Abstract
Avian Adeno viruses and Chicken Anemia Viruses cause serious economic losses to the poultry industry of Pakistan each year. Timely and efficient diagnosis of the viruses is needed in order to practice prevention and control strategies. In the first part of this study, we investigated broilers, breeder and Layer stocks for morbidity and mortality rates due to AAV and CAV infections and any co-infections by examining signs and symptoms typical of their infestation or post mortem examination. In the second part of the study, we developed a duplex PCR assay for the detection of AAV and CAV which is capable to simultaneously detect both the viral types prevalent in Pakistan with high sensitivity and 100% specificity.
Introduction
Adenoviruses are common infectious agents of poultry. The avian adenoviruses are divided into three groups i.e. group Ι, group Π and group III. Group Ι is composed of 5 species (A to E) and 12 serotypes (FAdV-1 to FAdV-12) of avian adenoviruses of chickens, turkeys, goose and duck [1-3]. The group Ι adenoviruses are famous for causing hydropericardium-syndrome in chickens (caused by FAdV-4 strains), Quail Bronchitis in quail (caused by FAdV-1) and inclusion body hepatitis [4-6]. Group Π of avian adenoviruses causes hemorrhagic enteritis in turkeys, marble spleen disease in pheasants and splenomegaly in chickens. Group Π viruses have a common group antigen that differentiates them from group Ι [7]. Group III viruses are famous for egg drop syndrome in chickens and a similar virus is believed to infect duck [8].
The first avian adenovirus was isolated in 1949 when material from a case of lumpy skin disease in cattle was inoculated into embryonated chicken eggs [9]. Other early unintentional isolates of fowl adenoviruses were the chicken embryo lethal orphan (CELO) isolates made in embryonated eggs [10] and the GAL viruses from chicken cell cultures [11]. The first isolate of an avian adenovirus from diseased birds was from an outbreak of respiratory disease in bobwhite quail (Colinus virginianus) by Olson [12].
The avian adenovirus infections cause high economic losses by increasing mortality in chickens, poor feed conversion, drop in egg production, eggs of poor quality and also diminished weight gain. The avian adenoviruses are also involved in immune suppression which may lead to secondary infections [13-15].
Chicken anaemia virus, previously known as chicken anaemia agent (CAA), was first isolated by Yuasa et al in 1979 [16]. The virus is small, non-enveloped, icosahedral, and contains a circular, single-stranded DNA genome [17,18]. It has recently been classified as the sole member of the genus Gyrovirus [19]. It causes severe aplastic anaemia in young chickens [16,20-22], depletion of lymphoid organs, subcutaneous and intramuscular haemorrhages, and destruction of erythroblastoid cells in bone marrow [23-25]. In addition to the above, some specific symptoms are also observed like haemorrhages in leg and chest muscles, focal necrosis in liver, ulcerative erosions in gizzard, and necrosis of wing skin [26].
Both AV and CAV have been implicated to cause serious economic losses to the poultry Industry of Pakistan [27]. Adenoviruses and Chicken Anaemia viruses are diagnosed in a number of ways including Electron microscopy, Insitu hybridization, Virus neutralization, ELISA, Immunofluorescence and Immunoperoxidase tests [28-33]. Majority of the existing methods used for the detection of adenovirus and chicken anaemia viruses are either highly expensive, time consuming or less reliable for the qualitative detection of different viral genotypes prevalent in Pakistan. Due to limitations of the Multiplex approach [34] for the efficient diagnosis of the viral genotypes prevalent in Pakistan, we conducted this study in order to develop an efficient, less time consuming and cost effective duplex PCR assay for the detection of adenovirus and chicken anaemia viruses in poultry birds which is capable to detect the prevalent viral types in Pakistan. Moreover, we also investigated broiler, breeders and Layer birds from various poultry farms of the country for the presence of AAV or CAV infection.
Materials and Methods
Field Samples and Experimental birds
The current study was carried out at GP laboratories Lahore after having approved by the Board of study of the Institute of Biotechnology and Genetic Engineering, Peshawar. The study was conducted in accordance with internationally accepted guidelines. Experimental birds and field samples were provided by various poultry farms from across the country. Chicks were artificially infected with various genotypes of AV and CAV and slaughtered after the signs and symptoms of the disease appeared. Liver, thymus and bone marrow samples were collected from diseased chickens at Grand Parents Laboratory (GP Lab) Lahore, where chickens and other birds are brought for the diagnosis of various diseases from different commercial farms from all over the country. Apparently healthy and effected Broiler, Layer and breeder stocks in various poultry farms across the country were also investigated for the presence of AAV and CAV infections by appearance of the clinical signs and symptoms of the diseases or post mortem examination. The morbidity and mortality rates in the case of AAV and CAV were also recorded. Determination of any co-infection was carried out microbiologically using standard procedures.
