Abstract
Understanding the immunological correlates associated with protective immunity following HCV re-exposure is a prerequisite for the design of effective HCV vaccines and immunotherapeutics. In this study we performed a comprehensive analysis of the innate and adaptive immunity following HCV re-exposure of two chimpanzees that had previously recovered from HCV-JFH1 infection. One of the chimpanzees, CH10274, became protected from active viremia by repeatedly challenges with homologous JFH1cc and developed neutralizing antibodies, but was later infected with high-level viremia by a heterologous challenge with H77 virus that persisted for more than one year. The other chimpanzee, CH10273 was protected from a similar, heterologous H77 challenge without any evidence of neutralizing antibodies. Peripheral HCV-specific T cell responses were present in both chimpanzees after challenges and interestingly the overall magnitude of response was lower in uninfected CH10273, which, however, exhibited a more robust CD8+ T cell response. CH10273 showed higher hepatic expression of CD8 and CD56 (natural killer) markers than CH10274 did shortly after inoculation with H77. The heightened T cell response was associated with an enhanced hepatic production of interferons (both type I and II) and interferon-stimulated genes (ISGs) in CH10273. Therefore protection or clearance of HCV reinfection upon heterologous re-challenge depends on the activation of both intrahepatic innate and cellular immune responses. Furthermore, our results suggest that serum neutralizing antibodies may contribute to early control of viral replication and spread after homologous HCV re-challenges but may not be sufficient for long-term protective immunity.
Conclusion
Our study shows that protective immunity against HCV re-infection is orchestrated by a complex network of innate and adaptive immune responses.
Keywords: HCV rechallenge, interferon response, T cell immunity, neutralizing antibodies
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a major public health burden worldwide because of the persistent infection and chronic liver disease that are hallmarks of the infection. There is no vaccine to prevent HCV infection and only a subset of patients respond to antiviral therapy 1. HCV infection is a highly dynamic process, with a viral half-life of only a few hours and average daily virion production of estimated 1012 particles in a given individual. This high replicative activity together with the lack of a proofreading function of the viral polymerase leads to a high genetic variability of HCV 2.
Approximately 30% of individuals spontaneously clear acute HCV infection. Clearance of HCV infection has been associated with a strong and sustained T-cell response targeting multiple HCV regions (for review, see 3). Although HCV-specific memory T-cells remain detectable for decades in patients with resolved HCV infection they appear not to be sufficient to prevent HCV infection upon re-exposure to the virus. However, the reduced risk of developing persistent HCV infection upon viral re-exposure in frequently exposed subjects indicates that the immune system can develop some degree of protective immunity against HCV 4–6. Thus, vaccine approaches that are capable of converting an evolving chronic infection into an acute self-limiting infection would have a substantial impact for protection of disease 7, 8.
Experimental HCV infection in chimpanzees is currently the only established in vivo model for the study of HCV infection. In contrast to humans, chimpanzees clear HCV infection more frequently (50–60%) 9, making it an attractive model to study immunological determinants involved in HCV clearance and protection. Several studies in chimpanzees demonstrated that protective immunity upon viral re-challenge with HCV of the same genotype and even with other genotypes is associated with a rapid and vigorous HCV-specific T-cell response and the induction of intrahepatic IFN-γ 10–13. But other studies showed that chimpanzees are not consistently protected even upon homologous re-challenge and in the presence of primed T cells 14, 15. Many studies in HCV-infected humans supported the importance of T cell-response in viral clearance either during primary infection or re-infection (for review, see 3). However, these studies investigated the peripheral immune response and did not explore intrahepatic immune responses in a comprehensive manner. These findings indicate that the immunological determinants mediating protective immunity are quite complex and not completely understood, and studies of intrahepatic immune responses may be crucial to understand these protective determinants.
To identify immunological determinants associated with protective immunity upon HCV re-exposure, we performed an extensive analysis of the innate and adaptive immune responses following HCV re-challenge in two chimpanzees that had previously recovered from primary HCV-JFH1 infection 16. Chimpanzee 10274 was repeatedly exposed to HCV-JFH1 to determine correlates of protective immunity against a homologous HCV strain. The chimpanzee then underwent a heterologous challenge with the HCV H77 strain (HCV genotype 1a). In contrast, chimpanzee 10273 was re-challenged with the HCV H77 strain in order to compare the quantity and quality of the induced immune responses. Following homologous and heterologous HCV re-challenges, we prospectively analyzed the intrahepatic immune response, the peripheral T-cell response, and the induction of neutralizing antibodies in relation to the clinical and virologic course of the animals.
MATERIALS AND METHODS
Chimpanzee and experimental infection
The housing, maintenance, and care of the chimpanzees (Pan troglodytes) in this study were in compliance with the Institutional Animal Care and Use Committee of the Centers for Disease Control and Prevention. Chimpanzee 10273 (CH10273 age 5, 20 kg,) a recovered animal initially infected intravenously in 2005 with 100 μl serum (9.6 × 106 copies) from a patient with fulminant hepatitis C, from whom the JFH-1 strain was isolated 17. Chimpanzee 10274 (CH10274, age 5, 22 kg) a recovered animal initially infected intravenously in 2005 with cell-culture derived HCV (JFH1cc, 1.4 × 107 copies) 16. Both animals had been tested negative for HCV RNA by RT-PCR in serum to and at the time of re-challenge. CH10274 was then experimentally re-challenged three times with cell-culture derived HCV (JFH1cc, 2x107 HCV copies) at 6-week interval (homologous challenges). At week 22, CH10274 was re-challenged with HCV H77 1a inoculum (CH1536 serum, 330 CID50) 18. CH10273 received a heterologous challenge with HCV 1a inoculum. All re-challenge inocula were given intravenously. Serum samples were collected at 3–4 days interval and tested for HCV RNA by quantitative real-time PCR and qualitative nested RT-PCR (detection limit: Cobas Monitor quantitative: 600 IU/ml, Cobas qualitative assay, 50 IU/ml). Serum samples were tested for HCV antibodies with the ORTHO version 3.0 enzyme-linked immunosorbent assay test system.
