than do wild-type viruses. One study reported rtA194T as a tenofovir resistance mutation; however, this pattern was not confirmed in other studies.
Protease Inhibitors for the Treatment of CHC
The current standard treatment of CHC is peginterferon-α in combination with ribavirin for 24 weeks (for HCV genotype 2 or 3) or 48 weeks (for HCV genotype 1). The major aim of treatment is to achieve SVR, which is defined as undetectable HCV RNA at 24 weeks after completion of treatment. A combination regimen of peginterferon-α and ribavirin results in SVR rates between 38% and 46%, and the rate is even lower among black patients. Hence, major efforts have been made to develop novel therapies for CHC. Recently, 2 serine protease inhibitors were approved as novel therapies for CHC due to genotype 1 infection. The addition of serine protease inhibitors to the backbone therapies of peginterferon-α and ribavirin will emerge as the standard of care for the HCV genotype 1 infection, both in treatment-naive and treatment-experienced patients.
Boceprevir. Boceprevir is a linear peptidomimetic ketoamide serine protease inhibitor that was recently approved for the treatment of CHC, particularly for genotype 1.194 It is available in oral formulation, and the time to peak concentration after oral administration is 2 hours. Food enhances its absorption. Boceprevir is metabolized primarily in the liver. It has an elimination half-life of 3 hours and is excreted mostly in the feces.194
Boceprevir exerts anti-HCV properties by binding reversibly to the HCV nonstructural 3 protein, ultimately inhibiting viral replication. In a recently conducted phase 3 international randomized placebo-controlled trial that enrolled previously untreated black and nonblack adults with HCV genotype 1 infection (SPRINT-2 [serine protease inhibitor therapy 2] trial), the addition of boceprevir for 22 weeks or 44 weeks to standard therapy (peginterferon-α-2b and ribavirin) resulted in significantly higher SVR rates compared with standard therapy alone for the nonblack cohort (67% and 68% vs 40%, respectively) and the black cohort (42% and 53% vs 23%, respectively).195 The relative increases in SVR rates for the nonblack cohort were 68% and 70%, respectively, compared with the standard therapy.195
The HCV RESPOND-2 (Retreatment with HCV Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol 2) trial evaluated boceprevir for the treatment of patients who had experienced a relapse or who had not achieved SVR to peginterferon-ribavirin treatment.196 In this randomized open-label trial that enrolled 403 patients, the SVR rates were significantly higher for patients who received peginterferon-ribavirin plus boceprevir treatment for 32 weeks (59%) or 44 weeks (66%) compared with standard peginterferon-ribavirin treatment alone (21%).196 In a multivariable stepwise logistic regression analysis, the baseline factors associated with SVR were boceprevir use, previous relapse (compared with previous nonresponder), low viral load at baseline, and absence of cirrhosis.196
Boceprevir (800 mg 3 times daily) was approved by the FDA as the first HCV protease inhibitor for the treatment of CHC, specifically for genotype 1; it should be combined with peginterferon and ribavirin. The most common adverse effects of boceprevir are flulike illness, fatigue, nausea, dysgeusia, and anemia.194 The addition of boceprevir nearly doubled the rate of anemia compared with the use of standard peginterferon and ribavirin therapy, with many patients requiring the use of erythropoietin.195
Telaprevir. Telaprevir is an orally available inhibitor specific to the HCV nonstructural 3/4A serine protease.197 It inhibits HCV replication by binding reversibly to nonstructural 3 serine protease. After oral administration, telaprevir achieves peak plasma concentrations in 4 to 5 hours. It is metabolized primarily in the liver and it has an elimination half-life of 4 to 5 hours. Most of the drug is excreted in the feces.
Early-phase studies demonstrated the potent anti-HCV properties of telaprevir.198-200 In a recent phase 3 international randomized double-blind placebo-controlled clinical trial, the addition of telaprevir to the standard treatment of peginterferon-ribavirin was associated with significantly higher SVR rates compared with standard peginterferon-ribavirin alone in a cohort of 1088 patients with previously untreated HCV genotype 1 infections.201 Specifically, the group of patients who received 12 weeks of telaprevir combined with peginterferon-ribavirin, followed by peginterferon-ribavirin for 12 weeks (if HCV RNA was undetectable at weeks 4 and 12) or 36 weeks (if HCV RNA was still detectable at weeks 4 and 12), had SVR rates of 75% vs 44% with standard therapy.201 The SVR rates were also significantly higher compared with standard therapy among patients who received only 8 weeks of telaprevir combined with peginterferon-ribavirin (69% vs 44%).201 In the second randomized phase 3 trial that evaluated telaprevir in treatment-experienced patients with HCV genotype 1 infection, the addition of telaprevir to the standard treatment regimen of peginterferon-α and ribavirin was associated with significantly higher SVR rates compared with the standard regimen of peginterferon-ribavirin alone.202
Collectively, these studies indicate that the addition of telaprevir to standard peginterferon-ribavirin therapy can significantly improve SVR rates in treatment-naive patients infected with HCV genotype 1 and in those who did not benefit from initial treatment with peginterferon-α-2a and ribavirin. As a result of these findings, the FDA approved telaprevir (750 mg 3 times daily) for this treatment indication. The most common adverse effects are anemia, neutropenia, leukopenia, and rash.201 In one study, 41% to 60% of patients reported some kind of rash.199 Rashes can be mild to severe, and Stevens-Johnson syndrome and drug rash with eosinophilia and systemic symptoms have been reported. Telaprevir therapy should be discontinued if these dermatologic complications occur, especially in cases of severe rash or even mild to moderate rash if accompanied by systemic symptoms. The mechanism underlying rash development is unknown.199 Fatigue, pruritus, and gastrointestinal sympotoms (eg, nausea, diarrhea, and taste disturbance) may also be observed.199
CONCLUSION
This review has highlighted the pharmacokinetics, mechanisms of action, clinical indications, and adverse effects of clinically available drugs for the management of viruses other than HIV. The currently available antiviral drugs target 3 main groups of viruses: herpes, hepatitis, and influenza viruses. The antiviral therapeutic armamentarium has evolved over the years and is rapidly expanding. Some of the “old” antiviral drugs retain their clinical utility for most infections, such as acyclovir for herpes simplex virus and ganciclovir for CMV. However, other of these “old” antiviral drugs (eg, amantadine and rimantadine for influenza virus infections) have lost their clinical utility because of the rapid and widespread development of resistance. This serves as a catalyst for the development of novel therapies and, more importantly, should urge the medical community to use these drugs optimally in the clinical setting. Indeed, increased resistance has been observed to the neuraminidase inhibitors for the treatment of influenza viruses and the nucleos(t)ide analogues for the treatment of CHB. As novel therapies develop (eg, the serine protease inhibitors for the treatment of CHC), care must be taken to optimize their use so that the clinical life span of these drugs is not abbreviated by the development of resistance.
