Abstract
Postsynaptic membrane rafts are believed to play important roles in synaptic signaling, plasticity, and maintenance. However, their molecular identities remain elusive. Further, how they interact with the well-established signaling specialization, the postsynaptic density (PSD), is poorly understood. We previously detected a number of conventional PSD proteins in detergent-resistant membranes (DRMs). Here, we have performed LC-MS/MS (liquid chromatography coupled with tandem mass spectrometry) analyses on postsynaptic membrane rafts and PSDs. Our comparative analysis identified an extensive overlap of protein components in the two structures. This overlapping could be explained, at least partly, by a physical association of the two structures. Meanwhile, a significant number of proteins displayed biased distributions to either rafts or PSDs, suggesting distinct roles for the two postsynaptic specializations. Using biochemical and electron microscopic methods, we directly detected membrane raft-PSD complexes. In vitro reconstitution experiments indicated that the formation of raft-PSD complexes was not due to the artificial reconstruction of once-solubilized membrane components and PSD structures, supporting that these complexes occurred in vivo. Taking together, our results provide evidence that postsynaptic membrane rafts and PSDs may be physically associated. Such association could be important in postsynaptic signal integration, synaptic function, and maintenance.
Keywords: membrane rafts, DRM, postsynaptic density, PSD
Introduction
Membrane rafts, or lipid rafts (Pike 2006) are small, heterogeneous, and cholesterol- and sphingolipids-enriched domains thought to compartmentalize cellular processes. They are short-lived and highly dynamic. Very small rafts (in the order of 10 nm or less) are dispersed throughout the membrane of cells at steady states (Kusumi & Suzuki 2005). However, in response to certain stimuli, they are believed to be rearranged into large, stable membrane rafts or large platforms, and recruit downstream signaling molecules (Pike 2006, Lingwood & Simons 2010, Brown & London 2000, Shogomori & Brown 2003). Increasing evidence indicates that membrane rafts may play important roles in various cellular processes, such as signal transduction, cell adhesion, membrane trafficking and molecular sorting (Simons & Ikonen 1997, Masserini et al. 1999, Pierini & Maxfield 2001, Nebl et al. 2002). Components closely associated with membrane rafts (or raft-philic proteins) have been isolated as detergent-resistant membranes (DRMs), which are recovered as a cold detergent-insoluble floating material. Although inappropriate for characterizing the size and dynamics of membrane rafts in vivo, DRMs can provide insights into the molecular compositions of membrane rafts.
Membrane rafts in postsynaptic neurons, along with postsynaptic densities (PSDs), are considered major sites of synaptic signaling, function, and maintenance (Suzuki 2002, Allen et al. 2007). Proteomics analyses have identified hundreds of protein components in the PSD. (Jordan et al. 2004, Li et al. 2004a, Peng et al. 2004, Satoh et al. 2002, Yoshimura et al. 2004, Cheng et al. 2006). In contrast, direct profiling of postsynaptic membrane rafts has not been reported, but these lipid microdomains are believed to contribute to dendritic spine morphogenesis, receptor trafficking, and synaptic maintenance (Hering et al. 2003). There is evidence that postsynaptic rafts are associated with PSDs, which can account for some of their roles in coordinating synaptic signaling. For instance, some PSD components possess lipid modifications typical for raft-associated proteins (e.g., PSD-95). Using Western blot analyses, we and others found that DRMs prepared from SPM or synaptosomes share a large number (~ 30) of proteins with the PSD (Suzuki 2002, Suzuki et al. 2001, Suzuki et al. 2008, Besshoh et al. 2005). The reason for this extensive overlapping is not clear, but is unlikely due to mutual contamination, because the two are completely separated by their densities (Suzuki 2002).
The molecular identities of postsynaptic membrane rafts important for synaptic signaling processing have not been determined. The potential direct association between PSDs and postsynaptic membrane rafts has not been directly examined. Here, we massively identified protein components of SPM-derived DRMs (SPM-DRMs) using LC/MS/MS, providing the first proteomic profile of postsynaptic membrane rafts at the scale comparable to that of PSDs. We also observed postsynaptic membrane raft-PSD complexes using electron microscopy, which, together with our LC-MS/MS and biochemical data, support in vivo association of the two structures. This study improves our understanding of the roles and the physiological significance of postsynaptic membrane rafts.
In this paper, “membrane rafts” and “DRMs” are differentially used to refer “in vivo domains” and “in vitro materials”, respectively, as recommended by the Keystone Symposium on Lipid Rafts and Cell Function (Pike 2006).
Experimental Procedures
Materials
Triton X-100 (TX-100), polyoxyethylene (Hering et al. 2003), and octylphenyl ether (T9284, lot no. 188-14808) were purchased from WAKO Pure Chemical Industries. Ltd. (Osaka, Japan); n-octyl-β-glucoside was from Dojindo Laboratories (Kumamoto, Japan); 12 tungsto (VI) phosphoric acid n-hydrate (phosphotungstic acid, PTA) was from Nakarai tesque (Tokyo, Japan); cholesterol assay kit was from Cayman Chemical Company (Ann Arbor, MI, USA); protease inhibitor cocktail (P8340), methyl-β-cyclodextrin (MβCD) and horseradish peroxidase-conjugated cholera toxin B subunit were from Sigma-Aldrich (St Louis, MO, USA). Antibody sources were described previously (Suzuki et al. 2008). All other chemicals were of reagent grade.
Preparation of SPM, PSD and SPM-DRM, and analysis of protein distributions on sucrose gradients
Animals were handled in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication no. 80-23). Wistar rats (6-week-old males) were killed by decapitation and forebrains were dissected and stored at −80°C until use. SPM was prepared from the forebrain as described previously (Suzuki et al. 2001, Suzuki 2011). SPM obtained from the interface between 1.0–1.2 M sucrose layers without washing-out sucrose was suspended in HEPES-KOH buffer (pH 7.4, 5 mM) containing 50% glycerol and stored unfrozen at −30°C, until use. SPM-derived PSDs (m-PSDs) were purified from the forebrain using a conventional method (the long procedure), which treated SPM with TX-100 (Cohen et al. 1977, Suzuki 2011). The TX-100 concentration was 0.5% and detergent vs. protein ratio was averagely 8.9 for m-PSD purification. The crude m-PSD fraction recovered at the interface between 1.5 and 2.1 M sucrose layers after TX-100 treatment and subsequent ultracentrifugation was washed once with TX-100/KCl, as stated in the original protocol. PSD fractions obtained as such have been verified biochemically and morphologically (Suzuki et al. 1994, Suzuki et al. 2001, Suzuki et al. 2008, Li et al. 2001). DRMs were isolated from the SPM (500 µg protein) after treatment with TX-100 and sucrose density gradient centrifugation (SDG) as described previously (Du et al. 2006). MβCD treatment was carried out as described previously (Du et al. 2006). Both SPM-DRM (0.15% TX-100) and m-PSD were prepared more than 15 times with highly reproducible protein profiles.
Analyses of protein, GM1 ganglioside and cholesterol
For protein profiling, 15 µL of each SDG fraction was applied to SDS-PAGE and stained with silver. For immunoblotting, proteins from each fraction was precipitated with trichloroacetic acid (TCA) and resuspended in the solution consisting of 150 µL of TNE buffer and 50 µL of SDS-PAGE sample buffer. Equal volume (usually 20 µL) of each fraction was analyzed by SDS-PAGE and immunoblotting. Western blotting was carried out using a chemiluminescent substrate (Pierce) and visualized with a CCD video camera system. GM1 ganglioside was detected by dot blot, as described previously (Du et al. 2006). For quantification of GM1 ganglioside, Bio-Dot (Bio-Rad) dot blot apparatus was used. Proteins and GM1 signals were quantified using NIH Image. Cholesterol was measured by using a cholesterol assay kit (Cayman Chemical Company) following manufacturer’s instruction.
Mass spectrometry
Protein complexes were separated by SDS-PAGE and stained by Coomassie Blue. Each lane was cut into 18 gel pieces, de-stained, reduced with dithiothreitol, alkylated with iodoacetamide, and digested in-gel with trypsin. The resultant peptide mixtures were extracted and lyophilized. The extracted peptide mixtures were re-suspended, loaded on a 10 cm × 75 µm Picofrit column (New Objective Inc.) packed with Magic C18-AQ, 200Å 5µ beads (Michrom Bioresources). Peptide digests eluted were sprayed directly into a hybrid Linear Ion Trap (LTQ)-Fourier Transform-Ion Cyclotron Resonance (FT-ICR) mass spectrometer (Thermo Electron Corp.) Typically top five most abundant ions for MS/MS fragmentation in the LTQ were employed for mass spectrometry analysis. The mass spectral data were analyzed using Bioworks 3.2 and SEQUEST version 28 (Thermo Electron Corp.) using an inhouse compilation of >60,000 human, mouse, rat and control proteins obtained from the UniProtKB database.
