Abstract
Diffuse alveolar hemorrhage is an uncommon yet often fatal complication of systemic lupus erythematosus (SLE). Advances in the treatment of alveolar hemorrhage have been hampered due to the heterogeneity of clinical findings and the lack of suitable animal models. A single intraperitoneal injection of pristane induces a lupus-like syndrome characterized by lupus-related autoantibodies and glomerulonephritis in non-autoimmune prone strains of mice. In addition, C57BL/6 (B6) mice frequently develop alveolar hemorrhage within a few weeks of pristane injection. Immunopathogenesis of pristane-induced alveolar hemorrhage was investigated in the present study. Early (2-4 weeks after injection) mortality due to hemorrhage was unique to C57BL/6 and C57BL/10 strains of mice. Recruitment of the macrophages and neutrophils preceded the hemorrhage by several days and hemorrhage started 3-7 days after pristane injection in some mice, peaked at 2 weeks (84% in B6) and then resolved by 4 weeks in a majority of mice. Alveolar hemorrhage was independent of MyD88-, or TLR7 pathways, in contrast to autoantibody production and glomerulonephritis, and also was independent of FcγR or Fas. Rag1-/- mice had a reduced prevalence of alveolar hemorrhage compared to B6 (P = 0.01) congenics. However, T-cell receptor deficient mice developed alveolar hemorrhage at a rate comparable to wild type controls, while B6 Igμ-/- mice surprisingly had a strikingly reduced prevalence (7% vs 84% in B6, P < 0.0001). Reconstitution of B6 Igμ-/- mice with wild type B cells increased the prevalence to 50% (P = 0.028).
Pristane-induced alveolar hemorrhage is a useful model to study the pathogenesis and develop new therapy for this underappreciated and often life-threatening complication of SLE.
Keywords: diffuse alveolar hemorrhage, systemic lupus erythematosus, pristane, animal model, B-cells
Pulmonary manifestations in SLE include pleural, parenchymal, vascular, and airway disease (1). Pleuritis is relatively characteristic of SLE and included in the classification criteria. Although pulmonary parenchymal manifestations are not frequently seen or pathognomonic of SLE, pneumonitis, acute respiratory distress syndrome (ARDS), diffuse alveolar hemorrhage (DAH), chronic interstitial pneumonitis, and diaphragmatic dysfunction/shrinking lung syndrome, can develop. Among these, DAH is one of the most serious pulmonary complications of SLE (1). While DAH occurs infrequently [average 1.9%, reviewed in (2)] (3, 4) in lupus patient cohorts, it is responsible for 1.5-3.7% of all SLE associated hospital admissions (3, 4), and 10 - 20% of mortality in SLE. Most patients that develop DAH are young females within 5 years of diagnosis of SLE (2, 3). Patients that develop DAH present with high fever, chest pain, coughing, rales, dyspnea, tachypnea and hemoptysis. In the acute setting of a systemically ill patient, DAH is frequently difficult to differentiate from bacterial or other opportunistic pulmonary infections (2, 4). Treatment for patients with DAH typically includes high doses of steroids alone or in combination with other immunosuppressive drugs and plasmapheresis. Mechanical ventilation is also required in over 50% of the cases of SLE associated DAH (5). Despite these interventions, DAH can often recur while the patient is already on immunosuppressive therapies, and SLE associated DAH is fatal in over 50% of cases (2, 3, 5, 6). More effective treatment is needed, but advances in understanding the pathogenesis and testing potential new therapeutic options have been hindered by the heterogeneity of the clinical and immunopathological findings (2-5, 7, 8).
Pristane (2, 6, 10, 14-tetramethylpentadecane) is a hydrocarbon oil that can induce a lupus-like autoimmune syndrome that includes several of the pathognomonic characteristics of human SLE in non-autoimmune prone strains of mice (9, 10). After a single intraperitoneal injection of pristane, mice develop anti-U1RNP/Sm, -Su, -dsDNA, and -chromatin autoantibodies, immune complex-mediated glomerulonephritis and arthritis (9-11). While most of these characteristics are also present in several genetic models of SLE, the ability of pristane to induce type I interferons (12-15) and alveolar hemorrhage (16, 17) in mice on a C57BL/6 (B6) background are unique to this chemically induced model of SLE. In this study we report that mice on the B6/B10 background frequently develop DAH, and characterize the sequence of events and the immune cells and mechanisms that are important for the development of this underappreciated and often fatal complication of SLE.