Supplementary Material
Footnotes
The author has no conflicts of interest to declare.
On completion of this article, you should be able to (1) discuss the different regimens for the prevention and treatment of human herpesviruses; (2) discuss options for the prevention and treatment of influenza virus, including infections with resistant strains; and (3) discuss antiviral drugs for the treatment of chronic hepatitis B and C infections, including novel nucleos(t)ide analogues and serine protease inhibitors, respectively.
REFERENCES
- 1. Corey L, Fife KH, Benedetti JK, et al. Intravenous acyclovir for the treatment of primary genital herpes. Ann Intern Med. 1983;98:914-921 [DOI] [PubMed] [Google Scholar]
- 2. Nilsen AE, Aasen T, Halsos AM, et al. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet. 1982;2:571-573 [DOI] [PubMed] [Google Scholar]
- 3. Serota FT, Starr SE, Bryan CK, Koch PA, Plotkin SA, August CS. Acyclovir treatment of herpes zoster infections: use in children undergoing bone marrow transplantation. JAMA. 1982;247:2132-2135 [PubMed] [Google Scholar]
- 4. Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127-2137 [DOI] [PubMed] [Google Scholar]
- 5. Reichman RC, Badger GJ, Mertz GJ, et al. Treatment of recurrent genital herpes simplex infections with oral acyclovir: a controlled trial. JAMA. 1984;251:2103-2107 [PubMed] [Google Scholar]
- 6. Luby JP, Gnann JW, Jr, Alexander WJ, et al. A collaborative study of patient-initiated treatment of recurrent genital herpes with topical acyclovir or placebo. J Infect Dis. 1984;150:1-6 [DOI] [PubMed] [Google Scholar]
- 7. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144 [DOI] [PubMed] [Google Scholar]
- 8. Zuckerman R, Wald A. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107 [DOI] [PubMed] [Google Scholar]
- 9. De Clercq E. Antivirals for the treatment of herpesvirus infections. J Antimicrob Chemother. 1993;32(suppl A):121-132 [DOI] [PubMed] [Google Scholar]
- 10. Valencia I, Miles DK, Melvin J, et al. Relapse of herpes encephalitis after acyclovir therapy: report of two new cases and review of the literature. Neuropediatrics. 2004;35:371-376 [DOI] [PubMed] [Google Scholar]
- 11. Balfour HH, Jr, Kelly JM, Suarez CS, et al. Acyclovir treatment of varicella in otherwise healthy children. J Pediatr. 1990;116:633-639 [DOI] [PubMed] [Google Scholar]
- 12. Wallace MR, Bowler WA, Murray NB, Brodine SK, Oldfield EC., III Treatment of adult varicella with oral acyclovir: a randomized, placebo-controlled trial. Ann Intern Med. 1992;117:358-363 [DOI] [PubMed] [Google Scholar]
- 13. Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347 [DOI] [PubMed] [Google Scholar]
- 14. Other herpesviruses: HHV-6, HHV-7, HHV-8, HSV-1 and -2, VZV. Am J Transplant. 2004;4(suppl 10):66-71 [DOI] [PubMed] [Google Scholar]
- 15. Bean B, Aeppli D. Adverse effects of high-dose intravenous acyclovir in ambulatory patients with acute herpes zoster. J Infect Dis. 1985;151:362-365 [DOI] [PubMed] [Google Scholar]
- 16. Perazella MA. Crystal-induced acute renal failure. Am J Med. 1999;106:459-465 [DOI] [PubMed] [Google Scholar]
- 17. Helldén A, Lycke J, Vander T, Svensson JO, Odar-Cederlof I, Stahle L. The aciclovir metabolite CMMG is detectable in the CSF of subjects with neuropsychiatric symptoms during aciclovir and valaciclovir treatment. J Antimicrob Chemother. 2006;57:945-949 [DOI] [PubMed] [Google Scholar]
- 18. Ernst ME, Franey RJ. Acyclovir- and ganciclovir-induced neurotoxicity. Ann Pharmacother. 1998;32:111-113 [DOI] [PubMed] [Google Scholar]
- 19. Wade JC, Meyers JD. Neurologic symptoms associated with parenteral acyclovir treatment after marrow transplantation. Ann Intern Med. 1983;98:921-925 [DOI] [PubMed] [Google Scholar]
- 20. Buck ML, Vittone SB, Zaglul HF. Vesicular eruptions following acyclovir administration. Ann Pharmacother. 1993;27:1458-1459 [DOI] [PubMed] [Google Scholar]
- 21. Amos RJ, Amess JA. Megaloblastic haemopoiesis due to acyclovir. Lancet. 1983;1:242-243 [DOI] [PubMed] [Google Scholar]
- 22. Danve-Szatanek C, Aymard M, Thouvenot D, et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42:242-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16:114-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malvy D, Treilhaud M, Bouee S, et al. A retrospective, case-control study of acyclovir resistance in herpes simplex virus. Clin Infect Dis. 2005;41:320-326 [DOI] [PubMed] [Google Scholar]
- 25. Superti F, Ammendolia MG, Marchetti M. New advances in anti-HSV chemotherapy. Curr Med Chem. 2008;15:900-911 [DOI] [PubMed] [Google Scholar]