DNA extraction
Adenovirus DNA was extracted from liver and Chicken Anemia Virus DNA from thymus and bone marrow samples using QIAampR DNA Mini Kit (Qiagen GmbH, D-40724, Hilden, Germany) according to the manufacturer's instruction. The DNA was then stored at -20°C until used.
Oligonucleotide Primers
Two sets of primers H1 (5'-TGGACATGGGGGCGACCTA-3') and H2 (5'-AAGGGATTGACGTTGTCCA3') for AAV, MK10 (5'-GACTGTAAGATGGCAAGACGAGCTC-3') and MK11 (5'-GGCTGAAGGATCCCTCATTC-3') for CAV [35,34] were synthesized by Invitrogen (Carlsbad, California) which were used to amplify 1219 bp and 675 bp from their respective viral genomes.
PCR amplification
Duplex PCR was conducted in a 25 μl reaction volume, containing 12.5 μl 2 × PCR Master Mix (Fermentas, Canada), 1 μl (10 μM) each of the Primer (H1, H2, MK10 and MK11), 1 μl each template DNA (from both AAV and CAV) and 6.5 μl nuclease free water.
PCR was performed in an automatic DNA thermal cycler (Techne® Endurance TC-312). The cycling protocol consisted of an initial denaturation at 95°C for 5 minutes followed by 35 cycles at 95°C for 45 sec, 55°C for 45 sec and 72°C for 1.5 min. Final extension was carried out at 72°C for 10 min. Positive and Negative controls were included each time the amplification was carried out.
Detection of PCR products
PCR products were detected on 1% agarose gel pre-stained with Ethidium Bromide at 110 V for 20 min. 100 bp ladder (Gibco BRL) was used as a DNA size marker.
Specificity and Sensitivity of the duplex assay
Specificity of the duplex PCR was assessed by examining its ability to amplify only AV and CAV genomes. Each duplex assay performed included two simplex reactions in which AAV primers were used to amplify the CAV DNA while the CAV primers were mixed with AAV DNA in another reaction tube to check if there was any non-specific amplification. The AAV and CAV-specific primers were also used to amplify other viral and bacterial samples available at GP Lab. The sensitivity of the duplex assay was investigated by serially diluting viral DNA (10 ng, 8 ng, 6 ng, 4 ng, 2 ng .04 ng, .03 ng) stock of both AAV and CAV DNA.
Results
In current study we observed avian adenoviral infection in broilers and breeders while no case of avian adenovirus in layers was observed. The mortality due to AAV varied in various broiler farms from 3% to 90%. In contrast we observed no incidence of CAV in breeders while in case of broiler we found CAV in stunted birds having no morbidity and significant mortality. In layers (6-8 weeks) we observed up to 30% mortality. The mortality was enhanced by secondary bacterial infection especially E. coli and Staphylococcus.
Results of the duplex PCR assay in the case of both the experimental birds and field samples indicated that the assay is highly specific for the detection of both AAV and CAV. A total of 300 samples each, taken from the experimental birds, in the case of AAV and CAV, were used for DNA extraction and subsequently subjected to the duplex assay. The specificity of the duplex assay was 100% for the detection of both the viruses (Table 1, 2). Field samples collected from poultry farms across the country were also used for the detection of AAV or CAV using the duplex assay. All the birds samples used for DNA extraction had clinical manifestations of AAV or CAV infections. A total of 100 field samples in the case of AAV revealed that 96 out of 100 were positive for AAV DNA by duplex PCR. Only 4 samples which showed the signs and symptoms typical of AAV infection turned out to be negative by the duplex PCR.
Table 1.
Adenoviral samples tested by duplex PCR
| Source of Samples | No. of samples | PCR positive | Organ used for DNA Isolation |
|---|---|---|---|
| Field samples | 100 | 96 (96%) | Liver |
| Experimental samples | 300 | 300 (100%) | Liver |
| Total | 400 | 396 (96%) | |
Table 2.