HCV proteins and peptides
Recombinant HCV core, helicase, NS5A and NS5B of genotype 1 were purchased from Mikrogen (Neuried, Germany). 15-mer peptides overlapped by 10 amino acids of the H77 strain (genotype 1a) were provided by the NIH AIDS Reagent Program and were pooled to generate one HCV core pool (27 peptides), two HCV NS3 pools (each with 30 peptides), two HCV NS5A pools (each with 33 peptides) and two HCV NS5B pools (each with 44 peptides). 15-mer peptides overlapped by 10 amino acids of the JFH-1 strain (genotype 2a) were purchased from Mimotopes (Richmond, VA) and were pooled to generate one HCV core pool (38 peptides), two HCV NS3 pools (each with 48 peptides) and two HCV NS5B pools (each with 48 peptides).
Neutralizing anti-HCV assay, ELISPOT, Intracellular cytokine staining (ICS) and quantitative RT-PCR
These methods are described in detail in Supporting Information. Primers and probes for qPCR of various genes are listed in Supporting Information.
RESULTS
Clinical and virologic outcome of HCV infection following HCV re-challenges
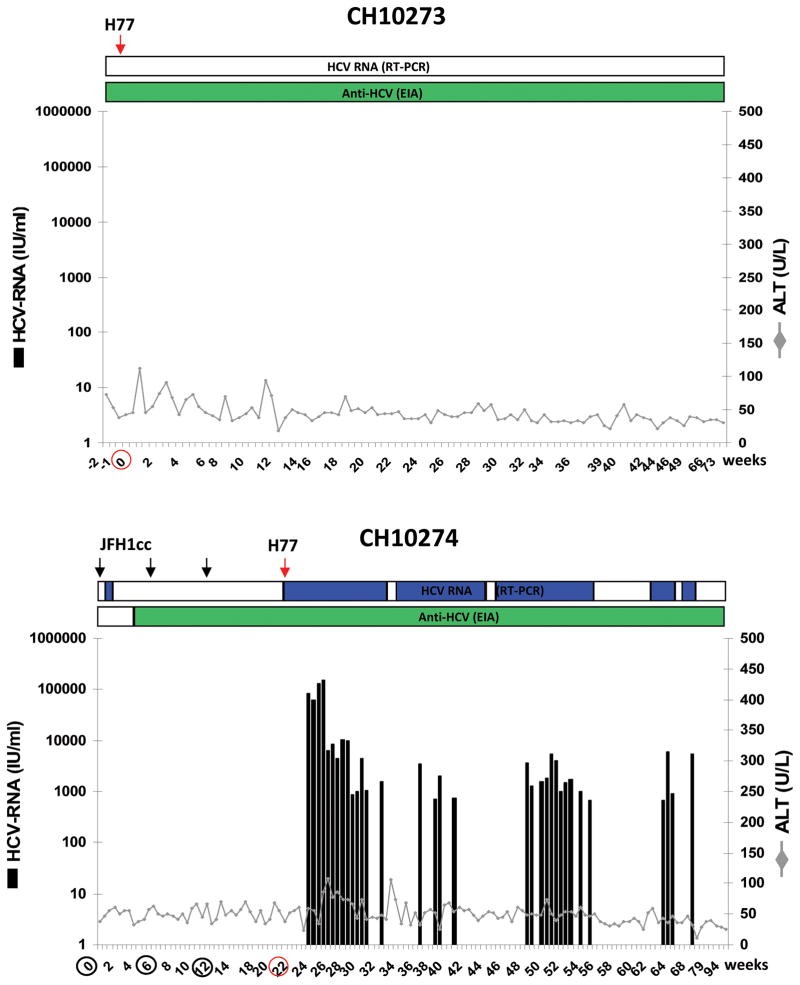
The chimpanzees CH10273 was part of a previous virologic study to assess the infectivity of cell-culture derived HCV (JFH1cc) and the corresponding HCV serum from the Japanese patient with fulminant hepatitis C. CH10273 was previously inoculated with HCV JFH-1 patient’s serum and became infected with low level of viremia. The HCV RNA in serum fluctuated and persisted until week 34, and HCV seroconversion was detected from week 20 after inoculation 16. In the present study, CH10273 negative for HCV RNA and anti-HCV positive was re-challenged with the H77 virus 23 months after the primary inoculation. Following the heterologous challenge, CH10273 did not become viremic but demonstrated mild elevation of liver enzyme values in two time points only (Figure 1). HCV RNA was also undetectable in the liver biopsy samples of the chimpanzee after the challenge, indicating that the chimpanzee was able to effectively control the infection if it were infected at all.
Figure 1. Clinical and virologic course of homologous and heterologous HCV re-challenges.
CH10273 was re-challenged with heterologous H77 (genotype 1a) at week 0. CH10274 was re-challenged three times with homologous JFH1-HCVcc (genotype 2a) at week 0, 6, and 12 and heterologous H77 (genotype 1a) at week 22. The course of infection was monitored by testing for HCV RNA (qualitative RT-PCR: top horizontal bar, blue as positive; real-time quantitative RT-PCR: black bars), HCV antibodies by ELISA, and ALT levels. Green horizontal bar indicates seroconversion. Arrows and circles indicate the time points of the re-challenges. ALT normal values (determined by using ten ALT determinations prior to the study): CH10273 < 76 U/L; CH10274 < 73 U/L.