- 26. De Clercq E. Discovery and development of BVDU (brivudin) as a therapeutic for the treatment of herpes zoster. Biochem Pharmacol. 2004;68:2301-2315 [DOI] [PubMed] [Google Scholar]
- 27. Lea AP, Bryson HM. Cidofovir. Drugs. 1996;52:225-230 [DOI] [PubMed] [Google Scholar]
- 28. Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2:2740-2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolf DL, Rodriguez CA, Mucci M, Ingrosso A, Duncan BA, Nickens DJ. Pharmacokinetics and renal effects of cidofovir with a reduced dose of probenecid in HIV-infected patients with cytomegalovirus retinitis. J Clin Pharmacol. 2003;43:43-51 [DOI] [PubMed] [Google Scholar]
- 30. Cundy KC. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet. 1999;36:127-143 [DOI] [PubMed] [Google Scholar]
- 31. Kendle JB, Fan-Havard P. Cidofovir in the treatment of cytomegaloviral disease. Ann Pharmacother. 1998;32:1181-1192 [DOI] [PubMed] [Google Scholar]
- 32. Cherrington JM, Fuller MD, Lamy PD, et al. In vitro antiviral susceptibilities of isolates from cytomegalovirus retinitis patients receiving first- or second-line cidofovir therapy: relationship to clinical outcome. J Infect Dis. 1998;178:1821-1825 [DOI] [PubMed] [Google Scholar]
- 33. Jabs DA, Enger C, Forman M, Dunn JP; The Cytomegalovirus Retinitis and Viral Resistance Study Group Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. Antimicrob Agents Chemother. 1998;42:2240-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Snoeck R, De Clercq E. Role of cidofovir in the treatment of DNA virus infections, other than CMV infections, in immunocompromised patients. Curr Opin Investig Drugs. 2002;3:1561-1566 [PubMed] [Google Scholar]
- 35. Cha S, Johnston L, Natkunam Y, Brown J. Treatment of verruca vulgaris with topical cidofovir in an immunocompromised patient: a case report and review of the literature. Transpl Infect Dis. 2005;7:158-161 [DOI] [PubMed] [Google Scholar]
- 36. Lamoth F, Pascual M, Erard V, Venetz JP, Nseir G, Meylan P. Low-dose cidofovir for the treatment of polyomavirus-associated nephropathy: two case reports and review of the literature. Antivir Ther. 2008;13:1001-1009 [PubMed] [Google Scholar]
- 37. Toro JR, Sanchez S, Turiansky G, Blauvelt A. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-309 [DOI] [PubMed] [Google Scholar]
- 38. Lalezari JP, Drew WL, Glutzer E, et al. Treatment with intravenous (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine of acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with AIDS. J Infect Dis. 1994;170:570-572 [DOI] [PubMed] [Google Scholar]
- 39. Bryant P, Sasadeusz J, Carapetis J, Waters K, Curtis N. Successful treatment of foscarnet-resistant herpes simplex stomatitis with intravenous cidofovir in a child. Pediatr Infect Dis J. 2001;20:1083-1086 [DOI] [PubMed] [Google Scholar]
- 40. Kopp T, Geusau A, Rieger A, Stingl G. Successful treatment of an aciclovir-resistant herpes simplex type 2 infection with cidofovir in an AIDS patient. Br J Dermatol. 2002;147:134-138 [DOI] [PubMed] [Google Scholar]
- 41. Garvey L, Thomson EC, Taylor GP. Progressive multifocal leukoencephalopathy: prolonged survival in patients treated with protease inhibitors and cidofovir: a case series. AIDS. 2006;20:791-793 [DOI] [PubMed] [Google Scholar]
- 42. Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS. 2002;16:1791-1797 [DOI] [PubMed] [Google Scholar]
- 43. Razonable RR, Aksamit AJ, Wright AJ, Wilson JW. Cidofovir treatment of progressive multifocal leukoencephalopathy in a patient receiving highly active antiretroviral therapy. Mayo Clin Proc. 2001;76:1171-1175 [DOI] [PubMed] [Google Scholar]
- 44. Segarra-Newnham M, Vodolo KM. Use of cidofovir in progressive multifocal leukoencephalopathy. Ann Pharmacother. 2001;35:741-744 [DOI] [PubMed] [Google Scholar]
- 45. Viallard JF, Lazaro E, Ellie E, et al. Improvement of progressive multifocal leukoencephalopathy after cidofovir therapy in a patient with a destructive polyarthritis. Infection. 2007;35:33-36 [DOI] [PubMed] [Google Scholar]
- 46. Cesaro S, Hirsch HH, Faraci M, et al. Cidofovir for BK virus-associated hemorrhagic cystitis: a retrospective study. Clin Infect Dis. 2009;49:233-240 [DOI] [PubMed] [Google Scholar]
- 47. Kottke MD, Parker SR. Intravenous cidofovir-induced resolution of disfiguring cutaneous human papillomavirus infection. J Am Acad Dermatol. 2006;55:533-536 [DOI] [PubMed] [Google Scholar]
- 48. Kazory A, Singapuri S, Wadhwa A, Ejaz AA. Simultaneous development of Fanconi syndrome and acute renal failure associated with cidofovir. J Antimicrob Chemother. 2007;60:193-194 [DOI] [PubMed] [Google Scholar]
