Abstract
Purpose
To describe bone status and analyse bone mass in adolescent cyclists.
Methods
Male road cyclists (n = 22) who had been training for a minimum of 2 years and a maximum of 7 years with a volume of 10 h/w, were compared to age-matched controls (n = 22) involved in recreational sports activities. Subjects were divided in 2 groups based on age: adolescents under 17 yrs (cyclists, n = 11; controls, n = 13) and over 17 yrs (cyclists, n = 11; controls, n = 9). Peak oxygen uptake (VO2max) was measured on a cycloergometer. Whole body, lumbar spine, and hip bone mineral content (BMC), density (BMD) and bone area were assessed using dual x-ray absorptiometry (DXA). Volumetric BMD (vBMD) and bone mineral apparent density (BMAD) were also estimated.
Results
The BMC of cyclists was lower for the whole body, pelvis, femoral neck and legs; BMD for the pelvis, hip, legs and whole body and legs bone area was lower but higher in the hip area (all, P≤0.05) after adjusting by lean mass and height. The BMC of young cyclists was 10% lower in the leg and 8% higher in the hip area than young controls (P≤0.05). The BMC of cyclists over 17 yrs was 26.5%, 15.8% and 14.4% lower BMC at the pelvis, femoral neck and legs respectively while the BMD was 8.9% to 24.5% lower for the whole body, pelvis, total hip, trochanter, intertrochanter, femoral neck and legs and 17.1% lower the vBMD at the femoral neck (all P≤0.05). Grouped by age interaction was found in both pelvis and hip BMC and BMD and in femoral neck vBMD (all P≤0.05).
Conclusion
Cycling performed throughout adolescence may negatively affect bone health, then compromising the acquisition of peak bone mass.
Introduction
The role of exercise in regulating bone health is still not well understood. However healthy bone is typically related to increased mechanical loading [1]. The magnitude of the strain could prevent and treat low bone mineral density (BMD) [2], [3] or increase the acquisition of bone mass during growth [4].
Cycling can be considered a healthy sport because it improves physical fitness and prevents fat accumulation [5], [6]. Adolescence is a sensitive phase for the acquisition of bone mass [7], [8]. Around 90% of bone mass is present at the end of the skeletal maturation phase [9]. Many professional and master cyclists can be classified as osteopenic [10], [11], [12]. Professional road cyclists have significantly lower bone mineral density (BMD) than the non-active population [10]. It is assumed that this non weight-bearing activity an insufficient stimulus to generate osteogenesis in clinically relevant bone sites [12], [13].
Rico et al. [14] did not find differences in the BMC in total or any regional site between adolescent cyclists and age-matched sedentary controls. Similarly, Duncan et al. [7] observed that female adolescent cyclists had similar BMD values for the whole body, lumbar, femoral neck, legs and arms than non-athlete controls. These researchers also found no differences among groups in mid-femur for the BMC and volumetric bone mineral density (vBMD) measured by computed tomography [15]. However, when these adolescent cyclists were compared with a group of runners, female cyclists showed significant lower values for BMD for the whole body, femoral neck and legs, and lower bone strength [15].
We hypothesized that cycling during adolescence is associated with lower bone mass acquisition compared a healthy adolescent population. Therefore the main aim of this investigation was to describe the bone status in adolescent cyclists compared to a healthy age-matched group. A secondary aim was to analyse the effect of years of cycling practice on the on the acquisition of bone mass.
Materials and Methods
Ethics statement
Written informed consent was obtained from parents and adolescents [16]. The study was performed following the ethical guidelines of the Declaration of Helsinki 1961 (revision of Edinburgh 2000). The protocol study was approved by the Ethics Committee of Clinical Research from the Government of Aragón (CEICA; Spain).
Subjects
Forty-four healthy male adolescents agreed to participate in the study (Table 1). To be included, subjects had to be bellow 21 years of age, healthy, without any chronic disease and free of musculoskeletal conditions, bone fractures, medications or habits affecting bone development. Twenty-two adolescent male road cyclists (CY) were recruited from different cycling teams from Aragon (Spain). All cyclists were regular participants in regional competitions, and had been participating in training sessions and competitions a mean 10 hours per week (h/week) for a minimum of 2 and a maximum of 7 years prior to the study. Twenty-two controls (CG), physically-active boys, were recruited among high school and physical education course university students. Control subjects were enrolled in recreational sports (rugby, tennis, handball, soccer) 2 h a week with occasional match at the weekend but none cycled more than 1 h per week. Cyclists and controls were divided into two subgroups, younger (<17 yr: n = 24, 11 cyclists and 13 controls) and older (>17 yr: n = 20, 11 cyclists and 9 controls), (Table 2). Subjects were asked to answer a medical and physical activity questionnaire and the parents gave additional information regarding physical activity, medical information such as past injuries, medication, known diseases and smoking habits.
Table 1. Subject characteristics.
| Controls (n = 22) | Cyclists (n = 22) | |||||
| Mean | SD | Mean | SD | |||
| Age (years) | 16.7 | ± | 2.1 | 16.9 | ± | 1.9 |
| Height (cm) | 176.1 | ± | 8.9 | 173.2 | ± | 6.7 |
| Body mass (kg) | 74.4 | ± | 16.8 | 61.3 | ± | 7.7* |
| Body fat mass (kg) | 15.6 | ± | 8.7 | 8.8 | ± | 3.0* |
| % total body fat | 21.8 | ± | 8.5 | 15.7 | ± | 4.5* |
| Total lean mass (kg) | 56.9 | ± | 10.7 | 50.7 | ± | 6.6* |
| VO2peak (mL kg−1 min−1) | 41.7 | ± | 9.0 | 56.6 | ± | 9.3* |
| Years of cycling training (yr) | 3.7 | ± | 1.9 | |||
| Hours of cycling training (h/sem) | 10.2 | ± | 1.8 | |||
Values are presented as mean ± SD.
*P<0.05 compared to controls. Peak oxygen uptake (VO2max).
