Abstract
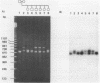
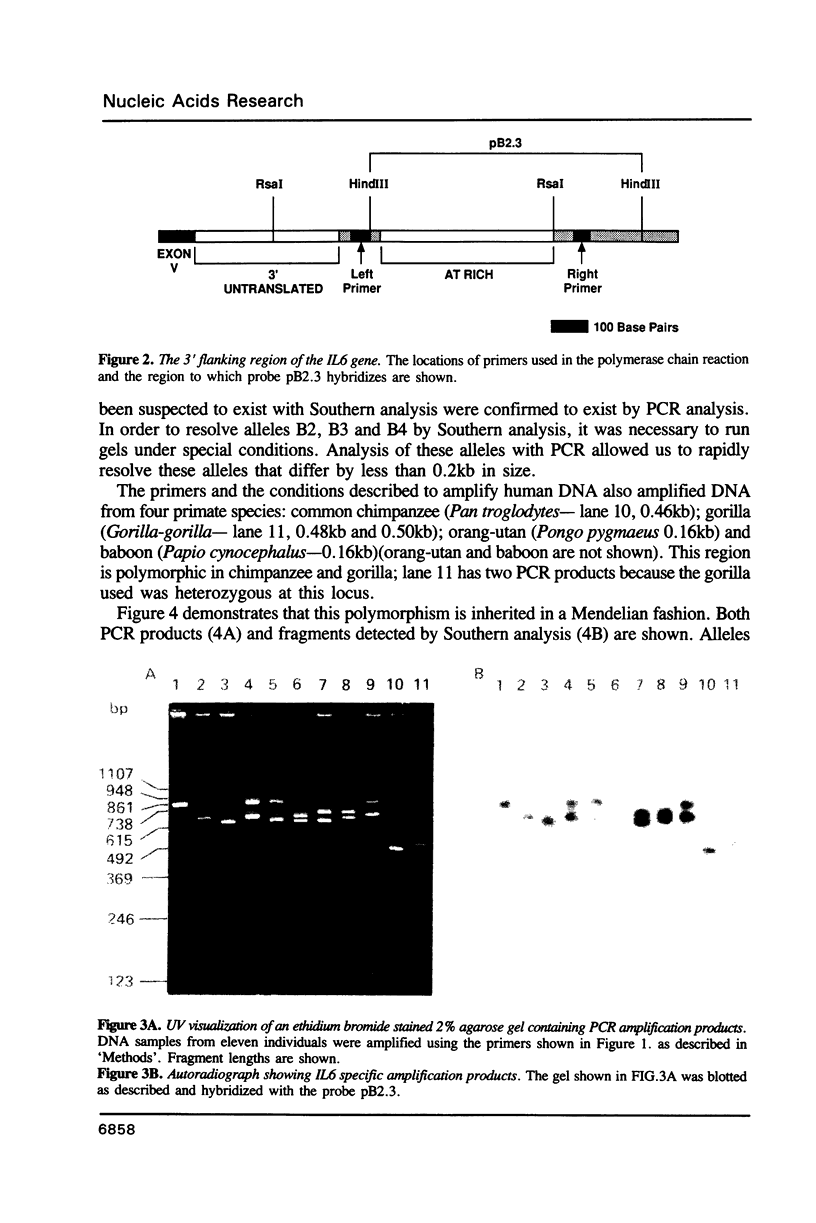
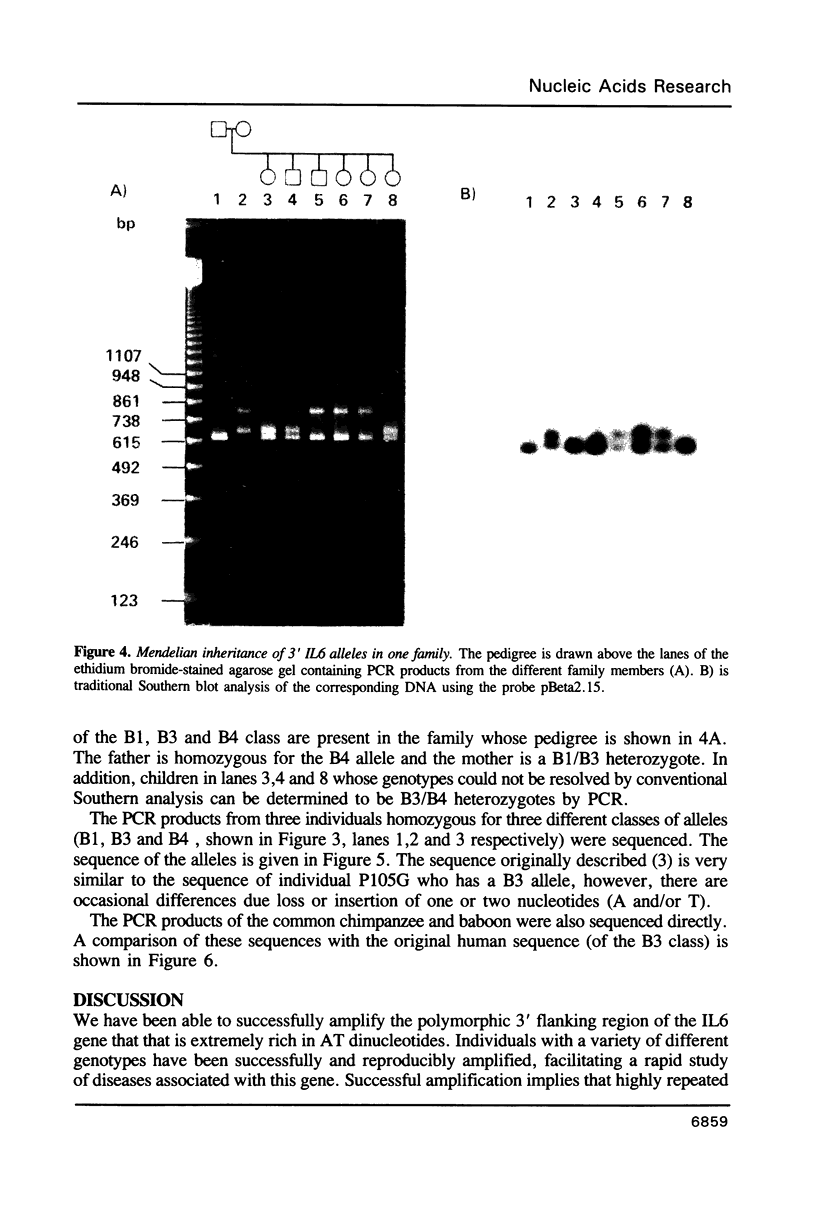
The 3' flanking region of the interleukin 6 gene is polymorphic due to insertions of different size. Within this region lies a sequence of approximately 500 base pairs that is AT rich. Based on flanking sequence information we have constructed oligonucleotides which prime the polymerase chain reaction (PCR) and amplify this AT rich region. The amplification products visualized by agarose gel electrophoresis gave fragment sizes for both homozygous and heterozygous individuals that were concordant with those observed by conventional genomic blotting techniques. Alleles that could not be typed by Southern analysis were resolved with this approach. These results illustrate the value of PCR for the rapid detection of length polymorphisms such as those due to variable numbers of tandem repeats. In contrast to RFLP analysis this procedure takes less than a day to perform, is cheaper, avoids the use of radioactivity and requires far less substrate DNA. Three different human alleles were sequenced, and differences were detected that were due to both large duplications and loss of one or two bases, suggesting that AT rich regions identify highly polymorphic loci. The same primers also amplified non-human primate DNA, allowing a comparison of the human sequence with that of the common chimpanzee and baboon.