Materials and Methods
Mice
Four to six week old A.SW, BALB/cJ, C3H/HeJ, CBA/CaJ, DBA/1J, SJL/J, C57BL/10J (B10) and C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), housed and bred in barrier cages in a conventional facility at the Malcolm Randall VA Medical Center (Gainesville, FL, USA). Four to six week old B6;129S7-Rag1tm1Mom/J (B6 RAG1-/-), B6.129P2-Tcrbtm1MomTcrdTm1Mom (B6 TCR-/-), B6.129S2-Igh-6tm1Cgn/J (B6 Igμ-/-), B6.MRL-Faslpr/J (B6 lpr), and B6 MyD88-/-, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). B6.129P2-Fcer1gtm1Ra N12(B6 FcγR-/) were purchased from Taconic Laboratories (Hudson, NY, USA). These mice were housed and bred in barrier cages in a specific pathogen-free (SPF) facility at the Malcolm Randall VA Medical Center (Gainesville, FL, USA). B6 MyD88-/-, Toll-like Receptor (TLR)7-/- and TLR9-/- [described previously (18)] were bred and maintained in a SPF facility at the Research Institute for Microbial Diseases at Osaka University (Osaka, Japan). At 3-4 months of age, mice received a single 0.5ml intraperitoneal injection of sterile 2,6,10,14-tetramethylpentadecane (pristane, Sigma-Aldrich, St. Louis, MO, USA). After 1, 3, 7, 14 or 28 days the spleen, lungs, kidneys, and liver were harvested as described previously (15). In reconstitution studies, splenocytes from age- and sex-matched donor B6 mice were positively selected for either CD19+ B cells, CD4+ T cells, or CD8+ T cells using magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA) as per the manufacturer’s instructions. B6 Rag1-/- mice were reconstituted by intravenous injection of either ten million splenic CD4+ T cells, CD8+ T cells or total T cells (5×106 of each CD4+ T cells and CD8+ T cells). B6 Igμ-/- mice were reconstituted by intravenous injection of 20×106 CD19+ B cells. Three days after receiving the transfers the recipient B6 Rag1-/- and B6 Igμ-/- mice were injected i.p. with 0.5ml of pristane and their organs harvested on day 14. In other experiment, potential role of antibodies and other factors in serum was tested by transferring serum from mice with DAH. Sera were collected from two B6 mice that received pristane 2 weeks earlier and developed DAH. Two-hundred microliter of serum was intravenously injected into four naïve B6 mice and their lung pathology was examined 2 weeks later. All studies were approved by the Institutional Animal Care and Use Committee at the Malcolm Randall VA Medical Center (Gainesville, FL, USA).
Histology
Spleen, kidney, liver and lung tissue were harvested and fixed in 4% paraformaldehyde overnight. The fixed tissues were embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and eosin.
Bronchoalveolar Lavage
Bronchoalveolar lavage was performed on euthanized B6 mice by making a small incision in the trachea. An 18G needle fitted with a blunted p20 pipette tip was attached to a 1 ml syringe filled with 1.2 ml of sterile Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech, Manassas, VA, USA). The trachea was securely clamped around the inserted 18G needle. The lungs were then flushed repeatedly, and the recovered fluid was collected in a 15 ml conical tube on ice. This process was repeated as needed until a total of 3-4 ml of lavage fluid was collected. The collected cells were analyzed by flow cytometry.
Detection of Apoptosis
Lung tissue sections were stained for apoptotic cells by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Apoptag Red in situ apoptosis detection kit, Millipore, Billerica, MA, USA) as per the manufacturer’s instructions.
Culturing Endothelial Cells with Pristane
A human umbilical cord endothelial cell line (EaHy926, a gift from Dr. F. Southwick, University of Florida, Gainesville, FL, USA) was cultured in a 24 well transwell plate (8.0μm pore size, Costar, Corning, NY, USA) with DMEM supplemented with 10% FCS until the cells were 70% confluent. Media was removed and replaced with either fresh DMEM with 10% FCS alone or 10% FCS that had been previously mixed with pristane (12). At three time points (0, 6, and 24h after replacement of medium), an electric current was passed over the cells and the total transepithelial electrical resistance (conductance, 1/Ω) was measured with a STX2 manual electrode with meter (World Precision Instruments, Sarasota, FL).
Flow Cytometry
The following conjugated antibodies from BD Biosciences (San Jose, CA, USA) were used: anti-CD3 complex-PE, anti-CD4-FITC, anti-CD5-FITC and -PE, anti-CD8-FITC, anti-CD11b-FITC and -PerCP5.5, anti-CD11c-FITC and -PE, anti-Ly6C-FITC, anti-Ly6G-PE. Anti-CD4-APC, anti-CD11b-APC and -PerCP5.5 were purchased from eBioscience (San Diego, CA, USA). Pacific blue-conjugated antibodies to CD3 complex, CD4, CD11b, CD19 and Ly6G were purchased from Biolegend (San Diego, CA, USA). FITC, PE, Pacific Blue, APC and PerCP5.5 conjugated isotype controls were used to determine background fluorescence. Isolated spleen, lung and peritoneal cells were washed with staining buffer (PBS supplemented with 0.5% bovine serum albumin, 0.05% NaN3). A half million cells/well were added to a 96 well round bottom polystyrene plate (Costar, Corning, NY, USA) and were incubated with 25μl of Fc block. Cells were incubated with fluorescently labeled antibodies for 20 minutes at 4°C and were then washed twice with staining buffer. The cells were fixed in 1% paraformaldehyde in PBS, run on a Cyan ADP flow cytometer (Dako Cytomation, Carpinteria, CA, USA), and data for twenty thousand live cell gated events were collected for each sample with Summit acquisition software where. Analyses of the data were done with Flowjo version 7.2.5 (Ashland, OR, USA).
Statistical Analysis
For differences in the prevalence of alveolar hemorrhage, Fisher’s exact test was used. For the phenotypic characterization of the immune cells found within the lungs, differences between groups were analyzed by Mann-Whitney U test with P < 0.05 considered to be significant. Data are presented as mean±SEM. Statistical analyses were performed with Prism 4.0 (GraphPad Software, San Diego, CA, USA).