CH10274 was previously inoculated with JFH1cc and became infected with low level of viremia. Serum HCV RNA disappeared at 9 weeks after inoculation and anti-HCV seroconversion was not observed 16. In the present study, CH10274 was re-challenged three times with homologous JFH1cc in 6-week intervals 18 months after the primary infection. HCV RNA became detectable in serum by RT-PCR 3 days after the first of three JFH1cc re-challenges and disappeared after 2 weeks. Anti-HCV antibodies were detected from week 4 after the first re-challenge (Figure 1). After a second JFH1cc re-challenge, CH10274 remained negative for HCV RNA by RT-PCR. Interestingly, ten weeks after the third challenge at week 22 of the experiment, low-level (<15 IU/mL) serum HCV RNA (JFH-1 sequences) was detected at the time when the chimpanzee was re-challenged with the heterologous H77 virus. The animal became viremic with H77 (JFH-1 sequence no longer detectable) and a peak titer of ~ 105 IU/ml at week 4 post-challenge and showed mild elevation of liver enzymes. Throughout the follow up, CH10274 had fluctuating, periodically non-quantifiable viremia. About 11 months after the heterologous HCV challenge, the animal cleared H77 infection and tested repeatedly negative for HCV RNA by RT-PCR (Figure 1).
Neutralizing antibody response following HCV re-challenge
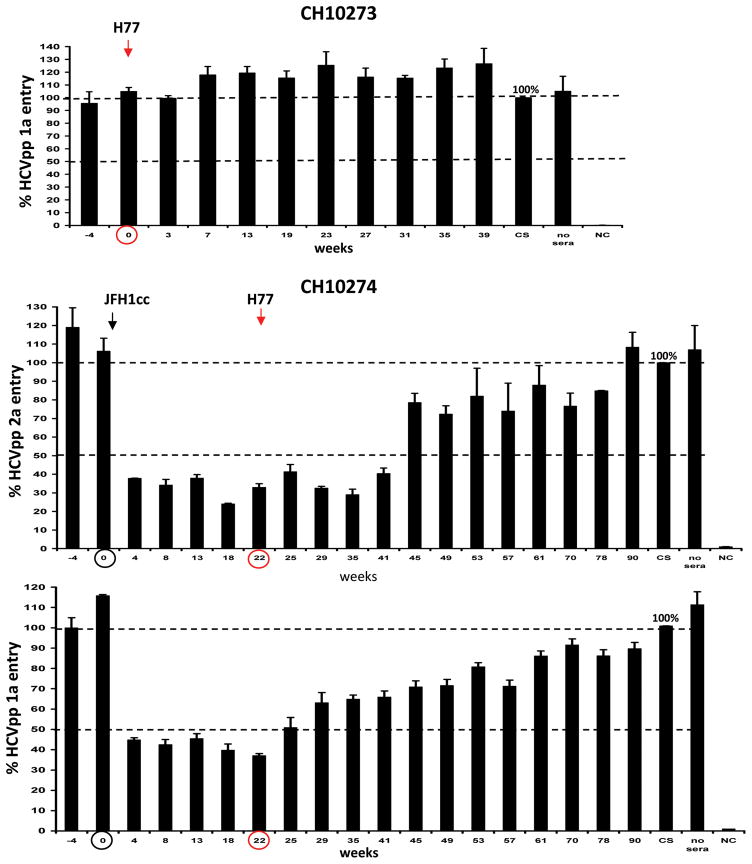
To evaluate determinants critical for protective immunity, serum samples of both chimpanzees were assessed for the presence of antibodies with neutralizing activity in an HCV pseudoparticle (HCVpp) assay. The protective immunity to HCV observed in CH10273 following heterologous challenge with the H77 virus was not associated with the induction of neutralizing antibodies against H77 HCVpp (Figure 2). In CH10274, the observed immunity to homologous rechallenge correlated with the development of neutralizing antibodies against the homologous HCVpp strain (JFH-1 HCVpp neutralization >50%). Serum samples not only neutralized HCVpp bearing the envelope glycoproteins of JFH1 (genotype 2a) but also H77 (genotype 1a) indicating the induction of cross-genotype neutralizing antibodies. Although cross-neutralizing antibodies were present at week 22, they did not prevent infection by the heterologous H77 virus (Figure 2). The heterologous challenge of CH10274 at week 22 with the H77 virus did not further induce neutralizing antibodies against either JFH-1 or H77 HCVpp and the antibodies gradually disappeared after the onset of H77 viremia (Figure 2).
Figure 2. Induction of cross-reactive neutralizing antibodies following homologous HCV re-challenge.
CH10273 was challenged with heterologous H77 at week 0. CH10274 was re-challenged three times with homologous JFH1-HCVcc (genotype 2a) at week 0, 6, and 12 and heterologous challenge with H77 (genotype 1a) at week 22. Serum samples of both chimpanzees were tested at the indicated weeks for the presence of neutralizing antibodies. For the determination of neutralizing antibodies, the percent of pseudoparticle infection bearing the JFH1 (genotype 2a) or the H77 (genotype 1a) envelope proteins was measured and compared to pseudoparticle infection in the presence of human control sera (=100%). Experiments were done in triplicates, and the standard deviation is indicated. The neutralizing activity was defined as ≥ 50% reduction in HCVpp entry indicated by a dashed line. The arrows and circle indicate the time points of the first homologous and heterologous HCV re-challenges.
