Abstract
The tellurium oxyanion tellurite induces oxidative stress in most microorganisms. In Escherichia coli, tellurite exposure results in high levels of oxidized proteins and membrane lipid peroxides, inactivation of oxidation-sensitive enzymes and reduced glutathione content. In this work, we show that tellurite-exposed E. coli exhibits transcriptional activation of the zwf gene, encoding glucose 6-phosphate dehydrogenase (G6PDH), which in turn results in augmented synthesis of reduced nicotinamide adenine dinucleotide phosphate (NADPH). Increased zwf transcription under tellurite stress results mainly from reactive oxygen species (ROS) generation and not from a depletion of cellular glutathione. In addition, the observed increase of G6PDH activity was paralleled by accumulation of glucose-6-phosphate (G6P), suggesting a metabolic flux shift toward the pentose phosphate shunt. Upon zwf overexpression, bacterial cells also show increased levels of antioxidant molecules (NADPH, GSH), better-protected oxidation-sensitive enzymes and decreased amounts of oxidized proteins and membrane lipids. These results suggest that by increasing NADPH content, G6PDH plays an important role in E. coli survival under tellurite stress.
Introduction
The tellurium oxyanion, tellurite (TeO3 2−), is especially harmful to prokaryotic cells mainly because of the generation of reactive oxygen species (ROS) [1]–[5]. In particular, tellurite-exposed Escherichia coli exhibits oxidative stress-sensitive [Fe-S] cluster-containing enzyme inactivation, increased protein carbonylation and lipid membrane oxidation, as well as activation of superoxide-responsive genes [3], [6]. In addition, tellurite causes thiol depletion, especially glutathione (GSH), that in turn causes oxidative stress [7], [8].
In response to superoxide-mediated stress, E. coli triggers a coordinated expression of a number of genes (soxRS regulon) whose biological role includes three different response levels: (i) prevention of oxidative damage, (ii) xenobiotic removal and recycling of damaged macromolecules and (iii) nicotinamide adenine dinucleotide phosphate (NADPH) regeneration [9]–[12]. In this context, intracellular NADPH levels are critical for maintaining a balanced redox status and therefore for survival [13].
Previous work from our laboratory has shown that NADPH metabolism is affected in cells exposed to the toxicant potassium tellurite. We observed that tellurite (Te4+) can be enzymatically reduced to elemental tellurium (Te0) by different microorganisms in a NAD(P)H-dependent manner [14]–[16]. Preliminary experiments have also indicated that the antioxidant response caused by tellurite-activated soxRS regulon might influence NADPH synthesis [3]. NADPH levels can be also affected because of non-enzymatic tellurite reduction by GSH or other intracellular reducing agents [7], [8].
Using a collection of mutants impaired in NADPH synthesis, we found that cells lacking glucose-6-phosphate dehydrogenase (G6PDH) were the most sensitive to tellurite. To a lesser extent, cells deficient in genes encoding isocitrate dehydrogenase (ICDH) or glutamate dehydrogenase (GDH) were also sensitive to the toxicant. Tellurite-exposed E. coli exhibited increased zwf expression which was paralleled by augmented G6PDH (protein amount and activity) and NADPH synthesis. Thus, upon zwf overexpression bacteria seems to be better protected against tellurite-induced stress.
Results and Discussion
Tellurite exposure results in augmented NADPH synthesis
Little is known about the E. coli antioxidant response when grown in the presence of tellurite. Previous reports by our group and others have shown increased superoxide dismutase activity in tellurite-exposed cells [3], [5]. In this context and since the metabolism of dinucleotides results altered in response to the oxidative stress-generating compounds gallium and menadione [17], [18] both NADP(H) and NAD(H) concentrations were determined to analyze whether tellurite exposure results in similar effects. Table 1 shows that while NADPH levels increased ∼30% NADH content was halved in tellurite-exposed wild type E. coli. Although decreased NADH concentrations may be counterproductive for energy generation [18], it may also represent a response to reduce the overall oxidative status of the cell. In fact, several NAD+-dependent enzymes such as α-ketoglutarate- and pyruvate-dehydrogenase complexes from prokaryotic or eukaryotic origins are selectively inhibited upon tellurite exposure ([16], [19], [20] Vásquez unpublished results). Surprisingly, the amount of oxidized dinucleotides (NAD+, NADP+) was not modified in the presence of the toxicant, a result that may be explained by NAD+ kinase activation in response to an oxidative stress-induced temporal NADPH depletion [18], [21], [22]. As expected, similar results were observed upon cell exposure to the superoxide-generating drug menadione (Table 1).
Table 1. Tellurite exposure results in augmented NADPH levels in E. coli.
| Treatment | |||
| Cofactor | Control | K2TeO3 (2 µM) | Menadione (100 µM) |
| NADPHa | 119.1±1.4 | 151.0±6.6* | 155.2±9.1* |
| NADP+ | 46.9±7.8 | 47.7±5.0 | 44.4±6.9 |
| NADH | 53.0±3.1 | 27.0±4.9* | 22.3±2.1* |
| NAD+ | 48.4±0.4 | 43.9±6.4 | 55.0±2.3 |
NADP(H) (nmol mg prot−1) and NAD(H) (mmol mg prot−1) concentration in E. coli BW25113 extracts was determined spectrophotometrically at 340 nm as described in Methods. Values are the mean of 3 independent trials ± SD.
*P≤0.05 as compared to controls.