Results
Pristane Induces Pulmonary Hemorrhage in C57BL/6 Mice
Previous work reported early mortality within the first 4 weeks after intraperitoneal pristane injection in C57BL/6 (B6) mice, presumably due to pulmonary hemorrhage (16). Survival curves for 3 groups of B6 and BALB/cByJ mice after pristane injection are shown (Fig.1A). Although early mortality within 4 weeks, mainly between 2 and 4 weeks, was observed in most groups of mice on the B6 background, the mortality rates varied from group to group. None of the pristane-treated BALB/c mice (Table 1) or control PBS-treated B6 mice (data not shown) died during the same period. Mortality of various non-autoimmune-prone strains of mice during the first 4 months after pristane injection is summarized (Table 1). Death within 4 months following pristane injection was very rare in all strains except those on B6 or B10 background. Most of the mortality occurred between 2 and 4 weeks after pristane injection, and further deaths after 4 weeks were rare. A high rate of mortality was observed in SJL/J mice between 4 and 6 months after pristane injection. However, the cause of death in the SJL/J mice appeared to be from mediastinal lymphadenopathy and nephritis and not DAH, since no pulmonary hemorrhage was observed at autopsy (19) (Satoh M et al., unpublished observation). Therefore, pristane induces early mortality, presumably due to alveolar hemorrhage only in mice derived from the B6/B10 strains. Although we do not have autopsy data on all the mice that died, it seems highly likely that the mortality in these mice is due to DAH based on the high prevalence of this complication, as we show later in the time course experiment. It should also be noted that we do not have autopsy data of all strains of mice between 2 and 4 weeks when B6 mice frequently develop DAH. Thus, it is possible that some strains of mice listed in Table 1 had DAH without observed mortality.
Figure 1.
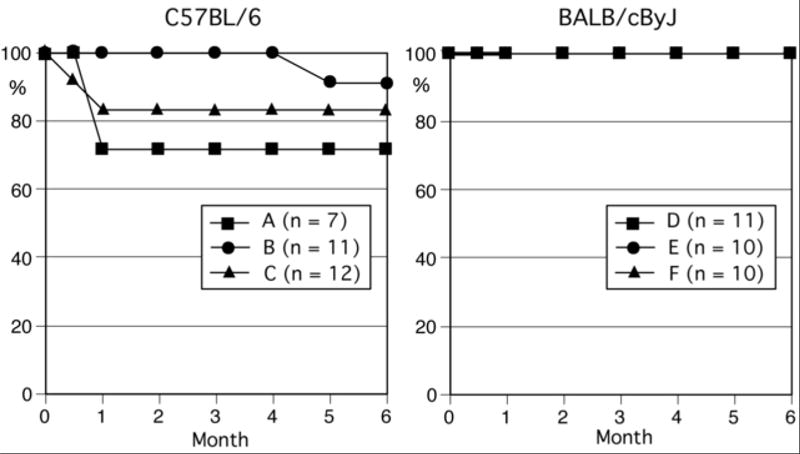
Survival curves of C57BL/6 and BALB/cByJ mice after pristane treatment. Survival curves for C57BL/6 (left, 3 groups, n = 7, 11, and 12) and BALB/cByJ (right, 3 groups, n = 11, 10, and 10) mice following a single 0.5 ml intraperitoneal injection of pristane are shown.
Table 1.
Mortality of non-autoimmune strains of mice following pristane injection
| Mortality (%) after pristane injection | ||||
|---|---|---|---|---|
|
| ||||
| Strain | n | 0-2 weeks | 2-4 weeks | 4-16 weeks |
| BALB/cByJ | 31 | 0 | 0 | 0 |
| BALB/cJ | 12 | 0 | 0 | 0 |
| C57BL/6J | 30 | 3 | 10 | 0 |
| B6 CH2bm12 | 12 | 0 | 42 | 0 |
| C57BL/J | 25 | 0 | 13 | 0 |
| B10.S | 15 | 0 | 12 | 0 |
| DBA/1J | 16 | 0 | 0 | 0 |
| C3H/HeJ | 16 | 0 | 0 | 0 |
| C3H/HeOuJ | 16 | 0 | 0 | 0 |
| C3HeB/FeJ | 8 | 0 | 0 | 0 |
| A.SW | 8 | 0 | 0 | 0 |
| SJL/J | 8 | 0 | 0 | 0 |
| CBA/CaJ | 24 | 0 | 0 | 0 |
Pristane-induced Hemorrhage is Strain and Organ Specific
The mechanisms of pristane-induced alveolar hemorrhage are poorly understood. To determine whether the hemorrhage observed in the lungs of B6 mice was organ specific and not due to a change in general vascular permeability in vivo, B6 and control BALB/c mice were injected with pristane and several organs were examined for evidence of hemorrhage. The lungs from pristane-treated B6 mice were filled with blood and heavily infiltrated with macrophages and neutrophils (Fig.2A, bottom left) two weeks after injection. No hemorrhage or inflammatory cellular infiltrates were seen in the lungs of untreated B6 mice (Fig.2A, top left). Consistent with the lack of early mortality in BALB/c mice following i.p. injection of pristane (Table 1), the lungs of BALB/c mice were free of any signs of hemorrhage (Fig.2A, right). We also confirmed that the hemorrhage in pristane treated mice was limited to the lungs and not seen in the spleen, liver and kidneys (Fig.2B), suggesting that pristane is not causing a generalized leakiness of endothelial cells in B6 mice.
