Abstract
Background
Previous studies have demonstrated impaired relational memory in schizophrenia. Here we studied eye-movement behavior as an indirect measure of relational memory, together with forced-choice recognition as an explicit measure.
Methods
Thirty-five patients with schizophrenia and thirty-five healthy participants were trained to associate a face with a background scene. During testing, scenes were presented as a cue and then overlaid with three previously studied faces. Participants were asked to recall the matching face, and both eye-movements and forced-choice recognition were recorded. During Non-Match trials, no faces matched the scene. During Match trials, one of the faces had previously been paired with the scene.
Results
On Non-Match trials, when no relational memory trace was present, both groups viewed the three faces equally. In contrast, on Match trials, control participants quickly (within 500 msec) and consistently (70–75% of test trial viewing) showed preferential viewing of the matching face. Viewing of the matching face was significantly delayed and reduced in schizophrenia participants. Forced-choice recognition of the matching face was also impaired in the patient group. An analysis of all correct Match trials revealed that preferential viewing was significantly reduced and delayed in participants with schizophrenia.
Conclusions
This study provides novel evidence for a specific relational memory impairment in schizophrenia. Patients showed deficits in their forced-choice recognition responses as well as abnormal eye-movement patterns during memory recall, even on trials when behavioral responses were accurate. We propose that eye-movements provide a promising new avenue for studying relational memory in schizophrenia.
Keywords: relational memory, eye-movement behavior, recognition memory, hippocampus, schizophrenia
Introduction
Memory impairment is a robust finding in studies of schizophrenia (1–5). In contrast to dementia or amnesia, however, schizophrenia is associated with less pronounced and more specific memory deficits (2, 6–9). Patients with schizophrenia exhibit the most severe impairments in episodic memory, which require that an item be bound to a particular temporal-spatial context (10–13). This “binding” aspect of memory is most directly assessed with tests of associative or relational memory, and several groups have demonstrated specific relational memory deficits in schizophrenia, above and beyond memory impairments for individual items (11, 12, 14–19). For example, patients with schizophrenia are especially impaired when asked to encode and retrieve the relationship between items (e.g., item hierarchy in a sequence (20), or learned pairs of items (19)). In healthy individuals, relational memory abilities are supported by the hippocampus (21–26), a region known to be abnormal in schizophrenia (27–38). Relational memory is also a core feature of conscious recollection, episodic memory retrieval and autobiographic memory, the disruption of which may play a critical role in the generation of psychotic symptoms (11).
Previous studies of relational memory in schizophrenia have employed only direct outcome measures, such as explicit recall and reaction times, as indices of memory ability (10). However, explicit responses represent an aggregate measure of a series of cognitive processes, including memory recall itself, supported by interactions between the medial temporal lobe and the frontal cortices (39), and response selection, supported by pre-frontal regions and the anterior cingulate cortex (40). As such, relational memory impairments may be confounded by a more generalized deficit of cognition. Since patients with schizophrenia have well documented deficits in executive function and response selection mechanisms (41), memory deficits observed in previous studies likely reflect the cumulative effect of impairments at multiple stages, from memory retrieval to response execution.
The current study addresses this limitation by employing a new experimental approach to assess relational memory. In addition to testing the conscious recollection of previously learned stimulus relationships, we also recorded eye-movements, which allows one to index immediate access to stored information without reliance on verbal reports, and may detect memory traces that do not reach conscious awareness (9, 42–45). Such experimental paradigms can quantify a participants’ ability to bind distinct elements of experience into new relational memory representations and to rapidly access them, in order to guide successful performance. The inclusion of eye-movement measures allows for assessment of very early memory processes in patients with schizophrenia, in relative isolation from additional domains of impairment.
The experimental paradigm employed here was used by Hannula et al. to demonstrate a marked relational memory deficit in patients with hippocampal amnesia (43). The current study extends this paradigm to schizophrenia. We tracked eye-movements during relational memory encoding and recall, and collected subsequent forced-choice recognition data for the trained associations. In the context of previous findings of impaired relational memory abilities in schizophrenia, we expected patients to show reduced preferential viewing of the matching face during testing, as well as impaired explicit memory performance in the subsequent recognition test.
Materials and Methods
Participants
We obtained written informed consent from 43 healthy control participants and 42 patients with schizophrenia (n = 28) and schizoaffective disorder (n = 14) after approval of the study protocol by the Vanderbilt University Institutional Review Board, Nashville, TN. Patients were recruited from the inpatient unit and outpatient clinic of the Vanderbilt Department of Psychiatry, as well as surrounding psychiatric caregiver communities. All participants underwent a detailed interview including the Structured Clinical Interview for DSM-IV (46) and were administered the North American Adult Reading Test as a measure of premorbid IQ (NAART; (47). All schizophrenia patients were assessed with the 17 item Hamilton Depression Rating Scale (48), the Young Mania Rating Scale (49), and the Positive and Negative Syndrome Scale (50). When available, the assessments of our research team were supplemented with clinical information obtained from the treating physicians. All participants with significant medical or neurological illness, significant head injury or a history of drug dependence were excluded. Control participants with a significant history of psychiatric illness or treatment with psychotropic medication were also excluded. Only participants who reported normal or corrected-to-normal eyesight and intact color vision were included. After task administration, 8 control participants and 7 patients with schizophrenia were excluded from further analysis due to either technical problems during the data collection (n = 5 control and 4 schizophrenia participants) or insufficient adherence to task instructions (n = 3 control and 3 schizophrenia participants). Our final study group (Table 1) included 35 control participants and 35 patients with schizophrenia (n = 25) and schizoaffective disorder (n = 10). We will refer to the patient group as the schizophrenia group. The two groups were closely matched for gender, age, handedness and parental education. One patient received haloperidol and two patients were taking no psychotropic medication at time of participation. All other patients received atypical antipsychotic medication. Chlorpromazine (CPZ) equivalent doses were calculated according to Woods (51).