Aiming to identify genes whose products could participate in such a response, several strains lacking enzymes involved in NADPH synthesis were tested for tellurite sensitivity. While cells deficient in gnd, maeB, pntA, pntB or udhA genes were not affected by the toxicant, cells lacking ICDH (ΔicdA) or GDH (ΔgdhA) were ∼40–60% more sensitive when compared to the wild type strain. Interestingly, cells devoid of G6PDH activity (Δzwf) exhibited ∼2-fold more sensitivity to tellurite, H2O2 and diamide (Fig. S1). In addition, ICDH, GDH and G6PDH activity increased ∼30, 50 and 60% when wild type cells were exposed to tellurite (Table 2), suggesting that these activities -mainly G6PDH- are most probably involved in increasing NADPH levels to face tellurite stress. This last assumption was confirmed by determining ICDH and GDH activity in extracts from tellurite-exposed Δzwf cells: while ICDH activity decreased by ∼25% regarding the untreated control, GDH activity results almost undetectable (Table 2). In the absence of tellurite, the Δzwf strain showed decreased (∼20%) NADPH levels regarding the wild type control and tellurite exposure did not modify them significantly (not shown).
Table 2. Tellurite induces NADPH-dependent enzymatic activities in E. coli.
| BW25113 | Δzwf | |||
| Enzyme | Control | K2TeO3 | Control | K2TeO3 |
| ICDH | 0.095±0.01 | 0.121±0.01* | 0.011±0.01 | 0.084±0.04* |
| GDH | 7.7±0.7 | 11.8±1.3* | 0.3±0.07 | 0.6±0.1* |
| G6PDH | 7.0±1.4 | 11.3±1.7* | ND | ND |
Enzymatic activity (µmol NADPH min−1 mg prot−1) was determined spectrophotometrically at 340 nm as described in Methods. Values are the mean of 4 independent trials ± SD.
*P≤0.05 as compared to controls. ND, not detected.
ROS generation and not thiol depletion is the primary signal for tellurite-induced zwf expression
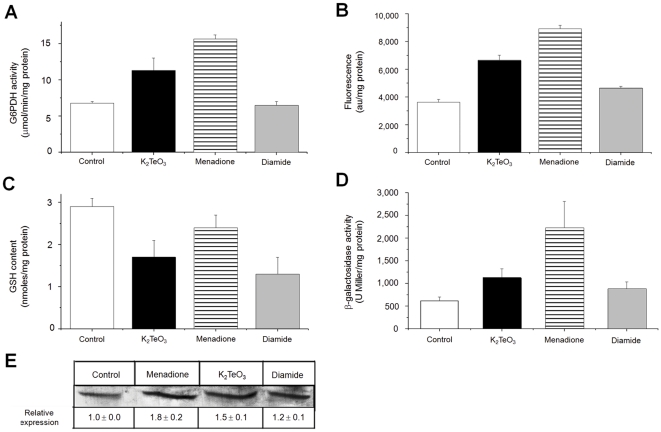
To determine if tellurite-mediated ROS generation [3] or thiol depletion [7], [8] is responsible of G6PDH activation, the effect of the superoxide-generating compound menadione [23] or the thiol-specific reagent diamide [24] was determined. Clearly G6PDH activity was induced by tellurite and menadione but not by diamide (Fig. 1A); this was true also for ROS production (Fig. 1B). In spite that tellurite-exposed cells exhibited a decreased GSH content (∼50%), this effect does not seem to be the primary signal inducing G6PDH activity (Figs. 1C). To assess if the observed tellurite-mediated increase of G6PDH activity was related to zwf induction, β-galactosidase activity was determined in the E. coli reporter strain zwf::lacZ. Fig. 1D shows that β-galactosidase activity increased 2- and 4-fold in extracts from tellurite- or menadione-exposed cells, respectively, when compared to untreated controls. No augment of β-galactosidase activity was observed in diamide-exposed cells (Fig. 1D). Increased G6PDH activity was paralleled by an augmented G6PDH protein, as shown by Western blotting (Fig. 1E). These results are in agreement with the soxRS regulon induction occurring at the onset of oxidative stress [25]. No induction of G6PDH activity was observed in tellurite-exposed ΔsoxRS E. coli (not shown), again indicating that the underlying signal for G6PDH induction is related to tellurite-induced ROS generation.
Figure 1. Tellurite induces G6PDH because of ROS formation and not thiol depletion in E. coli.
(A) G6PDH activity was determined spectrophotometrically at 340 nm as described [49]. (B) Intracellular ROS levels were analyzed using the oxidation-sensitive probe H2DCFDA (2′,7′-dichlorofluorescein diacetate) using an Applied Biosystems equipment CytoFluor 4000 Fluorescence Multi-well Plate Reader (excitation 490 nm, emission 519 nm) and normalized to protein concentration. (C) GSH content was assessed as described previously [56] with modifications. (D) β-galactosidase activity was determined in extracts of the reporter E. coli GC4468 zwf::lacZ strain [42] by monitoring the hydrolysis of o-nitrophenyl-β-D-galactopyranoside as described [43]. (E) Western blotting of G6PDH was analyzed using a specific in-house made antiserum. Band intensities were analyzed using the Gel-Pro Analyzer Program software, version 3.1. Relative expression was referred to that of control cells. The strain used in A–C and E was E. coli BW25113. E. coli cells were left untreated (control, white) or treated with 2 µM tellurite (black), 100 µM menadione (horizontal stripes) or 500 µM diamide (grey) for 30 min. Values are the mean ± SD of 3–4 independent trials. au, arbitrary units.