Table 2. Subject characteristics.
| Controls | Cyclists | |||||||||||
| <17 yr | ≥17 yr | <17 yr | ≥17 yr | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Age (years) | 15.2 | ± | 1.2 | 18.7 | ± | 1.2 | 15.5 | ± | 0.9 | 18.4 | ± | 1.4 |
| Height (cm) | 173.3 | ± | 9.8 | 180.2 | ± | 5.7 | 171.1 | ± | 5.9 | 175.3 | ± | 7.0 |
| Body mass (kg) | 72.7 | ± | 18.4 | 76.9 | ± | 14.9 | 56.9 | ± | 5.1$ | 65.6 | ± | 7.8 |
| Body fat mass (kg) | 17 | ± | 8.8 | 16 | ± | 9.4 | 9.4 | ± | 3.5$ | 10.1 | ± | 2.6 |
| % total body fat | 22.9 | ± | 8.4 | 20.1 | ± | 7.5 | 16.6 | ± | 5.6 | 15.5 | ± | 2.2 |
| Total lean mass (kg) | 54.9 | ± | 12.6 | 59.8 | ± | 6.8 | 46.8 | ± | 5.1 | 54.6 | ± | 5.5 |
| VO2peak (mL kg−1 min−1) | 40.2 | ± | 9.5 | 43.5 | ± | 8.6 | 55.2 | ± | 11.7$ | 57.9 | ± | 6.4* |
| Years of cycling training (yr) | 2.7 | ± | 1.3 | 4.4 | ± | 1.9λ | ||||||
| Hours of cycling training (h/sem) | 8.6 | ± | 1.0 | 11.4 | ± | 1.3λ | ||||||
Values are presented as mean ± SD.
*P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group <17 yr.
P<0.05 compared to Cyclists <17 yr.
Peak oxygen uptake (VO2max).
Cardiorespiratory test
Cardiorespiratory tests were performed at the same time of the day (09∶00–13∶00 h) on an electrically braked cycle-ergometer (Ergoselect 200 K, Ergoline; Bitz, Alemania). Subjects refrained from performing physical activity during the 24 h-period before the tests. After a warm-up period of 3-min with no load, power output was increased from an initial value of 30 W with 30 W increments every minute. Subjects maintained pedal cadence within the range of 60–80 rev·min−1. The cadence monitor was placed in view of the subject during each test and a designated investigator ensured that they maintained the required pedalling cadence throughout the duration of the test. The tests were terminated upon volitional exhaustion of the subjects and/or when cadence could not be maintained at a minimum of 60 rev·min−1. Peak oxygen consumption (VO2peak) was determined with a breath by breath gas analyzer (Oxycon Pro, Jaeger/Viasys, Germany). The gas analyzer was calibrated with a known gas prior to the first test each day, as recommended by the company. Electrocardiogram (ECG) was recorded by heart rate, utilizing a 12-lead system at rest, during the whole test and the first 3 minutes of recovery.
Bone, lean and fat mass
Subjects were scanned in order to obtain bone measurements of the whole body, pelvis, hip, lumbar spine, head and average of arms and legs. The bone mass and lean mass [body mass – (fat mass + bone mass)] were measured using DXA (paediatric version of the software QDR-Explorer, Hologic Corp., Software version 12.4, Waltham, MA, USA). DXA equipment was calibrated using a lumbar spine phantom as recommended by the manufacturer. Subjects were scanned in supine position and the scans were performed at high resolution [17]. Lean mass (g), fat mass (g), total area (cm2) and BMC (g) were calculated from total and regional analysis of the whole body scan. BMD (g · cm−2) was calculated using the formula BMD = BMC area−2. The regional analysis (upper and lower extremities and pelvic region) was performed as described elsewhere [17]. Also, examinations were conducted to estimate bone mass at the lumbar spine (L1–L4) and hip regions as previously described [18]. Two additional examinations were conducted to estimate bone mass at the lumbar spine (L1–L4) and proximal region of the femur (hip and femoral neck). Volumetric BMD (vBMD) was estimated for the lumbar spine and femoral neck using simple geometric cylindrical models [19]. Bone mineral apparent density (BMAD) was calculated as previously described [20], using the formula whole body BMAD = BMC/(area2/body height).
Statistics
As descriptive statistics, mean and standard deviation (SD) are given for raw data bone mass related variables and mean and standard error for bone mass adjusted results. Normality of data distribution was evaluated with Kolmogorov-Smirnov test.
To determine differences between age-groups in bone mass, one-way ANOVA with Bonferroni post hoc was applied. For adjusted results, one-way analysis of covariance (ANCOVA) with Bonferroni post hoc was used, including as covariates: body size (height) and whole body lean mass. Effect size statistics using Cohen's d (standardized mean difference) were calculated [21]. Taking into account the cut-offs established by Cohen, the effect size can be small (∼0.2), medium (∼0.5) or large (∼0.8). SPSS version 15.0 was used for the analysis. The probability value for the significance level was fixed at 0.05.
Results
Table 1 and Table 2 summarize the descriptive characteristics of the participants. Cyclists and control groups were comparable in age and height. Cyclists had significantly lower body mass, total lean mass, body fat percentage, total body fat and BMI (all, P<0.01). VO2peak was higher in cyclists than in controls (P<0.01).
Raw data for bone related variables is presented in Table 3. In general, lower BMC, BMD and bone area was observed in cyclists compared to controls (P<0.05, Table 3). Bone values adjusted for the combined influence of height and total lean mass are presented in table 4. Compared to controls, cyclists had lower BMC for the whole-body, pelvis, femoral neck and legs (P<0.01). BMD at the legs, pelvis, total hip and in the trochanter and inter-trochanteric subregion was lower in cyclists than in controls (P<0.01). Cyclists had lower bone area than controls at the whole-body, legs and femoral neck site (P<0.01). Total hip area was greater in cyclists than in controls (P<0.01).
Table 3. Bone mineral content (BMC), density (BMD) and area in control and cyclists group.