Table 1.
Sociodemographic and clinical characteristics of participants
| Normal Control | Schizophrenia | |
|---|---|---|
| N=35 | N=35 | |
| Gender (male/female) | 19/16 | 18/17 |
| Age mean years (SD) | 35.1 (10.44) | 38.9 (11.13) |
| Participants Education mean years (SD) | 15.6 (2.21) | 13.1 (2.58)* |
| Parental Education mean years (SD) | 13.3 (2.26) | 12.6 (3.40) |
| Edinburgh Handedness (right/left/ambidextrous) | 28/3/4 | 27/3/5 |
| Premorbid IQ | 112 (5.93) | 105.7 (9.03)* |
| NAART (SD) | ||
| GAF (SD) | - | 44.2 (13.73) |
| HAM-D (SD) | - | 7.3 (6.91) |
| YMRS (SD) | - | 3.2 (3.31) |
| PANSS (SD) | - | 57.1 (13.75) |
| Duration of Illness mean years (SD) | - | 16.6 (11.08) |
| CPZ (SD) | - | 366 (310.59) |
NAART = North American Adult Reading Test scores
GAF = Global Assessment of Functioning
HAM-D = Hamilton Depression Rating Scale
YMRS = Young Mania Rating Scale scores
PANSS = Positive and Negative Syndrome Scale scores
CPZ = Chlorpromazine Equivalent doses
Significance threshold defined at p<0.001
Experimental paradigm
Apparatus
Eye position and movement was monitored at a rate of 60 Hz using an ISCAN (ISCAN, Inc., Woburn, MA, www.iscaninc.com) RK-630PCI remote eye tracker. Stimuli were presented on a 17-inch color display controlled by a Windows-based computer using Presentation Software (version 12.2; Neurobehavioral Systems Inc, Albany, CA, www.neurobs.com/presentation).
Stimuli
For each stimulus list, face stimuli consisted of 18 male and 18 female full-color face images, sized 224×224 pixels, on a 244×244 pixel uniform gray background. Background-scene stimuli were 36 full-color images of real-world scenes sized 640×480 pixels. Images were obtained from an existing database of 144 face and 144 background images (43).
Experimental design
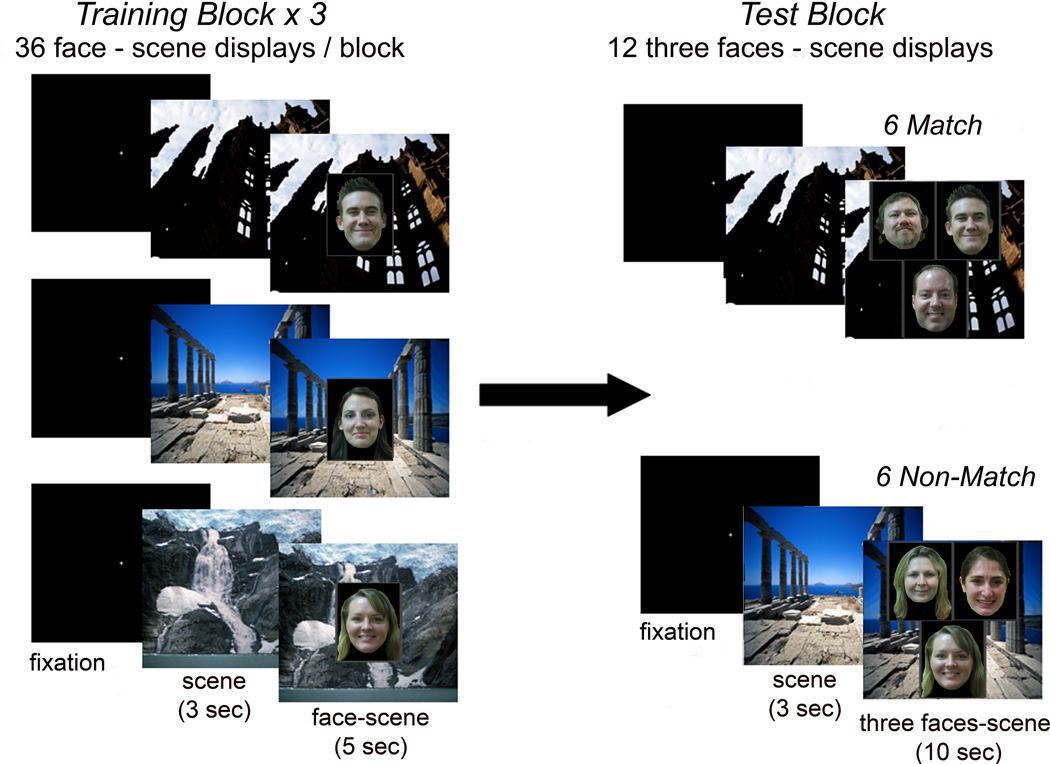
During training, participants viewed three consecutive, randomized study blocks composed of the same 36 face-scene pairs (Figure 1). The test phase followed immediately after completion of training and included 12 trials, each consisting of three faces overlaid on one scene. On the 6 Match trials, one of the three faces had been paired with the scene during the study phase, whereas on the 6 Non-Match trials none of the faces had been paired with that scene during training (Figure 1). All faces were equally familiar from the study period, and on Match trials the matching face was assigned equally often to the three display positions (i.e. upper left, upper right and bottom). Lists of stimuli were rotated and counterbalanced across participants to ensure each scene was paired equally often with each face across the study.