Electron microscopy
Fractions of SDG were fixed with 1 or 2% glutaraldehyde, post-fixed with 1% osmiumtetroxide, dehydrated, and embedded in Epon. An ultrathin section was cut and stained with uranyl acetate and lead citrate. To observe synaptic components, fractions were fixed with 2% glutaraldehyde and stained with 1% ethanolic phosphotungstic acid (E-PTA) without osmiumtetroxide fixation and staining with lead citrate and uranium acetate (Bloom & Aghajanian 1966). Specimens were examined under a JEOL JEM-1400EX electron microscope at 80 kV (Tokyo, Japan).
In vitro reconstitution
SPM (500 µg protein) was treated with 0.15% TX-100 at 4°C for 30 min in the absence or presence purified PSDs, and protein profiles were analyzed after SDG. Fraction No. 12 obtained after treatment of SPM with 1% octyl glucoside was used as purified PSDs to liberate raft lipids as much as possible from PSDs (Shogomori & Brown 2003, Garner et al. 2008). Proteins were stained with silver under nearly the same conditions between samples.
Results
Identification of protein components in SPM-DRM by LC-MS/MS
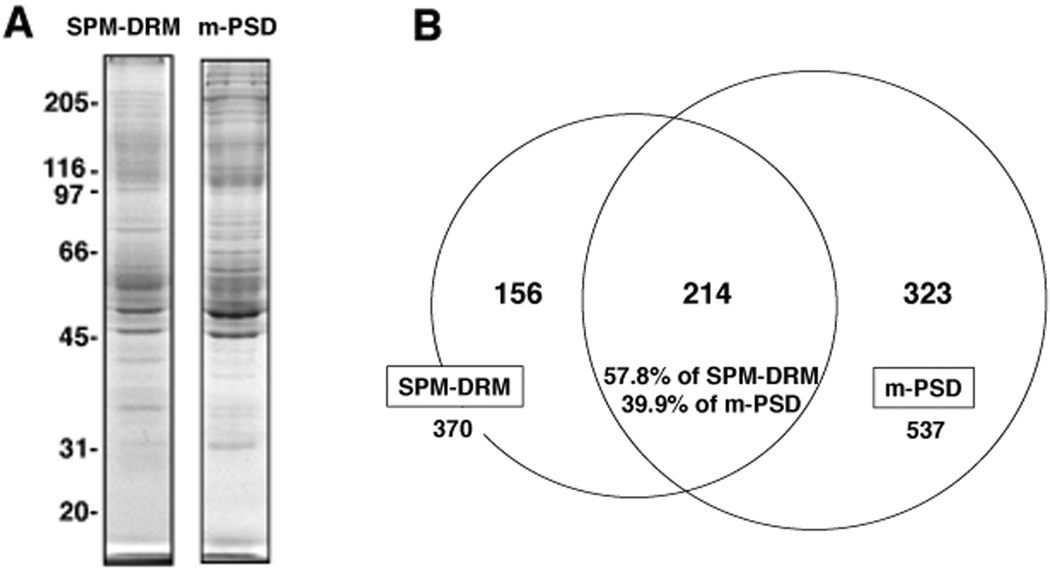
We used LC-MS/MS to identify protein components in postsynaptic rafts. SPM-DRM (fraction No. 5, see below) was prepared with 0.15% TX-100 as described (Suzuki et al. 2008, Du et al. 2006). For the purpose of direct comparisons, we also prepared PSDs from the SPM (m-PSDs) to differentiate them from PSDs isolated directly from synaptosomes, because the two PSD preparations are not completely the same (Matus et al. 1980, Suzuki 2011). Protein profiles of SPM-DRM and m-PSD fractions were shown in Fig. 1A. Proteomic analyses of the two fractions identified a total of 693 proteins, 370 in SPM-DRM and 537 in m-PSD (Fig. 1B). Detailed information for these proteins is provided in Supplementary Tables 1 and 2. Among these, 214 proteins (30.9% of total) were present in both fractions, accounting for 39.9% of proteins in the m-PSD and 57.8% of proteins in the SPM-DRM. 156 (22.5% of total) and 323 (46.6%) proteins were recovered preferentially in the SPM-DRM and m-PSD, respectively. Based on their distribution profiles, proteins in the three groups were designated “SPM-DRM only”, “Both” or “m-PSD only”. These results suggest that the two postsynaptic structures contain different but overlapping protein components.
Fig. 1. Mass spectrometry analyses of SPM-derived DRM and m-PSD fractions.
(A) Protein profiles of SPM-DRM and m-PSD fractions used for LC-MS/MS. SPM-DRM (fraction No. 5) was prepared using 0.15% TX-100 followed by SDG. Proteins (~5 µg) were separated by SDS-PAGE and stained with coomassie brilliant blue R-250. (B) Venn diagram summarizing protein distributions in SPM-DRM and m-PSD fractions based on LC-MS/MS analyses. Numbers of protein species identified and the percentages of proteins found in both fractions are indicated.
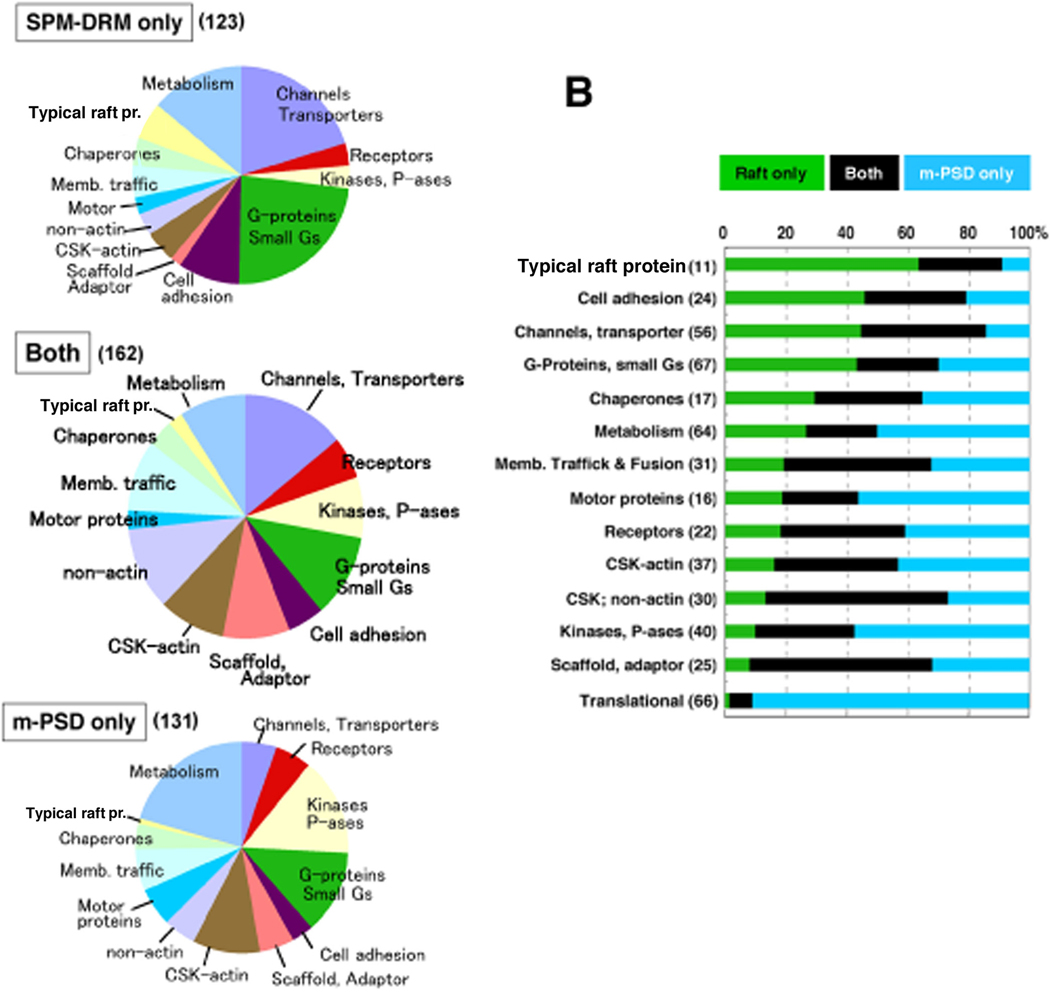
To gain initial insights into potential functions of postsynaptic rafts and their relationship to the PSD, we classified each protein in the three groups into different functional categories (Fig. 2). Proteins found in “SPM-DRM only” were mostly channels/transporters, G-proteins and related molecules, cell adhesion molecules; in comparison, proteins found in “m-PSD only” or “Both” were largely kinases/phosphatases, scaffold/cytoskeleton/motor proteins, membrane traffic proteins and metabolism-related proteins (Fig. 2A). In a different view, how each protein category distributed in SPM-DRM and m-PSD fractions was analyzed (Fig. 2B). Nearly or more than half of proteins classified as typical raft proteins, cell adhesion molecules, channels/transporters and G-proteins/small G-proteins resided only in the SPM-DRM. In contrast, approximately half of kinases/phosphatases, motor proteins, and metabolism-related proteins were distributed only in the m-PSD. Many proteins in the categories of scaffolds/adaptors, non-actin cytoskeletal proteins and those involved in membrane trafficking and fusion were recovered in both SPM-DRMs and PSDs. Translation-related proteins detected were mostly ribosomal subunit proteins, 94.7% (54 out of 57) of which were identified in “m-PSD only” group. Taken together, the distribution profiles of various protein categories in SPM-DRMs and m-PSDs suggest distinct yet related functions between the two postsynaptic specializations.