- 49. Ambati J, Wynne KB, Angerame MC, Robinson MR. Anterior uveitis associated with intravenous cidofovir use in patients with cytomegalovirus retinitis. Br J Ophthalmol. 1999;83:1153-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tseng AL, Mortimer CB, Salit IE. Iritis associated with intravenous cidofovir. Ann Pharmacother. 1999;33:167-171 [DOI] [PubMed] [Google Scholar]
- 51. Gill KS, Wood MJ. The clinical pharmacokinetics of famciclovir. Clin Pharmacokinet. 1996;31:1-8 [DOI] [PubMed] [Google Scholar]
- 52. Faro S. A review of famciclovir in the management of genital herpes. Infect Dis Obstet Gynecol. 1998;6:38-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chacko M, Weinberg JM. Famciclovir for cutaneous herpesvirus infections: an update and review of new single-day dosing indications. Cutis. 2007;80:77-81 [PubMed] [Google Scholar]
- 54. Saltzman R, Jurewicz R, Boon R. Safety of famciclovir in patients with herpes zoster and genital herpes. Antimicrob Agents Chemother. 1994;38:2454-2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grillone LR, Lanz R. Fomivirsen. Drugs Today (Barc). 2001;37:245-255 [DOI] [PubMed] [Google Scholar]
- 56. Vitravene Study Group A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol. 2002;133:467-474 [DOI] [PubMed] [Google Scholar]
- 57. Highleyman L. Fomivirsen. BETA. 1998;29:31 [PubMed] [Google Scholar]
- 58. Chrisp P, Clissold SP. Foscarnet: a review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs. 1991;41:104-129 [DOI] [PubMed] [Google Scholar]
- 59. Balfour HH, Jr, Fletcher CV, Erice A, et al. Effect of foscarnet on quantities of cytomegalovirus and human immunodeficiency virus in blood of persons with AIDS. Antimicrob Agents Chemother. 1996;40:2721-2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779-795 [DOI] [PubMed] [Google Scholar]
- 61. Humar A, Snydman D. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S78-S86 [DOI] [PubMed] [Google Scholar]
- 62. Jacobson MA, Causey D, Polsky B, et al. A dose-ranging study of daily maintenance intravenous foscarnet therapy for cytomegalovirus retinitis in AIDS. J Infect Dis. 1993;168:444-448 [DOI] [PubMed] [Google Scholar]
- 63. Beaufils H, Deray G, Katlama C, et al. Foscarnet and crystals in glomerular capillary lumens. Lancet. 1990;336:755 [DOI] [PubMed] [Google Scholar]
- 64. Lor E, Liu YQ. Neurologic sequelae associated with foscarnet therapy. Ann Pharmacother. 1994;28:1035-1037 [DOI] [PubMed] [Google Scholar]
- 65. Nichols WG, Boeckh M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000;16:25-40 [DOI] [PubMed] [Google Scholar]
- 66. Frank KB, Chiou JF, Cheng YC. Interaction of herpes simplex virus-induced DNA polymerase with 9-(1,3-dihydroxy-2-propoxymethyl)guanine triphosphate. J Biol Chem. 1984;259:1566-1569 [PubMed] [Google Scholar]
- 67. Razonable RR, Paya CV. The impact of human herpesvirus-6 and -7 infection on the outcome of liver transplantation. Liver Transpl. 2002;8:651-658 [DOI] [PubMed] [Google Scholar]
- 68. Spector SA, Weingeist T, Pollard RB, et al. ; AIDS Clinical Trials Group; Cytomegalovirus Cooperative Study Group A randomized, controlled study of intravenous ganciclovir therapy for cytomegalovirus peripheral retinitis in patients with AIDS. J Infect Dis. 1993;168:557-563 [DOI] [PubMed] [Google Scholar]
- 69. Drew WL, Ives D, Lalezari JP, et al. ; Syntex Cooperative Oral Ganciclovir Study Group Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. N Engl J Med. 1995;333:615-620 [DOI] [PubMed] [Google Scholar]
- 70. Gane E, Saliba F, Valdecasas GJ, et al. ; Oral Ganciclovir International Transplantation Study Group Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients [published correction appears in Lancet. 1998;351(9100):454]. Lancet. 1997;350:1729-1733 [DOI] [PubMed] [Google Scholar]
- 71. Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin Transplant. 2008;22:162-170 [DOI] [PubMed] [Google Scholar]
- 72. Sarisky RT, Bacon TH, Boon RJ, et al. Profiling penciclovir susceptibility and prevalence of resistance of herpes simplex virus isolates across eleven clinical trials. Arch Virol. 2003;148:1757-1769 [DOI] [PubMed] [Google Scholar]
- 73. Bacon TH, Boyd MR. Activity of penciclovir against Epstein-Barr virus. Antimicrob Agents Chemother. 1995;39:1599-1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spruance SL, Rea TL, Thoming C, Tucker R, Saltzman R, Boon R; Topical Penciclovir Collaborative Study Group Penciclovir cream for the treatment of herpes simplex labialis: a randomized, multicenter, double-blind, placebo-controlled trial. JAMA. 1997;277:1374-1379 [PubMed] [Google Scholar]
- 75. Acosta EP, Fletcher CV. Valacyclovir. Ann Pharmacother. 1997;31:185-191 [DOI] [PubMed] [Google Scholar]