CAV (Chicken Anemia Virus) samples tested by duplex PCR
| Source of Samples | No. of samples | PCR positive | Clinical Manifestation/Organ (Used for DNA Isolation) |
|---|---|---|---|
| Field samples | 100 | 95 (95%) | +/bone marrow, thymus, spleen |
| Experimental samples | 300 | 300 (100%) | +/bone marrow, thymus, spleen |
| Total | 400 | 395 (95%) | |
Almost similar results were obtained after the clinically validated organ/Tissue samples were investigated for CAV DNA by the duplex assay. All the 300 experimental samples were positive for CAV DNA by the duplex assay while out of the 100 field samples, 95 turned out to have active CAV infection (Table 2).
Specificity of the duplex PCR was determined by examining its ability to detect and differentiate only AV and CAV. Each time the duplex assay was carried out, two simplex reactions were performed in such a way that AAV primers were used to amplify the CAV DNA and the CAV primers were mixed with AAV DNA in another tube to check if there appeared any amplified product. Apart from this, the primers were used to amplify other viral and bacterial samples available at GP Lab. In all the cases, we found out that the respective AAV and CAV primers were highly specific (100%) by amplifying only their respective genomes.
To know about the sensitivity of both primer sets (H1-H2 and MK10-MK11) and its affinity for their target DNA, PCRs using each primer set were applied to the serially diluted DNAs of avian adenovirus and chicken anemia virus. Results of the duplex assay indicated that, 30 pg of AAV DNA and 40 pg of the CAV DNA was successfully amplified (Figure 1).
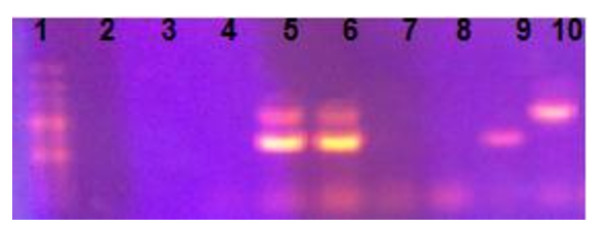
Figure 1.
Gel photograph of duplex PCR. Lane 1 contains 100 bp Plus DNA ladder, Lane 2 contains AAV DNA with CAV primers, Lane 3 contains CAV DNA with AAV primers, Lane 4 is negative field sample, Lanes 5 & 6 are positive field samples for AAV (1219 bp) and CAV (675 bp), Lane 7 & 8 are negative controls, Lane 9 is CAV positive control while Lane 10 is AAV positive control.
Discussion
Avian adenovirus and chicken anemia virus cause heavy economic losses to the poultry industry in Pakistan. The mortality due to avian adenovirus has been reported up to 80% [36] while chicken anemia virus cause fewer deaths but dual infection are however very serious when the chickens are infected with other virus like IBD, Merek's disease, reovirus and adenovirus in addition to chicken anemia virus [37,38].
In this study we observed that Layer stocks at various poultry farms were free of AAV infection while the breeder and broiler stocks were infected showing 3-90% mortality in various poultry farms causing heavy economic losses on the farmers across the country. Investigation of the flocks for CAV infection indicated that the breeder stocks were free of the infection broiler birds were found to be infected. Interestingly, no morbidity was recorded but mortality was significantly high. In young Layers, mortality was recorded to be 30%. We also observed in this study that co-infections of birds with E.Coli and Staphylococci enhanced the rates of mortality among various flocks of birds.
Serological tests for the diagnosis of AAV and CAV like Indirect Immunoflourescent Assay, Enzyme-linked Immunosorbant Assay, Dot ELISA and Double Immuno-diffusion are difficult to be interpreted because antibodies against these viruses are found in both healthy and infected birds. These tests are also highly laborious, time consuming and also having low sensitivity and specificity [39] According to previous data available the sensitivity and specificity of the dot ELISA for the detection of avian adenovirus was 67.1% and 96.9% [40]. Similarly Indirect Immunofluorescence (IIFA) that takes only few hours is also reported to have poor sensitivity and specificity. Antibody-based tests are not informative about active infection [41] and therefore PCR is used to detect nucleic acids of the pathogens in order to confirm the status of infection.