Fig. 2. Categorizations of SPM-DRM and m-PSD proteins.
(A) Differential distributions of protein categories in SPM-DRMs and/or m-PSDs. Proteins found only in the m-PSD (m-PSD only), only in the SPM-DRM (SPM-DRM only) and in both fractions (Both) were classified based on their functional categories. Proteins in the same category were grouped and represented in pie graphs. Note that proteins classified as “Translation”, “Other compartments” and “Unclassified or Unknown” were not included. (B) Normalized representations of various protein categories in SPM-DRM and m-PSD fractions. Protein categories analyzed in (A) are shown. Proteins found in SPM-DRM only, m-PSD only and both fractions are color coded. Numbers of proteins in each category are indicated in parenthesis. CSK, P-ases and Small Gs refer to cytoskeleton, phosphatases and small G-proteins, respectively.
Effects of TX-100 concentrations on the isolation of SPM-DRM
We have previously determined that 0.15% (w/v) (the detergent: protein ratio is 5.25:1 (w/w)) is an optimal TX-100 concentration to prepare SPM-DRMs (Du et al. 2006). Higher detergent concentrations, such as 1%, solubilizes even the membrane raft components (Ostermeyer et al. 1999, Shogomori & Brown 2003, Murphy et al. 2004, Chamberlain 2004), whereas too low of a detergent concentration is inadequate to dissolve non-raft components. To explore the reason for the extensive protein overlaps between SPM-DRMs and m-PSDs, we examined the effects of various TX-100 concentrations more systematically on the SPM-DRM components.
The distribution profiles of SPM proteins from various SDG fractions after treatment with increasing concentrations of TX-100 (0.05, 0.15, 0.25, 1.0 and 5.0%) are shown in Fig. 3A. Fraction No. 5 was judged to be a main DRM fraction based on: (i) it has high cholesterol content (Mean ± S.E. = 751 ± 49 µg/mg, n=3; comparable to (Suzuki et al. 2001)) at 0.15% TX-100; and (2) it is most enriched with the membrane raft marker GM1 ganglioside at low TX-100 concentrations. The amount of total proteins in this fraction was decreased as the TX-100 concentration was increased, and became undetectable after solubilization with 1% and 5% TX-100 under our staining and visualizing conditions. A rough estimate suggested that about 80% of DRM proteins recovered in fraction No. 5 was lost when TX-100 concentration was raised from 0.15% to 1.0%. Thus, 1% TX-100 is too high of a concentration to prepare SPM-DRMs (from 0.5 mg SPM proteins in 1.75 mL of TNE buffer, in which the detergent : protein ratio was 35.0 : 1 (w/w)). The effects of high TX-100 concentrations are in good agreement with a previous report showing that excessively high detergent vs. protein ratio destroys membrane rafts (Murphy et al. 2004). The protein profiles also suggested that the amount of proteins recovered in the pellet fraction (No. 12), which contained PSDs, increased in parallel with the increasing TX-100 concentrations up to 1.0% (Fig. 3A). Quantification of the pellet protein amounts confirmed that the protein yield after 1% TX-100 treatment was approximately twice as that after 0.15% TX-100 treatment (Fig. 3B). These results suggest that the TX-100 concentration is critical for preparation and analyses of SPM-DRMs and PSDs.
Fig. 3. Effects of increasing concentrations of TX-100 and cholesterol extraction on SDG isolation of SPM-DRM.
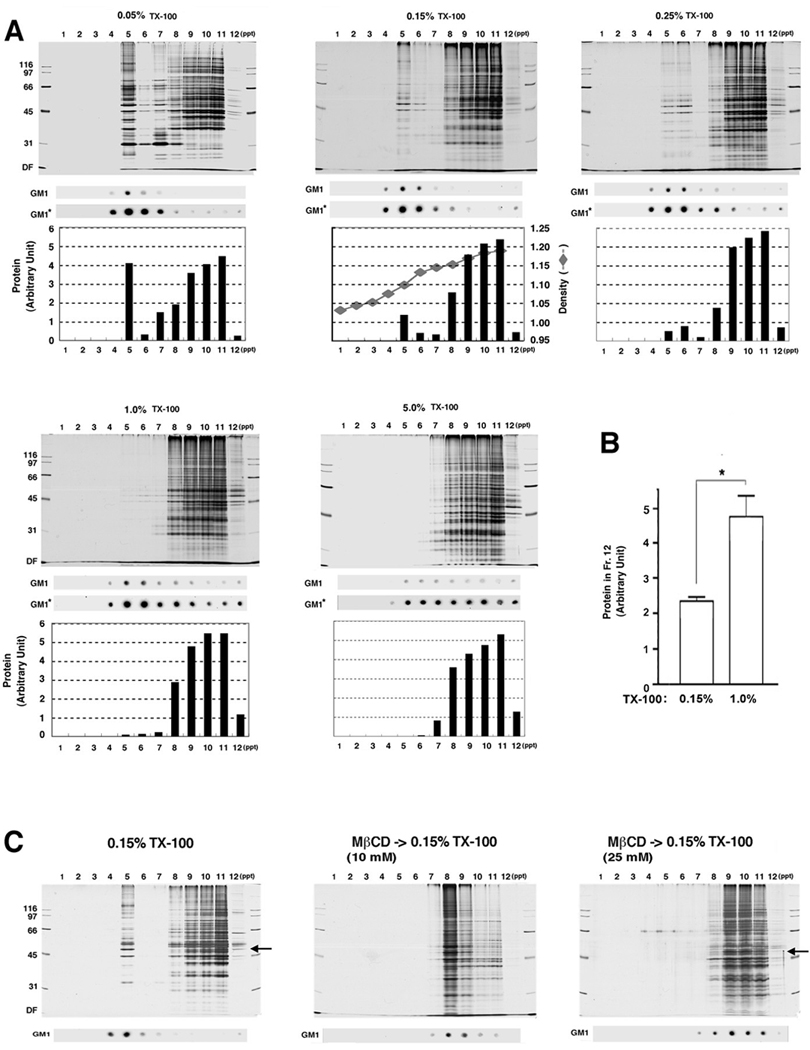
(A) Protein and GM1 ganglioside distributions under different concentrations of TX-100. SPM proteins were solubilized with TX-100 at 4°C at the concentrations indicated and subjected to SDG. Proteins separated by SDS-PAGE were silver-stained (upper), quantified with NIH Image and the total amount of proteins in each lane is plotted (lower). Total amounts of proteins in each of the 5 graphs were normalized to be equal for comparisons. GM1* dot blots indicate enhanced images of the above GM1 blots by increasing exposure time. Sucrose density is also plotted for 0.15% TX-100. (B) Comparison of protein recovery in the pellet between 0.15% and 1% TX-100. The pellet (ppt, fraction No. 12) obtained after treatment with 0.15% or 1.0% TX-100 was separated on SDS-PAGE, silver-stained, and total amount of proteins in each lane was measured by NIH Image. n = 3. (C) Effects of MβCD (10 or 25 mM) pretreatment (prior to TX-100) on protein profiles of each fraction. Proteins in the SDS-PAGE gel were silver-stained. CaMKIIα bands are indicated by arrows. The experiment was repeated three times with similar results. In this and following figures, numbers to the left of gel images indicated molecular weights in kDa. DF, dye front. Bars, SE. * p<0.05, unpaired Student's t-test.
To further ensure that fraction No. 5 on the sucrose gradient was indeed DRM-enriched, we treated SPM with MβCD, a cholesterol-depleting agent (Christian et al. 1997) prior to solubilization with 0.15% TX-100 (Fig. 3C). The prior MβCD treatment completely abolished proteins and GM1 ganglioside from fraction No. 5. Protein bands were undetectable in fractions No. 4–6 even with enhanced silver staining (data not shown). Instead, GM1 ganglioside, as well as proteins, distributed mostly at fraction No. 8 after treatment with 10 mM MβCD and further shifted to higher fractions at 25 mM MβCD. These results suggested that molecular components and subcellular structures from which fraction No. 5 was derived were tethered to cholesterol-enriched microdomains. Unexpectedly, less proteins were recovered in the pellet fraction after MβCD pretreatment (both 10 and 25 mM), including CaMKIIα, a typical PSD protein (Suzuki et al. 1994), suggesting that extracting PSDs from surrounding structures by TX-100 became more difficult after cholesterol depletion. Together, these cholesterol depletion experiments confirmed that postsynaptic membrane rafts are enriched in fraction No. 5.