- 76. Spruance SL, Tyring SK, DeGregorio B, Miller C, Beutner K; Valaciclovir HSV Study Group A large-scale, placebo-controlled, dose-ranging trial of peroral valaciclovir for episodic treatment of recurrent herpes genitalis. Arch Intern Med. 1996;156:1729-1735 [PubMed] [Google Scholar]
- 77. Pergam SA, Limaye AP. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lowance D, Neumayer HH, Legendre CM, et al. ; International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N Engl J Med. 1999;340:1462-1470 [DOI] [PubMed] [Google Scholar]
- 79. Razonable RR, Paya CV. Valganciclovir for the prevention and treatment of cytomegalovirus disease in immunocompromised hosts. Expert Rev Anti Infect Ther. 2004;2:27-41 [DOI] [PubMed] [Google Scholar]
- 80. Brown F, Banken L, Saywell K, Arum I. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin Pharmacokinet. 1999;37:167-176 [DOI] [PubMed] [Google Scholar]
- 81. Wiltshire H, Hirankarn S, Farrell C, et al. Pharmacokinetic profile of ganciclovir after its oral administration and from its prodrug, valganciclovir, in solid organ transplant recipients. Clin Pharmacokinet. 2005;44:495-507 [DOI] [PubMed] [Google Scholar]
- 82. Caldes A, Colom H, Armendariz Y, et al. Population pharmacokinetics of ganciclovir after intravenous ganciclovir and oral valganciclovir administration in solid organ transplant patients infected with cytomegalovirus. Antimicrob Agents Chemother. 2009;53:4816-4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346:1119-1126 [DOI] [PubMed] [Google Scholar]
- 84. Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611-620 [DOI] [PubMed] [Google Scholar]
- 85. Khoury JA, Storch GA, Bohl DL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6:2134-2143 [DOI] [PubMed] [Google Scholar]
- 86. Snydman DR. Use of valganciclovir for prevention and treatment of cytomegalovirus disease [editorial]. Clin Infect Dis. 2008;46:28-29 [DOI] [PubMed] [Google Scholar]
- 87. Einsele H, Reusser P, Bornhauser M, et al. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood. 2006;107:3002-3008 [DOI] [PubMed] [Google Scholar]
- 88. Len O, Gavalda J, Aguado JM, et al. Valganciclovir as treatment for cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis. 2008;46:20-27 [DOI] [PubMed] [Google Scholar]
- 89. Asberg A, Humar A, Rollag H, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106-2113 [DOI] [PubMed] [Google Scholar]
- 90. Wilhelmus KR. Therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2008;CD002898 [DOI] [PubMed] [Google Scholar]
- 91. De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115-133 [DOI] [PubMed] [Google Scholar]
- 92. Tappenden P, Jackson R, Cooper K, et al. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technol Assess. 2009;13(11):iii,ix-xii, 1-246 [DOI] [PubMed] [Google Scholar]
- 93. Betts RF. Amantadine and rimantadine for the prevention of influenza A. Semin Respir Infect. 1989;4:304-310 [PubMed] [Google Scholar]
- 94. Krumbholz A, Schmidtke M, Bergmann S, et al. High prevalence of amantadine resistance among circulating European porcine influenza A viruses. J Gen Virol. 2009;90:900-908 [DOI] [PubMed] [Google Scholar]
- 95. Higgins RR, Eshaghi A, Burton L, Mazzulli T, Drews SJ. Differential patterns of amantadine-resistance in influenza A (H3N2) and (H1N1) isolates in Toronto, Canada. J Clin Virol. 2009;44:91-93 [DOI] [PubMed] [Google Scholar]
- 96. Hayden FG, Hay AJ. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr Top Microbiol Immunol. 1992;176:119-130 [DOI] [PubMed] [Google Scholar]
- 97. Hayden FG, Sperber SJ, Belshe RB, Clover RD, Hay AJ, Pyke S. Recovery of drug-resistant influenza A virus during therapeutic use of rimantadine. Antimicrob Agents Chemother. 1991;35:1741-1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother. 2005;55(suppl 1):i5-i21 [DOI] [PubMed] [Google Scholar]
- 99. Sugaya N, Tamura D, Yamazaki M, et al. Comparison of the clinical effectiveness of oseltamivir and zanamivir against influenza virus infection in children. Clin Infect Dis. 2008;47:339-345 [DOI] [PubMed] [Google Scholar]
- 100. Khazeni N, Bravata DM, Holty JE, Uyeki TM, Stave CD, Gould MK. Systematic review: safety and efficacy of extended-duration antiviral chemoprophylaxis against pandemic and seasonal influenza. Ann Intern Med. 2009;151:464-473 [DOI] [PubMed] [Google Scholar]
- 101. Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759-765 [DOI] [PubMed] [Google Scholar]
- 102. de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667-2672 [DOI] [PubMed] [Google Scholar]
- 103. Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108 [DOI] [PubMed] [Google Scholar]
- 104. Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301:1034-1041 [DOI] [PubMed] [Google Scholar]