Peripheral immune response following HCV re-challenge
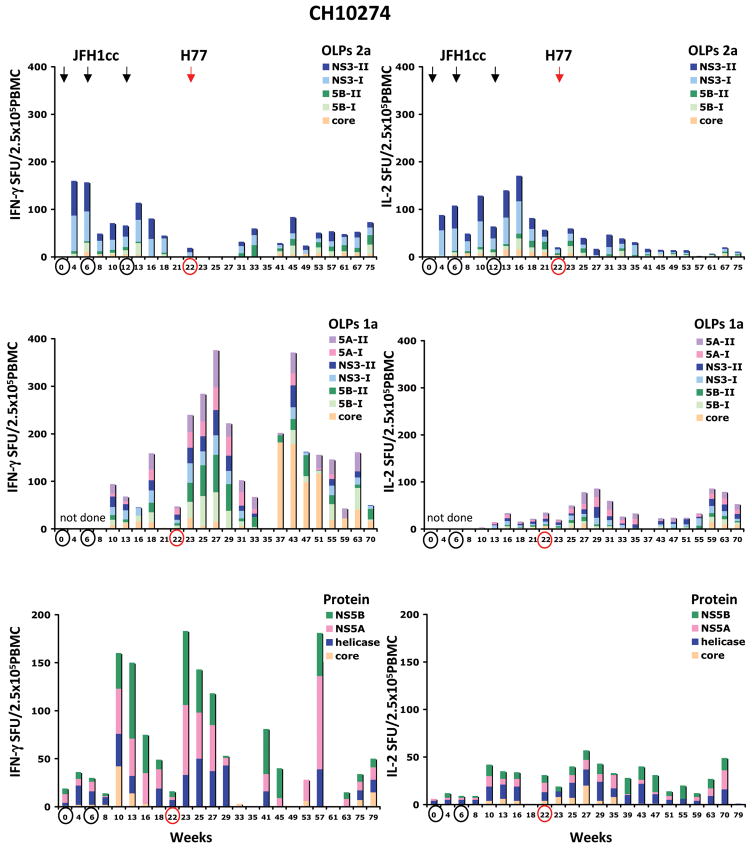
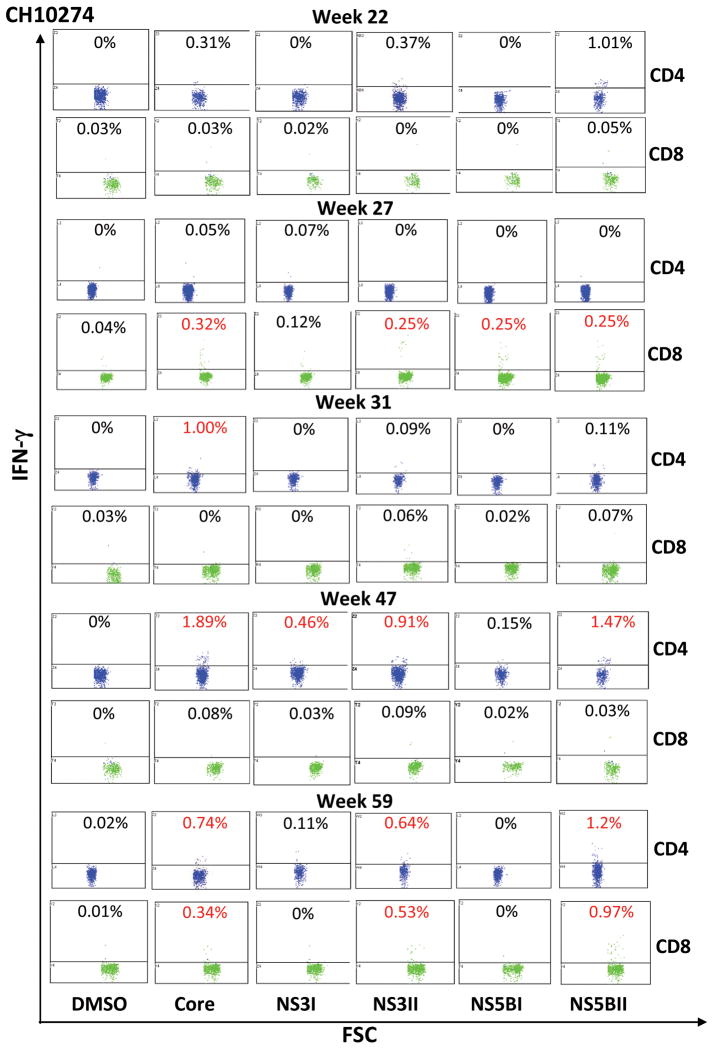
To investigate the kinetic of the HCV-specific T cell response following HCV re-challenge, PBMCs of both chimpanzees were incubated with a panel of overlapping peptide pools of genotype 2a (core, NS3, NS5B), genotype 1a (core, NS3, NS5A, NS5B) and HCV proteins of genotype 1 (core, helicase, NS5A, NS5B). The HCV-specific T cell response was quantified by IFN-γ and IL-2 ELISPOT analysis and intracellular cytokine staining (ICS) of IFN-γ. Prevention of re-infection of CH10273 following heterologous re-challenge with polyclonal H77 virus was associated with an enhanced frequency of IFN-γ producing HCV-specific T cells in response to multiple HCV peptides (genotype 1a) and HCV proteins (genotype 1). Interestingly, the magnitude of the induced HCV-specific T cell response was markedly lower compared to CH10274 who became re-infected following the heterologous re-challenge (Figure 3). Circulating HCV-specific T cells of CH10273 decreased progressively at week 11 after re-challenge. In contrast, IL-2 producing HCV-specific T cells following heterologous re-challenge were very weak or absent (Figure 3). Intracellular IFN-γ staining identified CD8+ T cells as the responding T cell population in CH10273 as evidenced by the enhanced frequency of IFN-γ producing CD8+ T cells specific for NS3 and NS5B peptides after the challenge (Figure 4).