Figure 2.
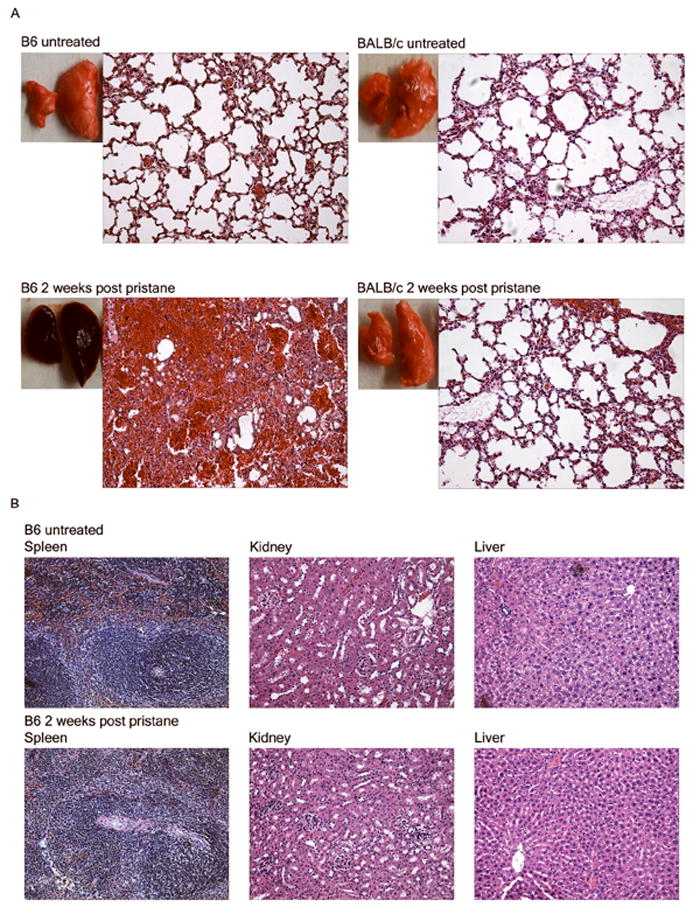
Pathology of lung, spleen, liver, and kidneys. A. Lung pathology of untreated vs. pristane-treated C57BL/6 (B6) and BALB/c mice. Representative gross pathology and H&E stained tissue sections of lungs from untreated (top) or pristane-treated (bottom) C57BL/6 (left) and BALB/c (right) mice are shown (200x magnification). BALB/c (n=7) and C57BL/6 (n=24) mice received a single 0.5ml i.p. injection of pristane. B. Histology of the spleen, liver and kidneys from pristane-treated and untreated control C57BL/6 mice. Tissues were harvested from mice that received an i.p. injection of pristane 14 days earlier or left untreated (200x magnification, H&E).
As one possibility, we hypothesized that pristane may directly change the permeability of the endothelial cells in the lungs. To examine this possibility, EaHy926 cells were cultured with pristane, and conductivity was measured. The total conductance was measured immediately after changing the media and after 6 and 24 hours. At all time points there was no difference in the total conductance across the cultured EaHy926 cells in the presence or absence of pristane (data not shown), suggesting that pristane did not directly affect permeability in human endothelial cells. Although we consider it unlikely, the possibility that pristane has different effects on murine pulmonary endothelial cells or that there may even be unique effects on B6-derived cells cannot be formally excluded.
Pristane-Induced Diffuse Alveolar Hemorrhage Occurs Independently of MyD88-, TLR7, FcγR Signaling, or Fas-Mediated Apoptosis
Type I interferons (IFN) play a critical role in the pathogenesis of SLE. The expression of type I IFN correlates with active disease, certain autoantibodies to RNA-protein complexes, and increased incidence of renal involvement in human SLE (20, 21). The interferon signature is also present in pristane-treated B6 mice and type I IFNs are produced by Ly6Chi monocytes in MyD88 and TLR7 dependent manner (12). Additionally, the recruitment of Ly6Chi monocytes, Ly6Clo macrophages and granulocytes to the peritoneal cavity following i.p. injection of pristane depends on TLR7 and MyD88, respectively (12). Thus, whether B6 mice deficient in TLR7 or MyD88 were resistant to pristane-induced DAH was examined 14 days after injection. Unexpectedly, both MyD88 -/- (n = 10, 60% hemorrhage) and TLR7 -/- (n = 4, 100%) B6 mice developed DAH at prevalence similar to that of wild type B6 mice. FcγR signaling did not play a role in the recruitment of immature monocytes and neutrophils to the peritoneal cavity following an i.p. injection of pristane (12). Although it is possible that FcγR is involved in the recruitment of inflammatory cells to the lungs, B6 FcγR-/- mice also developed DAH (n = 5, 80% at day 14). Based on these findings, we concluded that the induction of DAH is independent of these pathways and different from recruitment of myeloid cells to the peritoneal cavity after pristane injection (12),
While deficiencies in Fas or its ligand (lpr or gld mutations) accelerate the development of a lupus-like syndrome in mice (22), it was paradoxically found that B6/lpr mice were resistant to pristane-induced lupus (16). To determine the role of Fas-mediated apoptosis in the DAH induced by pristane, lungs from B6/lpr mice 14 days after pristane injection were examined. The prevalence of DAH in pristane-treated B6/lpr (80% hemorrhage, n = 5) was not reduced vs. wild type mice (83%, n = 24, Fig.3), unlike the production of pristane-induced anti-U1RNP/Sm autoantibodies (16). Moreover, very few apoptotic cells were detected in lung tissue by TUNEL assay prior to hemorrhage (data not shown). Thus the pathways involved in pristane-induced DAH are independent of Fas-mediated apoptosis.