Tellurite treatment induces G6P accumulation in E. coli
Several lines of evidence suggest that the metabolic adaptation model of Singh et al. [18] is associated with flux changes in central metabolic pathways. In this context, cell exposure to oxidants results in altered NADH/NADPH content [18], soxRS-mediated zwf activation [25] and a shifting of glucose catabolic flux from glycolysis to the pentose phosphate pathway (PPP) [26]–[28]. To determine if a similar event could explain the observed tellurite-mediated increase of NADPH levels (Table 1), the intracellular concentration of glucose-6-phosphate (G6P) was assessed. The G6P content increased ∼50% in tellurite-exposed cells as compared to untreated controls (Table 3). As expected, the activity of the G6P suppliers PtsG (glucose-specific transporter of the phosphotransferase system) and Pgi (phosphoglucose isomerase), involved in a soxRS-controlled antioxidant mechanism [28], increased ∼2–3 fold under tellurite stress (Table 3). Preliminary results from our laboratory also indicate that while augmented pgi transcription occurs upon tellurite exposure, the activity of the enzymatic regulators phosphofructokinase and pyruvate kinase is significantly decreased, suggesting that the glycolytic pathway is down-regulated in these conditions (Vásquez, unpublished data).
Table 3. Tellurite exposure induces G6P accumulation in E. coli.
| Treatment | |||
| Control | K2TeO3 | Menadione | |
| G6Pa | 6.1±0.5 | 9.2±2.3* | 5.4±0.2 |
| PtsGb | 11.9±3.3 | 29.6±7.9* | 28.1±9.1* |
| Pgib | 0.26±0.1 | 0.56±0.1* | 0.46±0.1* |
Intracellular G6P concentration (nmol mg prot−1);
Enzymatic activity (µmol NADPH min−1 mg prot−1) was determined spectrophotometrically at 340 nm as described in Methods. Values are the mean of 3 independent trials ± SD.
*P≤0.05 as compared to controls.
zwf expression is involved in the E. coli response to tellurite stress
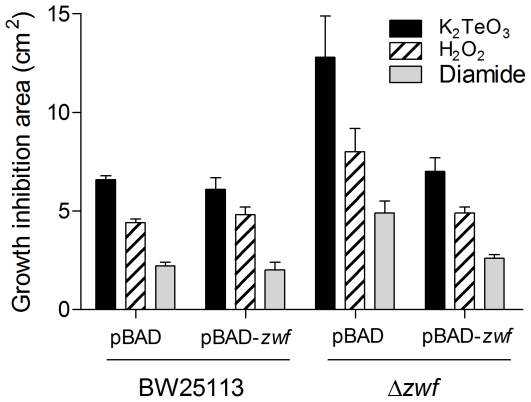
To further unveil the role of G6PDH in the cellular response to tellurite, the effect of overexpressing or eliminating the E. coli zwf gene was carried out (see Table S1 for strain genotypes). Curiously, cells overexpressing zwf did not show increased tolerance to tellurite and resistance levels similar to those exhibited by wild type controls were observed in genetically-complemented strains (Fig. 2). Similar results were obtained when hydrogen peroxide or diamide were used. Since the absence of zwf results in increased sensitivity to tellurite and other stress-generating compounds (Figs. 2 and S1), it was expected that inducing zwf expression would reverse this effect, which was the case in zwf-complemented cells (Fig. 2). Overexpressing zwf did not generate increased resistance to these toxicants in either LB (Fig. 2) or M9-minimal medium (not shown). Similar results have been observed in Salmonella enterica serovar Typhimurium and E. coli exposed to H2O2, S-nitroso-glutathione or paraquat [29], [30].
Figure 2. Effect of zwf expression on the E. coli sensitivity to oxidative stress elicitors.
Growth inhibition zones were determined for wild type (BW25113 pBAD), zwf-overexpressing (pBAD-zwf), mutant (BW25113 Δzwf::kan) and genetically complemented (BW25113 Δzwf::kan pBAD-zwf) strains as described [41]. Briefly, cells were grown to OD600∼0.5, diluted and spread on LB plates. After air drying, ten microliters of tellurite (40 mM), H2O2 (10 M) or diamide (100 mM) were deposited on sterile disks in the centers of the plates. Results were determined after 24 h. Values are the mean of 4–5 independent trials ± SD.
In agreement with results of Table 1, tellurite-treated E. coli carrying pBAD vector alone also showed increased (∼30%) NADPH synthesis regarding the respective controls (Table 4). In pBAD-carrying Δzwf cells NADPH levels decreased ∼20% while those of NADP+ increased ∼50%, an effect that was unchanged in the presence of the toxicants (Table 4). In turn, upon zwf overexpression NADPH levels increased ∼30% in the absence of toxicants while genetically-complemented Δzwf cells exhibited dinucleotide levels similar to those of the wild type strain (Table 4). These results suggest that the protective effect of G6PDH activity (or its product NADPH) occurs during the soxRS-mediated response in cells facing stress [22] and that any further increase of activity is not reflected in higher tellurite resistance. Restitution of the resistance phenotype in the complemented strain (Fig. 2 and Table 4), therefore seems to result from increased NADPH levels.
Table 4. Effect of zwf expression and toxicant exposure on NADPH concentration.
| Strain | Plasmid | Treatment | NADP+a | NADPHa | [NADP++NADPH] |
| BW25113 | pBAD | Control | 46.9±7.8 | 119.1±7.7 | 166.0 |
| K2TeO3 | 47.7±5.0 | 157.0±6.6* | 204.8 | ||
| Menadione | 44.4±6.9 | 155.2±9.1* | 199.6 | ||
| BW25113 | pBAD-zwf | Control | 35.0±3.5 | 156.8±13.5 | 191.8 |
| K2TeO3 | 47.1±1.9* | 218.3±6.6* | 265.4 | ||
| Menadione | 42.6±4.6 | 229.6±10.0* | 272.2 | ||
| Δzwf | pBAD | Control | 68.7±3.2 | 100.4±2.8 | 169.1 |
| K2TeO3 | 70.2±8.2 | 124.4±10.0 | 194.6 | ||
| Menadione | 73.8±6.8 | 98.1±8.7 | 172.4 | ||
| Δzwf | pBAD-zwf | Control | 45.5±4.1 | 138.8±3.0 | 184.3 |
| K2TeO3 | 57.9±3.1 | 156.0±8.5* | 214.1 | ||
| Menadione | 46.8±8.3 | 141.9±4.0 | 188.7 |
nmol/mg protein. Values are the mean of 3 independent trials ± SD.