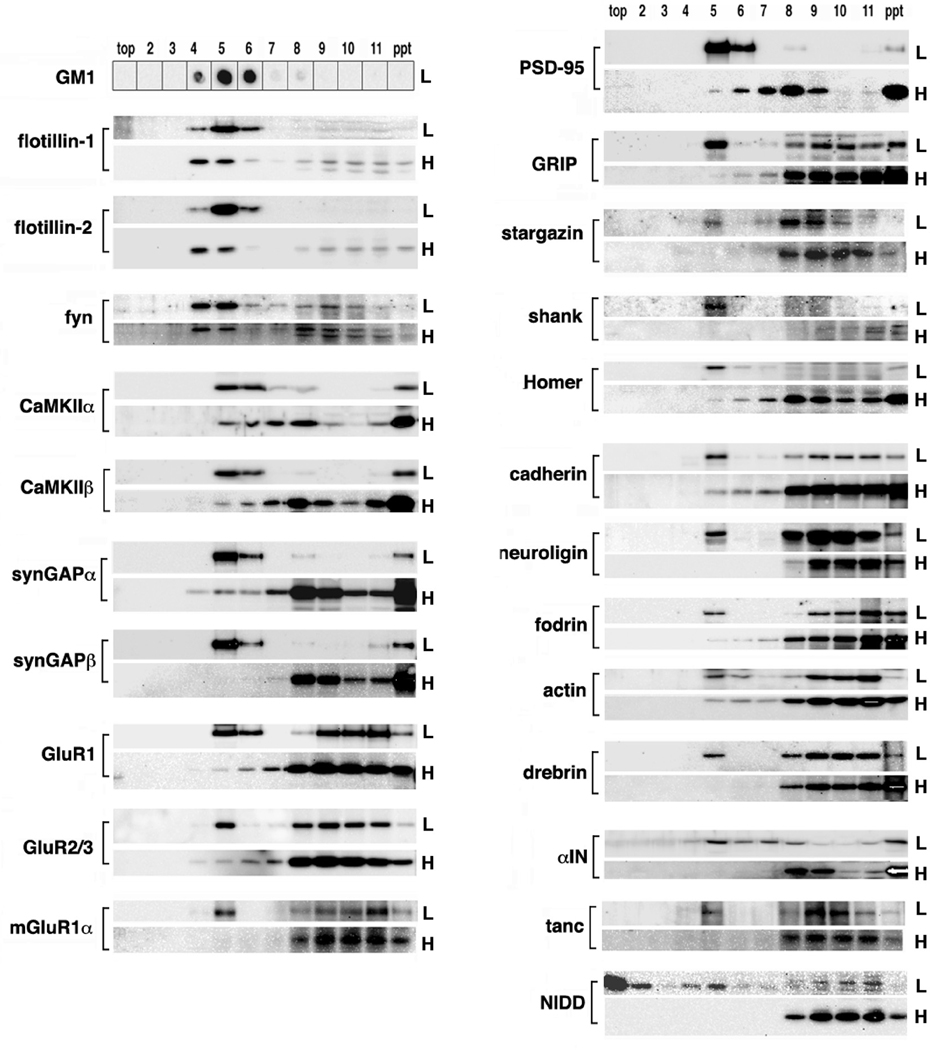
We have previously shown that many “PSD proteins” are present in the SPM-DRM (Suzuki et al. 2008). Here, we have extended this finding by examining the effects of low (0.15%) and high (1.0%) TX-100 concentrations on the SDG distribution profile of an additional number of PSD and raft marker proteins, using Western blotting (Fig. 4). All proteins tested showed enrichment in fraction No. 5 after 0.15% TX-100 treatment. After 1% TX-100 treatment, the membrane raft marker proteins, flotillin-1, 2 and fyn, showed a relatively small shift from DRM fractions to the heavier fractions, whereas other traditional PSD proteins showed a marked shift toward the heavier fractions, the pellet fraction in particular. This shift for PSD proteins was in good agreement with the profile change observed in Fig. 3A. The extent of the shift varied from proteins to proteins; for example, significant portions of CaMKII and PSD-95 remained in DRM fractions whereas many other PSD proteins were greatly diminished in the DRM coupled with a corresponding increase in the pellets. These Western blotting data confirmed the presence of a large number of conventional PSD proteins in the SPM-DRM and demonstrated their shifts from DRMs to pellets following high concentrations of TX-100 treatment.
Fig. 4. Distribution profiles of representative PSD proteins in the SPM-DRM at low and high TX-100 concentrations.
Proteins in the SPM fraction were solubilized with 0.15% (L) or 1.0% (H) TX-100 at 4°C and subjected to SDG. Selected proteins were detected by Western blotting. α-IN, α-internexin. Some of distributions after treatment with 0.15% TX-100 have been previously reported (Suzuki et al. 2008).
Electron microscopic examination of DRM and other SDG fractions
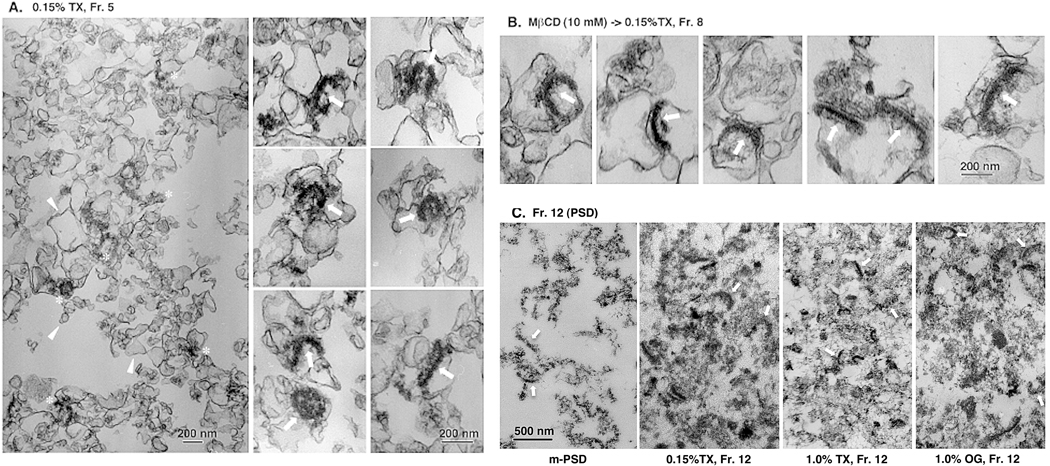
To further test the hypothesis that PSDs and postsynaptic rafts are integrally associated, we attempted to directly visualize structures contained in various SDG fractions by electron microscopy. Fig. 5A shows representative electron microscopic images of SPM-DRMs prepared by 0.15% TX-100. The SPM-DRM was packed with large amounts of membrane fragments or sacs and small amounts of electron-dense fuzzy structures. At a higher magnification, some of these structures were revealed to be PSDs associated with membrane fragments. Electron-dense PSD structures were also found in fraction No. 8 obtained after treatment with MβCD (10 mM, 4°C for 30 min) prior to 0.15% TX-100 treatment and SDG (Fig. 5B). Compared with those without MβCD pretreatment, the morphologies of PSD structures and PSD-associated membranes appeared more intact in MβCD-pretreated samples. For comparison, PSD structures contained in the m-PSD and fractions No. 12 (pellet) prepared under different detergent conditions are shown in Fig. 5C.
Fig. 5. Electron microscopic observation of raft-PSD complexes and PSD fractions.
(A) Electron micrographs of SPM-DRMs (fraction No. 5) obtained after 0.15% TX-100 treatment. One low (left) and six high (right panels) magnification photographs are shown. (B) Electron micrographs of fraction No. 8 obtained after treatment of SPM with MβCD (10 mM) prior to 0.15% TX-100 treatment and SDG. (C) Electron micrographs of m-PSD (fraction No. 12). The sharpness of images was enhanced using unsharp mask in Photoshop. Representative electron-dense fuzzy materials, membrane sacs of various sizes, and PSD structures are indicated by asterisks, arrowheads and arrows, respectively.