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boerwinkle E., Xiong W. J., Fourest E., Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3' hypervariable region. Proc Natl Acad Sci U S A. 1989 Jan;86(1):212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowcock A. M., Kidd J. R., Lathrop G. M., Daneshvar L., May L. T., Ray A., Sehgal P. B., Kidd K. K., Cavalli-Sforza L. L. The human "interferon-beta 2/hepatocyte stimulating factor/interleukin-6" gene: DNA polymorphism studies and localization to chromosome 7p21. Genomics. 1988 Jul;3(1):8–16. doi: 10.1016/0888-7543(88)90152-8. [DOI] [PubMed] [Google Scholar]
- Feder J., Yen L., Wijsman E., Wang L., Wilkins L., Schroder J., Spurr N., Cann H., Blumenberg M., Cavalli-Sforza L. L. A systematic approach for detecting high-frequency restriction fragment length polymorphisms using large genomic probes. Am J Hum Genet. 1985 Jul;37(4):635–649. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G. T., Richards B., Klinger K. W. Amplification of a highly polymorphic VNTR segment by the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2140–2140. doi: 10.1093/nar/17.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. S., Breslow J. L. A unique AT-rich hypervariable minisatellite 3' to the ApoB gene defines a high information restriction fragment length polymorphism. J Biol Chem. 1987 Jul 5;262(19):8952–8955. [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Cheah K. S., Griffin J. R., Pope F. M., Solomon E. A highly polymorphic region 3' to the human type II collagen gene. Nucleic Acids Res. 1985 Jul 11;13(13):4613–4622. doi: 10.1093/nar/13.13.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wolff R. K., Nakamura Y., White R. Molecular characterization of a spontaneously generated new allele at a VNTR locus: no exchange of flanking DNA sequence. Genomics. 1988 Nov;3(4):347–351. doi: 10.1016/0888-7543(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]