, P≤0.05 as compared to control.
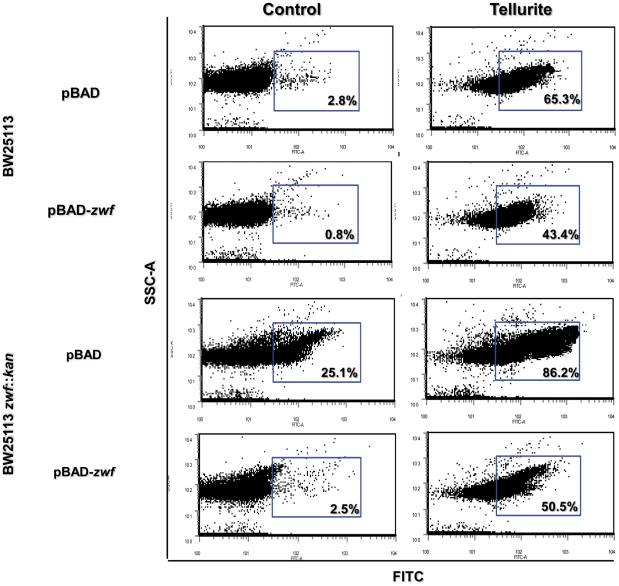
Next, the effect of zwf expression on several markers of tellurite-triggered oxidative stress was investigated. All tested strains showed increased ROS content in the presence of tellurite as compared to controls (Fig. 3). While Δzwf cells showed a significant ROS increase (∼20%) even in the absence of the toxicant, the genetically-complemented strain showed ROS levels comparable to unexposed wild type controls. Also, zwf overexpression reduced tellurite-induced ROS content by ∼20–30% in BW25113 pBAD-zwf and Δzwf pBAD-zwf cells. These results suggest a direct relationship between zwf expression (resulting in NADPH generation) and intracellular ROS content (Fig. 3). A putative explanation for these findings may lay in NADPH acting -apart from its role in maintaining the cellular redox state- as scavenger of various radical species [31], [32].
Figure 3. Effect of zwf expression on ROS content.
ROS content was assessed in the indicated strains by flow cytometry using the oxidation-sensitive probe H2DCFDA. Expression of zwf was induced in the presence of L-arabinose (0.2%). Cells were incubated for 30 min in the absence or in the presence of 2 µM tellurite, washed and incubated with 2 mM H2DCFDA for 30 min in the dark, washed again and diluted 1∶10 with PBS buffer. Fluorescence intensity was determined using a Becton Dickinson model FacsCanto II equipment equipped with an argon laser (excitation 490 nm, emission 519 nm) [45]. The per cent of cell population that was positive for fluorescence is indicated (blue rectangles). A representative dot plot of 3 independent trials is shown. FITC, fluorescence intensity; SSC-A, cell complexity.
Since tellurite-mediated ROS generation causes oxidative damage in several macromolecules (protein carbonylation, membrane lipid peroxidation) [3], we tested whether zwf expression had a potential effect on these oxidation markers (Fig. S2). In the presence of the toxicant, both classes of damage were induced in Δzwf cells, probably as consequence of diminished antioxidant ability (lower NADPH content) or the high basal ROS content displayed by these cells (Table 4 and Fig. 3). In zwf-expressing mutants, carbonylated proteins and lipid peroxides levels were restored to those observed in wild type controls (Fig. S2). Evidence about the role of G6PDH and/or NADPH in regulating the oxidation status of protein and lipid macromolecules in bacterial systems is scarce. However, it has been reported that Saccharomyces cerevisiae lacking Δidp2 and Δzwf1, encoding the cytoplasmic isoforms of ICDH and G6PDH, respectively, displays increased levels of membrane protein oxidation [33], [34].
On the other hand, it has been previously shown that cell exposure to tellurite also affects essential [Fe-S] cluster-containing enzymes as aconitase and fumarase [6] and redox equivalents from G6PDH-synthesized NADPH can be first transferred to NADPH-dependent ferrodoxin/flavodoxin reductase and then to oxidatively-damaged [Fe-S] clusters [30]. In this context, it was found that zwf overexpression results in augmented fumarase activity, even in the presence of toxicants (Fig. S3). Increased NADPH levels (in zwf-overexpressing cells, Table 4) could probably protect and/or repair more efficiently [Fe-S] cluster-containing enzymes during the soxRS response [22], [30].
Finally, since tellurite also triggers GSH oxidation resulting in important changes in the cell's redox status [7], [8], [35], it was assessed if zwf expression influences intracellular GSH levels. As expected, the GSH content decreased (∼45%) in the presence of tellurite and diamide, suggesting that zwf expression and thus NADPH (Table 4), could participate in regulating GSH levels (not shown). Preliminary results indicate that the observed increase in GSH levels is not related to glutathione reductase activity (not shown). Experiments aiming to determine which route is being used to recover GSH levels in tellurite-exposed E. coli [35] are under way in our laboratory.
Materials and Methods
Bacterial strains and plasmids
E. coli strains and plasmids used in this study are listed in Table S1. E. coli BW25113 chromosomal DNA and the specific primers indicated was used to amplify the zwf gene. The PCR product was ligated to pBAD TOPO (Invitrogen) vector resulting in plasmid pBAD-zwf. Insert orientation was confirmed by SalI digestion and PCR. Plasmids pBAD and pBAD-zwf were transformed into E. coli BW25113 and Δzwf strains.