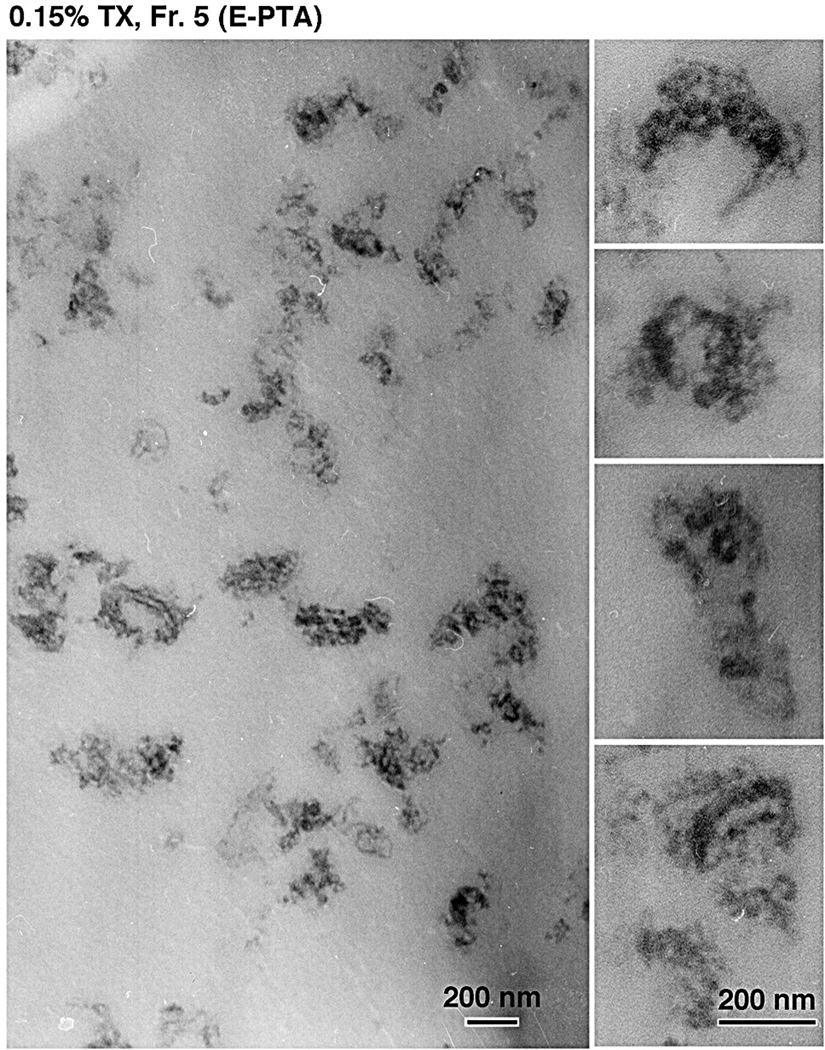
To ensure that the structures we observed were PSDs, we examined the DRM fraction by staining with E-PTA, which selectively stains presynaptic dense projections, cleft materials, PSDs and subsynaptic webs without staining membrane components (Bloom & Aghajanian 1966). E-PTA- stained synaptic complexes, though unfolded by the detergent action, were found to be scattered in the SPM-DRM (0.15% TX-100; Fig. 6), confirming the presence of PSD structures in SPM-DRMs. Fraction No. 5 prepared after 1.0% TX-100 also contained fuzzy electron-dense materials and membrane sacs, but not intact PSD structures (data not shown). We also confirmed the enrichment of PSD structures in the pellets obtained after 0.15% and 1% TX-100 treatments by the E-PTA method (data not shown).
Fig. 6. Verification of synaptic complexes in the SPM-DRM by E-PTA staining.
The SPM-DRM fraction obtained as in Fig. 5 was fixed with glutaraldehyde alone and stained with E-PTA. A low magnification (left) and four high magnification (right) photographs are shown. Structures stained were judged to be part of synaptic complexes (Bloom & Aghajanian 1966).
Absence of artificial reconstitution of once-solubilized membrane components and PSD structures
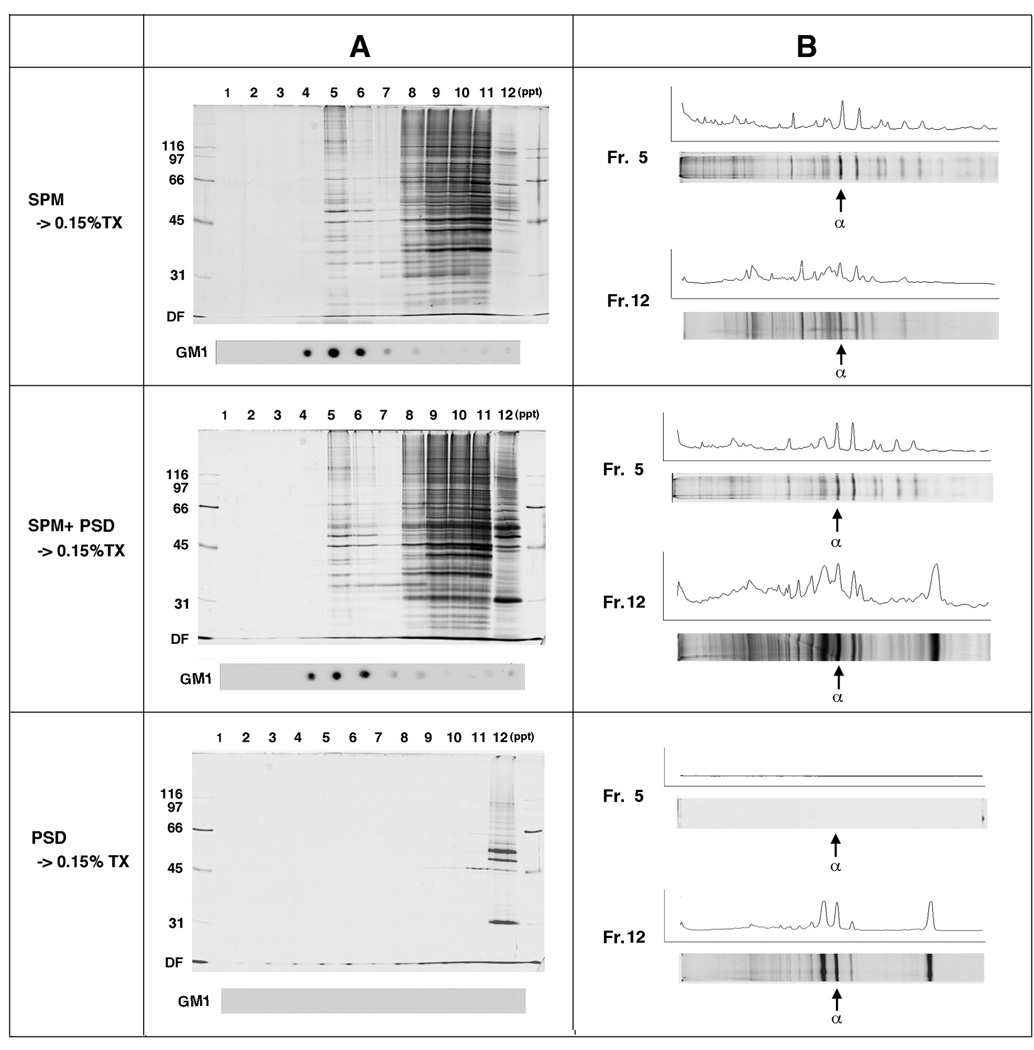
It is possible that once solubilized proteins or dissociated proteinaceous structures and membranes reassemble and form certain structures in vitro during the detergent-based DRM preparation procedures. Should this be the case, raft-PSD complexes found in the SPM-DRM could be artificially assembled structures. To test this possibility, we performed a reconstitution experiment (Fig. 7). SPMs were treated with 0.15% TX-100 in the presence or absence of purified PSDs, and the effect of PSD addition was analyzed. Densitometric profiles showed that adding PSDs only increased proteins in fraction No. 12 (PSD-containing pellet) without apparent changes in protein levels in other fractions. Thus, the exogenously added PSDs were not incorporated into the DRM fraction (No. 5) during detergent treatment. This experiment ruled out the possibility that PSD structures once dissociated from raft domains by detergent could reassemble with the raft domains. The result suggests that the PSD-membrane complexes found in the SPM-DRM are not artifacts formed in vitro; rather, they are derived in vivo.
Fig. 7. Retention of conventional PSD proteins in postsynaptic rafts is not due to the artificial reconstitution of once-solubilized membrane components and PSD structures.
(A) Protein profiles after SDG. Same amounts of SPM were treated with 0.15% TX-100 in the presence or absence of purified PSDs followed by SDG (upper 2 panels). For this experiment, PSD obtained by treatment of SPM with 1% octyl glucoside was used. The PSD alone was also treated in the same way and served as a control (bottom panel). Silver staining was carried out under approximately the same conditions. (B) Densitometric protein profile analyses of the lanes shown in (A). Arrows indicate CaMKIIα bands. The experiment was repeated twice with similar results.