| Controls | Cyclists | ||||||
| Mean | SD | Mean | SD | P * | |||
| BMC (g) | |||||||
| Whole body | 2103.4 | ± | 461.6 | 1665.3 | ± | 318.3 | 0.001 |
| Pelvis | 312.7 | ± | 87.1 | 233.1 | ± | 48.8 | 0.001 |
| Hip | 41.6 | ± | 9.3 | 36.6 | ± | 6.0 | 0.041 |
| Trochanter | 10.2 | ± | 2.5 | 8.4 | ± | 1.7 | 0.007 |
| Inter- trochanter | 26.0 | ± | 6.6 | 23.6 | ± | 4.1 | 0.158 |
| Femoral neck | 5.3 | ± | 0.9 | 4.5 | ± | 0.7 | 0.030 |
| Lumbar spine | 64.4 | ± | 16.1 | 53.3 | ± | 11.4 | 0.011 |
| Average arms | 167.0 | ± | 41.1 | 140.0 | ± | 33.4 | 0.022 |
| Average legs | 535.6 | ± | 108.3 | 419.5 | ± | 77.9 | 0.001 |
| Head | 491.3 | ± | 97.9 | 465.1 | ± | 109.1 | 0.405 |
| BMD (g cm−2) | |||||||
| Whole body | 1.061 | ± | 0.115 | 0.933 | ± | 0.084 | 0.001 |
| Pelvis | 1.243 | ± | 0.202 | 0.998 | ± | 0.115 | 0.001 |
| Hip | 1.072 | ± | 0.132 | 0.949 | ± | 0.088 | 0.001 |
| Trochanter | 0.877 | ± | 0.095 | 0.741 | ± | 0.086 | 0.001 |
| Inter-trochanter | 1.201 | ± | 0.164 | 1.073 | ± | 0.941 | 0.001 |
| Femoral-neck | 0.985 | ± | 0.132 | 0.873 | ± | 0.112 | 0.004 |
| Lumbar spine | 0.998 | ± | 0.157 | 0.875 | ± | 0.111 | 0.005 |
| Average arms | 0.762 | ± | 0.078 | 0.717 | ± | 0.617 | 0.001 |
| Average legs | 1.325 | ± | 0.144 | 1.161 | ± | 0.119 | 0.001 |
| Head | 2.030 | ± | 0.335 | 1.965 | ± | 0.383 | 0.553 |
| vBMD (g·cm−3) | |||||||
| Femoral neck | 0.354 | ± | 0.051 | 0.324 | ± | 0.044 | 0.050 |
| Lumbar spine | 0.274 | ± | 0.032 | 0.257 | ± | 0.029 | 0.087 |
| BMAD | 0.130 | ± | 0.171 | 0.091 | ± | 0.006 | 0.291 |
| Area (cm−2) | |||||||
| Whole body | 1963.43 | ± | 258.89 | 1771.89 | ± | 181.99 | 0.001 |
| Pelvis | 248.03 | ± | 43.52 | 231.7 | ± | 26.26 | 0.139 |
| Hip | 38.44 | ± | 5.80 | 38.37 | ± | 3.77 | 0.160 |
| Trochanter | 11.57 | ± | 1.88 | 11.27 | ± | 1.19 | 0.529 |
| Inter-trochanter | 21.52 | ± | 4.25 | 21.96 | ± | 2.80 | 0.688 |
| Femoral-neck | 5.35 | ± | 0.44 | 5.14 | ± | 0.37 | 0.091 |
| Lumbar spine | 63.90 | ± | 9.11 | 60.48 | ± | 6.58 | 0.959 |
| Average arms | 216.82 | ± | 36.79 | 193.12 | ± | 30.14 | 0.024 |
| Average legs | 401.7 | ± | 50.29 | 359.31 | ± | 33.79 | 0.002 |
| Head | 240.92 | ± | 14.20 | 235.52 | ± | 14.18 | 0.213 |
Values as mean and SD.
P<0.05 compared with control group. Volumetric bone mineral density (vBMD); bone mineral apparent density (BMAD).
Table 4. Adjusted bone mineral content (BMC), density (BMD) and area in control.
| Controls | Cyclists | ||||||
| Mean | SD | Mean | SD | P * | |||
| BMC (g) | |||||||
| Whole body | 2438.1 | ± | 39.3 | 2287.0 | ± | 39.3 | 0.012 |
| Pelvis | 291.2 | ± | 7.8 | 254.6 | ± | 7.8 | 0.003 |
| Hip | 39.2 | ± | 0.9 | 38.9 | ± | 0.9 | 0.777 |
| Trochanter | 9.7 | ± | 0.3 | 9.0 | ± | 0.3 | 0.135 |
| Inter-trochanter | 24.5 | ± | 0.7 | 25.2 | ± | 0.7 | 0.533 |
| Femoral neck | 5.1 | ± | 0.1 | 4.7 | ± | 0.1 | 0.054 |
| Lumbar spine | 60.8 | ± | 2.1 | 56.9 | ± | 2.1 | 0.200 |
| Average arms | 154.7 | ± | 3.2 | 152.3 | ± | 3.2 | 0.606 |
| Average legs | 506.1 | ± | 8.4 | 449.0 | ± | 8.4 | 0.001 |
| Head | 464.6 | ± | 15.7 | 491.9 | ± | 15.7 | 0.241 |
| BMD (g cm−2) | |||||||
| Whole body | 1.132 | ± | 0.016 | 1.089 | ± | 0.016 | 0.065 |
| Pelvis | 1.200 | ± | 0.026 | 1.042 | ± | 0.026 | 0.001 |
| Hip | 1.045 | ± | 0.190 | 0.976 | ± | 0.190 | 0.015 |
| Trochanter | 0.860 | ± | 0.160 | 0.760 | ± | 0.160 | 0.001 |
| Inter-trochanter | 1.170 | ± | 0.023 | 1.104 | ± | 0.023 | 0.054 |
| Femoral neck | 0.956 | ± | 0.200 | 0.903 | ± | 0.020 | 0.082 |
| Lumbar spine | 0.961 | ± | 0.200 | 0.912 | ± | 0.200 | 0.110 |
| Average arms | 0.742 | ± | 0.090 | 0.738 | ± | 0.090 | 0.787 |
| Average legs | 1.287 | ± | 0.018 | 1.199 | ± | 0.018 | 0.002 |
| Head | 1.940 | ± | 0.060 | 2.050 | ± | 0.060 | 0.214 |
| vBMD (g cm−3) | |||||||
| Femoral neck | 0.347 | ± | 0.008 | 0.331 | ± | 0.008 | 0.160 |
| Lumbar spine | 0.268 | ± | 0.006 | 0.264 | ± | 0.006 | 0.579 |
| BMAD | 0.127 | ± | 0.027 | 0.094 | ± | 0.027 | 0.412 |
| Area (cm−2) | |||||||
| Whole body | 2133.25 | ± | 15.65 | 2078.5 | ± | 15.65 | 0.021 |
| Pelvis | 238.44 | ± | 3.97 | 241.29 | ± | 3.97 | 0.626 |
| Hip | 37.22 | ± | 0.54 | 39.59 | ± | 0.54 | 0.004 |
| Trochanter | 11.50 | ± | 0.02 | 11.80 | ± | 0.02 | 0.115 |
| Inter-trochanter | 20.75 | ± | 0.53 | 22.73 | ± | 0.53 | 0.014 |
| Femoral neck | 5.30 | ± | 0.06 | 5.19 | ± | 0.06 | 0.249 |
| Lumbar spine | 62.46 | ± | 1.13 | 61.92 | ± | 1.13 | 0.746 |
| Average arms | 206.34 | ± | 3.33 | 203.60 | ± | 3.33 | 0.576 |
| Average legs | 390.78 | ± | 4.98 | 370.22 | ± | 4.98 | 0.007 |
| Head | 238.12 | ± | 2.17 | 238.31 | ± | 2.17 | 0.952 |
Values as mean and SD.