- 105. Hatakeyama S, Sugaya N, Ito M, et al. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA. 2007;297:1435-1442 [DOI] [PubMed] [Google Scholar]
- 106. Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug Saf. 1999;21:267-281 [DOI] [PubMed] [Google Scholar]
- 107. Gaur AH, Bagga B, Barman S, et al. Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza [letter]. N Engl J Med. 2010;362(1):88-89 [DOI] [PubMed] [Google Scholar]
- 108. Kidd IM, Down J, Nastouli E, et al. H1N1 pneumonitis treated with intravenous zanamivir. Lancet. 2009;374:1036 [DOI] [PubMed] [Google Scholar]
- 109. Gravenstein S, Johnston SL, Loeschel E, Webster A. Zanamivir: a review of clinical safety in individuals at high risk of developing influenza-related complications. Drug Saf. 2001;24:1113-1125 [DOI] [PubMed] [Google Scholar]
- 110. Zeuzem S, Welsch C, Herrmann E. Pharmacokinetics of peginterferons. Semin Liver Dis. 2003;23(suppl 1):23-28 [DOI] [PubMed] [Google Scholar]
- 111. Pedder SC. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin Liver Dis. 2003;23(suppl 1):19-22 [DOI] [PubMed] [Google Scholar]
- 112. Wong DK, Cheung AM, O’Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B: a meta-analysis. Ann Intern Med. 1993;119:312-323 [DOI] [PubMed] [Google Scholar]
- 113. Haria M, Benfield P. Interferon-alpha-2a: a review of its pharmacological properties and therapeutic use in the management of viral hepatitis. Drugs. 1995;50:873-896 [DOI] [PubMed] [Google Scholar]
- 114. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662 [DOI] [PubMed] [Google Scholar]
- 115. Marcellin P, Asselah T, Boyer N. Treatment of chronic hepatitis B. J Viral Hepat. 2005;12:333-345 [DOI] [PubMed] [Google Scholar]
- 116. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695 [DOI] [PubMed] [Google Scholar]
- 117. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217 [DOI] [PubMed] [Google Scholar]
- 118. Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated Interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-129 [DOI] [PubMed] [Google Scholar]
- 119. Farci P, Mandas A, Coiana A, et al. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med. 1994;330:88-94 [DOI] [PubMed] [Google Scholar]
- 120. Farci P, Roskams T, Chessa L, et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740-1749 [DOI] [PubMed] [Google Scholar]
- 121. Soriano V, Puoti M, Bonacini M, et al. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel. AIDS. 2005;19:221-240 [PubMed] [Google Scholar]
- 122. Alberti A, Clumeck N, Collins S, et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005;42:615-624 [DOI] [PubMed] [Google Scholar]
- 123. Poynard T, Leroy V, Cohard M, et al. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778-789 [DOI] [PubMed] [Google Scholar]
- 124. Zeuzem S, Feinman SV, Rasenack J, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666-1672 [DOI] [PubMed] [Google Scholar]
- 125. Perry CM, Jarvis B. Peginterferon-alpha-2a (40 kD): a review of its use in the management of chronic hepatitis C. Drugs. 2001;61:2263-2288 [DOI] [PubMed] [Google Scholar]
- 126. Heathcote EJ, Shiffman ML, Cooksley WG, et al. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680 [DOI] [PubMed] [Google Scholar]
- 127. Brok J, Gluud LL, Gluud C. Ribavirin plus interferon versus interferon for chronic hepatitis C. Cochrane Database Syst Rev. 2010;(1):CD005445 [DOI] [PubMed] [Google Scholar]
- 128. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982 [DOI] [PubMed] [Google Scholar]
- 129. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965 [DOI] [PubMed] [Google Scholar]
- 130. Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839-2848 [DOI] [PubMed] [Google Scholar]
- 132. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438-450 [DOI] [PubMed] [Google Scholar]
- 133. Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452-1457 [DOI] [PubMed] [Google Scholar]
- 135. Santantonio T, Fasano M, Sinisi E, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005;42:329-333 [DOI] [PubMed] [Google Scholar]
- 136. Eron LJ, Judson F, Tucker S, et al. Interferon therapy for condylomata acuminata. N Engl J Med. 1986;315:1059-1064 [DOI] [PubMed] [Google Scholar]
- 137. Leung DT, Sacks SL. Current recommendations for the treatment of genital herpes. Drugs. 2000;60:1329-1352 [DOI] [PubMed] [Google Scholar]
- 138. Huang SS, Skolasky RL, Dal Pan GJ, Royal W, III, McArthur JC. Survival prolongation in HIV-associated progressive multifocal leukoencephalopathy treated with alpha-interferon: an observational study. J Neurovirol. 1998;4:324-332 [DOI] [PubMed] [Google Scholar]