In Pakistan, due to scarcity of technological expertise in modern diagnostics, avian infections are diagnosed either clinically or microbiologically; which very often leads to faulty or untimely diagnosis of deadly infections making the management of birds not only uneconomical but also sometimes threatening for the public health. Due to the economic importance of AAV and CAV for the poultry industry, we attempted in this study to develop a cost-effective duplex assay for the diagnostic set ups in our country and elsewhere. Serial dilutions of the AAV and CAV DNA extracted from the effected organs indicated that as low as 30 pg DNA could be efficiently detected by the duplex assay in the case of AAV while 40 pg of CAV DNA was enough for amplification of the 672 bp product. Specificity of the duplex assay was assessed by using the primers specific for AAV and CAV to amplify other viral and bacterial DNA isolated from tissues of birds at GP Lab. We observed that the primers used did not amplify non-specifically in any of the cases. The sensitivity and specificity of the assay developed is high enough to be used for screening stocks of birds for CAV and AAV infections in Pakistan. The duplex assay could also be used as a confirmatory test for the determination of active AAV or CAV infection in effected birds.
Conclusion
Avian Adeno Virus and Chicken Anemia Virus infections cause high morbidity and morbidity in poultry birds across Pakistan which ultimately transpires in the form of heavy economic losses. Duplex PCR assay developed in this study has 100% specificity and high sensitivity to detect AAV and CAV infections in poultry birds and is a major improvement in poultry diagnostics of Pakistan in terms of its reliability and efficiency to detect viral types. It will not only save time to carry out timely diagnosis of the infections in Pakistan but will also save precious foreign exchange spent on confirmation of diagnosis from other countries.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IA, BA and MAB designed the study and advised about the protocols. LR carried out sampling and experimental procedures. ATJ, AI, SUK, KUZ, MUR and NUK helped with experimental procedures and manuscript preparation. JB and ZAS critically reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Latif U Rehman, Email: latif_ibge@yahoo.com.
Bakht Sultan, Email: bakhtsultan@hotmail.com.
Ijaz Ali, Email: bachakhan35@yahoo.com.
Muhammad A Bhatti, Email: draleem5@hotmail.com.
Sana U Khan, Email: sanaullahkust@gmail.com.
Khaliq U Zaman, Email: khaleeqibge_07@yahoo.com.
Anila T Jahangiri, Email: anila.jahangiri@yahoo.com.
Najib U Khan, Email: naji_banni@yahoo.com.
Aqib Iqbal, Email: Aqib_72@yahoo.com.
Jehan Bakht, Email: jehanbakht@yahoo.co.uk.
Zahoor A Swati, Email: drzaswati@yahoo.com.
Muti U Rehman, Email: mutirehman@yahoo.com.
Acknowledgements
The corresponding author highly acknowledges the facilities and funding provided by GP Laboratories Lahore, Pakistan.
References
- Kawamura H, Shimizu E, Tsuba-hara H. Avian adenovirus: its properties and serological classification. Natl Inst Anim Health Q (Tokyo) 1964;4:183–193. [PubMed] [Google Scholar]
- Mcferran JB, Adair B, Connor TJ. Adenoviral antigens (CELO, QBV, GAL) Am J Vet Res. 1975;36:527–529. [PubMed] [Google Scholar]
- Zsak L, Kisary J. Characterization of adeno-virus isolated from geese. Avian Pathol. 1984;13:253–264. doi: 10.1080/03079458408418529. [DOI] [PubMed] [Google Scholar]
- Hess M, Raue R, Prusas C. Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol. 1999;28:433–439. doi: 10.1080/03079459994443. [DOI] [PubMed] [Google Scholar]
- Dutta SK, Pomeroy BS. Electron microscopic studies of quail bronchitis virus. Am J Vet Res. 1967;28:296–299. [Google Scholar]
- Guy JS, Barnes HJ. Characterization of an avian adenovirus associated with inclusion body hepatitis in day-old turkeys. Avian Dis. 1997;41:726–731. doi: 10.2307/1592167. [DOI] [PubMed] [Google Scholar]
- Domermuth CH, Weston CR, Cowen BS, Colwell WM, Gross WB, DuBose WB. Incidence and distribution of avian adeno-virus group II splenomegaly of chickens. Avian Dis. 1980;24:591–594. doi: 10.2307/1589794. [DOI] [PubMed] [Google Scholar]
- Mcferran JB, Connor TJ, Adair BM. Studies on the antigenic relationship between an isolate (127) from the egg drop syndrome 1976 and a fowl adenovirus. Avian Patho. 1978;7:629–636. doi: 10.1080/03079457808418315. [DOI] [PubMed] [Google Scholar]
- Van den EMP, Don PA, Kipps A. The isolation in eggs of a new filterable agent which may be the cause of bovine lumpy skin disease. J Gen Microbiol. 1949;3:174–182. doi: 10.1099/00221287-3-2-174. [DOI] [PubMed] [Google Scholar]
- Yates VJ, Fry DE. Observations on a chicken embryo lethal orphan (CELO) virus. Am J Vet Res. 1957;18:657–660. [PubMed] [Google Scholar]
- Burmester BR, Sharpless GR, Fontes AK. Virus isolated from avian lymphomas unrelated to lymphomatosis virus. J Natl Cancer Inst. 1960;24:1443–1447. [PubMed] [Google Scholar]
- Olson NO. A respiratory disease (bronchitis) of quail caused by a virus. Proc 54th Annu Meet US Livestock Sanit Assoc. 1950. pp. 171–174.