Figure 1.
Experimental paradigm
During each of three training blocks participants are presented with 36 face-scene pairs. Immediately following the conclusion of training, participants view 12 test displays, consisting of one of the background scenes from training, and three familiar faces.
Half of the test trials contain one face previously paired with the scene (‘Match’); the other half present three faces that were not paired with the scene during training (‘Non-Match’). Each trial is preceded by a 3-sec presentation of the scene in isolation.
Stimulus presentation
Eye tracking was performed under consistent room lighting conditions with participants sitting 35–40 inches from the computer screen facing the desktop eye tracking system. After visualization of the pupil and corneal reflection of the right eye, the eye tracker was calibrated using a 5-point spatial calibration procedure (center and 4 corners of the screen), which was repeated prior to each experimental block. Participants were allowed to take breaks between training blocks if necessary. An experimenter initiated each trial when the participant focused their eyes on a central fixation crosshair and reported they were ready to proceed. On training trials, a background scene was presented alone for 3-sec, followed by a 5-sec display of an individual face superimposed on the scene. Participants were instructed to carefully study and memorize face-scene pairings for a recognition test to follow (“I will begin showing you pictures of faces paired with background scenes. Please try to memorize which face goes with which scene because you will be tested on these pairings later”). Test trials began with a 3-sec presentation of a previously studied scene, followed by a 10-sec display of three faces superimposed on the scene. Participants were instructed to try to remember which of the three faces had been paired with the background scene during training, without giving an explicit response, and to keep their eyes focused on the computer screen, even if no matching face was detected.
Eye-movement recording
Eye-movements were recorded and analyzed for each test trial. Borders were defined around the face stimuli (244×244 pixel frame) to assign eye-movements to a particular display element (Training: Face or Background; Test: Face Upper Left, Face Upper Right, Face Bottom, or Background). Viewing measures included: 1) the duration of fixations on the display elements (faces and background), and 2) time-course measures of the proportion of time allocated to the different display elements across the 10-sec trial.
Explicit Memory Testing
To assess explicit recall of the face-scene pairings, we administered a subsequent 4-alternative forced choice memory test in 30 control and 31 schizophrenia participants after viewing of the 12 test trials was completed. No eye-movements were recorded in this phase. Participants viewed the 12 test displays in the same order as in the preceding test phase, and indicated the matching face by pressing the button corresponding to its position on the display, or pressing the space bar if they thought none of the faces had been paired with that scene during training.
Statistical analysis of behavioral and eye-movement data
Group differences in overall viewing patterns were tested using two statistical approaches, 1) an analysis of variance (ANOVA) for average viewing of individual faces and background during Match and Non-Match trials and 2) a regression analysis of total viewing of each display element across trial types using a generalized linear model. The time course of preferential viewing of the matching face during Match trials was compared between groups for the first two seconds of test display viewing using a repeated measures ANOVA including face type (matching, non-matching), time (8×250 ms bins) and group (control, schizophrenia). Explicit recall was compared between groups with 2-tailed, independent samples t-tests.
Results
Eye-movement behavior
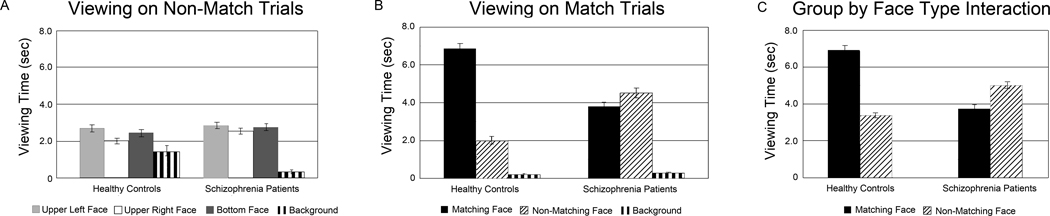
Participants spent most of the 10-sec trial viewing the display elements (Match: control subjects = 9.1 ±0.4 sec, schizophrenia subjects = 8.6 ±0.6 sec; Non-Match: control subjects = 8.6 ±0.6 sec, schizophrenia subjects = 8.4 ±0.8 sec), with minimal time spent on blinks or looking away from the computer monitor (Figure 3).
Figure 3.