Growth conditions and toxicant treatment
Bacteria were routinely grown in LB medium [36] at 37°C with vigorous shaking to OD600∼0.5. When required, ampicillin (100 µg ml−1) or kanamycin (50 µg ml−1) was added to the medium. Unless otherwise stated, compounds tested were used at final concentrations of 2.0 µM (tellurite), 100 µM (menadione), 1 mM (H2O2) and 500 µM (diamide). Gene induction was carried out in the presence of 0.2% L-arabinose.
Determination of growth inhibition zones
Growth inhibition zones were determined as described previously [37]. Briefly, cells were grown to OD600∼0.5, diluted and spread on LB plates (2%). After air drying, toxicants to be tested were deposited on sterile filter disks previously placed at the centers of the plates. Plates were incubated overnight at 37°C.
Enzyme purification
E. coli BL21(DE3) harboring plasmid pET17-G6PDH [30] was used to purify G6PDH. Cells were grown to OD600∼0.5 and induced with 1 mM IPTG for 5 h with vigorous agitation. After disrupting by sonication, crude extracts were prepared in 20 mM sodium phosphate buffer, pH 7.4, that contained 0.5 M NaCl and 20 mM imidazole. Proteins were purified by HisTrap HP (Amersham) affinity column chromatography as recommended by the vendor.
Transcriptional analysis
Overnight cultures of E. coli GC4468 carrying a chromosomal zwf::lacZ fusion [38] were diluted 1∶1000 with fresh LB medium and grown at 37°C to OD600∼0.2. Samples (in triplicate) were removed to assay for β-galactosidase by monitoring the hydrolysis of o-nitrophenyl-β-D-galactopyranoside as described [39].
Western blotting
E. coli cultures (10 ml) were centrifuged and suspended in 0.5 ml of 50 mM phosphate buffer, pH 7.4, which contained 0.1 mM phenylmethylsulfonyl fluoride. Cells were disrupted by sonication and G6PDH content was analyzed by SDS-PAGE and immunoblotting using a specific antiserum. Band intensity was analyzed using the Gel-Pro Analyzer Program software, version 3.1.
Detection of reactive oxygen species (ROS)
To determine intracellular ROS, the oxidation-sensitive probe H2DCFDA (2′,7′-dichlorofluorescein diacetate, Calbiochem) was used. Aerobically grown cells in LB medium (OD600∼0.5) were split up into 4 identical aliquots and treated individually for 30 min with the different compounds tested. Cultures (1 ml) were sedimented and cells washed with potassium phosphate buffer 10 mM, pH 7.0, and incubated for 30 min with an equal volume of buffer containing 20 µM H2DCFDA (in dimethylsulfoxide) in the dark. After washing, cells were disrupted by sonication and extracts (100 µl) were loaded in triplicate in 96-well plates. Fluorescence intensity was determined using an Applied Biosystems equipment CytoFluor 4000 Fluorescence Multi-well Plate Reader (excitation 490 nm, emission 519 nm) and normalized to protein concentration as described earlier [3], [40].
Assessing intracellular ROS by flow cytometry was performed in the same way with minor modifications. Tert-butylhydroperoxide (100 µM) was used as positive control (not shown). Cells were incubated with 2 mM H2DCFDA for 30 min in the dark, washed and diluted 1∶10 with PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) [41]. Fluorescence intensity was determined using a Becton Dickinson model FacsCanto II equipment equipped with an argon laser (excitation 490 nm, emission 519 nm).
Determination of protein carbonyl group content
Protein carbonyl group content was determined as previously described [42], [43]. Nucleic acid-free E. coli extracts were mixed with 4 volumes of 10 mM dinitrophenylhydrazine (dissolved in 2 M HCl) and incubated for 1 h at room temperature. Proteins were precipitated with 1 volume of cold 20% trichloroacetic acid and centrifuged at 10,000g for 10 min. After washing 3 times with ethanol∶ethyl acetate (1∶1), the pellet was dissolved with 450 µl of 50 mM dithiothreitol in 6 M guanidine-HCl. Carbonyl content was determined at 370 nm using a molar absorption coefficient of 22,000 M−1 cm−1 [42].
Determination of membrane lipid peroxides
Membrane lipid peroxides were determined as described previously [44]. Toxicant-treated E. coli was centrifuged and suspended in 0.5 ml of a solution that contained 50 mM Tris-HCl, pH 7.4, and 1% SDS. After sonication samples were washed with water and dried pellets were dissolved in methanol∶chloroform (2∶1) and kept at room temperature with agitation for 1 h. Samples were then treated with 300 µl of the FOX II reactant (0.1 mM xylenol orange, 0.25 mM ammonium ferrous sulfate, 25 mM H2SO4, 4 mM butylated hydroxytoluene, in 90% methanol), mixed and let to stand at room temperature for 1 h. Membrane lipid peroxide content was determined at 560 nm using a molar absorption coefficient of 45,200 M−1 cm−1 [44].
Determination of enzyme activity
Cells from 10 ml cultures were disrupted by sonication and extracts cleared by centrifugation. Aliquots of cell-free extracts were assayed for glucose-6-phosphate dehydrogenase [45], isocitrate dehydrogenase [46], NADP+-glutamate dehydrogenase [47], fumarase [48] and glutathione reductase [49]. Protein concentration was determined as described by Bradford using bovine serum albumin as standard [50].
Determination of dinucleotide concentration
Duplicated samples were used for the selective extraction of dinucleotides as described earlier [51]. Briefly, cells were centrifuged at 13,000g for 2 min and immediately frozen in a dry ice-ethanol bath. Samples were treated with 250 µl of 0.2 M HCl or 0.2 M NaOH for extracting NAD(P)+ or NAD(P)H, respectively. Dinucleotides were extracted after incubating for 10 min at 100°C and centrifuging at 5,000g for 5 min to remove the cell debris. Supernatants were transferred to fresh tubes and kept on ice until use. Both NADP+ and NADPH were assessed spectrophotometrically using commercially available G6PDH and glutathione reductase, respectively [52]. NADP+ and NADPH standards from 0.01–1.0 mM were used to calibrate the assays.