- 139. Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol. 2001;7:353-357 [DOI] [PubMed] [Google Scholar]
- 140. Renault PF, Hoofnagle JH, Park Y, et al. Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med. 1987;147:1577-1580 [PubMed] [Google Scholar]
- 141. Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon19 alpha: a review. Am J Psychiatry. 2000;157:867-876 [DOI] [PubMed] [Google Scholar]
- 142. Midturi J, Sierra-Hoffman M, Hurley D, Winn R, Beissner R, Carpenter J. Spectrum of pulmonary toxicity associated with the use of interferon therapy for hepatitis C: case report and review of the literature. Clin Infect Dis. 2004;39:1724-1729 [DOI] [PubMed] [Google Scholar]
- 143. Levenson JL, Fallon HJ. Fluoxetine treatment of depression caused by interferon-alpha. Am J Gastroenterol. 1993;88:760-761 [PubMed] [Google Scholar]
- 144. Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961-966 [DOI] [PubMed] [Google Scholar]
- 145. Durand JM, Kaplanski G, Portal I, Scheiner C, Berland Y, Soubeyrand J. Liver failure due to recombinant alpha interferon. Lancet. 1991;338:1268-1269 [DOI] [PubMed] [Google Scholar]
- 146. Averbuch SD, Austin HA, III, Sherwin SA, Antonovych T, Bunn PA, Jr, Longo DL. Acute interstitial nephritis with the nephrotic syndrome following recombinant leukocyte a interferon therapy for mycosis fungoides. N Engl J Med. 1984;310:32-35 [DOI] [PubMed] [Google Scholar]
- 147. Agesta N, Zabala R, Diaz-Perez JL. Alopecia areata during interferon alpha-2b/ribavirin therapy. Dermatology. 2002;205:300-301 [DOI] [PubMed] [Google Scholar]
- 148. Wade JR, Snoeck E, Duff F, Lamb M, Jorga K. Pharmacokinetics of ribavirin in patients with hepatitis C virus. Br J Clin Pharmacol. 2006;62:710-714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kramer TH, Gaar GG, Ray CG, Minnich L, Copeland JG, Connor JD. Hemodialysis clearance of intravenously administered ribavirin. Antimicrob Agents Chemother. 1990;34:489-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gish RG. Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006;57:8-13 [DOI] [PubMed] [Google Scholar]
- 151. Gluud LL, Marchesini E, Iorio A. Peginterferon plus ribavirin for chronic hepatitis C in patients with human immunodeficiency virus. Am J Gastroenterol. 2009;104:2335-2341 [DOI] [PubMed] [Google Scholar]
- 152. Ventre K, Randolph AG. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2007;CD000181 [DOI] [PubMed] [Google Scholar]
- 153. Pelaez A, Lyon GM, Force SD, et al. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant. 2009;28:67-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Knowles SR, Phillips EJ, Dresser L, Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis. 2003;37:1139-1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Muller MP, Dresser L, Raboud J, et al. Adverse events associated with high-dose ribavirin: evidence from the Toronto outbreak of severe acute respiratory syndrome. Pharmacotherapy. 2007;27:494-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. McJunkin JE, Khan R, de los Reyes EC, et al. Treatment of severe La Crosse encephalitis with intravenous ribavirin following diagnosis by brain biopsy. Pediatrics. 1997;99:261-267 [DOI] [PubMed] [Google Scholar]
- 157. Chong HT, Kamarulzaman A, Tan CT, et al. Treatment of acute Nipah encephalitis with ribavirin. Ann Neurol. 2001;49:810-813 [DOI] [PubMed] [Google Scholar]
- 158. McCormick JB, King IJ, Webb PA, et al. Lassa fever: effective therapy with ribavirin. N Engl J Med. 1986;314:20-26 [DOI] [PubMed] [Google Scholar]
- 159. Huggins JW, Hsiang CM, Cosgriff TM, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119-1127 [DOI] [PubMed] [Google Scholar]
- 160. Fisher-Hoch SP, Khan JA, Rehman S, Mirza S, Khurshid M, McCormick JB. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet. 1995;346:472-475 [DOI] [PubMed] [Google Scholar]
- 161. Mardani M, Jahromi MK, Naieni KH, Zeinali M. The efficacy of oral ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Iran. Clin Infect Dis. 2003;36:1613-1618 [DOI] [PubMed] [Google Scholar]
- 162. Kilgore PE, Ksiazek TG, Rollin PE, et al. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin Infect Dis. 1997;24:718-722 [DOI] [PubMed] [Google Scholar]
- 163. Prochoda K, Mostow SR, Greenberg K. Hantavirus-associated acute respiratory failure. N Engl J Med. 1993;329:1744 [DOI] [PubMed] [Google Scholar]
- 164. Dando T, Plosker G. Adefovir dipivoxil: a review of its use in chronic hepatitis B. Drugs. 2003;63:2215-2234 [DOI] [PubMed] [Google Scholar]
- 165. Rivkin AM. Adefovir dipivoxil in the treatment of chronic hepatitis B. Ann Pharmacother. 2004;38:625-633 [DOI] [PubMed] [Google Scholar]