Average viewing time of display elements for each group during Non-Match trials (Figure 3A), Match trials (Figure 3B) and across all trials (Figure 3C)
On Non-Match trials, when no relational memory trace is present, both groups view faces at the three screen locations equally. On Match trials, healthy control participants spend the majority of the 10-sec trial viewing the matching face, relative to the two non-matching faces. This preferential viewing is much reduced in schizophrenia patients, resulting in a significant group by face type interaction in the Match condition ((F(1,67) = 60.1, p < .001). A similar interaction between group and face type also emerges from a regression analysis of all test trials (Wald χ2 = 82.8, p < .001).
The Non-Match trials allowed us to study viewing patterns when no relational memory of face-scene pairs could guide eye-movements. The two groups did not differ significantly in how they viewed the three faces in the Non-Match trials (F(2,66) = 0.9, p = .42 for the interaction of the two main effects face location (upper left, upper right, bottom) and group (control and schizophrenia); Figure 3A). In contrast, the two groups differed significantly when viewing face-scene pairs during Match trials. The healthy control subjects spent significantly more time (6.9±2.0 sec) than the schizophrenia subjects (3.8±2.0 sec) viewing the matching face (F(1,67) = 60.1, p < .001 for the interaction of the two main effects face type (matching, non-matching) and group (control and schizophrenia); Figure 3B). Patients with schizophrenia and patients with schizoaffective disorder did not significantly different in their average viewing of the matching face (t(33) = 0.7, p = 0.51).
We entered all Match and Non-Match trials into a regression analysis of total viewing time, using a generalized linear model. This yielded a significant face type by group interaction (Wald χ2 = 82.8, p < .001; Figure 3C), in addition to significant main effects of group (Wald χ2 = 78.7, p < .001), face type (Wald χ2 = 218.2, p < .001), and face location (Wald χ2 = 13.4, p < .001; slightly greater viewing of face in upper left location, no significant group by location interaction). This provides compelling evidence for a selective relational memory deficit in schizophrenia: healthy participants demonstrated markedly greater viewing preference of the matching face compared to the non-matching face, whereas schizophrenia patients did not (Figure 3C).
Time-course analysis of proportional viewing time
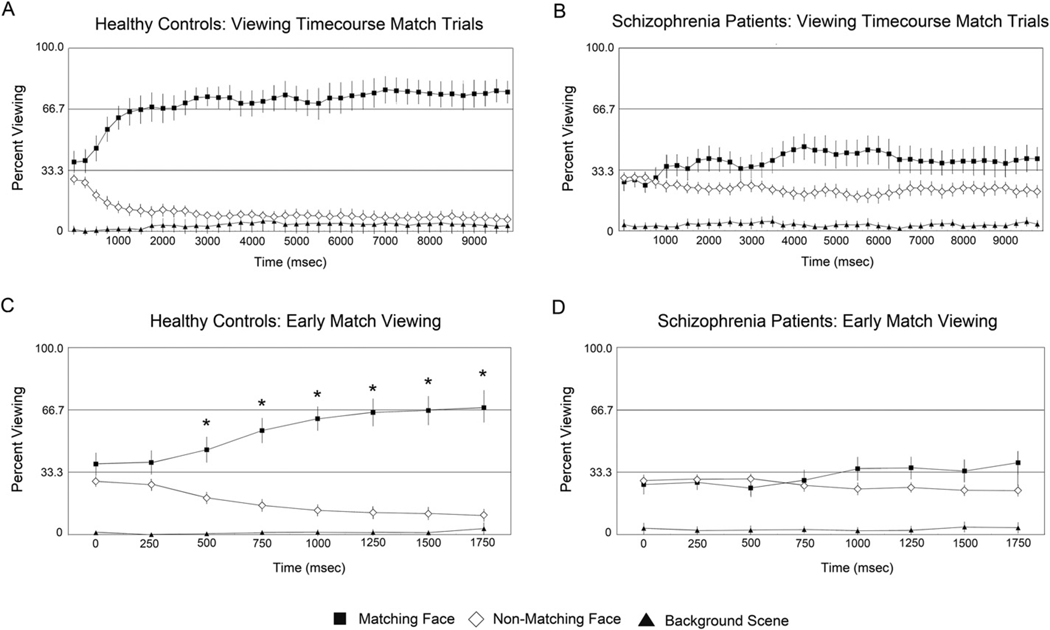
To better understand these differences between groups in overall viewing pattern, the average proportion of time spent on each of the different display elements (Matching face, Non-matching face, Background) was compared in 250 ms bins for Match trials (Figures 4A and 4B). This bin size was selected based on previous studies with this paradigm which demonstrated this organization is sufficient to capture the rapid onset of preferential viewing of the matching face (43, 45). To determine the onset of preferential viewing, we compared the percent viewing of the Matching Face to chance (i.e., 33.33%) at each time bin during the first 2 seconds of test stimulus viewing (Figures 4C and 4D). In control participants, preferential viewing of the matching face emerged within 500 ms (t(34) = 3.5, p<.05, corrected, Figure 4C), and remained well above chance levels (i.e, 70–75%) throughout (Figure 4A). In contrast, schizophrenia subjects failed to reach greater than chance preferential viewing for any time bin during the first 2 seconds (Figure 4D). Later time bins (between 4 and 6 seconds) revealed preferential viewing of the matching face, but this pattern was not as consistent and robust, never exceeding 50% (Figure 4B). This marked difference in proportional viewing time was confirmed by a significant 3 way interaction of face type (matching, non-matching) time (8×250 ms bins) and group (control, schizophrenia) during the first 2 seconds (F(7,62) = 2.4, p = .03).