Figure 3.
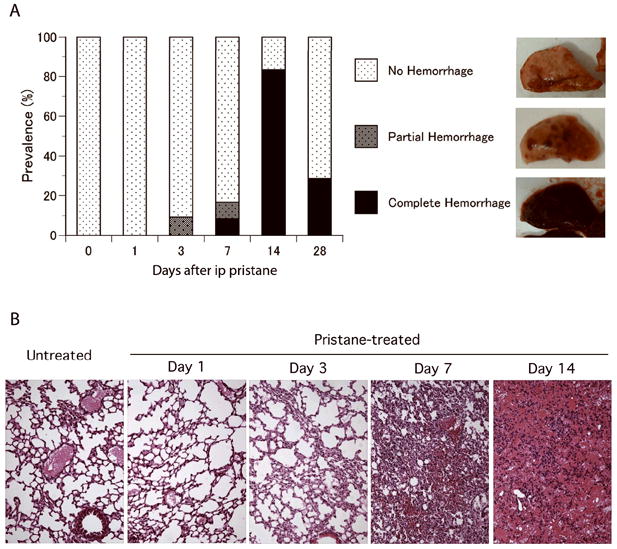
Time course of pristane-induced alveolar hemorrhage. A. Incidence and types of gross pathology. C57BL/6 mice received a single intraperitoneal injection of pristane. Lungs were examined at day 0 (untreated, n = 7), day 1 (n = 9), day 3 (n = 11), day 7 (n = 12), day 14 (n = 24), and day 28 (n = 7). Lung gross pathology was classified into no hemorrhage, partial hemorrhage, and complete hemorrhage. B. Pathology of lung tissue from pristane-treated C57BL/6 mice over time. Representative hematoxylin-eosin staining of lung tissues from pristane-treated mice at untreated (day 0), day 1, day 3, day 7, and day 14 are shown (original magnification 200x).
B1 cells are a self-renewing population of immune cells that are found predominantly in the peritoneal and pleural cavities and have an increased presence in both humans and mice that develop SLE (23, 24). They produce most of the naturally occurring autoantibodies in a T-independent fashion and can also function as antigen presenting cells, especially in response to bacterial infections (23). We examined whether the reduction of B1 cells in the peritoneal cavity would affect the susceptibility to DAH by depleting peritoneal B1 cells prior to and over the course of pristane treatment. An intraperitoneal injection of pristane alone reduces the CD19+ CD11b+ B1 cells to less than a few percent in the peritoneal cavity (13, 15). The injection of distilled water into the peritoneal cavity decreased the percentage of CD19 and CD11b positive B1 cells within the peritoneal cavity from 30-40% to 5-15% (data not shown). Two-thirds of the B6 mice that had their B1 cells reduced were still susceptible to pristane-induced DAH (n = 15, 66%). The loss of peritoneal B1 cells following pristane is dramatic and their fate unclear. Although the role of B-1 cells in pristane-induced DAH is not entirely conclusive, since B-1 cell depletion in this experiment was incomplete, this experiment was very informative, as it strongly suggests that B1 cells are not integral to the pathogenesis of pristane-induced DAH and likely unrelated to the pathogenesis of pristane-induced autoimmunity.
Cellular Infiltration of the Lungs Precedes the Development of Hemorrhage
Since B6 mice start to die around 2 weeks after pristane injection and do not have further mortality after week 4, we performed time course studies focusing on the first 4 weeks after injection to determine the sequence of pathology of DAH induced by pristane. The lungs of pristane-treated B6 mice were examined after 1, 3, 7, 14 and 28 days. Prior to analysis, all B6 mice were asymptomatic and were otherwise indistinguishable from their PBS injected littermates. The first signs of alveolar hemorrhage were observed starting at 3 days after pristane injection (Fig.3A). While over 90% of pristane-treated B6 mice were without any signs of alveolar hemorrhage (Fig.3B) a subpopulation of B6 mice developed small yet distinct foci of hemorrhage that were uniformly distributed in all lobes of the lungs (Fig. 3A). On day 7, some B6 mice developed complete/diffuse alveolar hemorrhage while others had focal/partial hemorrhage. The peak incidence of DAH was at two weeks after pristane injection (Fig.3A); 84% of the pristane-treated B6 mice had lungs that were completely filled with blood. The incidence of DAH was reduced to less than 30% at week 4, indicating that the majority of mice that had hemorrhage at day 14 spontaneously recovered and cleared the hemorrhage from their lungs. Since the lungs of human SLE patients with DAH are filled with not only erythrocytes but also proinflammatory cells such as macrophages and neutrophils, the lungs were examined histologically to determine if these cells were also being recruited to the lungs of pristane-treated B6 mice prior to alveolar hemorrhage. Twenty-four hours after injection, no difference in the histology of the lungs was observed between pristane-treated compared to untreated B6 mice. At day 3, the lungs began to fill with proinflammatory cells (Fig. 3B) and by day 7 the lungs were heavily infiltrated by inflammatory cells. It was also on day 7 that the first mouse with DAH was observed (Fig.3B). Both the hemorrhage and recruitment of inflammatory cells progressively worsened so that by day 14, nearly all the alveolar space was replaced with erythrocytes and infiltrating immune cells (Fig.3B).