*P<0.05 compared to control group. Volumetric bone mineral density (vBMD); bone mineral apparent density (BMAD).
Table 5, 6 & 7 summarize raw data for bone based on whether cyclists and controls were younger or older than 17 years. Cyclists under 17 had lower BMC in the arms and legs and lower BMD in the whole body, pelvis, throchanter, arms and legs; and cyclists over 17 had lower BMC in the pelvis, femoral neck and legs, and lower BMD in the pelvis, hip, throchanter, femoral neck and legs (all P<0.05, Table 5, 6). Table 8, 9 & 10 present the bone values adjusted by height and total lean mass. When differences between groups were compared by age, cyclists under 17 had 10% lower BMC in the legs and 8% higher total hip area than age-matched controls (P<0.05). In groups over 17, cyclists had 26.5%, 15.8% and 14.4% lower BMC than controls at the pelvis, femoral neck and legs, respectively (P<0.05). BMD was lower in cyclists over 17 than in age-matched controls at the pelvis, hip, trochanter, inter-trochanter, femoral neck and legs (the percentages being 24.5, 10.5, 16.2, 7.5, 15.8 and 14.3, respectively, P<0.05). In addition, cyclists had a vBMD 17.1% lower than controls at the femoral neck (P<0.05, Table 9).
Table 5. Bone mineral content (BMC), density (BMD) and area in control and cyclist group under 17 (<17 yr) and equal or over (≥17 yr).
| Controls | Cyclists | |||||||||||||
| <17 yr | ≥17 yr | P $ | <17 yr | ≥17 yr | P * | |||||||||
| BMC (g) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Whole body | 2469.0 | ± | 606.5 | 2776.5 | ± | 409.7 | 0.043 | 1934.7 | ± | 285.9 | 2325.9 | ± | 436.6 | 0.213 |
| Pelvis | 286.3 | ± | 93.1 | 351.0 | ± | 64.2 | 0.077 | 214.8 | ± | 46.3 | 251.4 | ± | 46.0 | 0.012 |
| Hip | 38.2 | ± | 9.3 | 46.4 | ± | 7.5 | 1 | 34.4 | ± | 5.8 | 38.7 | ± | 5.8 | 0.146 |
| Trochanter | 9.6 | ± | 2.4 | 11.2 | ± | 2.5 | 0.393 | 8.0 | ± | 1.5 | 8.8 | ± | 1.8 | 0.101 |
| Inter-trochanter | 23.6 | ± | 7.0 | 29.5 | ± | 4.4 | 1.000 | 22.1 | ± | 4.2 | 25.2 | ± | 3.5 | 0.400 |
| Femoral neck | 5.0 | ± | 0.8 | 5.7 | ± | 0.9 | 0.287 | 4.3 | ± | 0.5 | 4.7 | ± | 0.9 | 0.032 |
| Lumbar spine | 59.2 | ± | 16.3 | 72.0 | ± | 13.1 | 0.394 | 49.0 | ± | 9.8 | 57.7 | ± | 11.6 | 0.114 |
| Average arms | 160.2 | ± | 47.9 | 176.9 | ± | 28.4 | 0.048 | 120.2 | ± | 24.0 | 159.9 | ± | 30.8 | 1 |
| Average legs | 515.0 | ± | 120.7 | 565.4 | ± | 85.0 | 0.012 | 390.1 | ± | 57.6 | 449.0 | ± | 86.8 | 0.043 |
| Head | 468.0 | ± | 101.3 | 525.2 | ± | 87.0 | 1 | 412.7 | ± | 76.2 | 517.4 | ± | 114.8 | 1 |
Values as mean and SD. P values calculated with ANOVA.
*P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group <17 yr.
Table 6. Bone mineral density (BMD) and volumetric density (vBMD) in control and cyclist group under 17 (<17 yr) and equal or over (≥17 yr).
| Controls | Cyclists | |||||||||||||
| <17 yr | ≥17 yr | P $ | <17 yr | ≥17 yr | P * | |||||||||
| BMD (g cm−2) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Whole body | 1.133 | ± | 0.127 | 1.215 | ± | 0.119 | 0.036 | 1.002 | ± | 0.926 | 1.106 | ± | 0.097 | 0.204 |
| Pelvis | 1.183 | ± | 0.164 | 1.33 | ± | 0.229 | 0.110 | 0.966 | ± | 0.109 | 1.03 | ± | 0.118 | 0.001 |
| Hip | 1.04 | ± | 0.115 | 1.119 | ± | 0.148 | 0.174 | 0.937 | ± | 0.096 | 0.96 | ± | 0.083 | 0.017 |
| Trochanter | 0.859 | ± | 0.83 | 0.903 | ± | 0.11 | 0.020 | 0.741 | ± | 0.931 | 0.742 | ± | 0.826 | 0.002 |
| Inter-trochanter | 1.166 | ± | 0.146 | 1.251 | ± | 0.185 | 0.290 | 1.055 | ± | 0.101 | 1.091 | ± | 0.087 | 0.063 |
| Femoral neck | 0.946 | ± | 0.09 | 1.042 | ± | 0.166 | 0.713 | 0.867 | ± | 0.109 | 0.879 | ± | 0.119 | 0.027 |
| Lumbar spine | 0.957 | ± | 0.136 | 1.057 | ± | 0.174 | 0.118 | 0.528 | ± | 0.113 | 0.923 | ± | 0.09 | 0.164 |
| Average arms | 0.753 | ± | 0.894 | 0.776 | ± | 0.062 | 0.076 | 0.753 | ± | 0.18 | 0.74 | ± | 0.073 | 1 |
| Average legs | 1.288 | ± | 0.151 | 1.377 | ± | 0.124 | 0.005 | 1.219 | ± | 0.112 | 1.243 | ± | 0.155 | 0.046 |
| Head | 1.953 | ± | 0.346 | 2.141 | ± | 0.301 | 1 | 1.788 | ± | 0.329 | 2.142 | ± | 0.363 | 1 |
| vBMD (g cm−3) | ||||||||||||||
| Femoral neck | 0.346 | ± | 0.036 | 0.364 | ± | 0.068 | 1 | 0.334 | ± | 0.051 | 0.315 | ± | 0.036 | 0.171 |
| Lumbar spine | 0.27 | ± | 0.022 | 0.28 | ± | 0.044 | 0.268 | 0.244 | ± | 0.027 | 0.271 | ± | 0.026 | 1 |
| BMAD | 0.091 | ± | 0.006 | 0.185 | ± | 0.265 | 1 | 0.092 | ± | 0.007 | 0.093 | ± | 0.005 | 0.551 |
Values as mean and SD. P values calculated with ANOVA.