Intracellular concentrations of NAD+ and NADH were assessed spectrophotometrically using NADH-dependent alcohol dehydrogenase as described previously [53] with modifications [54]. Standards of NAD+ and NADH from 0.05–0.75 mM were used to construct a calibration curve.
Determination of GSH content
After tellurite or diamide treatment, cells were washed twice with ice-cold phosphate-buffered saline and centrifuged at 4°C for 2 min at 10,000g. Pellets were suspended in 100 µl of 5-sulfosalicylic acid (SSA) (5%, w/v), frozen in liquid nitrogen, thawed twice, centrifuged at 4°C and kept at −80°C until use. Total glutathione (GSH+GSSG) was determined as described previously [55]. Reduced GSH was calculated from total glutathione to which oxidized glutathione (GSSG) was subtracted. GSSG was determined using 2-vinylpyridine (M2VP) as described earlier [56] with minor modifications. Both GSH and GSSG standards from 0 to 0.5 mM were used to calibrate the assay.
Determination of G6P concentration
Cultures (1 ml) -in duplicate- were centrifuged at 13,000g at 4°C for 2 min, washed and sonicated. Cell lysates were cleared by centrifugation at 13,000g for 10 min at 4°C. Extracts were boiled for 10 min, chilled and centrifuged at 13,000g for 10 min at 4°C. Supernatants were used immediately. Samples (50–200 µl) were incubated in a reaction buffer that contained 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 0.7 mM NADP+ and 0.5 U ml−1 G6PDH. G6P concentration was assessed spectrophotometrically at 340 nm as described [57]. Standards of G6P from 0.005 to 0.25 mM were used to calibrate the assay.
Data analysis
In general, results were expressed as the mean ± the standard deviation. Differences between experimental groups were analyzed using one-way ANOVA. P values less than 0.05 were considered statistically significant.
Supporting Information
Sensitivity of various E. coli strains impaired in NADPH synthesis to oxidative stress elicitors. Growth inhibition zones (cm2) were determined for wild type and several strains deficient in NADPH synthesis essentially as described in Fig. 2. Results were determined after 24 h. Values are the mean of 4–5 independent trials ± SD. BW25113 (wild type), Δzwf (glucose-6-phosphate dehydrogenase), Δgnd (6-phosphogluconate dehydrogenase), ΔicdA (isocitrate dehydrogenase), ΔmaeB (NADP+-dependent malic enzyme), ΔgdhA (glutamate dehydrogenase), ΔpntA (pyridine nucleotide transhydrogenase, α-subunit), ΔpntB (pyridine nucleotide transhydrogenase, β-subunit), ΔudhA (soluble pyridine nucleotide transhydrogenase).
(TIF)
Effect of zwf expression on macromolecule oxidation. Oxidized cytoplasmatic proteins (A) and total membrane lipid peroxides (B) were assessed in the indicated strains. Cells were grown in LB-arabinose in the absence of toxicant (white bars) or exposed to 2 µM tellurite (black bars) or 100 µM H2O2 (stripes) for 30 min. Values are the average of 3 independent trials ± SD.
(TIF)
Effect of zwf expression on fumarase activity. Total fumarase activity was assessed as described in Methods. The indicated strains were grown in LB-arabinose in the absence of toxicants (white bars) or exposed for 30 min to 2 µM tellurite (black bars) or 100 µM menadione (stripes) for 30 min. Values are the average of 3 independent trials ± SD.
(TIF)
E. coli strains, plasmids and primers used in this study.
(DOCX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants # 1090097 from Fondecyt (Fondo Nacional de Investigación Científica y Tecnológica) and Dicyt (Dirección de Investigación Científica y Tecnológica)-USACH (Universidad de Santiago de Chile) to C.C.V. J.M.S. was supported by a doctoral fellowship from MECESUP (Mejoramiento de la Calidad y Equidad de la Educación Superior) UCH407, Chile. F.A.A. received doctoral fellowships from CONICYT (Comisión Nacional de Ciencia y Tecnología) and from MECESUP UCH607, Chile. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Borsetti F, Tremaroli V, Michelacci F, Borghese R, Winterstein C, et al. Tellurite effects on Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res Microbiol. 2005;156:807–813. doi: 10.1016/j.resmic.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Chasteen TG, Fuentes DE, Tantaleán JC, Vásquez CC. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev. 2009;33:820–832. doi: 10.1111/j.1574-6976.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- 3.Pérez JM, Calderón IL, Arenas FA, Fuentes DE, Pradenas GA, et al. Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS ONE. 2007;2:e211. doi: 10.1371/journal.pone.0000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoval JM, Levêque P, Gallez B, Vásquez CC, Buc Calderón P. Tellurite-induced oxidative stress leads to cell death of murine hepatocarcinoma cells. Biometals. 2010;23:623–632. doi: 10.1007/s10534-010-9316-2. [DOI] [PubMed] [Google Scholar]
- 5.Tremaroli V, Fedi S, Zannoni D. Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch Microbiol. 2007;187:127–135. doi: 10.1007/s00203-006-0179-4. [DOI] [PubMed] [Google Scholar]
- 6.Calderón IL, Elías AO, Fuentes EL, Pradenas GA, Castro ME, et al. Tellurite-mediated disabling of [4Fe-4S] clusters of Escherichia coli dehydratases. Microbiology. 2009;155:1840–1846. doi: 10.1099/mic.0.026260-0. [DOI] [PubMed] [Google Scholar]
- 7.Turner RJ, Weiner JH, Taylor DE. Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology. 1999;145:2549–2557. doi: 10.1099/00221287-145-9-2549. [DOI] [PubMed] [Google Scholar]