Additionally based on their morphology, the majority of the immune cells recruited to the lungs of pristane-treated B6 mice were identified as macrophages and neutrophils. The bleeding and accumulation of macrophages and neutrophils in the lungs of pristane-treated B6 mice in this study is consistent with previously published work in B10 mice (17) and reports of human SLE associated DAH (4, 7, 25, 26). The DAH begins as early as one week after the pristane injection and that the influx of macrophages and neutrophils precedes the DAH by several days.
Since prevalence of DAH peaks at 2 weeks after an i.p. pristane injection, potential role of antibodies and other factors in serum at 2 weeks in the development of DAH was tested by a serum transfer experiment. None of four naïve B6 mice that received serum from B6 mice with pristane-induced DAH, developed DAH (data not shown).
Monocytes, Granulocytes, B-cells and T-cells Accumulate Over the Course of Pristane-induced Alveolar Hemorrhage
Macrophages and neutrophils accumulated in the lungs of B6 mice 2 weeks after the pristane injection. To confirm the presence of these innate immune cells and to determine when and what types of immune cells accumulate in the lungs of pristane treated B6 mice, cells in bronchoalveolar lavage (BAL) fluid collected from the mice over a 14 day time course study were analyzed. The percentage of Ly6Clo monocytes, Ly6Chi monocytes and granulocytes in BAL remained constant over the first 3 days after injection of pristane (Fig.4A-4C). One week after the injection all three populations were increased (Fig.4A-4C), indicating that infiltration of these cells precedes the hemorrhage. The Ly6Clo and Ly6Chi monocytes and granulocytes populations in the BAL isolated 7 and14 days after an i.p. pristane injection appeared to be increased, however, the difference was not statistically significant (Mann-Whitney) due to high variability in individual mice and small numbers per group. When the percentage of Ly6Chi monocytes and granulocytes in BAL from hemorrhagic mice (n = 8) vs non-hemorrhagic mice (n = 3) was compared, the percentage in the former was significantly higher (P = 0.0121) than that of the latter. However, the percentage of Ly6Clo monocytes was not significantly different. The B- and T-cells in BAL also increased after day 3-7 (Fig.4D-4F). Percentage of B-cells at day 7 and 14 was significantly higher than that of day 1 (P = 0.036 and P = 0.021 respectively by Mann-Whitney). CD4+ T-cells at day 7 ad 14 were significantly increased vs day 1 and 3 (P = 0.036 and P = 0.028, respectively), and CD8+ T-cells at day 7 and 14 were higher than day 1 (P = 0.036 and P = 0.049, respectively). Nearly all of the pristane-treated B6 mice that developed alveolar hemorrhage within 2 weeks had at least 8% of B cells in their BAL (Fig.4D). It is unclear what role CD4+ T-cells (Fig.4E) or CD8+ T-cells (Fig.4F,) plays in pristane-induced alveolar hemorrhage since the number is small and the presence of T cells did not necessarily coincide with the development of DAH.
Figure 4.
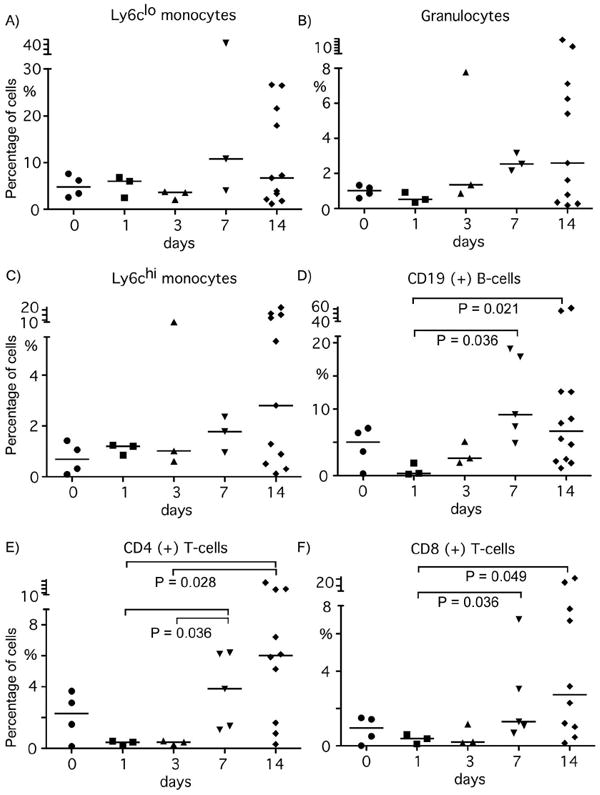
Flow cytometry analysis of cells in the bronchoalveolar lavage fluid of pristane treated B6 mice. B6 mice were either left untreated (day 0, n=4) or were injected with 0.5ml of pristane intraperitoneally and had their lungs lavaged after 1 day (n=3), 3 days (n=3), 7 days (n=5) or 14 days (n=11). Percentage of Ly6Clo monocytes (A), Ly6g+ granulocytes (B), Ly6Chi monocytes (C), CD19+ B cells (D), CD4+ T cells (E) and CD8+ T cells (F) are shown. P values between different time points were by Mann-Whitney U-test.