*P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group <17 yr.
Bone mineral apparent density (BMAD).
Table 7. Bone mineral area in control and cyclist group under 17 (<17 yr) and equal or over (≥17 yr).
| Controls | Cyclists | |||||||||||||
| <17 yr | ≥17 yr | P $ | <17 yr | ≥17 yr | P * | |||||||||
| Area (cm−2) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Whole body | 2152.31 | ± | 314.47 | 2279.52 | ± | 176.06 | 0.117 | 1925.74 | ± | 147.61 | 2089.6 | ± | 203.38 | 0.418 |
| Pelvis | 236.67 | ± | 50.27 | 264.45 | ± | 25.86 | 1 | 220.31 | ± | 26.73 | 243.09 | ± | 21.16 | 1 |
| Hip | 36.32 | ± | 6.09 | 41.52 | ± | 3.81 | 1 | 36.58 | ± | 3.53 | 40.156 | ± | 3.21 | 1 |
| Trochanter | 11.08 | ± | 2.02 | 12.28 | ± | 1.46 | 1 | 10.7 | ± | 0.95 | 11.83 | ± | 1.17 | 1 |
| Inter-trochanter | 19.98 | ± | 4.43 | 23.75 | ± | 2.94 | 1 | 20.87 | ± | 2.96 | 23.04 | ± | 2.26 | 1 |
| Femoral neck | 5.26 | ± | 0.48 | 5.49 | ± | 0.39 | 0.742 | 5.01 | ± | 0.32 | 5.28 | ± | 0.39 | 1 |
| Lumbar spine | 60.82 | ± | 9.42 | 68.35 | ± | 6.85 | 1 | 58.83 | ± | 5.46 | 62.124 | ± | 7.43 | 0.449 |
| Average arms | 209.51 | ± | 42.75 | 227.37 | ± | 24.49 | 0.062 | 175.22 | ± | 22.28 | 211.01 | ± | 26.29 | 1 |
| Average legs | 395.88 | ± | 57.38 | 410.09 | ± | 39.55 | 0.115 | 352.58 | ± | 30.76 | 366.03 | ± | 36.77 | 0.175 |
| Head | 238.28 | ± | 15.47 | 244.75 | ± | 11.93 | 1 | 231.13 | ± | 12.56 | 239.9 | ± | 14.91 | 1 |
Values as mean and SD. P values calculated with ANOVA.
P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group < 17 yr.
Volumetric bone mineral density (vBMD); bone mineral apparent density (BMAD).
Table 8. Adjusted Bone mineral content (BMC) in control and cyclists group under 17 (<17 yr) and equal or over (≥17 yr).
| Controls | Cyclists | ||||||||||||||
| <17 yr | ≥17 yr | P $ | <17 yr | ≥17 yr | P * | P £ | |||||||||
| BMC (g) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Whole body | 2413.9 | ± | 51.7 | 2474.8 | ± | 64.5 | 0.781 | 2288.8 | ± | 59.9 | 2283.8 | ± | 54.8 | 0.171 | 0.565 |
| Pelvis | 277.2 | ± | 9.5 | 310.9 | ± | 11.9 | 1 | 264.1 | ± | 11.0 | 245.7 | ± | 10.1 | 0.001 | 0.017 |
| Hip | 37.3 | ± | 1.1 | 42.1 | ± | 1.4 | 1 | 39.6 | ± | 1.3 | 38.1 | ± | 1.2 | 0.216 | 0.015 |
| Trochanter | 9.4 | ± | 0.4 | 10.1 | ± | 0.5 | 1 | 9.3 | ± | 0.5 | 8.7 | ± | 0.4 | 0.214 | 0.123 |
| Inter-trochanter | 23.1 | ± | 0.9 | 26.7 | ± | 1.1 | 0.515 | 25.5 | ± | 1.0 | 24.8 | ± | 1.0 | 1 | 0.033 |
| Femoral neck | 4.9 | ± | 0.2 | 5.3 | ± | 0.2 | 1 | 4.8 | ± | 0.2 | 4.6 | ± | 0.2 | 0.055 | 0.084 |
| Lumbar spine | 58.0 | ± | 2.6 | 65.3 | ± | 3.3 | 1 | 56.9 | ± | 3.1 | 56.7 | ± | 2.8 | 0.312 | 0.199 |
| Average arms | 155.6 | ± | 4.2 | 154.6 | ± | 5.2 | 1 | 147.0 | ± | 4.8 | 156.8 | ± | 4.4 | 1 | 0.248 |
| Average legs | 506.4 | ± | 11.0 | 503.7 | ± | 13.8 | 0.055 | 459.1 | ± | 12.8 | 440.5 | ± | 11.7 | 0.007 | 0.516 |
| Head | 458.8 | ± | 20.3 | 477.9 | ± | 25.4 | 1 | 468.8 | ± | 23.6 | 510.8 | ± | 21.6 | 1 | 0.611 |
Values as mean and SD. P values calculated with repeated measures ANCOVA adjusted with total lean mass and height.
*P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group <17 yr.
P<0.05 interaction group × age.