Figure 4.
Average proportion of viewing time allocated to display elements over the 10-sec test trial for control participants (Figure 4A) and schizophrenia patients (Figure 4B) as well as the first two seconds of test display viewing for controls (Figure 4C) and patients (4D)
Bars plotted around the means represent 95% confidence intervals. For C and D, starred values indicate greater than chance (33%) viewing of matching face for an individual time bin during the first two seconds of display viewing, p < 0.05, Bonferroni corrected. In the control group, preferential viewing of the matching face emerges within 500 msec after presentation of the test face triad (Figure 4C). A strong preferential viewing pattern is maintained throughout the trial, with controls spending 70–75% of total viewing time on the matching face (Figure 4A). In contrast, for the schizophrenia group viewing of the matching face never exceeds chance levels during early display viewing (Figure 4D), and is not as robust as the control participants, never exceeding 50% for any individual time bin (Figure 4B).
Explicit relational memory testing
Explicit relational memory was assessed in a separate test block immediately following the recording of eye-movements in the majority of our sample (n = 30 healthy controls, 31 schizophrenia patients). Healthy controls were significantly more accurate than schizophrenia subjects on Match trials (mean accuracy and SD: control 94 ±10%, schizophrenia 51±28%, t(59) = 7.8, p < .001), Non-Match trials (control 76 ±28%, schizophrenia 25±30%, t(59) = 6.8, p < .001), and testing overall (control 85±15%, schizophrenia 38±27%, t(59) = 8.3, p < .001). While explicit relational memory of the schizophrenia subjects was impaired, they performed significantly better than chance (51% correct versus 25% chance performance) on the 4-alternative forced choice test (t(30) = 5.24, p < .001). This indicates that explicit relational memory for face-scene pairings was impaired, but not absent, in the schizophrenia group.
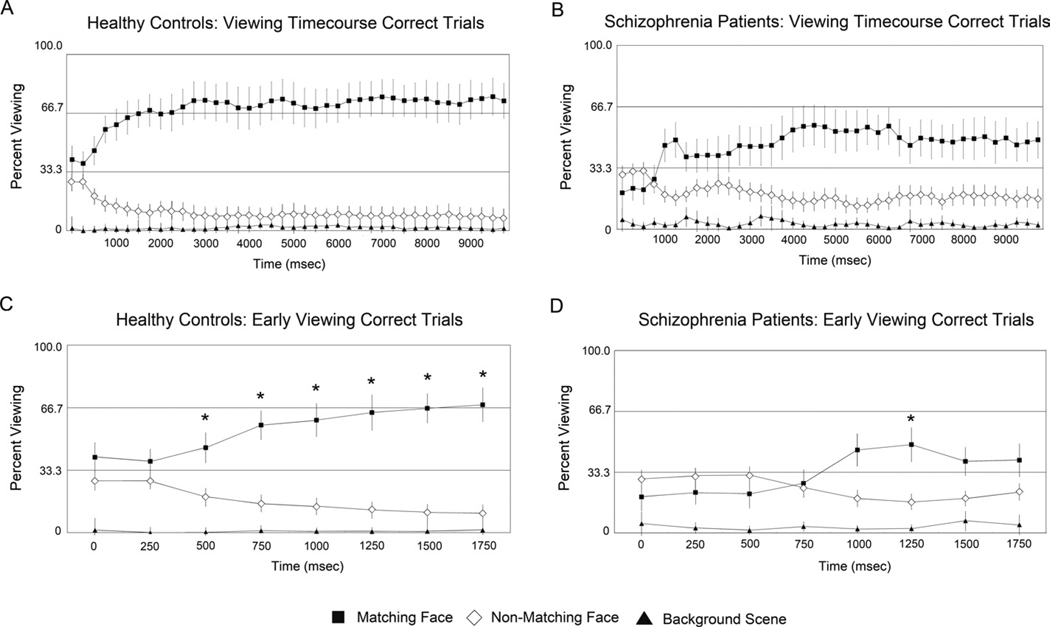
Correct trial analysis
We also analyzed eye-movements during all Match trials for which the face-scene pairings were subsequently correctly identified (168, or 93% of trials in control subjects and 93, or 50% in schizophrenia subjects). Both groups viewed the matching face preferentially, but the magnitude of this preference was much greater in the control subjects (6.8±1.6 sec) than the schizophrenia subjects (4.5±1.8 sec), resulting in a significant face type by diagnosis interaction (F(1,58) = 24.9, p < .001). In the control group, the time course for all (Figure 4) and correct (Figure 5) Match trials did not differ. In contrast, schizophrenia subjects showed more preferential viewing of the matching face in the correct trials, but viewing preference did not reach the normal pattern (Figure 5B). In schizophrenia subjects, only the 1250 ms time bin reached significantly greater than chance level (t(28) = 2.9, p < .05 corrected: Figure 5D). As seen in the analysis of all Match trials, the 3 way interaction of face type (matching, non-matching), time (8×250 ms bins) and group (control, schizophrenia) remained significant (F (7,51) = 2.8, p = .02). These results indicate that the early preferential viewing of the matching face was diminished in the schizophrenia group, even when subjects subsequently identified the face-scene pairing correctly.