Figure 3. Peripheral HCV-specific T cell response following HCV re-challenge.
Frequencies of IFN-γ and IL-2 producing T cells in response to HCV genotype 2a, genotype 1a overlapping peptide pools (OLPs) and HCV proteins (genotype 1) are shown as spot-forming units (SFU) per 2.5 × 105 PBMCs. T cell responses to OLPs of HCV genotype 2a comprise core, NS3 and NS5B, and of HCV genotype 1a comprise core, NS3, NS5A and NS5B. T cell responses to HCV proteins comprise core, helicase, NS5A and NS5B. Antigen-specific SFU was calculated by subtracting the average of background values (typically fewer than 10 spots) from that of the antigen-stimulated sample. Arrows and circles indicate the time points of the re-challenges. The weeks analyzed are indicated at the bottom of each graph. Weeks that are circled in black represent repeated JFH inoculations and week circled in red represents H77 challenge.
Figure 4. Intracellular cytokine staining of IFN-γ+ CD4 and CD8 T cells after HCV re-challenge.
The upper histogram shows the percentage of IFN-γ secreting CD4 (blue) and the bottom CD8 (green) cells in the CD3+ lymphocyte population. The percentage in red indicates value that is significantly above the DMSO background sample (>0.25%) and at least twice of the baseline value (week 0 for CH10273 and week 22 for CH10274). FSC - forward scatter.
The protective immune responses capable of controlling active viremia in CH10274 following homologous JFH1cc re-challenges correlated with the induction of IFN-γ and IL-2 producing T cells in response to HCV genotype 2a peptide pools with a preferred response to NS3. In addition, a response to multiple HCV proteins was detected following homologous JFH1cc re-challenge although HCV proteins of genotype 1 were used in this assay (Figure 3). The two subsequent re-challenges with homologous JFH1cc did not change the pattern of the HCV-specific T cell response in CH10274. The frequency of HCV-specific T cells decreased progressively but remained detectable during the follow-up. During the heterologous challenge with the H77 virus at week 22, CH10274 became viremic. CH10274 rapidly displayed IFN-γ producing T cells in response to multiple HCV peptides (genotype 1a but not 2a) and HCV proteins (genotype 1). Similarly, there was a slight increase in the frequency of IL-2 producing HCV-specific T cells (Figure 3). Analysis of CD4+ and CD8+ T cells by intracellular cytokine staining of IFN-γ at week 47 (25 weeks post-infection) identified CD4+ T cells as the responding population (Figure 4). During the follow-up, the frequency of IFN-γ and IL-2 producing HCV-specific T cells gradually disappeared probably due to the absence of viremia. With the re-appearance of viremia at week 57 (35 weeks post-infection), circulating IFN-γ producing HCV-specific T cells with a preferred response to HCV core emerged (Figure 3). Intracellular IFN-γ staining confirmed the specificity of the T cells for HCV core and again identified CD4+ T cells as the responding population (Figure 4). The frequency of HCV-specific T cells decreased progressively during the follow-up but remained detectable.
Intrahepatic immune response following HCV re-challenge
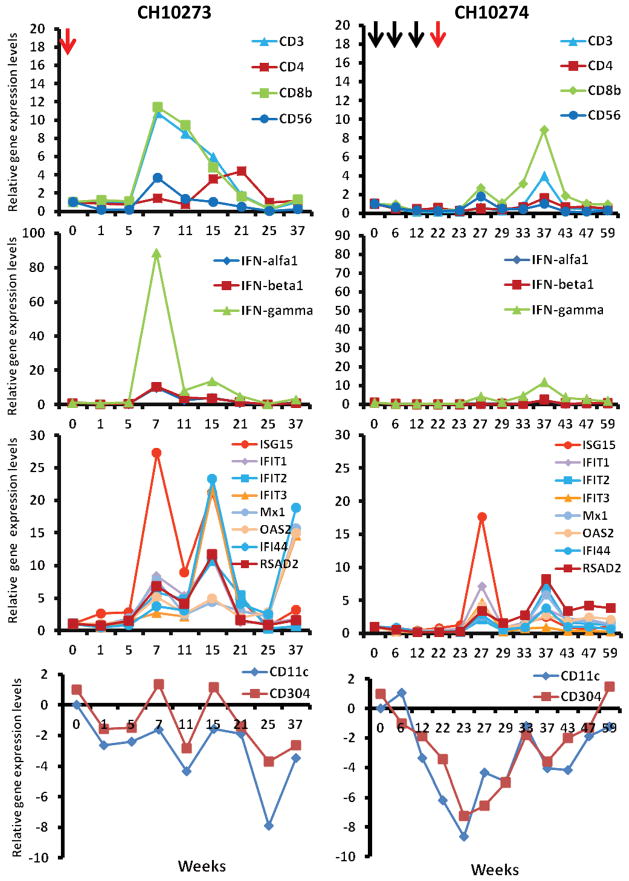
To assess the nature and kinetics of the intrahepatic immune response following HCV re-challenge, liver biopsies from both chimpanzees were obtained and assessed for the presence of a broad spectrum of immunological markers. In total, 17 markers were analyzed by real-time quantitative RT-PCR, such as markers for T-cells (CD3, CD4, CD8b), NK cells (CD56) and dendritic cells (DCs) (CD11c, CD304), interferons (IFN-α, IFN-β, and IFN-γ), and ISGs (OAS2, Mx1, ISG15, IFIT1-3, IFI44, RSAD2).
