Abstract
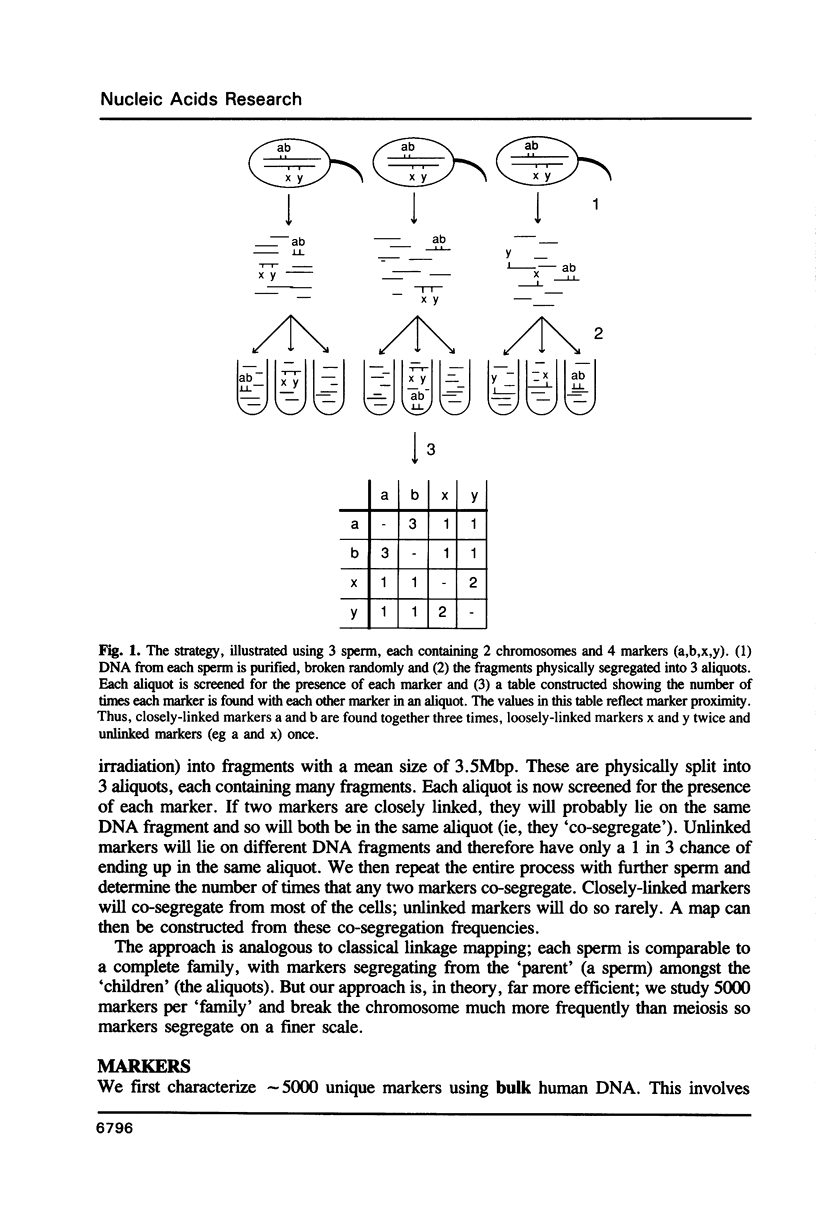
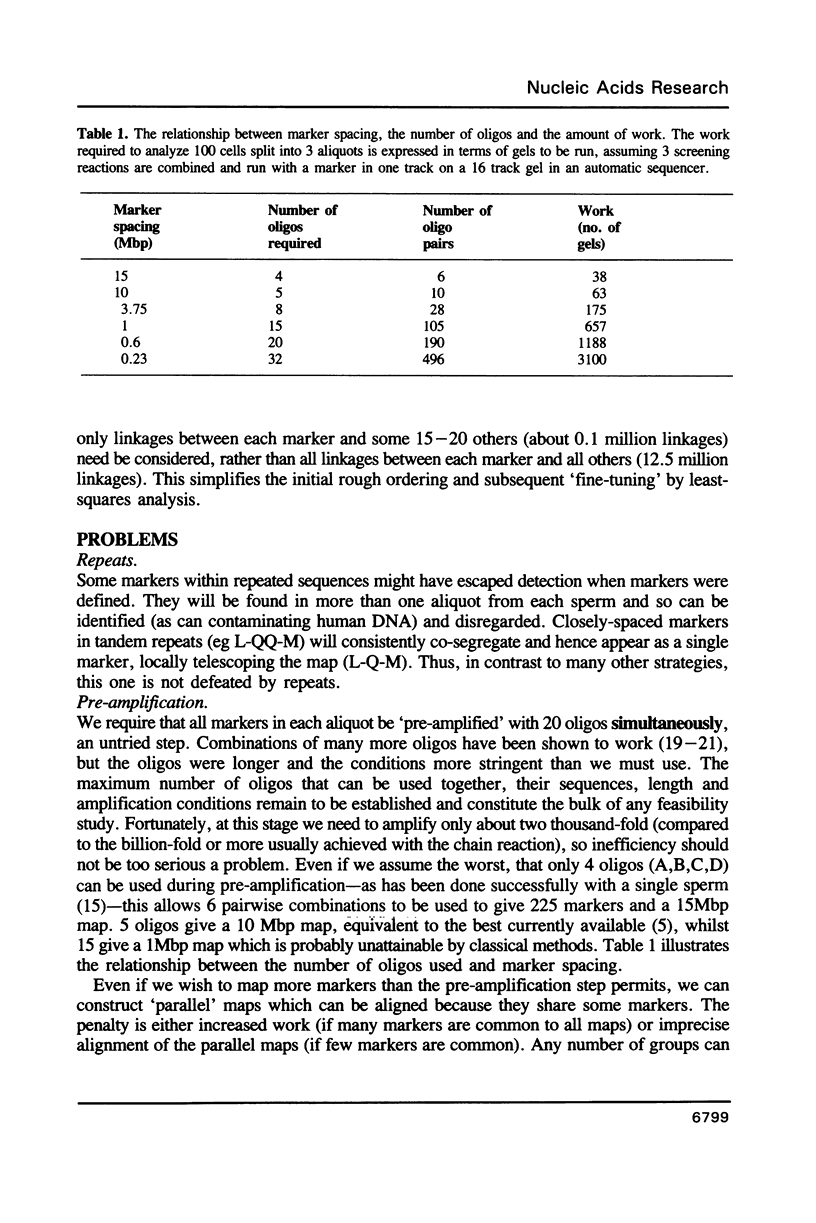
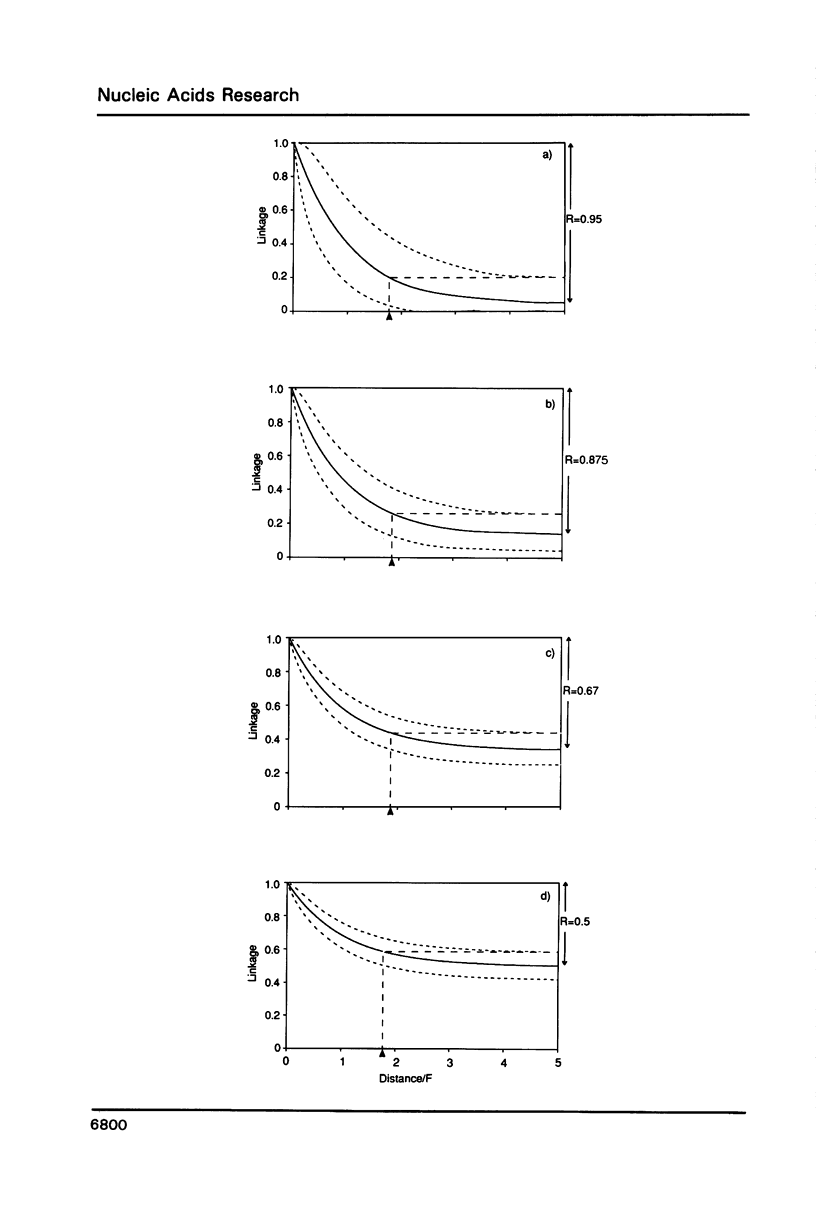
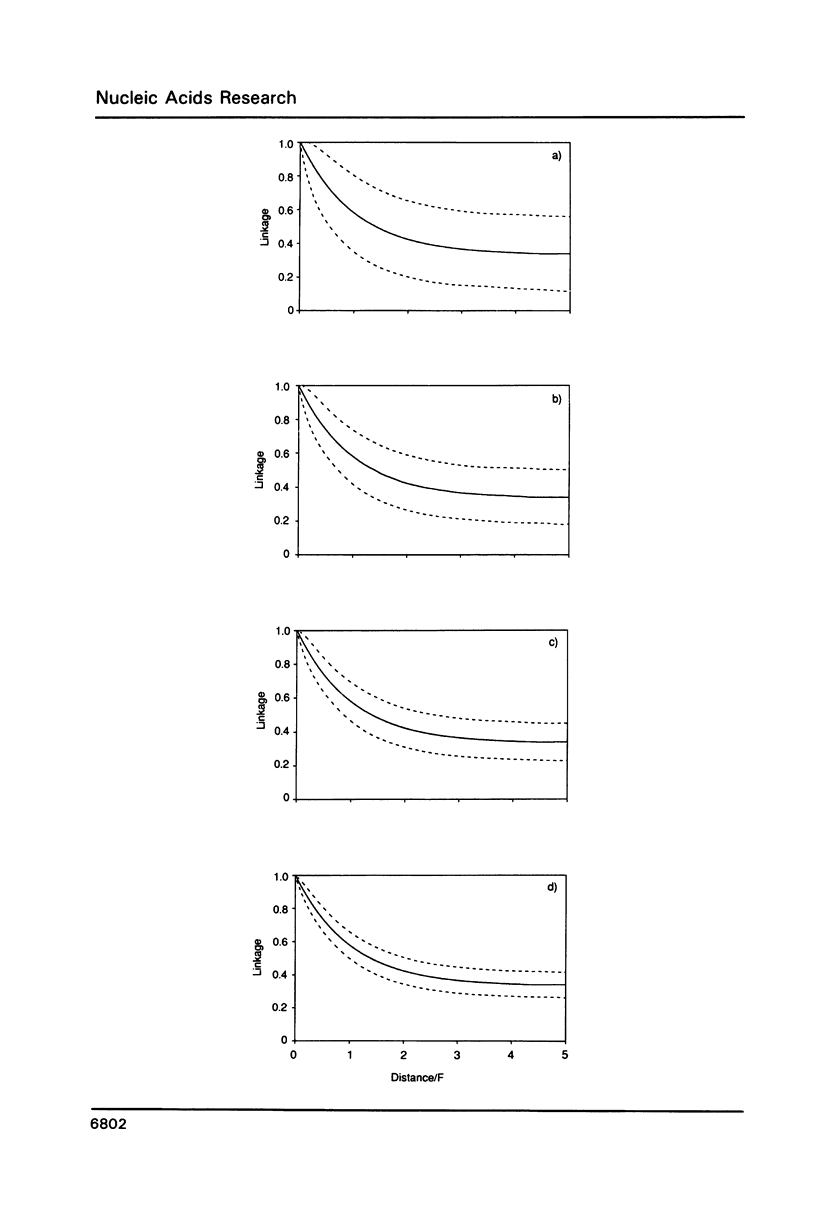
A theoretical approach for linkage mapping the genome of any higher eukaryote is described. It uses the polymerase chain reaction, oligonucleotides of random sequence and single haploid cells. Markers are defined and then the DNA of a single sperm is broken at random (eg by gamma-rays) and physically split into 3 aliquots. Each aliquot is screened for the presence of each marker. Closely-linked markers are more likely to be found in the same aliquot than unlinked markers. The entire process is repeated with further sperm and the frequency that any two markers co-segregate determined. Closely-linked markers co-segregate from most cells; unlinked markers do so rarely. A map can then be constructed from these co-segregation frequencies. A specific application for determining the order and distance between sets of closely-linked and previously-defined markers is also described.