Figure 5.
Correct trials only analysis. Average proportion of viewing time allocated to display elements for all correct trials, over the entire 10-sec trial for control participants (Figure 5A) and schizophrenia patients (Figure 5B) as well as the first two seconds of test display viewing for controls (Figure 5C) and patients (Figure 5D)
Bars plotted around the means represent 95% confidence intervals. For C and D, starred values indicate greater than chance (33%) viewing of matching face for an individual time bin during the first two seconds of display viewing, p < 0.05, Bonferroni corrected. Similar to the analysis of all trials, control participants show preferential viewing of the matching face within 500 msec of test display presentation (Figure 5C), with consistent viewing of the matching face at 70 – 75% throughout the 10-sec trial (Figure 5A). For schizophrenia patients, one time bin (1250 ms) does reach significantly greater than chance viewing (Figure 5D), but this preference is not a strong or as consistent as the control group, with viewing of the matching face never exceeding 60% for any individual time bin across the entire trial (Figure 5B). These data indicate that for the schizophrenia group, eye-movement measures of relational memory are abnormal relative to healthy controls, even for trials on which the face-scene pair is successfully identified during a subsequent recognition memory test.
Predictors of relational memory performance during testing
Relational memory performance during testing was quantified for all participants on explicit (percent correct on all trials, Match trials, and Non-Match trials) and eye-movement measures (average viewing of matching face on all trials, and correct trials only). For the control group, no demographic variable significantly predicted test performance. For schizophrenia patients, both premorbid IQ and parental education were strong predictors of all explicit measures of relational memory (IQ: all r > .47, all p < .008; parental education: all r > .40, all p < .04). In contrast, these factors did not strongly relate to eye-movement behavior, with only one significant correlation between parental education and viewing of the matching face on all trials (r = .35, p = .046). Relational memory performance was not significantly correlated with any other demographic variable, current medication (chlorpromazine equivalent doses), duration of illness, or current psychotic symptoms (PANSS). This pattern of results supports the idea that these forced-choice recognition and eye-movement measures index two distinct abilities, with the former being more tied to general intelligence and executive function than to pure memory ability.
Eye-movements during training
We did not find any significant difference between the two groups in the exploration of the face-scene pairs during training. On training trials, the groups spent equal time viewing the display (controls 4.2±0.6 sec, schizophrenia subjects 4.5±2.2 sec, F(1,64) = 0.9, p = .36), the faces (controls 3.8±0.6 sec, schizophrenia subjects 4.1±2.1 sec, F(1,64) = 0.6, p = .46) and the background scene (controls 0.4±0.3 sec, schizophrenia subjects 0.5±0.5 sec, F(1,64) = 0.7, p = .41). Groups also did not differ in the number of transitions between face and scene (controls 1.5±0.9, schizophrenia patients 1.6±1.4, F(1,64) = 0.2, p = .64) and both groups decreased the number of transitions over the course of training (significant main effect of training block F(2,63) = 4.6, p < .05, no training block by group interaction). These findings indicate that patients in the schizophrenia patient group did not show any global deficits in inspecting the visual displays, in distributing attention between faces and background scenes, or in showing the usual benefits of repeated presentations in their eye-movement patterns. Together with the findings of normal viewing times in the schizophrenia patient group for Non-match test trials, it appears the eye-movement abnormalities observed during Match trials are truly specific to the relational memory condition and are not driven by any gross abnormalities of eye-movements
Discussion
The results of the current study provide new and compelling evidence for a selective relational memory impairment in schizophrenia. We were able to demonstrate this deficit by studying eye-movements as an indirect measure of relational memory and by recording the explicit, forced-choice recognition of previously learned face-scene pairs. Control participants were able to search and find, with their eyes, the one face –among 3 equally familiar faces – that matched (i.e., had been previously studied with) the scene within 500 msec of viewing. In contrast, preferential viewing of the matching face was significantly delayed and reduced in magnitude for the schizophrenia participants, despite normal viewing patterns of the Non-Match displays which contain no relational memory information. This pattern of results was also present in an analysis of correct trials only, indicating the observed eye-movement abnormalities persisted even for trials on which the face-scene pair was correctly identified. Such a dissociation between explicit and eye-movement measures may indicate that schizophrenia patients invoke compensatory mechanisms to recognize the trained associations, as early, automatic relational memory processes are impaired relative to healthy controls.