- 8.Turner RJ, Aharonowitz Y, Weiner JH, Taylor DE. Glutathione is a target in tellurite toxicity and is protected by tellurite resistance determinants in Escherichia coli. Can J Microbiol. 2001;47:33–40. [PubMed] [Google Scholar]
- 9.Farr SB, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudú P, Moon N, Weiss B. Regulation of the soxRS oxidative stress regulon. Reversible oxidation of the Fe-S centers of SoxR in vivo. J Biol Chem. 1997;272:5082–5086. doi: 10.1074/jbc.272.8.5082. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard JL, Wholey WY, Conlon EM, Pomposiello PJ. Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks. PLoS ONE. 2007;2:e1186. doi: 10.1371/journal.pone.0001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomposiello PJ, Bennik MH, Demple B. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol. 2001;183:3890–3902. doi: 10.1128/JB.183.13.3890-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 14.Chiong M, González E, Barra R, Vásquez C. Purification and biochemical characterization of tellurite-reducing activities from Thermus thermophilus HB8. J Bacteriol. 1988;170:3269–3273. doi: 10.1128/jb.170.7.3269-3273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderón IL, Arenas FA, Pérez JM, Fuentes DE, Araya MA, et al. Catalases are NAD(P)H-dependent tellurite reductases. PLoS ONE. 2006;1:e70. doi: 10.1371/journal.pone.0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro ME, Molina R, Díaz W, Pichuantes SE, Vásquez CC. The dihydrolipoamide dehydrogenase of Aeromonas caviae ST exhibits NADH dependent tellurite reductase activity. Biochem Biophys Res Commun. 2008;375:91–94. doi: 10.1016/j.bbrc.2008.07.119. [DOI] [PubMed] [Google Scholar]
- 17.Bériault R, Hamel R, Chenier D, Mailloux RJ, Joly H, et al. The overexpression of NADPH-producing enzymes counters the oxidative stress evoked by gallium, an iron mimetic. Biometals. 2007;20:165–176. doi: 10.1007/s10534-006-9024-0. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Mailloux RJ, Puiseux-Dao S, Appanna VD. Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J Bacteriol. 2007;189:6665–6675. doi: 10.1128/JB.00555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siliprandi D, De Meio RH, Toninello A, Zoccarato F. The action of tellurite, a reagent for thiol groups, on mitochondria oxidative processes. Biochem Biophys Res Commun. 1971;45:1071–1075. doi: 10.1016/0006-291x(71)90446-3. [DOI] [PubMed] [Google Scholar]
- 20.Siliprandi D, Storey DT. Interaction of tellurite with the respiratory chain in rat liver mitochondria. FEBS Lett. 1973;29:101–104. doi: 10.1016/0014-5793(73)80535-6. [DOI] [PubMed] [Google Scholar]
- 21.Grose JH, Joss L, Velick SF, Roth JR. Evidence that feedback inhibition of NAD kinase controls responses to oxidative stress. Proc Natl Acad Sci USA. 2006;103:7601–7606. doi: 10.1073/pnas.0602494103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krapp AD, Humbert MV, Carrillo N. The soxRS response of Escherichia coli can be induced in the absence of oxidative stress and oxygen by modulation of NADPH. Microbiology. 2011;157:957–965. doi: 10.1099/mic.0.039461-0. [DOI] [PubMed] [Google Scholar]
- 23.Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem. 2006;281:40485–40492. doi: 10.1074/jbc.M607704200. [DOI] [PubMed] [Google Scholar]
- 24.Kosower NS, Kosower EM, Wertheim B, Correa WS. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969;37:593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- 25.Liochev SI, Benov L, Touati D, Fridovich I. Induction of the soxRS regulon of Escherichia coli by superoxide. J Biol Chem. 1999;274:9479–9481. doi: 10.1074/jbc.274.14.9479. [DOI] [PubMed] [Google Scholar]
- 26.Ralser M, Wamelink MM, Kowald A, Gerisch B, Heeren G, et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J Biol. 2007;6:10. doi: 10.1186/jbiol61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rui B, Shen T, Zhou H, Liu J, Chen J, et al. A systematic investigation of Escherichia coli central carbon metabolism in response to superoxide stress. BMC Syst Biol. 2010;4:122. doi: 10.1186/1752-0509-4-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rungrassamee W, Liu X, Pomposiello PJ. Activation of glucose transport under oxidative stress in Escherichia coli. Arch Microbiol. 2008;190:41–49. doi: 10.1007/s00203-008-0361-y. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg BE, Wolf RE, Jr, Dinauer MC, Xu Y, Fang FC. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect Immun. 1999;67:436–438. doi: 10.1128/iai.67.1.436-438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giró M, Carrillo N, Krapp AR. Glucose-6-phosphate dehydrogenase and ferredoxin-NADP(H) reductase contribute to damage repair during the soxRS response of Escherichia coli. Microbiology. 2006;152:1119–1128. doi: 10.1099/mic.0.28612-0. [DOI] [PubMed] [Google Scholar]
- 31.Kirsch M, de Groot H. NAD(P)H, a directly operating antioxidant? FASEB J. 2001;15:1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 32.Petrat F, Pindiur S, Kirsch M, de Groot H. NAD(P)H, a primary target of 1O2 in mitochondria of intact cells. J Biol Chem. 2003;278:3298–3307. doi: 10.1074/jbc.M204230200. [DOI] [PubMed] [Google Scholar]
- 33.Johnson RM, Ravindranath Y, el-Alfy M, Goyette G., Jr Oxidant damage to erythrocyte membrane in glucose-6-phosphate dehydrogenase deficiency: correlation with in vivo reduced glutathione concentration and membrane protein oxidation. Blood. 1994;83:1117–1123. [PubMed] [Google Scholar]