Following heterologous H77 challenge, liver biopsy samples of CH10273 displayed a markedly enhanced expression of CD3, CD4, CD8, and CD56 mRNA levels 7 weeks after re-challenge (Figure 5). In parallel, a strong up-regulation of IFN-γ mRNA level and a moderate induction of IFN-α and -β mRNA levels were observed (Figure 5), suggesting a prominent infiltration of activated T and NK/NKT cells into the liver. Peak levels of these markers coincided with the significant induction of several ISGs. A marked enhancement was observed for ISG15, IFI44, IFIT1, IFIT2, IFIT3, and RSAD2. Moderately increased expression levels were observed for Mx1 and OAS2. In contrast, we observed a decrease in the expression of CD11c and CD304 mRNA levels which are markers for myeloid and plasmacytoid DCs, respectively, suggesting a constant efflux of resident DCs from the liver to the draining lymph nodes in both chimpanzees (Figure 5). Next, we measured IFN-α serum levels to see whether the induction of liver type I IFN and IGSs is reflected in an enhanced serum level of IFN-α. However, IFN-α serum levels increased only marginally over the detection limit of the assay (> 10 pg/ml) following rechallenge (data no shown), probably because of very short serum half-life and rapid clearance of IFN-α.
Figure 5. Analyses of intrahepatic immune response during HCV re-challenge by quantitative real-time PCR.
Serial samples of liver biopsies from both chimpanzees were obtained and used to isolated total RNA. cDNA synthesis and TaqMan real-time PCR was performed as described in Methods. Relative levels of 17 different gene expression, markers for T cells (CD3, CD4, CD8b), NK cells (CD56) and dendritic cells (CD11c, CD304), interferons (IFN-α, IFN-β, and IFN-γ), and ISGs (OAS, Mx1, ISG15, IFIT1-3, IFI44, RSAD2), were analyzed. The y-axis illustrates the relative gene expression levels where each gene expression was normalized to GAPDH and determined relatively to week 0 value that is set as 1.
Despite the presence of peripheral HCV-specific T cells (Figure 3) and the induction of neutralizing antibodies (Figure 4), no hepatic gene induction was observed in CH10274 following the three homologous JFH-1cc re-challenges. Following heterologous challenge with the H77 virus at week 22, a weak induction of CD3, CD8, IFN-γ mRNA levels occurred at week 27 indicating a lesser degree of T cell infiltration into the liver in CH10274 when compared to CH10273. Likewise, IFN-γ mRNA expression level was only slightly induced (Figure 5). Peak levels of the indicated T cell marker coincided with a moderate induction of several ISGs that might be responsible for the initial control of viremia (Figure 5). Furthermore, the DC specific markers CD11c and CD304 were down-regulated similar to that observed in CH10273. During the follow-up, the chimpanzee developed at week 37 a pronounced increase in intrahepatic CD8 mRNA levels, which coincided with an increase in peripheral HCV-specific T cell response (Figure 3, weeks 37–43). This increase was accompanied by an intrahepatic induction of IFN-γ and several ISGs and then followed by disappearance of viremia after week 42 (Figure 5). The viremia, however, returned with a fluctuating course until the virus was ultimately cleared.
DISCUSSION
The development of an HCV vaccine is challenged by the fact that HCV can infect patients that previously recovered from HCV infection 19, 20 suggesting that complete protection appears difficult to achieve. Likewise, studies in chimpanzees demonstrated that animals re-challenged with homologous or heterologous strains of HCV are not consistently protected against re-infection following acute resolving infection 15. Aiming to better understand the immunological determinants of protective immune responses to HCV infection, we performed an extensive analysis of the innate and adaptive immune response in two chimpanzees that had previously cleared HCV and were re-challenged with homologous and/or heterologous strains of HCV.
Chimpanzee 10274 was re-challenged three times with the JFH1 homologous virus derived from cell culture. The first challenge produced detectable HCV RNA lasting only 2 weeks. The chimpanzee was not infected following the subsequent challenge. The chimpanzee became virus-positive at a low level 10 weeks after the third re-challenge. Unfortunately the chimpanzee was re-challenged with the H77 virus on the same day per protocol and we were not able to follow this course of viremia. The homologous JFH1cc re-challenges were associated with the development of neutralizing antibodies and the induction of HCV-specific T cells, probably contributing to the rapid control of viral infection. The viral clearance was not associated with a significant increase of serum ALT level suggesting that cytolytic mechanisms were not involved in viral clearance or that the number of virus-infected cells in the liver was very low. We also did not detect any intrahepatic innate immune response in this animal. Our results are in line with several previous studies in chimpanzees demonstrating the importance of T cells in viral clearance and protection after re-challenge 11–13, 15, 21. It is interesting to note that the H77 virus overcame the protective immune responses against JFH1, dominated over the concurrent low-level JFH1 viremia, and developed into a high-viremic infection, suggesting that the protective immunity noted above was rather strain-specific.
The role of the humoral immune response in protection against HCV infection is less well defined. Prospective studies demonstrated that viral clearance in acute HCV infection did not correlate with the development of neutralizing antibodies in chimpanzees 15, 22. In our study, CH10274 sero-converted and developed neutralizing antibodies against the homologous re-challenge strain (JFH-1, genotype 2a) and a heterologous strain (genotype 1a), indicating the production of genotype cross-reactive antibodies. However such antibodies were not able to prevent re-infection with the H77 strain. Thus, neutralizing antibodies may be capable of preventing low-level subclinical infection, such as the JFH-1cc infection 16, but they are not sufficient to control robust high-viremic infection like the H77 infection 18.