Our findings provide novel support for the hypothesis that schizophrenia is associated with episodic and relational memory deficits (15, 16, 20, 52, 53). Whereas previous studies have employed traditional experimental approaches to study accuracy and reaction time during explicit memory tests (10), here we expanded the study of relational memory in schizophrenia to include indirect measures of memory via assessment of eye-movement patterns, using a paradigm that is sensitive to relational memory deficits in amnesia patients with medial temporal lobe (MTL) damage (43, 44). These measures are a useful addition to the study of relational memory in patients with schizophrenia as they do not rely on explicit verbal reports, and allow for the quantification of very early memory retrieval abilities in relative isolation from additional impairments in response selection and execution. The absence of a strong correlation between eye-movement measures and premorbid IQ/ parental education in our schizophrenia group further supports the notion that this indirect metric captures a memory ability that can be separated from forced-choice recognition, which is highly correlated with these general intelligence variables. The vast majority of previous eye-movement studies in schizophrenia have focused on either saccadic or smooth pursuit eye-movements (54) which have been linked to several cognitive processes, including attention, selection, expectation, working memory, prediction and mismatch detection (55). Here we provide evidence that the study of eye-movement behavior can reveal relational memory deficits in schizophrenia.
Impaired relational memory in schizophrenia may be due to deficits during encoding or retrieval of relational information. Lepage et al. (17) suggested that schizophrenia patients have difficulties forming relations between items during encoding, which manifest in impaired conscious recollection at test. According to our view of relational memory (25, 26, 56) as well as other memory frameworks (57), the binding of elements of episodes must be captured in hippocampal-dependent representations during encoding in order for successful retrieval of the relevant episodic content to occur during test. In our experimental paradigm, the background-scene preview presented during the first 3 sec of each test trial provides the opportunity for reactivation of the face-scene associations acquired during training (58). Retrieval of the relevant associations can occur very rapidly, as captured in the early onset of preferential viewing of the matching face in control participants. The impaired performance in the schizophrenia group observed here, including delayed and only modest preferential viewing of the matching face on Match trials, may be caused by insufficient relational memory binding during encoding, insufficient reactivation of relational representations during retrieval, or both.
Though this behavioral paradigm cannot directly index the neural basis of these between group differences, convergent lines of evidence indicate both the explicit and eye-movement measures of relational memory rely on the hippocampus. For one, patients with lesions to the MTL tested on this paradigm fail to develop preferential viewing of the matching face, in the context of impaired explicit relational memory retrieval (43). In addition, a recent fMRI study using this paradigm finds that the cued retrieval process utilized during the test trials involves the hippocampus (45), with evidence for increased hippocampal activation during the background-scene preview for trials on which the matching face is subsequently viewed preferentially (successful retrieval) relative to trials on which a non-matching face is viewed (unsuccessful retrieval). However, it is not known whether these eye-movement abnormalities are specific to hippocampal damage, or are also present in other patient populations with known memory impairments, such as patients with pre-frontal lesions. Further studies are needed to explore whether the well-known hippocampal abnormalities in schizophrenia (27–34) lead to the behavioral deficits observed in our study.
Face recognition has been studied extensively in patients with schizophrenia, typically revealing a deficit in processing of emotion information (59, 60), and the classification of a visual stimulus as a face (61). However, increasing information content and strength of facial signals, as well as prolonging the delay between presentation of stimuli can improve performance in face differentiation (62). In the current study, each face was presented for 5 sec during encoding, allowing for consolidation of individual images, making it unlikely that the observed relational memory deficits are due to impaired recognition of the face stimuli.
The current experiment revealed a specific relational memory impairment in schizophrenia, and demonstrated good correspondence between the eye-movement and explicit recognition measures despite several limitations. The number of test trials was relatively modest, including only 6 Match and 6 Non-Match trials, though this is comparable to previous investigations of relational memory in schizophrenia (18, 20). Furthermore, forced-choice recognition testing occurred in a separate phase, after the initial eye-movement testing phase. Both of these limitations can be addressed in future studies by implementing experimental designs with more test trials, while also assessing explicit recall after each test trial (45). Finally, almost all participants with schizophrenia were chronic patients, treated with antipsychotic medication. Future studies should explore eye-movement behavior as an index of relational memory deficits in the early stages of schizophrenia.
In summary, our study provides novel and compelling evidence for relational memory impairment in schizophrenia, as indicated by abnormal eye-movement behavior, even when explicit recognition is successful. We propose that eye-movements provide a promising new avenue for the study of relational memory in schizophrenia, as they allow for the assessment of rapid, non-verbal memory processes that are separable from, but likely contribute to, patients’ explicit memory deficits.
Figure 2.
Typical eye-movement behavior for Match trials in the control group (Figure 2A) and in the schizophrenia group (Figure 2B); matching face is in the bottom position
Circles indicate regions of fixation, and the radius of the circle reflects fixation duration (larger circles represent longer fixations). Solid lines represent the path of eye-movements on the display. Normal controls spend preferentially more time fixating on the matching face with limited exploration of the two non-matching faces or the background. In contrast, preferential viewing of the matching face is markedly reduced in the schizophrenia group with more transitions between the display elements.
Acknowledgments
Supported by: R01 MH070560 (SH)
Footnotes
Financial Disclosures:
Dr. Williams reports no biomedical financial interests or potential conflicts of interest. Dr. Must reported no biomedical financial interests or potential conflicts of interest. Ms. Avery reported no biomedical financial interests or potential conflicts of interest. Mr. Woolard reported no biomedical financial interests or potential conflicts of interest. Dr. Woodward reported no biomedical financial interests or potential conflicts of interest. Dr. Cohen reported no biomedical financial interests or potential conflicts of interest. Dr. Heckers reported he has received funding from the National Institute of Mental Health (R01-MH070560), and that he has no potential conflicts of interest.