Discussion
Proteins associated with SPM-DRM: potential roles of postsynaptic membrane rafts
In this paper, we performed a large-scale proteomics analysis of SPM-DRM and PSD fractions and observed extensive protein overlaps in the two postsynaptic structures. The results extend our previous Western blotting studies showing that many conventional PSD proteins are also present in the membrane rafts (Suzuki et al. 2008). Our data suggest potential interplays between the two subcellular structures in coordinating synaptic signaling processes and functions. The large heterogeneity of protein categories found in the SPM-DRM and PSD fractions suggests that postsynaptic rafts and PSDs are involved in diverse functions at postsynaptic sites. On the other hand, a substantial portion of proteins is specifically associated with either synaptic rafts or PSDs, supporting the notion that the two postsynaptic structures possess different roles in synaptic functions. Below, we discuss several major protein categories identified in our proteomic profiling, in the context of their potential functions and comparison with prior literature. This discussion helps generating insights into the roles that postsynaptic membrane rafts may play at the synapse under the following restriction. The molecular composition of postsynaptic membrane rafts and PSDs in different brain regions may differ, therefore, our proteomic profiles represent “molecular signatures” of these structures from heterogenous neuron populations. Also, independent approaches, such as immunofluorescence or immunoelectron microscopy, are needed in the future to confirm the PSD and/or raft localization of individual proteins identified, because contamination from different compartments cannot be avoided during subcellular fractionation (Suzuki et al. 2007).
Our proteomic analyses identified that typical raft-associated proteins, multiple cell adhesion molecules, channel/transporters and G-protein/small G-proteins reside more preferentially in the SPM-DRM. SPFH proteins contain the stomatin/prohibitin/flotillin/HflK/C (SPFH) domain (also known as the prohibitin domain) and share similar properties such as DRM localization, possibly through cholesterol binding (Huber et al. 2006) or oligomer formation (Browman et al. 2007). However, distributions and roles of SPFH proteins, including prohibitin-1, -2, flotillin-1 and -2, stomatin, stomatin-like protein 3, podocin and SPFH1 and 2 (erlin-1 and -2), differ among the members. SPFH1 and 2 are localized to the membrane raft-like domains in the ER membrane (Pearce et al. 2009). Prohibitin is well-known as chaperones involved in the stabilization of mitochondrial proteins (Mishra et al. 2005). This protein is also enriched in the PSD and dendritic spines, may play a role in regulating spine morphology, and may be involved in schizophrenia (Smalla et al. 2008). Paralemmin is associated with the cytoplasmic face of the plasma membranes of postsynaptic specializations, axonal and dendritic processes, spines and perikarya, and intracellular vesicle pools (Kutzleb et al. 1998). Striatin family proteins (striatin, SG2NA and zinedin) are molecular scaffolds that are mainly expressed in the nervous system and localized to dendritic spines (Gaillard et al. 2006, Benoist et al. 2008), and may be involved in intraspine vesicular trafficking (Benoist et al. 2006). Immunogold electron microscopic studies demonstrated their localization in the cytoplasmic side of PSDs, either immediately close to or apart from the PSDs (Kachidian et al. 1998). This is consistent with our findings that striatin family proteins were recovered in the SPM-DRM, and supports the presence and roles of membrane raft domains at postsynaptic sites. Other typical raft-associated proteins in neuronal tissues, such as the calmodulin-binding NAP22 and growth associated protein 43 (GAP43), were also preferentially localized in the SPM-DRM (Maekawa et al. 1997). Among 24 cell adhesion-related molecules identified in our proteomics screening, many were either preferentially localized in the SPM-DRM or in both SPM and m-PSD fractions. Cell adhesion points and cell-cell contact regions are particularly enriched in membrane rafts (Gaus et al. 2003), consistent with a major role for cell adhesion regulation in membrane rafts.
Various channels/transporter family proteins and integral membrane proteins with multiple transmembrane domains were detected preferentially in the SPM-DRM or in both the SPM-DRM and m-PSD. Presence of H+-transporting ATPase in the SPM-DRM is in good agreement with a previous report (Yoshinaka et al. 2004). Na+, K+-ATPase, a Na pump, is enriched in the synapse and plays important roles in the maintenance of proper ionic gradients across the membrane and neuronal excitability (Zhang et al. 2009). Many membrane proteins, including integral multi-pass membrane proteins, are known to be DRM proteins (Blonder et al. 2004). Some of these proteins directly coalesce into raft via lipid shells surrounding specific proteins in the lipid bilayer (Anderson & Jacobson 2002). Our findings imply activities for channels and transporters in membrane rafts. Presence of membrane receptors, another type transmembrane proteins, in DRM were also in good agreement with the previous reports (Hou et al. 2008, Marchand et al. 2002, Li et al. 2007, Bruses et al. 2001, Allen et al. 2007).
Enrichment of G-proteins, small G-proteins and related proteins suggest roles for synaptic membrane rafts in G-protein-coupled signaling. Many G-proteins are palmitoylated and/or myristoylated, and reversibly associate with the cytoplasmic leaflet of the DRM bilayer (Blonder et al. 2004, Allen et al. 2007, Li et al. 2003). Various members of Rab family GTPases play roles in membrane trafficking processes (Zerial & McBride 2001). Rac, a Rho family GTPase, is a regulator of cytoskeletal organization and is also implicated in the regulation of endosome recycling (Ridley 2001, Ridley 2006). Several Rho family members are believed to direct actin assembly to membrane fusion sites. Rap-related molecules, which are involved in spine size regulation (Pak et al. 2001), are also present in the SPM-DRM. Membrane rafts may link actin-based vesicular trafficking (Li et al. 2003) and targeting to the PSD. Presence of actin, α-actinin (Pavalko et al. 1991), actin-related protein (ARP), Rho family proteins and Wiskott Aldrich syndrome protein (WASP) in SPM-DRM, m-PSD, or both suggests interplay between postsynaptic rafts and PSDs in the remodeling of actin cytoskeleton.
Proteins in the molecular chaperones category appear to be evenly distributed in the PSD and raft although some proteins are more preferentially associated with either structure than others (Supplemental Table 1). The association of heat shock proteins with membrane rafts is consistent with a previous report (Chen et al. 2005). Localization of Hsc70 and Hsp40 in spines, where both proteins are localized to cytoplasmic regions close to or apart from PSDs (Suzuki et al. 1999), may suggest that membrane rafts are localized at the cytoplasmic side at postsynaptic sites. Hsp70 and J-type chaperones have been reported to associate with ribosome-bound nascent polypeptides and help proper protein folding (Craig et al. 2003). In addition, chaperonin (Hsp60)-containing T-complex protein (TCP) may assist folding of newly synthesizing proteins or refolding of denatured proteins (Kubota 2002). Thus, the presence of these proteins in postsynaptic membrane rafts suggests that they may participate in protein folding during postsynaptic local protein synthesis. Other possible roles for postsynaptic chaperones are transport of newly synthesized cholesterol from the ER to rafts (Uittenbogaard et al. 1998), and targeting of G-proteins, raft-enriched proteins, to raft locations (Waheed & Jones 2002). Our data also reveal that the vast majority of ribosomal proteins are preferentially distributed in m-PSD fraction, supporting a role for PSD in local protein synthesis as well. Precisely how the postsynaptic membrane rafts and PSDs coordinate to regulate protein synthesis in dendritic spines await further investigations.
Association of and interplay between postsynaptic rafts and PSDs
Our biochemical analysis coupled with electron microscopic observation of SPM-DRMs obtained after treatments with varying concentrations of TX-100 reveals the existence of raft-PSD complexes in the SPM-DRM fraction (Fig. 5). The PSDs contained in the SPM-DRM are associated or surrounded with many membrane fragments, possibly consisting of fused membrane raft domains. No PSD structures free from membrane fragments are observed in the SPM-DRM fraction, as expected from the heavier density for PSD, thus excluding the possibility of SPM-DRM contamination by isolated PSD structures. Complex formation with membrane rafts nevertheless allows recovery of some membrane-associated PSDs in the lighter DRM fraction. The PSD-raft association is verified by electron microscopic examination of the synaptic complex-selective E-PTA staining (Fig. 6). The association of PSDs with raft membranes is dissociated by increased TX-100 concentration, shifting the dissociated PSDs to the pellet. The presence of raft-PSD complexes in the DRM explains the abundance of conventional PSD proteins as SPM-DRM components. However, a large number of PSD proteins are not detected in the SPM-DRM (Figs. 1, 2), possibly due mainly to the low abundance of raft-PSD complexes in the SPM-DRM.
The PSD-raft association is further supported by the cholesterol-depletion experiment, where PSD-membrane sacs association is unexpectedly retained (Fig. 5B). The association could also be supported by the general notion that membrane rafts are closely associated with cytoskeleton, which has been visualized in the DRM at the electron microscopic level (Suzuki et al. 2001, Olive et al. 1995, Chang et al. 1994, Bouillot et al. 1996, Lisanti et al. 1994, Nebl et al. 2002). Association with cytoskeleton has also been observed in caveolar membranes, a specialized form of membrane rafts (Smart et al. 1995). Presence of cytoskeleton-enriched DRM (H-DRM) (Nebl et al. 2002) and presence of many cytoskeleton-related proteins in the DRM (Allen et al. 2007, Nebl et al. 2002, Li et al. 2004b, Shaw & Li 2003) further support linkage of PSDs with membrane rafts.