B Cells Play a Pathogenic Role in Pristane-induced Alveolar Hemorrhage
To determine the role of B and T cells in DAH, we examined whether B6 deficient in both of these cell populations (B6 Rag1-/-) were resistant to DAH. Half of pristane-treated B6 Rag1-/- mice developed alveolar hemorrhage (Fig.5A) at 2 weeks, indicating that B and T-cells are not an absolute requirement. Nevertheless, prevalence of DAH in B6 RAG1-/- mice were lower than that in wild type B6 mice (P = 0.01 for complete hemorrhage, P = 0.06 for any hemorrhage by Fisher’s exact test), suggesting that B and/or T cells may accelerate the development of DAH. To clarify the role of T- vs. B-cells in DAH, B6 mice that were deficient in only T cells (B6 TCR-/-) or only B cells (B6 Igμ-/-) were treated with pristane. Two-thirds of B6 TCR-/- mice developed complete hemorrhage and one mouse developed partial hemorrhage (Fig.5A). In striking contrast to T-cell deficient mice, all of but one pristane-treated B6 Igμ-/- mice were resistant to alveolar hemorrhage (P < 0.0001 vs. B6 by Fisher exact test) (Fig.5A). The prevalence of pristane-induced alveolar hemorrhage between male and female B6, B6 RAG1-/-, B6 TCR-/- and B6 Igμ-/- mice was also analyzed. Female dominance as seen in many autoimmune diseases was not observed, and the prevalence between male and female in all strains examined was not statistically different (data not shown).
Figure 5.
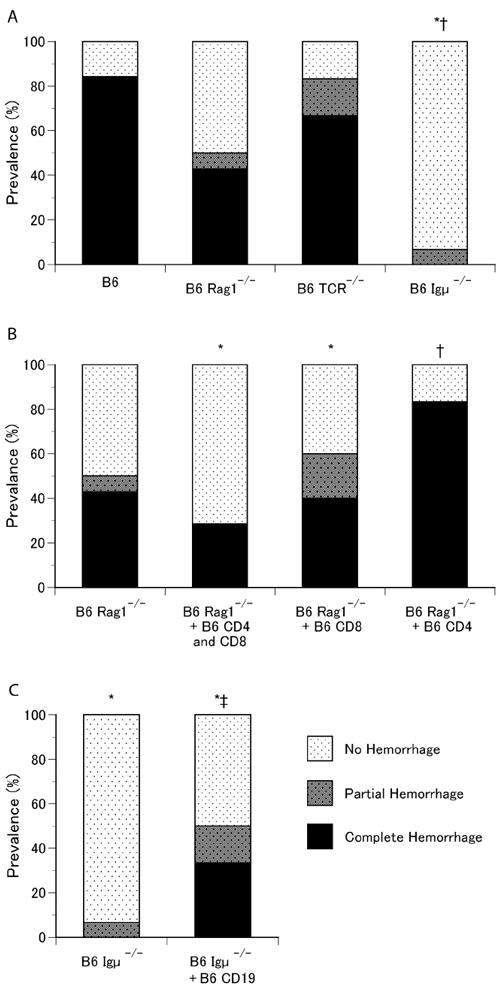
Role of T-cells and B-cells in pristane-induced alveolar hemorrhage. A. Prevalence of alveolar hemorrhage in wild-type C57BL/6, B6 RAG1-/-, B6 TCR-/-, and B6 Igμ-/- mice. Lungs from C57BL/6 (n = 24), B6 RAG1-/- (n=14), B6 TCR-/- (n=6) and B6 Igμ-/- (n=15) mice 14days after pristane treatment were examined. 1, P = 0.01 for complete hemorrhage and P = 0.06 for any hemorrhage, 2, B6 vs. B6 Igμ-/-, P < 0.0001 by Fisher’s exact test. B. Prevalence of alveolar hemorrhage in B6 RAG1-/- mice reconstituted with T-cells. B6 RAG1-/- mice were reconstituted with 10×106 wild type B6 splenic total T cells (n=7), 10×106 B6 CD8+ (n=5) or 10×106 B6 CD4+ spleen cells (n=12), 3 days prior to pristane treatment. Lungs were examined 14 days after i.p. pristane injection. 3, B6 Rag1-/- + B6 CD4+ and CD8+ vs. B6 Rag1-/- + B6 CD4+, P = 0.04; 4, B6 Rag1-/- vs. B6 Rag1-/- + B6 CD4+, P = 0.051 by Fisher’s exact test. C. Effects of B-cell reconstitution in B6 Igμ-/- mice on alveolar hemorrhage. B-cells were reconstituted in B6 Igμ-/- mice by intravenous transfer of CD19+ splenic B cells from wild-type B6 donors (20×106/recipient) 3 days prior to pristane injection. B6 Igμ-/-(n=15) and B-cell reconstituted B6 Igμ-/- mice (n = 12) received a single i.p. injection of pristane and lungs were examined 14 days later. ; 5, B6 Igμ-/- vs. B6 Igμ-/- + B6 CD19+, P = 0.028 for complete hemorrhage and P = 0.024 for any hemorrhage by Fisher’s exact test.