References
- 1.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 2.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 3.Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- 4.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 5.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 6.Crespo-Facorro B, Barbadillo L, Pelayo-Teran JM, Rodriguez-Sanchez JM. Neuropsychological functioning and brain structure in schizophrenia. Int Rev Psychiatry. 2007;19:325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- 7.Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- 8.Ornstein TJ, Sahakian BJ, McKenna PJ. Memory and executive impairment in schizophrenia: comparison with frontal and temporal brain damage. Psychol Med. 2008;38:833–842. doi: 10.1017/S0033291707001468. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- 10.Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danion JM, Huron C, Vidailhet P, Berna F. Functional mechanisms of episodic memory impairment in schizophrenia. Can J Psychiatry. 2007;52:693–701. doi: 10.1177/070674370705201103. [DOI] [PubMed] [Google Scholar]
- 12.Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychol Rev. 2009;19:312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- 13.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 14.Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in Schizophrenia? A meta-analysis. Brain Cogn. 2003;53:121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 15.Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- 16.Huron C, Danion JM, Giacomoni F, Grange D, Robert P, Rizzo L. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry. 1995;152:1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- 17.Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: an event-related fMRI study. Biol Psychiatry. 2006;60:1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr Res. 2004;68:235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- 19.Luck D, Montoya A, Menear M, Achim AM, Lal S, Lepage M. Selective pair recognition memory impairment with no response bias in schizophrenia. Psychiatry Res. 2009;169:39–42. doi: 10.1016/j.psychres.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63:356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 21.Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–517. [Google Scholar]
- 22.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 23.Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Konkel A, Cohen NJ. Relational Memory and the Hippocampus: Representations and methods. Front Neurosci. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 26.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems fo the Brain. New York, New York: Oxford University Press; 2001. [Google Scholar]
- 27.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 28.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 29.Wood SJ, Velakoulis D, Smith DJ, Bond D, Stuart GW, McGorry PD, et al. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res. 2001;52:37–46. doi: 10.1016/s0920-9964(01)00175-x. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Sitoh YY, Ho YC, Poon LY, Subramaniam M, Chan YH, et al. Hippocampal volumes in first-episode psychosis. J Neuropsychiatry Clin Neurosci. 2009;21:24–29. doi: 10.1176/jnp.2009.21.1.24. [DOI] [PubMed] [Google Scholar]
- 31.Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- 32.Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, et al. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall J, Whalley HC, Marwick K, McKirdy J, Sussmann J, Romaniuk L, et al. Hippocampal function in schizophrenia and bipolar disorder. Psychol Med. 2009:1–10. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- 34.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 35.Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- 36.Schobel SA, Kelly MA, Corcoran CM, Van Heertum K, Seckinger R, Goetz R, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr Res. 2009;114:110–118. doi: 10.1016/j.schres.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss AP, Goff D, Schacter DL, Ditman T, Freudenreich O, Henderson D, et al. Fronto-hippocampal function during temporal context monitoring in schizophrenia. Biol Psychiatry. 2006;60:1268–1277. doi: 10.1016/j.biopsych.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- 40.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 41.Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Althoff RR, Cohen NJ. Eye-movement-based memory effect: a reprocessing effect in face perception. J Exp Psychol Learn Mem Cogn. 1999;25:997–1010. doi: 10.1037//0278-7393.25.4.997. [DOI] [PubMed] [Google Scholar]
- 43.Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J Cogn Neurosci. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- 44.Ryan JD, Hannula DE, Cohen NJ. The obligatory effects of memory on eye movements. Memory. 2007;15:508–525. doi: 10.1080/09658210701391022. [DOI] [PubMed] [Google Scholar]
- 45.Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth, Text Revision ed. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 47.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. The Clinical Neuropsychologist. 1989;3 doi: 10.1080/13854049008401493. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 50.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 51.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 52.Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elvevag B, Egan MF, Goldberg TE. Paired-associate learning and memory interference in schizophrenia. Neuropsychologia. 2000;38:1565–1575. doi: 10.1016/s0028-3932(00)00074-9. [DOI] [PubMed] [Google Scholar]
- 54.O'Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 2008;68:359–370. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 55.Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 2008;68:309–326. doi: 10.1016/j.bandc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 56.Ryan JD, Cohen NJ. Evaluating the neuropsychological dissociation evidence for multiple memory systems. Cogn Affect Behav Neurosci. 2003;3:168–185. doi: 10.3758/cabn.3.3.168. [DOI] [PubMed] [Google Scholar]
- 57.Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- 58.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 59.Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br Med Bull. 2008;88:43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- 60.Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Norton D, Ongur D, Heckers S. Inefficient face detection in schizophrenia. Schizophr Bull. 2008;34:367–374. doi: 10.1093/schbul/sbm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y, Norton D, McBain R, Ongur D, Heckers S. Visual and cognitive processing of face information in schizophrenia: detection, discrimination and working memory. Schizophr Res. 2009;107:92–98. doi: 10.1016/j.schres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]