Table 9. Adjusted Bone mineral density (BMD) and volumetric density (vBMD) in control and cyclists group under 17 (<17 yr) and equal or over (≥17 yr).
| Controls | Cyclists | ||||||||||||||
| <17 yr | ≥17 yr | P $ | <17 yr | ≥17 yr | P * | P £ | |||||||||
| BMD (g cm−2) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Whole body | 1.113 | ± | 0.025 | 1.164 | ± | 0.025 | 1 | 1.075 | ± | 0.023 | 1.099 | ± | 0.021 | 0.316 | 0.543 |
| Pelvis | 1.151 | ± | 0.031 | 1.27 | ± | 0.039 | 0.489 | 1.063 | ± | 0.036 | 1.02 | ± | 0.033 | 0.001 | 0.023 |
| Hip | 1.016 | ± | 0.023 | 1.085 | ± | 0.029 | 1 | 1 | ± | 0.027 | 0.954 | ± | 0.024 | 0.008 | 0.03 |
| Trochanter | 0.843 | ± | 0.21 | 0.874 | ± | 0.026 | 0.650 | 0.789 | ± | 0.024 | 0.737 | ± | 0.22 | 0.001 | 0.076 |
| Inter-trochanter | 1.138 | ± | 0.029 | 1.216 | ± | 0.037 | 1 | 1.124 | ± | 0.034 | 1.085 | ± | 0.031 | 0.052 | 0.076 |
| Femoral neck | 0.918 | ± | 0.025 | 1.009 | ± | 0.031 | 1 | 0.934 | ± | 0.029 | 0.874 | ± | 0.026 | 0.01 | 0.008 |
| Lumbar spine | 0.928 | ± | 0.025 | 1.012 | ± | 0.032 | 1 | 0.906 | ± | 0.029 | 0.916 | ± | 0.027 | 0.148 | 0.191 |
| Average arms | 0.743 | ± | 0.012 | 0.742 | ± | 0.015 | 1 | 0.725 | ± | 0.014 | 0.749 | ± | 0.013 | 1 | 0.356 |
| Average legs | 1.269 | ± | 0.024 | 1.318 | ± | 0.03 | 0.182 | 1.186 | ± | 0.027 | 1.21 | ± | 0.025 | 0.045 | 0.633 |
| Head | 1.908 | ± | 0.077 | 2.012 | ± | 0.097 | 1 | 1.967 | ± | 0.09 | 2.123 | ± | 0.082 | 1 | 0.759 |
| vBMD (g cm−3) | |||||||||||||||
| Femoral neck | 0.331 | ± | 0.009 | 0.369 | ± | 0.011 | 1 | 0.348 | ± | 0.01 | 0.315 | ± | 0.009 | 0.004 | 0.001 |
| Lumbar spine | 0.263 | ± | 0.007 | 0.278 | ± | 0.009 | 1 | 0.255 | ± | 0.008 | 0.27 | ± | 0.007 | 1 | 0.992 |
| BMAD | 0.089 | ± | 0.035 | 0.186 | ± | 0.043 | 1 | 0.092 | ± | 0.04 | 0.093 | ± | 0.037 | 0.634 | 0.211 |
Values as mean and SD. P values calculated with repeated measures ANCOVA adjusted with total lean mass and height.
*P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group <17 yr.
P<0.05 interaction group × age. Bone mineral apparent density (BMAD).
Table 10. Adjusted Bone mineral area in control and cyclists group under 17 (<17 yr) and equal or over (≥17 yr).
| Controls | Cyclists | ||||||||||||||
| <17 yr | ≥17 yr | P $ | <17 yr | ≥17 yr | P * | P £ | |||||||||
| Area (cm−2) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Whole body | 2144.3 | ± | 20.37 | 2113.46 | ± | 25.42 | 0.695 | 2093.01 | ± | 23.59 | 2067.12 | ± | 21.58 | 1 | 0.543 |
| Pelvis | 236.15 | ± | 5.23 | 241.71 | ± | 6.53 | 1 | 242.5 | ± | 6.06 | 240.12 | ± | 5.55 | 1 | 0.912 |
| Hip | 36.39 | ± | 0.68 | 38.55 | ± | 0.84 | 0.053 | 39.31 | ± | 0.78 | 39.78 | ± | 0.72 | 1 | 0.262 |
| Trochanter | 11.03 | ± | 0.28 | 11.4 | ± | 0.35 | 1 | 11.59 | ± | 0.32 | 11.72 | ± | 0.29 | 1 | 0.695 |
| Inter-trochanter | 20.03 | ± | 0.68 | 21.87 | ± | 0.84 | 0.123 | 22.59 | ± | 0.78 | 22.8 | ± | 0.72 | 1 | 0.282 |
| Femoral neck | 5.32 | ± | 0.08 | 5.28 | ± | 0.1 | 0.797 | 5.13 | ± | 0.1 | 5.25 | ± | 0.09 | 1 | 0.357 |
| Lumbar spine | 61.43 | ± | 1.48 | 63.97 | ± | 1.85 | 1 | 62.22 | ± | 1.71 | 61.59 | ± | 1.57 | 1 | 0.335 |
| Average arms | 206.2 | ± | 4.28 | 207.67 | ± | 5.35 | 1 | 197.99 | ± | 4.96 | 208.28 | ± | 4.54 | 1 | 0.335 |
| Average legs | 396.16 | ± | 6.16 | 380.55 | ± | 7.69 | 0.641 | 380.23 | ± | 7.14 | 362.23 | ± | 6.53 | 0.448 | 0.86 |
| Head | 238.99 | ± | 2.86 | 236.93 | ± | 3.58 | 1 | 237.65 | ± | 3.32 | 238.93 | ± | 3.04 | 1 | 0.599 |
Values as mean and SD. P values calculated with repeated measures ANCOVA adjusted with total lean mass and height.
*P<0.05 compared to control group ≥17 yr.
P<0.05 compared to control group <17 yr.
P<0.05 interaction group × age.
Analyses were repeated including fat mass as covariable with no variation of the presented results.
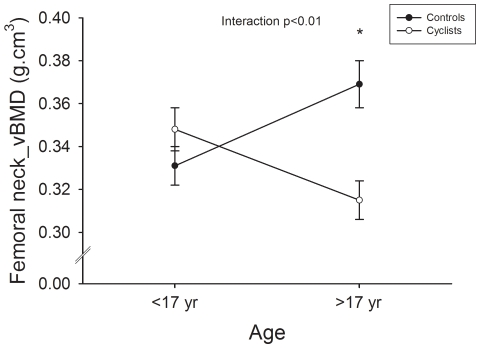
Group interaction by age was found for BMC at the pelvis, hip and inter-trochanter sub-regions, and for BMD at the pelvis, hip and femoral neck (all P≤0.05, Table 8 & 9), as well as for vBMD at the femoral neck (P≤0.001, Figure 1).
Figure 1. Group × age interaction for femoral neck volumetric bone mineral density (vBMD; P<0.01) in young and old adolescent cyclists and controls.
* P<0.05 between control and cyclists groups ≥17 years.
All the previous comparisons exhibited large effect sizes (Cohen's d ranged from 0.8 to 7), excepting the hip BMD which were low (Cohen's d = 0.3).
Discussion
The main finding herein is that adolescent cyclists had lower BMC and BMD compared with healthy age-matched controls in regions of clinical interest (hip, pelvis and femoral neck). Our study also shows that differences in BMC and BMD between cyclists and controls were higher in adolescents over 17 years old.