Following challenge with H77 virus in CH10274 and CH10273, we observed two distinct clinical courses. CH10273 had what appeared to be protective immunity because no viremia was detected. By contrast, CH10274 was infected with a fluctuating course of viremia and viral clearance almost a year later. In humans, chronic HCV infection is characterized by 1–2 log decrease in viral load followed by a viral load stabilization in most cases of persistent infection within several months. However, fluctuating viremia in both patients with resolution of infection and those with chronic infection including intermittent negative HCV RNA test results after initial viremia have been observed. Furthermore, although most patients with an acute and self-limited course of HCV infection clear infection within 6 months, viral clearance has been also reported 1 and 2 years after diagnosis of acute infection 23.
As discussed above, although CH10274 possessed antibodies with neutralizing activity against the re-challenging viral strain, the antibodies appeared insufficient to prevent re-infection. We did not observe any significant level of neutralizing antibodies in CH10273 following heterologous challenge suggesting that the observed sterilizing immunity was not associated with the development of neutralizing antibodies. Although both animals demonstrated HCV-specific T cell responses in the blood, the magnitude of the HCV-specific T cell response was higher in CH10274 who became re-infected. Since there is an ongoing redistribution and migration of T cells between blood, lymph nodes and liver, we examined the intrahepatic immune response in both animals. Compared to other organs, the liver is particularly enriched with cells of the innate immune system, including natural killer (NK), natural killer T (NKT) cells, Kupffer cells (KC) and dendritic cells (DCs), and T cells, which participate in adaptive immune responses 24. The protective immunity in CH10273 was associated with a rapid and durable increase of specific T, NK and NKT cell markers and increased level of IFN-γ mRNA in the liver suggesting an intense infiltration of activated T, NK and NKT cells into the liver and/or the activation of resident liver T, NK and NKT cells. CD8+ T cells, NK and NKT cells exert their effector functions in viral infection either by direct cytotoxicity or the release of IFN-γ which inhibits viral replication 24. Since we observed only a mild evaluation of ALT level following heterologous HCV re-challenge, control of HCV replication was probably mediated by non-cytolytic mechanisms. In contrast, CH10274 who became re-infected displayed a weak enhancement of T, NK and NKT cell markers with marginally induced IFN-γ mRNA in the liver. This relative inability of virus-specific T and innate immune cells to enter the liver and be activated may account initially for the inefficient control of HCV replication in this animal. However this animal did develop a strong secondary infiltration of a different T cell response much later, leading to eventual viral clearance. The underlying mechanism that contributes to the weak or delayed movement of HCV-specific T cells from the blood into the liver of CH10274 remains unknown. It would be of interest to examine and correlate the intrahepatic HCV-specific T cell responses in theses chimpanzees. However the currently available technique in studying intrahepatic T cells involves artificial T cell expansion and cloning, which is inadequate in providing a global analysis of the T cell response.
Distinct subsets of DCs, including myeloid and plasmacytoid DCs are present in the liver and there is a continuous influx of DCs from the blood into the liver 25. Analysis of DC markers revealed a decrease in plasmacytoid and myeloid DCs in both animals following re-challenges suggesting that liver resident DCs migrated to the draining lymph node. Recruitment of DCs to lymph nodes is pivotal for the initiation of adaptive immune responses 25.
Interferons (IFNs) are key mediators of the host innate antiviral immune response. Interferon stimulated gene (ISG) products can prevent the translation of viral RNAs and thereby limiting the initial viral spread in the liver until viral clearance occurs by HCV-specific T cells 26. In CH10273, prevention of re-infection was associated with an early and extensive induction of the ISGs in the liver, coinciding with the enhanced NK, NKT and T markers and IFN-γ. Infected hepatocytes are probably the primary cell types in the liver involved in type I IFN and ISG expression. However, since we did not dissect the cellular origin of the type I IFN production and ISG expression in the liver, DCs may also be involved in IFN-α/β production. Although, DCs appear not to be directly infected or stimulated by HCV to produce type I IFNs in vitro, recent studies demonstrated that HCV-infected hepatocyte cell lines have the capability to stimulate pDCs to produce large amounts of type 1 IFN via Toll-like receptor 7 (TLR7) signaling that is induced by direct cell-to-cell contact with HCV-infected cells 27. Gene expression analysis of liver biopsy samples from CH10273 revealed strong induction of interferon antiviral pathways, e.g. ISG15, Mx, RSAD2, IFI44, IFIT1 and OAS. Since these pathways are involved in blocking viral transcription, degrading viral RNA, inhibiting translation and modifying protein functions 26, the induced vigorous IFN response in CH10273 appeared to control virus replication and spread in the liver. The data are in line with previous reports that demonstrate the induction of the IFN response pathways in chimpanzees during acute resolving HCV infection 28–30.
CH10274 also exhibited induction of ISGs in the liver shortly after re-infection by H77 virus. However the magnitude and breadth was weaker than that of CH10273. This induction of ISGs occurred in the absence of a robust increase in intrahepatic T and NK cell markers, suggesting that this response is probably secondary to a high level of viral replication in the liver of this chimpanzee but insufficient to clear the viral infection. However this chimpanzee was able to mount a more vigorous T cell response with induction of ISGs in the liver later prior to viral clearance. These observations suggest that the timing and the breadth of the innate and adaptive intrahepatic immune responses is a critical factor in determining the outcome of HCV infection. It can be assumed that the earlier and robust ISG response observed in CH10273 inhibited HCV replication and spread in the liver. Furthermore, the ISG response in this animal was supported by a robust intrahepatic NK and T cell response which probably cleared infected cells. As observed in CH10274, the weak ISG response and intrahepatic immunity led to a continued HCV replication and a poor or inefficient activation of the intrahepatic T cell response. It was probably the second wave of the intrahepatic innate and cellular responses in CH10274 that finally controlled the heterologous HCV re-challenge. The reason for the variation in the immune response of the two animals is unknown. However, it could be due to the different re-challenges protocol but may also reflect inter-individual variability. As discussed above, CH10274 had a low-level subclinical infection with HCV JFH1cc at the time of the heterologous H77 re-challenge.