- 34.Minard KI, Carroll CA, Weintraub ST, Mc-Alister-Henn L. Changes in disulfide bond content of proteins in a yeast strain lacking major sources of NADPH. Free Radic Biol Med. 2007;42:106–117. doi: 10.1016/j.freeradbiomed.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smirnova GV, Oktyabrsky ON. Glutathione in bacteria. Biochemistry (Moscow) 2005;70:1199–1211. doi: 10.1007/s10541-005-0248-3. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a laboratory manual. 2nd Ed. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, N.Y; 1989. [Google Scholar]
- 37.Fuentes DE, Fuentes EL, Castro ME, Pérez JM, Araya MA, et al. Cysteine metabolism-related genes and bacterial resistance to potassium tellurite. J Bacteriol. 2007;189:8953–8960. doi: 10.1128/JB.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffith KL, Wolf RE., Jr Genetic evidence for pre-recruitment as the mechanism of transcription activation by SoxS of Escherichia coli: the dominance of DNA binding mutations of SoxS. J Mol Biol. 2004;344:1–10. doi: 10.1016/j.jmb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Miller JH. Experiments in Molecular Genetics, pp. 201–205, 352–355, and 431–433. New York, USA: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 40.Echave P, Tamarit J, Cabiscol E, Ros J. Novel antioxidant role of alcohol dehydrogenase E from Escherichia coli. J Biol Chem. 2003;32:30193–30198. doi: 10.1074/jbc.M304351200. [DOI] [PubMed] [Google Scholar]
- 41.Herrera G, Martínez A, O'Connor JE, Blanco M. Functional assays of oxidative stress using genetically engineered Escherichia coli strains. Curr Protoc Cytom. 2003:11.16.1–11.16.9. doi: 10.1002/0471142956.cy1116s24. [DOI] [PubMed] [Google Scholar]
- 42.Semchyshyn H, Bagnyukova T, Lushchak V. Involvement of soxRS regulon in response of Escherichia coli to oxidative stress induced by hydrogen peroxide. Biochemistry (Moscow) 2005;70:1238–1244. doi: 10.1007/s10541-005-0253-6. [DOI] [PubMed] [Google Scholar]
- 43.Contreras N del P, Vásquez CC. Tellurite-induced carbonylation of the Escherichia coli pyruvate dehydrogenase multienzyme complex. Arch Microbiol. 2010;192:969–973. doi: 10.1007/s00203-010-0624-2. [DOI] [PubMed] [Google Scholar]
- 44.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 45.Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol Regul Integr Comp Physiol. 2001;280:R100–107. doi: 10.1152/ajpregu.2001.280.1.R100. [DOI] [PubMed] [Google Scholar]
- 46.Murakami K, Tsubouchi R, Fukayama M, Ogawa T, Yoshino M. Oxidative inactivation of reduced NADP-generating enzymes in E. coli: iron-dependent inactivation with affinity cleavage of NADP-isocitrate dehydrogenase. Arch Microbiol. 2006;186:385–392. doi: 10.1007/s00203-006-0153-1. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto N, Kotre AM, Savageau MA. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975;124:775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liochev SI, Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci USA. 1992;89:5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asnis RE. A glutathione reductase from Escherichia coli. J Biol Chem. 1955;213:77–85. [PubMed] [Google Scholar]
- 50.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 51.Heber UW, Santarius KA. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1967;109:390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Yu J, Stanton RC. A method for determination of pyridine nucleotides using a single extract. Anal Biochem. 2000;285:163–167. doi: 10.1006/abio.2000.4701. [DOI] [PubMed] [Google Scholar]
- 53.Leonardo MR, Dailly Y, Clark DP. Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 55.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 56.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 57.Hasan MR, Rahman M, Jaques S, Purwantini E, Daniels L. Glucose-6-phosphate accumulation in mycobacteria: implications for a novel F420-dependent anti-oxidant defense system. J Biol Chem. 2010;285:19135–19144. doi: 10.1074/jbc.M109.074310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity of various E. coli strains impaired in NADPH synthesis to oxidative stress elicitors. Growth inhibition zones (cm2) were determined for wild type and several strains deficient in NADPH synthesis essentially as described in Fig. 2. Results were determined after 24 h. Values are the mean of 4–5 independent trials ± SD. BW25113 (wild type), Δzwf (glucose-6-phosphate dehydrogenase), Δgnd (6-phosphogluconate dehydrogenase), ΔicdA (isocitrate dehydrogenase), ΔmaeB (NADP+-dependent malic enzyme), ΔgdhA (glutamate dehydrogenase), ΔpntA (pyridine nucleotide transhydrogenase, α-subunit), ΔpntB (pyridine nucleotide transhydrogenase, β-subunit), ΔudhA (soluble pyridine nucleotide transhydrogenase).
(TIF)
Effect of zwf expression on macromolecule oxidation. Oxidized cytoplasmatic proteins (A) and total membrane lipid peroxides (B) were assessed in the indicated strains. Cells were grown in LB-arabinose in the absence of toxicant (white bars) or exposed to 2 µM tellurite (black bars) or 100 µM H2O2 (stripes) for 30 min. Values are the average of 3 independent trials ± SD.
(TIF)
Effect of zwf expression on fumarase activity. Total fumarase activity was assessed as described in Methods. The indicated strains were grown in LB-arabinose in the absence of toxicants (white bars) or exposed for 30 min to 2 µM tellurite (black bars) or 100 µM menadione (stripes) for 30 min. Values are the average of 3 independent trials ± SD.
(TIF)
E. coli strains, plasmids and primers used in this study.
(DOCX)