Our reconstitution experiment (Fig. 7) rules out the possibility that PSD structures previously dissociated from membrane rafts by detergent are incorporated into DRM in the presence of TX-100, suggesting that the raft-PSD association exists in vivo. This experiment was not intended to address whether detergent extraction of membranes could extract proteins from PSDs and artifactually deposit them in DRMs; rather, it was designed to test the possibility of potential artificial contamination of membrane rafts by once solubilized, morphologically intact PSD structures, because we observed these structures associated with SPM-DRMs under EM. The results presented in Fig. 7 strongly suggest that such contamination was unlikely. We also note that fusion and enlargement of raft membranes associated with PSDs are likely to occur, as is suggested in other DRMs prepared in the presence of detergent (Brown 2007). Formation of raft-PSD complexes in vivo suggests that association with membrane raft domains may be necessary for PSDs to function in the synapse in vivo.
It should be noted that some so called “PSD proteins” also reside in postsynaptic cytosolic compartments, apart from PSDs. These extrasynaptic “PSD proteins” can also associate with membrane raft domains if they have raft-philic properties (Brown 2007). Raft-philic “PSD proteins” may come and go among postsynaptic rafts, PSDs, other cellular structures or cytoplasm. Raft-philic proteins may bind to membrane rafts either directly or indirectly. Some proteins can become raft-philic by acquiring raft-targeting motif(s), or vise versa, by protein modifications such as palmitoylation/depalmitoylation and phosphorylation/dephosphorylation (Besshoh et al. 2005, Palestini et al. 2000, Wong & Schlichter 2004, Kang et al. 2008). Such modifications could shift the ratio of conventional PSD proteins between DRMs and PSDs, and may play a role in cellular processes that mediate development (Besshoh et al. 2007), transient global ischemia (Besshoh et al. 2005), synaptic activation (Gil et al. 2006) and spatial memory formation (Delint-Ramirez et al. 2008). Together, dynamic movements of raft-philic “PSD proteins” can facilitate interactions between postsynaptic membrane rafts and PSDs in fulfilling certain cellular functions.
In conclusion, our proteomics analysis demonstrates unique distribution profiles of large amount of synaptic proteins in the SPM-DRM and PSD fractions. Our electron microscopic study visualizes for the first time the raft-PSD complexes, and our in vitro reconstitution experiments suggest that these raft-PSD complexes represent in vivo interaction. Association of PSDs with postsynaptic membrane rafts may be necessary for the PSD to perform its function in synaptic transmission, signaling and plasticity.
Supplementary Material
Acknowledgment
We thank Ms. Yi-Ling Lu, Harvard Medical School and New England Primate Research Center, for her comments and help in typewriting the manuscript. This work was supported, in part, by a National Center for Research Resources grant RR000168 (to NEPRC), National Institute of Health grants NS057311 and DA021420 (W.-D.Y.), and a Harvard Milton Fund (W.-D. Y.).
Abbreviations used
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- DRM
detergent-resistant membrane
- m-PSD
PSD prepared from SPM
- PSD
postsynaptic density
- PTA
phosphotungstic acid
- SPM
synaptic plasma membrane
- SPM-DRM
SPM-derived DRM
- MβCD
methyl-β-cyclodextrin
- SDG
sucrose density gradient centrifugation
- TX-100
Triton X-100
Footnotes
The authors have no conflicts of interest to declare.
References
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Benoist M, Baude A, Tasmadjian A, Dargent B, Kessler JP, Castets F. Distribution of zinedin in the rat brain. J Neurochem. 2008;106:969–977. doi: 10.1111/j.1471-4159.2008.05448.x. [DOI] [PubMed] [Google Scholar]
- Benoist M, Gaillard S, Castets F. The striatin family: a new signaling platform in dendritic spines. J Physiol Paris. 2006;99:146–153. doi: 10.1016/j.jphysparis.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Chen S, Brown IR, Gurd JW. Developmental changes in the association of NMDA receptors with lipid rafts. J Neurosci Res. 2007;85:1876–1883. doi: 10.1002/jnr.21336. [DOI] [PubMed] [Google Scholar]
- Blonder J, Hale ML, Lucas DA, Schaefer CF, Yu LR, Conrads TP, Issaq HJ, Stiles BG, Veenstra TD. Proteomic analysis of detergent-resistant membrane rafts. Electrophoresis. 2004;25:1307–1318. doi: 10.1002/elps.200405891. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Aghajanian GK. Cytochemistry of synapses: selective staining for electron microscopy. Science. 1966;154:1575–1577. doi: 10.1126/science.154.3756.1575. [DOI] [PubMed] [Google Scholar]
- Bouillot C, Prochiantz A, Rougon G, Allinquant B. Axonal amyloid precursor protein expressed by neurons in vitro is present in a membrane fraction with caveolae-like properties. J Biol Chem. 1996;271:7640–7644. doi: 10.1074/jbc.271.13.7640. [DOI] [PubMed] [Google Scholar]
- Browman DT, Hoegg MB, Robbins SM. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17:394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Brown DA. Analysis of raft affinity of membrane proteins by detergent-insolubility. Methods Mol Biol. 2007;398:9–20. doi: 10.1007/978-1-59745-513-8_2. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Bruses JL, Chauvet N, Rutishauser U. Membrane lipid rafts are necessary for the maintenance of the (alpha)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J Neurosci. 2001;21:504–512. doi: 10.1523/JNEUROSCI.21-02-00504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH. Detergents as tools for the purification and classification of lipid rafts. FEBS Lett. 2004;559:1–5. doi: 10.1016/s0014-5793(04)00050-x. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Ying YS, Rothberg KG, et al. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994;126:127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR. Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res. 2005;81:522–529. doi: 10.1002/jnr.20575. [DOI] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- Cohen RS, Blomberg F, Berzins K, Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977;74:181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Eisenman HC, Hundley HA. Ribosome-tethered molecular chaperones: the first line of defense against protein misfolding? Curr Opin Microbiol. 2003;6:157–162. doi: 10.1016/s1369-5274(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I, Salcedo-Tello P, Bermudez-Rattoni F. Spatial memory formation induces recruitment of NMDA receptor and PSD-95 to synaptic lipid rafts. J Neurochem. 2008;106:1658–1668. doi: 10.1111/j.1471-4159.2008.05523.x. [DOI] [PubMed] [Google Scholar]
- Du F, Saitoh F, Tian QB, Miyazawa S, Endo S, Suzuki T. Mechanisms for association of Ca2+/calmodulin-dependent protein kinase II with lipid rafts. Biochem Biophys Res Commun. 2006;347:814–820. doi: 10.1016/j.bbrc.2006.06.162. [DOI] [PubMed] [Google Scholar]
- Gaillard S, Bailly Y, Benoist M, Rakitina T, Kessler JP, Fronzaroli-Molinieres L, Dargent B, Castets F. Targeting of proteins of the striatin family to dendritic spines: role of the coiled-coil domain. Traffic. 2006;7:74–84. doi: 10.1111/j.1600-0854.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Garner AE, Smith DA, Hooper NM. Visualization of detergent solubilization of membranes: implications for the isolation of rafts. Biophys J. 2008;94:1326–1340. doi: 10.1529/biophysj.107.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus K, Gratton E, Kable EP, Jones AS, Gelissen I, Kritharides L, Jessup W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100:15554–15559. doi: 10.1073/pnas.2534386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil C, Cubi R, Blasi J, Aguilera J. Synaptic proteins associate with a sub-set of lipid rafts when isolated from nerve endings at physiological temperature. Biochem Biophys Res Commun. 2006;348:1334–1342. doi: 10.1016/j.bbrc.2006.07.201. [DOI] [PubMed] [Google Scholar]
- Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Huang Y, Amato S, Snyder SH, Huganir RL, Man HY. Regulation of AMPA receptor localization in lipid rafts. Mol Cell Neurosci. 2008;38:213–223. doi: 10.1016/j.mcn.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Schermer B, Muller RU, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Vuillet J, Bartoli M, Castets F, Nieoullon A, Kerkerian-Le Goff L. Relationships between striatin-containing neurons and cortical or thalamic afferent fibres in the rat striatum. An ultrastructural study by dual labelling. Neuroscience. 1998;85:111–122. doi: 10.1016/s0306-4522(97)00593-9. [DOI] [PubMed] [Google Scholar]