- Rabbani M, Naeem K. Proceeding of International Symposium on Adenovirus and Reovirus Infections in Poultry. Rauischholzhausen, Germany; 1996. In vitro and in vivo evaluation of avian adenovirus isolates from out-breaks of hydro pericardium syndrome; pp. 26–31. [Google Scholar]
- Silk R, Suresh M, Neumann U, Sharma JM. Proc International Symposiumo on Adeno-virus and Reovirus Infections in Poultry. Rauischholzhausen, Germany; 1996. Pathogenic mechanisms of avian adenovirus type II; pp. 41–50. [Google Scholar]
- Van EJHH, Davelaar EG, Van den Heuvel-plesman TAM, Van KN, Kouwenhoven B, Guldie EHM. Dropped egg production, soft shelled and shell-less eggs associated with appearance of precipitins to adenovirus in flocks of laying fowl. Avian Pathol. 1976;5:261–272. doi: 10.1080/03079457608418195. [DOI] [PubMed] [Google Scholar]
- Yuasa N, Taniguchi T, Yoshida I. Isolation and some properties of an agent inducing anemia in chicks. Avian Diseases. 1979;23:366–85. doi: 10.2307/1589567. [DOI] [Google Scholar]
- Gelderblom H, Kling S, Lurz R, Tischer I, Bülow von V. Morphological characterization of chicken anemia agent (CAA) Archives of Virology. 1989;109:115–20. doi: 10.1007/BF01310522. [DOI] [PubMed] [Google Scholar]
- Pringle CR. Virus taxonomy at the XIth International Congress of Virology, Sydney, Australia. Arch Virol. 1999;144:2065–2069. doi: 10.1007/s007050050728. [DOI] [PubMed] [Google Scholar]
- Todd D, Creelan JL, Mackie DP, Rixon F, McNulty MS. Purification and biochemical characterization of chicken anemia agent. Journal of General Virology. 1990;71:819–23. doi: 10.1099/0022-1317-71-4-819. [DOI] [PubMed] [Google Scholar]
- Todd D, Mackie DP, Mawhinney KA, Conner TJ, McNeilly F, McNulty MS. Development of an enzyme-linked immunosorbent assay to detect serum antibody to chicken anemia agent. Avian Diseases. 1990;34:359–63. doi: 10.2307/1591419. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Yuasa N, Maeda M, Horiuchi T. Haematopathological changes in dead and transmoribund chicks induced by chicken anemia agent. National Institute of Animal Health Quarterly, Japan. 1982;22:61–9. [PubMed] [Google Scholar]
- Taniguchi T, Yuasa N, Maeda M, Horiuchi T. Chronological observations on haematopathological lesions in chicks inoculated with chicken anemia agent. National Institute of Animal Health Quarterly, Japan. 1983;23:12. [PubMed] [Google Scholar]
- Bulow V, von Fuchs B, Bertram M. Untersuchungen uber den Erreger der infektiosen Anamie bei Hu"hnerku"ken (CAA) in vitro: Vermehrung, Titration, Serumneutralisationstest und indirekter Immunofluoreszenztest. Journal of Veterinary Medicine. 1985;32:679–93. [PubMed] [Google Scholar]
- Yuasa N, Imai K, Watanabe K, Saito F, Abe M, Komi K. Aetiological examination of an outbreak of haemorrhagic syndrome in a broiler flock in Japan. Avian Pathology. 1987;16:521–6. doi: 10.1080/03079458708436401. [DOI] [PubMed] [Google Scholar]
- Noteborn MHM, Koch G. Chicken anemia virus infection: molecular basis of pathogenicity. Avian Pathology. 1995;24:11–31. doi: 10.1080/03079459508419046. [DOI] [PubMed] [Google Scholar]
- Smyth JA, Moffett DA, McNulty MS, Todd D, Mackie DP. A sequantial histopathologic and immunocytochemical study of chicken anemia virus infection at one day of age. Avian Dis. 1993;37:324–338. doi: 10.2307/1591656. [DOI] [PubMed] [Google Scholar]
- Jaffery MS. A treatise on Angara disease (hydropericardium pulmonary oedema - hepatonephritis syndrome) Pak Vet Med Assoc. 1988. pp. 1–33.