- 166. Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816 [DOI] [PubMed] [Google Scholar]
- 167. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807 [DOI] [PubMed] [Google Scholar]
- 168. Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673-2681 [DOI] [PubMed] [Google Scholar]
- 169. Delaney WE. Progress in the treatment of chronic hepatitis B: long-term experience with adefovir dipivoxil. J Antimicrob Chemother. 2007;59:827-832 [DOI] [PubMed] [Google Scholar]
- 170. Lim SG, Ng TM, Kung N, et al. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Intern Med. 2006;166:49-56 [DOI] [PubMed] [Google Scholar]
- 171. Sims KA, Woodland AM. Entecavir: a new nucleoside analog for the treatment of chronic hepatitis B infection. Pharmacotherapy. 2006;26:1745-1757 [DOI] [PubMed] [Google Scholar]
- 172. Matthews SJ. Entecavir for the treatment of chronic hepatitis B virus infection. Clin Ther. 2006;28:184-203 [DOI] [PubMed] [Google Scholar]
- 173. Scott LJ, Keating GM. Entecavir: a review of its use in chronic hepatitis B. Drugs. 2009;69:1003-1033 [DOI] [PubMed] [Google Scholar]
- 174. Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokinet. 1999;36:41-66 [DOI] [PubMed] [Google Scholar]
- 175. Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661 [DOI] [PubMed] [Google Scholar]
- 176. Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263 [DOI] [PubMed] [Google Scholar]
- 177. Lai CL, Chien RN, Leung NW, et al. ; Asia Hepatitis Lamivudine Study Group A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61-68 [DOI] [PubMed] [Google Scholar]
- 178. Honkoop P, de Man RA, Heijtink RA, Schalm SW. Hepatitis B reactivation after lamivudine. Lancet. 1995;346:1156-1157 [DOI] [PubMed] [Google Scholar]
- 179. Zhou XJ, Ke J, Sallas WM, Farrell C, Mayers DL, Pentikis HS. Population pharmacokinetics of telbivudine and determination of dose adjustment for patients with renal impairment. J Clin Pharmacol. 2009;49:725-734 [DOI] [PubMed] [Google Scholar]
- 180. Kim JW, Park SH, Louie SG. Telbivudine: a novel nucleoside analog for chronic hepatitis B. Ann Pharmacother. 2006;40:472-478 [DOI] [PubMed] [Google Scholar]
- 181. Jones R, Nelson M. Novel anti-hepatitis B agents: a focus on telbivudine. Int J Clin Pract. 2006;60:1295-1299 [DOI] [PubMed] [Google Scholar]
- 182. Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588 [DOI] [PubMed] [Google Scholar]
- 183. Chan HL, Heathcote EJ, Marcellin P, et al. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann Intern Med. 2007;147:745-754 [DOI] [PubMed] [Google Scholar]
- 184. Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43:595-612 [DOI] [PubMed] [Google Scholar]
- 185. Grim SA, Romanelli F. Tenofovir disoproxil fumarate. Ann Pharmacother. 2003;37:849-859 [DOI] [PubMed] [Google Scholar]
- 186. Gallant JE, Deresinski S. Tenofovir disoproxil fumarate. Clin Infect Dis. 2003;37:944-950 [DOI] [PubMed] [Google Scholar]
- 187. Wong SN, Lok AS. Tenofovir disoproxil fumarate: role in hepatitis B treatment. Hepatology. 2006;44:309-313 [DOI] [PubMed] [Google Scholar]
- 188. Reijnders JG, Janssen HL. Potency of tenofovir in chronic hepatitis B: mono or combination therapy? J Hepatol. 2008;48:383-386 [DOI] [PubMed] [Google Scholar]
- 189. Gitman MD, Hirschwerk D, Baskin CH, Singhal PC. Tenofovir-induced kidney injury. Expert Opin Drug Saf. 2007;6:155-164 [DOI] [PubMed] [Google Scholar]
- 190. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22:99-103 [DOI] [PubMed] [Google Scholar]
- 191. Schmid S, Opravil M, Moddel M, et al. Acute interstitial nephritis of HIV-positive patients under atazanavir and tenofovir therapy in a retrospective analysis of kidney biopsies. Virchows Arch. 2007;450:665-670 [DOI] [PubMed] [Google Scholar]
- 192. Kapitsinou PP, Ansari N. Acute renal failure in an AIDS patient on tenofovir: a case report. J Med Case Reports. 2008;2:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42:283-290 [DOI] [PubMed] [Google Scholar]
- 194. Foote BC, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C [Epub ahead of print August 9, 2011]. Ann Pharmacother. doi:10.1345/aph.1P744 [DOI] [PubMed]
- 195. Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979-1998 [DOI] [PubMed] [Google Scholar]
- 198. Sarrazin C, Kieffer TL, Bartels D, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767-1777 [DOI] [PubMed] [Google Scholar]
- 199. McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838 [DOI] [PubMed] [Google Scholar]
- 200. Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850 [DOI] [PubMed] [Google Scholar]
- 201. Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416 [DOI] [PubMed] [Google Scholar]
- 202. Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.