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., Bird A. P. Long-range restriction site mapping of mammalian genomic DNA. 1986 Jul 31-Aug 6Nature. 322(6078):477–481. doi: 10.1038/322477a0. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A. V., Lamerdin J., Ashworth L. K., Watkins B., Branscomb E., Slezak T., Raff M., de Jong P. J., Keith D., McBride L. A high-resolution, fluorescence-based, semiautomated method for DNA fingerprinting. Genomics. 1989 Feb;4(2):129–136. doi: 10.1016/0888-7543(89)90291-7. [DOI] [PubMed] [Google Scholar]
- Cook P. R. A general method for preparing intact nuclear DNA. EMBO J. 1984 Aug;3(8):1837–1842. doi: 10.1002/j.1460-2075.1984.tb02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Smith B. A., Cooke H. J., Niemann S., Schmidtke J. An estimate of unique DNA sequence heterozygosity in the human genome. Hum Genet. 1985;69(3):201–205. doi: 10.1007/BF00293024. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- De B. K., Srinivasan A. Multiple primer pairs for the detection of HTLV-I by PCR. Nucleic Acids Res. 1989 Mar 11;17(5):2142–2142. doi: 10.1093/nar/17.5.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Drmanac R., Petrović N., Glisin V., Crkvenjakov R. A calculation of fragment lengths obtainable from human DNA with 78 restriction enzymes: an aid for cloning and mapping. Nucleic Acids Res. 1986 Jun 11;14(11):4691–4692. doi: 10.1093/nar/14.11.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. G. Causes of early embryonic loss in human pregnancy. Hum Reprod. 1986 Apr;1(3):185–198. doi: 10.1093/oxfordjournals.humrep.a136378. [DOI] [PubMed] [Google Scholar]
- Girgis S. I., Alevizaki M., Denny P., Ferrier G. J., Legon S. Generation of DNA probes for peptides with highly degenerate codons using mixed primer PCR. Nucleic Acids Res. 1988 Nov 11;16(21):10371–10371. doi: 10.1093/nar/16.21.10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U., Kaiser R., Caskey C. T., Hood L. DNA diagnostics--molecular techniques and automation. Science. 1988 Oct 14;242(4876):229–237. doi: 10.1126/science.3051381. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Lin C. C., Balkan W., Burns K. Direct chromosomal analysis of human spermatozoa: preliminary results from 18 normal men. Am J Hum Genet. 1982 May;34(3):459–468. [PMC free article] [PubMed] [Google Scholar]
- Olson M. V., Dutchik J. E., Graham M. Y., Brodeur G. M., Helms C., Frank M., MacCollin M., Scheinman R., Frank T. Random-clone strategy for genomic restriction mapping in yeast. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7826–7830. doi: 10.1073/pnas.83.20.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustka A., Pohl T., Barlow D. P., Zehetner G., Craig A., Michiels F., Ehrich E., Frischauf A. M., Lehrach H. Molecular approaches to mammalian genetics. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):131–139. doi: 10.1101/sqb.1986.051.01.016. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- White R., Lalouel J. M. Sets of linked genetic markers for human chromosomes. Annu Rev Genet. 1988;22:259–279. doi: 10.1146/annurev.ge.22.120188.001355. [DOI] [PubMed] [Google Scholar]
- Wyman A. R., Wolfe L. B., Botstein D. Propagation of some human DNA sequences in bacteriophage lambda vectors requires mutant Escherichia coli hosts. Proc Natl Acad Sci U S A. 1985 May;82(9):2880–2884. doi: 10.1073/pnas.82.9.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]