- McFerran JB, Adair BM. Avian adenoviruses--A review. Avian Pathol. 1977;6:189–217. doi: 10.1080/03079457708418228. [DOI] [PubMed] [Google Scholar]
- Hess M. Detection and differentiation of avian adenoviruses: a review. Avian Pathol. 2000;29:195–206. doi: 10.1080/03079450050045440. [DOI] [PubMed] [Google Scholar]
- Bulow V, Von Fuchs B, Bertram M, Von-Bulow V. Investigations on CIA in vitro: multiplication, titration, SNT and IIFT. Zentralblattfur Veterinamedizin B. 1985;32(6):679–693. [Google Scholar]
- McNulty MS, Connor TJ, McNeilly F, Kirkpatrick KS, McFerran JB. A serological survey of domestic poultry in the United Kingdom for antibody to chicken anaemia agent. AvianPathol. 1988;17:315–324. doi: 10.1080/03079458808436450. [DOI] [PubMed] [Google Scholar]
- Brewer J, Saunders JM, Chettle NJ. In: Proceedings Int Symp On IBD & CIA: Kaleta EF, editor. Rauischholzhausen, Germany; 1994. The development of an enzyme linked immunosorbent assay to detect antibodies to chicken anaemia virus and its comparison with the indirect immunofluroesence antibody test; pp. 408–412. [Google Scholar]
- Nishan SS, Anjan D. Diagnosis and Molecular Characterization of Chicken Anaemia Virus. Veterinary World. 2009;2(4):156–160. [Google Scholar]
- Caterina KM, Frasca S Jr, Girshick T, Khan MI. Development of a multiplex PCR for detection of avian adenovirus, avian reovirus, infectious bursal disease virus, and chicken anemia virus. Mol Cell Probes. 2004;18(5):293–8. doi: 10.1016/j.mcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Raue R, Hess M. Hexon based PCRs combined with restriction enzyme analysis for rapid detection and differentiation of fowl adenoviruses and egg drop syndrome virus. J Virol Methods. 1998;73:211–217. doi: 10.1016/S0166-0934(98)00065-2. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chandra R, Shukla SK. Isolation of etiological agent of hydropericardium syndrome in chicken embryo liver cell culture and its serological characterization. Indian J Biol. 2003;41:821–826. [PubMed] [Google Scholar]
- Engstrom BE. Blue wing disease of chickens: isolation of avian reovirus and chicken anaemia agent. Avian Pathol. 1988;17:23–32. doi: 10.1080/03079458808436425. [DOI] [PubMed] [Google Scholar]
- Rosenberger JK, Cloud SS. The effects of age, route of exposure, and coinfection with infectious bursal disease virus on the pathogenicity and transmissibility of chicken anemia agent (CAA) Avian Dis. 1989;33:753–759. doi: 10.2307/1591156. [DOI] [PubMed] [Google Scholar]
- Shamim S, Rehmani SF, Siddiqi AA, Qureshi MA, Khan TA. Characterization of avian adenovirus type-4 causing hydro-pericardium syndrome in broilers in Karachi, Pakistan. Iranian Journal of Veterinary Research, Shiraz University. 2009;1:38–43. [Google Scholar]
- Subramanyam KV, Purushothaman V, MuraliManohar B, Ravikumar G, Manoharan S. Development of Dot - Enzyme Linked Immunosorbent Assay for Detection of Antibodies to Infectious Bursal Disease, Hydropericardium Syndrome and Chicken Anaemia Viruses. EJBS. 2010;4(1):8–18. http://www.ejarr.com/Volumes/Vol4/EJBS_4_02.pdf [Google Scholar]
- Najib UK, Ijaz A, Naeem UA, Aqib I, Latif UR, Iqbal M, Muti UR, Sanaullah K, Sajid A, Lubna S, Zahoor AS. Prevalence of active HCV infection among the blood donors of Khyber Pakhtunkwa and FATA region of Pakistan and evaluation of the screening tests for anti-HCV. Virol J. 2011;8:154. doi: 10.1186/1743-422X-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]