In conclusion, although the number of animals studied was limited and we used different re-challenge protocols, our study, which included multiple sequential samples of the liver and blood, demonstrates that protective immunity against HCV infection likely depends primarily on the activation of both intrahepatic innate and cellular immune responses. Our data indicate that regardless of the infection outcome following heterologous HCV re-challenge, peripheral T cell responses are present. However, a rapid onset of the complex and coordinated interplay between innate immune cells and T cells in the liver appears to be critical for protection against HCV infection after re-challenge with heterologous genotypes. Miscueing of this coordinated immune response in the liver leads to failure of viral control and favours persistent viral infection. In addition, our results suggest that neutralizing antibodies contribute to the initial protection after re-exposure with homologous HCV probably by interfering with the early steps of the HCV life cycle such as viral binding and entry. However, despite the evidence for cross-reactivity of these antibodies, they appear to not to provide protection against the heterologous HCV strain. Development of an effective preventive vaccine and immunotherapeutics would have to target multiple pathways of immune response for optimal effect.
Supplementary Material
Acknowledgments
Financial Support This work was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (BA 3643/1-1 to H.B.), the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, and programmatic support of Division of Viral Hepatitis, NCHHSTP, CDC, and the Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (2010-106; 2010-242 to H.B.), and Inserm, France.
The authors thank E. Soulier (Inserm U748, University Strasbourg, France) for excellent technical assistance.
Abbreviations
- ALT
alanine aminotransferase
- ELISpot
enzyme-linked immunosorbent spot
- HCV
hepatitis C virus
- HCVpp
HCV pseudoparticle
- IFN
interferon
- IL
interleukin
- ISGs
interferon-stimulated genes
- JFH1cc
cell-culture generated JFH-1 virus
- PBMC
peripheral blood mononuclear cell
- RT-PCR
reverse transcription polymerase chain reaction
Footnotes
Potential conflict of interest: Nothing to report
References
- 1.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 2.Argentini C, Genovese D, Dettori S, Rapicetta M. HCV genetic variability: from quasispecies evolution to genotype classification. Future Microbiol. 2009;4:359–373. doi: 10.2217/fmb.09.8. [DOI] [PubMed] [Google Scholar]
- 3.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 5.Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–1145. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 6.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmowalid GA, Qiao M, Jeong SH, Borg BB, Baumert TF, Sapp RK, Hu Z, et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci USA. 2007;104:8427–8432. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner AJ, Paliard X, Selby MJ, Medina-Selby A, Coit D, Nguyen S, Kansopon J, et al. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J Virol. 2001;75:7142–7148. doi: 10.1128/JVI.75.15.7142-7148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassett SE, Brasky KM, Lanford RE. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 12.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, et al. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prince AM, Brotman B, Lee DH, Pfahler W, Tricoche N, Andrus L, Shata MT. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. 2005;192:1701–1709. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- 15.Bukh J, Thimme R, Meunier JC, Faulk K, Spangenberg HC, Chang KM, Satterfield W, et al. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J Virol. 2008;82:8183–8195. doi: 10.1128/JVI.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato T, Choi Y, Elmowalid G, Sapp RK, Barth H, Furusaka A, Mishiro S, et al. Hepatitis C virus JFH-1 strain infection in chimpanzees is associated with low pathogenicity and emergence of an adaptive mutation. Hepatology. 2008;48:732–740. doi: 10.1002/hep.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato T, Furusaka A, Miyamoto M, Date T, Yasui K, Hiramoto J, Nagayama K, et al. Sequence analysis of hepatitis C virus isolated from a fulminant hepatitis patient. J Med Virol. 2001;64:334–339. doi: 10.1002/jmv.1055. [DOI] [PubMed] [Google Scholar]
- 18.Major ME, Mihalik K, Fernandez J, Seidman J, Kleiner D, Kolykhalov AA, Rice CM, et al. Long-term follow-up of chimpanzees inoculated with the first infectious clone for hepatitis C virus. J Virol. 1999;73:3317–3325. doi: 10.1128/jvi.73.4.3317-3325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai ME, Mazzoleni AP, Argiolu F, De Virgilis S, Balestrieri A, Purcell RH, Cao A, et al. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet. 1994;343:388–390. doi: 10.1016/s0140-6736(94)91224-6. [DOI] [PubMed] [Google Scholar]
- 20.Asselah T, Vidaud D, Doloy A, Boyer N, Martinot M, Vidaud M, Valla D, et al. Second infection with a different hepatitis C virus genotype in a intravenous drug user during interferon therapy. Gut. 2003;52:900–902. doi: 10.1136/gut.52.6.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meunier JC, Engle RE, Faulk K, Zhao M, Bartosch B, Alter H, Emerson SU, et al. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci USA. 2005;102:4560–4565. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busch MP, Shafer KA. Acute-phase hepatitis C virus infection: implications for research, diagnosis, and treatment. Clin Infect Dis. 2005;40:959–961. doi: 10.1086/428583. [DOI] [PubMed] [Google Scholar]
- 24.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 25.Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. A look behind closed doors: Interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8:350–360. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Major ME, Dahari H, Mihalik K, Puig M, Rice CM, Neumann AU, Feinstone SM. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology. 2004;39:1709–1720. doi: 10.1002/hep.20239. [DOI] [PubMed] [Google Scholar]
- 30.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.