The present study shows that adolescent cyclists had lower BMC and BMD than healthy age-matched controls. Cross-sectional and longitudinal studies have shown that weight-bearing and impact-loading sports improve bone mass, especially at weight-bearing sites [22], [23], [24]. However, athletes who perform activities in which the body weight load is diminished or without impacts, as in cycling, are associated with a lower bone mass compared with athletes who participated in weight-bearing sports [25], [26]. Male professional cyclists had lower BMD for the whole body (12%), legs (16%), pelvis (18%), femoral neck (25%) [22] and lumbar spine [28] than non-active controls of similar age.
The differences observed in BMC and BMD in our adolescent cyclists are similar to those observed previously in professional cyclists who trained 3 times as much [22]. Sanchis et al. [27] found that young tennis players had 69% of the inter-arm asymmetry in BMC observed in professional tennis players who trained nearly twice as much, and all the asymmetry in bone area. In the review literature, we have found only 3 studies evaluating the bone mass in adolescent cyclists [11], [14], [15]. Rico et al. [14] did not find differences in total or regional BMC between male adolescent cyclists with a similar training frequency than in the present study (10 h/week), and age-matched controls, when values were corrected by body weight; one possible explanation that may explain this discrepancy is that in our study we corrected the BMC and BMD by the total lean mass and the height, as they are the variables having the highest effect on bone growth [4]. Unfortunately Rico et al. [14] did not evaluate BMD.
Duncan at al. [11] observed that female cyclists had similar BMD at whole body, lumbar, femoral neck, legs and arms than non-active population, but lower BMD at whole body, femoral neck and legs than a group of female runners. The same researchers compared total and cortical vBMD at the femur bone in adolescent females from different sport disciplines (cyclists, triathletes, swimmers and runners) and a non-active control group of the same gender [15]. Duncan et al (2002) showed that BMC and vBMD in mid femur was similar in all groups, except for runners who showed higher BMD values and bone strength than cyclists [15]. Several aspects may cause the differences between this latter research and the present one, such as different control groups (sedentary vs. actives) [28], differences in lean mass [24], or the known gender dimorphism in the bone development [29].
Our results showed that differences in BMC and BMD between cyclists and active controls were greater in adolescents over 17 years old than in those under that age. We also found a negative association between age and BMC, and BMD, in the cyclists. Unfortunately we only can compare our results with longitudinal studies conducted in adults. Nichols et al. [29] described the tracked changes in BMD over a 7-year period in competitive male master cyclists and non-athletes. Their results showed that at the beginning of the study, cyclists had lower lumbar and hip BMD than the control group; interestingly at the end of the study master cyclists had lost more BMD than controls [32]. A previous study examined BMD over a one year season in amateur male cyclists and found 1–1.5% decrease in BMD at the proximal femur but no changes at the lumbar spine [33]. Nichols et al. [8] observed that master cyclists (>50 yr) had lower total, lumbar and hip BMD than younger cyclists (mean 31 yr).
Bone mineral density is the main variable used to determine osteoporosis [30]. There is a close relationship between BMD and bone mechanical strength [31]. Our study shows that adolescent cyclists developed lower BMD than controls at relevant clinical sites. This could increase the risk of bone fractures and/or osteoporosis. However, in spite of lower levels of BMD at clinical sites, adult cyclists develop a higher cortical thickness which can also increase bone strength [32].
A recent study showed that master professional cyclists (>50 yr) exhibited greater BMC and cortical area at the tibiae and radius which was associated with higher polar momentum of resistance [32]. Longitudinal studies should be conducted to corroborate this finding and to analyze whether this effect can be generalized to include other bones of greater clinical interest [33].
Some limitations should be recognized. One is the design, from which it cannot be concluded that the effect that is observed in older adolescents is due to the longer period (years) of practice of cycling rather than internal (i.e. genetic) or external (i.e. energy imbalance) factors. The absence of hormone and calcium intake data is another potential weakness of this study because this may affect bone acquisition; although it could explain the mechanisms behind these observations they should not change the found lower bone mass found in cyclists.
Nevertheless, the analysis of the interaction between bone mass, age and cycling training may indicate that the practice of cycling training is linked to the lower bone mass found in our adolescents. In the same line we have found a strong negative correlation, after taking into account the age, height, muscle mass and years of practice, between hours of practice and BMC and BMD in all the regions studied in older adolescent cyclists (r = −0.31 to r = −0.76) although none of them reached statistical significance, maybe because the low sample. Nonetheless, this hypothesis must be corroborated with longitudinal or intervention studies.
A strength of this study is that volumetric density was calculated: vBMD and BMAD have been proposed as a better reflection of the real bone density [20], [34]. We detect no other study where these bone parameters were estimated in adolescent cyclists. In our study, we found a 17% lower vBMD at the femoral neck of the older cyclists compared to older controls, which may be associated with an important risk of fracture in this relevant clinical zone. The BMAD in the cyclist group was 100% lower than that in the controls in the older adolescents; although of no statistical significance, maybe because of the sample size, this may imply important biological consequences reflected by the high effect size.
Conclusions
Our study shows that cycling training, may adversely affect bone mass during adolescence. Although this is a case control study and caution must be used in interpreting the results, the practice of cycling practice during adolescence may compromise the acquisition of bone mass.