- Kang R, Wan J, Arstikaitis P, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H. Function and regulation of cytosolic molecular chaperone CCT. Vitam Horm. 2002;65:313–331. doi: 10.1016/s0083-6729(02)65069-1. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Kutzleb C, Sanders G, Yamamoto R, Wang X, Lichte B, Petrasch-Parwez E, Kilimann MW. Paralemmin, a prenyl-palmitoyl-anchored phosphoprotein abundant in neurons and implicated in plasma membrane dynamics and cell process formation. J Cell Biol. 1998;143:795–813. doi: 10.1083/jcb.143.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KW, Hornshaw MP, Van Der Schors RC, et al. Proteomics analysis of rat brain postsynaptic density. Implications of the diverse protein functional groups for the integration of synaptic physiology. J Biol Chem. 2004a;279:987–1002. doi: 10.1074/jbc.M303116200. [DOI] [PubMed] [Google Scholar]
- Li N, Mak A, Richards DP, Naber C, Keller BO, Li L, Shaw AR. Monocyte lipid rafts contain proteins implicated in vesicular trafficking and phagosome formation. Proteomics. 2003;3:536–548. doi: 10.1002/pmic.200390067. [DOI] [PubMed] [Google Scholar]
- Li N, Shaw AR, Zhang N, Mak A, Li L. Lipid raft proteomics: analysis of in-solution digest of sodium dodecyl sulfate-solubilized lipid raft proteins by liquid chromatography-matrix-assisted laser desorption/ionization tandem mass spectrometry. Proteomics. 2004b;4:3156–3166. doi: 10.1002/pmic.200400832. [DOI] [PubMed] [Google Scholar]
- Li W, Okano A, Tian QB, Nakayama K, Furihata T, Nawa H, Suzuki T. Characterization of a novel synGAP isoform, synGAP-beta. J Biol Chem. 2001;276:21417–21424. doi: 10.1074/jbc.M010744200. [DOI] [PubMed] [Google Scholar]
- Li X, Serwanski DR, Miralles CP, Bahr BA, De Blas AL. Two pools of Triton X-100-insoluble GABA(A) receptors are present in the brain, one associated to lipid rafts and another one to the post-synaptic GABAergic complex. J Neurochem. 2007;102:1329–1345. doi: 10.1111/j.1471-4159.2007.04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid Rafts As a Membrane-Organizing Principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S, Kumanogoh H, Funatsu N, Takei N, Inoue K, Endo Y, Hamada K, Sokawa Y. Identification of NAP-22 and GAP-43 (neuromodulin) as major protein components in a Triton insoluble low density fraction of rat brain. Biochim Biophys Acta. 1997;1323:1–5. doi: 10.1016/s0005-2736(96)00222-2. [DOI] [PubMed] [Google Scholar]
- Marchand S, Devillers-Thiery A, Pons S, Changeux JP, Cartaud J. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J Neurosci. 2002;22:8891–8901. doi: 10.1523/JNEUROSCI.22-20-08891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserini M, Palestini P, Pitto M. Glycolipid-enriched caveolae and caveolae-like domains in the nervous system. J Neurochem. 1999;73:1–11. doi: 10.1046/j.1471-4159.1999.0730001.x. [DOI] [PubMed] [Google Scholar]
- Matus A, Pehling G, Ackermann M, Maeder J. Brain postsynaptic densities: the relationship to glial and neuronal filaments. J Cell Biol. 1980;87:346–359. doi: 10.1083/jcb.87.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Murphy LC, Nyomba BL, Murphy LJ. Prohibitin: a potential target for new therapeutics. Trends Mol Med. 2005;11:192–197. doi: 10.1016/j.molmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Murphy SC, Samuel BU, Harrison T, et al. Erythrocyte detergent-resistant membrane proteins: their characterization and selective uptake during malarial infection. Blood. 2004;103:1920–1928. doi: 10.1182/blood-2003-09-3165. [DOI] [PubMed] [Google Scholar]
- Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- Olive S, Dubois C, Schachner M, Rougon G. The F3 neuronal glycosylphosphatidylinositol-linked molecule is localized to glycolipid-enriched membrane subdomains and interacts with L1 and fyn kinase in cerebellum. J Neurochem. 1995;65:2307–2317. doi: 10.1046/j.1471-4159.1995.65052307.x. [DOI] [PubMed] [Google Scholar]
- Ostermeyer AG, Beckrich BT, Ivarson KA, Grove KE, Brown DA. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. methyl-beta-cyclodextrin does not affect cell surface transport of a GPI-anchored protein. J Biol Chem. 1999;274:34459–34466. doi: 10.1074/jbc.274.48.34459. [DOI] [PubMed] [Google Scholar]
- Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Palestini P, Pitto M, Tedeschi G, Ferraretto A, Parenti M, Brunner J, Masserini M. Tubulin anchoring to glycolipid-enriched, detergent-resistant domains of the neuronal plasma membrane. J Biol Chem. 2000;275:9978–9985. doi: 10.1074/jbc.275.14.9978. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Otey CA, Simon KO, Burridge K. Alpha-actinin: a direct link between actin and integrins. Biochem Soc Trans. 1991;19:1065–1069. doi: 10.1042/bst0191065. [DOI] [PubMed] [Google Scholar]
- Pearce MM, Wormer DB, Wilkens S, Wojcikiewicz RJ. An endoplasmic reticulum (ER) membrane complex composed of SPFH1 and SPFH2 mediates the ER-associated degradation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2009;284:10433–10445. doi: 10.1074/jbc.M809801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Pierini LM, Maxfield FR. Flotillas of lipid rafts fore and aft. Proc Natl Acad Sci U S A. 2001;98:9471–9473. doi: 10.1073/pnas.181353098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Satoh K, Takeuchi M, Oda Y, Deguchi-Tawarada M, Sakamoto Y, Matsubara K, Nagasu T, Takai Y. Identification of activity-regulated proteins in the postsynaptic density fraction. Genes Cells. 2002;7:187–197. doi: 10.1046/j.1356-9597.2001.00505.x. [DOI] [PubMed] [Google Scholar]
- Shaw AR, Li L. Exploration of the functional proteome: lessons from lipid rafts. Curr Opin Mol Ther. 2003;5:294–301. [PubMed] [Google Scholar]
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smalla KH, Mikhaylova M, Sahin J, et al. A comparison of the synaptic proteome in human chronic schizophrenia and rat ketamine psychosis suggest that prohibitin is involved in the synaptic pathology of schizophrenia. Mol Psychiatry. 2008;13:878–896. doi: 10.1038/mp.2008.60. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Lipid rafts at postsynaptic sites: distribution, function and linkage to postsynaptic density. Neurosci Res. 2002;44:1–9. doi: 10.1016/s0168-0102(02)00080-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Isolation of synapse-subdomains by subcellular fractionation using sucrose density gradient centrifugation. In: Li KW, editor. Neuroproteomics “Springer Protocols Neuromethods. Vol. 57. Humana Press; 2011. pp. 47–61. [Google Scholar]
- Suzuki T, Du F, Tian QB, Zhang J, Endo S. Ca2+/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. J Neurochem. 2008;104:596–610. doi: 10.1111/j.1471-4159.2007.05035.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ito J, Takagi H, Saitoh F, Nawa H, Shimizu H. Biochemical evidence for localization of AMPA-type glutamate receptor subunits in the dendritic raft. Brain Res Mol Brain Res. 2001;89:20–28. doi: 10.1016/s0169-328x(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Okumura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+/calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J Neurochem. 1994;63:1529–1537. doi: 10.1046/j.1471-4159.1994.63041529.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tian QB, Kuromitsu J, Kawai T, Endo S. Characterization of mRNA species that are associated with postsynaptic density fraction by gene chip microarray analysis. Neurosci Res. 2007;57:61–85. doi: 10.1016/j.neures.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Usuda N, Murata S, Nakazawa A, Ohtsuka K, Takagi H. Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain. Brain Res. 1999;816:99–110. doi: 10.1016/s0006-8993(98)01083-x. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Waheed AA, Jones TL. Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J Biol Chem. 2002;277:32409–32412. doi: 10.1074/jbc.C200383200. [DOI] [PubMed] [Google Scholar]
- Wong W, Schlichter LC. Differential recruitment of Kv1.4 and Kv4.2 to lipid rafts by PSD-95. J Biol Chem. 2004;279:444–452. doi: 10.1074/jbc.M304675200. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Yamauchi Y, Shinkawa T, Taoka M, Donai H, Takahashi N, Isobe T, Yamauchi T. Molecular constituents of the postsynaptic density fraction revealed by proteomic analysis using multidimensional liquid chromatography-tandem mass spectrometry. J Neurochem. 2004;88:759–768. doi: 10.1046/j.1471-4159.2003.02136.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaka K, Kumanogoh H, Nakamura S, Maekawa S. Identification of V-ATPase as a major component in the raft fraction prepared from the synaptic plasma membrane and the synaptic vesicle of rat brain. Neurosci Lett. 2004;363:168–172. doi: 10.1016/j.neulet.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci. 2009;29:4498–4511. doi: 10.1523/JNEUROSCI.6094-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.