Acknowledgments
We wish to thank the controls, cyclists and their coaches for their diligence and cooperation throughout this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work has been funded by Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (DPS2008-06999) and Presidencia del Gobierno de España, Consejo Superior de Deportes (21/UPB20/10). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goulet GC, Halonen NR, Koch LG, Britton SL, Zernicke RF, et al. Osteoblast Response to Ovariectomy Is Enhanced in Intrinsically High Aerobic-Capacity Rats. Calcif Tissue Int. 2011 doi: 10.1007/s00223-010-9457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119:S3–S11. doi: 10.1016/j.amjmed.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 4.Vicente-Rodriguez G. How does exercise affect bone development during growth? Sports Med. 2006;36:561–569. doi: 10.2165/00007256-200636070-00002. [DOI] [PubMed] [Google Scholar]
- 5.de Geus B, Van Hoof E, Aerts I, Meeusen R. Cycling to work: influence on indexes of health in untrained men and women in Flanders. Coronary heart disease and quality of life. Scand J Med Sci Sports. 2008;18:498–510. doi: 10.1111/j.1600-0838.2007.00729.x. [DOI] [PubMed] [Google Scholar]
- 6.Matthews CE, Jurj AL, Shu XO, Li HL, Yang G, et al. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–1350. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]
- 7.Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, et al. Bone mineral density in adolescent female athletes: relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002;34:286–294. doi: 10.1097/00005768-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Slemenda CW, Reister TK, Hui SL, Miller JZ, Christian JC, et al. Influences on skeletal mineralization in children and adolescents: evidence for varying effects of sexual maturation and physical activity. J Pediatr. 1994;125:201–207. doi: 10.1016/s0022-3476(94)70193-8. [DOI] [PubMed] [Google Scholar]
- 10.Medelli J, Lounana J, Menuet JJ, Shabani M, Cordero-MacIntyre Z. Is osteopenia a health risk in professional cyclists? J Clin Densitom. 2009;12:28–34. doi: 10.1016/j.jocd.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 11.Nichols JF, Palmer JE, Levy SS. Low bone mineral density in highly trained male master cyclists. Osteoporos Int. 2003;14:644–649. doi: 10.1007/s00198-003-1418-z. [DOI] [PubMed] [Google Scholar]
- 12.Rector RS, Rogers R, Ruebel M, Hinton PS. Participation in road cycling vs running is associated with lower bone mineral density in men. Metabolism. 2008;57:226–232. doi: 10.1016/j.metabol.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Warner SE, Shaw JM, Dalsky GP. Bone mineral density of competitive male mountain and road cyclists. Bone. 2002;30:281–286. doi: 10.1016/s8756-3282(01)00704-9. [DOI] [PubMed] [Google Scholar]
- 14.Rico H, Revilla M, Hernandez ER, Gomez-Castresana F, Villa LF. Bone mineral content and body composition in postpubertal cyclist boys. Bone. 1993;14:93–95. doi: 10.1016/8756-3282(93)90233-z. [DOI] [PubMed] [Google Scholar]
- 15.Duncan CS, Blimkie CJ, Kemp A, Higgs W, Cowell CT, et al. Mid-femur geometry and biomechanical properties in 15- to 18-yr-old female athletes. Med Sci Sports Exerc. 2002;34:673–681. doi: 10.1097/00005768-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Beghin L, Castera M, Manios Y, Gilbert CC, Kersting M, et al. Quality assurance of ethical issues and regulatory aspects relating to good clinical practices in the HELENA Cross-Sectional Study. Int J Obes (Lond) 2008;32(Suppl 5):S12–18. doi: 10.1038/ijo.2008.179. [DOI] [PubMed] [Google Scholar]
- 17.Calbet JA, Dorado C, Diaz-Herrera P, Rodriguez-Rodriguez LP. High femoral bone mineral content and density in male football (soccer) players. Med Sci Sports Exerc. 2001;33:1682–1687. doi: 10.1097/00005768-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Vicente-Rodriguez G, Ara I, Perez-Gomez J, Dorado C, Calbet JA. Muscular development and physical activity as major determinants of femoral bone mass acquisition during growth. Br J Sports Med. 2005;39:611–616. doi: 10.1136/bjsm.2004.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravholt CH, Lauridsen AL, Brixen K, Mosekilde L, Heickendorff L, et al. Marked disproportionality in bone size and mineral, and distinct abnormalities in bone markers and calcitropic hormones in adult turner syndrome: a cross-sectional study. J Clin Endocrinol Metab. 2002;87:2798–2808. doi: 10.1210/jcem.87.6.8598. [DOI] [PubMed] [Google Scholar]
- 20.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 22.Bassey EJ, Ramsdale SJ. Weight-bearing exercise and ground reaction forces: a 12-month randomized controlled trial of effects on bone mineral density in healthy postmenopausal women. Bone. 1995;16:469–476. doi: 10.1016/8756-3282(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 23.Dorado C, Sanchis Moysi J, Vicente-Rodriguez G, Serrano JA, Rodriguez LR, et al. Bone mass, bone mineral density and muscle mass in professional golfers. J Sports Sci. 2002;20:591–597. doi: 10.1080/026404102320183149. [DOI] [PubMed] [Google Scholar]
- 24.Vicente-Rodriguez G, Dorado C, Ara I, Perez-Gomez J, Olmedillas H, et al. Artistic versus rhythmic gymnastics: effects on bone and muscle mass in young girls. Int J Sports Med. 2007;28:386–393. doi: 10.1055/s-2006-924397. [DOI] [PubMed] [Google Scholar]
- 25.Morel J, Combe B, Francisco J, Bernard J. Bone mineral density of 704 amateur sportsmen involved in different physical activities. Osteoporos Int. 2001;12:152–157. doi: 10.1007/s001980170148. [DOI] [PubMed] [Google Scholar]
- 26.Nevill A, Holder R, Stewart A. Do sporting activities convey benefits to bone mass throughout the skeleton? J Sports Sci. 2004;22:645–650. doi: 10.1080/02640410310001655769. [DOI] [PubMed] [Google Scholar]
- 27.Sanchis-Moysi J, Dorado C, Olmedillas H, Serrano-Sanchez JA, Calbet JA. Bone and lean mass inter-arm asymmetries in young male tennis players depend on training frequency. Eur J Appl Physiol. 2010;110:83–90. doi: 10.1007/s00421-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 28.Vicente-Rodriguez G, Dorado C, Perez-Gomez J, Gonzalez-Henriquez JJ, Calbet JA. Enhanced bone mass and physical fitness in young female handball players. Bone. 2004;35:1208–1215. doi: 10.1016/j.bone.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Nichols JF, Rauh MJ. Longitudinal Changes in Bone Mineral Density in Male Master Cyclists and Nonathletes. J Strength Cond Res. 2010 doi: 10.1519/JSC.0b013e3181c6a116. [DOI] [PubMed] [Google Scholar]
- 30.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 31.Lauritzen JB. Hip fractures: incidence, risk factors, energy absorption, and prevention. Bone. 1996;18:65S–75S. doi: 10.1016/8756-3282(95)00382-7. [DOI] [PubMed] [Google Scholar]
- 32.Wilks DC, Gilliver SF, Rittweger J. Forearm and tibial bone measures of distance- and sprint-trained master cyclists. Med Sci Sports Exerc. 2009;41:566–573. doi: 10.1249/MSS.0b013e31818a0ec8. [DOI] [PubMed] [Google Scholar]
- 33.Ducher G. Bone Health in Cyclists: Discrepancies Between the Axial and Peripheral Skeletons. Med Sci Sports Exerc. 2009;41:1975. doi: 10.1249/MSS.0b013e3181af2764. [DOI] [PubMed] [Google Scholar]
- 34.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]