The role of T and B cells in pristane-induced alveolar hemorrhage was examined through a series of reconstitution studies. B6 Rag1-/- mice were intravenously reconstituted with either wild type B6 CD4+ spleen T-cells, CD8+ spleen T-cells or a combination of both populations 3 days prior to pristane treatment. It is possible that the prevalence of DAH is affected by the transfer of T cells, and CD4+ T-cells may enhance the alveolar hemorrhage (B6 Rag1-/- + B6 CD4+ and CD8+ vs. B6 Rag1-/- + B6 CD4, P = 0.04; B6 Rag1-/- vs. B6 Rag1-/- + B6 CD4+, P = 0.051 by Fisher’s exact test (Fig.5B). Reconstitution of B6 Igμ-/- mice with spleen CD19+ B cells prior to pristane injection dramatically increased the prevalence of alveolar hemorrhage from 7% to 50% (B6 Igμ-/- vs. B6 Igμ-/- + B6 CD19+, P = 0.024 for any hemorrhage, P = 0.028 for complete hemorrhage by Fisher’s exact test) (Fig.5C). These results strongly suggest that the presence of B-cells is pro-inflammatory in this model and that the presence of T-cells in the absence of B-cells (B6 Igμ-/- mice) is protective.
Discussion
Alveolar hemorrhage is an uncommon but serious complication of SLE. However, despite many reports on case series of patients with DAH, the pathogenesis remains to be clarified. While the infiltration of the lungs by inflammatory cells is common and the deposition of immune complexes and capillaritis have been also described in some reports (4, 25, 27, 28), alveolar hemorrhage can also occur in the absence of some or all of these manifestations (7, 8). Advances in our understanding on the pathogenesis of alveolar hemorrhage in SLE have been hampered by the inconsistency of immunopathological and clinical findings within the literatures. Most of the information regarding tissue immunopathology is from autopsy cases and sequential analysis of tissue is unavailable. Thus, the order and time course of immunopathological events are not known. In addition, the lack of an appropriate animal model makes it difficult to understand the immunopathogenetic mechanisms and to develop a new treatment.
A single intraperitoneal injection of pristane induces a lupus-like syndrome that includes production of lupus-related autoantibodies, glomerulonephritis and arthritis in several non-autoimmune prone strains of mice (9-11, 29). In addition, up to 50% of pristane-treated B6 mice die within the first month of injection due to alveolar hemorrhage, while this early mortality is not seen in other strains of mice (Table 1, Fig.1) (16). The present study demonstrates that the induction of pristane-induced DAH is unique to mice on the B6 background including B10, which was originally separated from B6 mice and essentially identical in terms of genetics. This is consistent with the previous work that demonstrated that B10 mice were also susceptible to DAH (17). Chowdhary et al found that 2 weeks after i.p. pristane injection the BAL fluid from B10 mice was consistently bloody and filled predominantly with macrophages and neutrophils (17). However, the histopathology of the lungs showed only areas of focal hemorrhage (17). Our data not only recapitulated what is seen in human patients in terms of severity of bleeding and recruitment of macrophages and neutrophils to the lungs, but we were also able to show by BAL and lung histology that inflammatory cells preceded any signs of hemorrhage by several days (Fig.4).
The production of autoantibodies to RNA-protein complexes and DNA is one of the hallmarks of the pristane-induced model of SLE (9, 10). The autoantigens are thought to be released from dead cells and can stimulate MyD88-dependent TLR signaling via TLR7 and TLR9 (30). Induction of autoantibodies to snRNPs and nephritis in pristane-induced lupus was shown to be TLR-7 dependent (12). MyD88 and TLR7 were also recently reported to be absolute requirements for the recruitment of monocytes and granulocytes to the peritoneal cavity following intraperitoneal pristane injection (14). Unexpectedly, B6 mice deficient in MyD88 or TLR7 were both susceptible to pristane-induced alveolar hemorrhage, indicating that the development of DAH is not dependent on either of these signaling pathways and thus is different from other features of lupus seen in the pristane-induced model. The susceptibility of FcγR-/- or B6/lpr mice to alveolar hemorrhage was not entirely unexpected. The recruitment of Ly6Chi CD11b+ monocytes to the peritoneal cavity in response to pristane was shown to be independent of FcγR signaling (14). Pristane exposure and defective Fas-mediated apoptosis induce lupus-associated autoantibodies by different pathways that do not appear to be synergistic (16).
B1 cells are the major population of immune cells in the peritoneal and pleural cavities and have been linked to the development of autoimmune disease (24, 31). Since pristane is injected intraperitoneally in this model of SLE, peritoneal B1 cells would be among the first immune cells to be exposed to this hydrocarbon oil. Peritoneal B-1 cells nearly completely disappear within a few days after a single pristane injection despite a sharp increase of serum IgM peaking at 2 weeks (32). Intraperitoneal injections of distilled water reduced the number of B-1 cells in the peritoneal cavity to 5-15% but did not affect the prevalence of DAH. While the cause for the disappearance of B-1 cells following pristane is unclear, it is known that distilled water depletes B-1 cells through osmotic lysis. Thus, our data suggest that B-1 cells are not pivotal to the development of pristane-induced DAH.
Abnormalities in B and T cell functions have been well described in both human and animal models of SLE (33). When the cells in BAL from pristane-treated B6 mice were examined, in addition to the monocytes and granulocytes (Fig.4A-4C), the numbers of CD19+ B cells as well as CD4+ and CD8+ T cells were increased over time (Fig.4D-4F). B6 Rag1-/- mice that lack both B and T cells had significantly reduced prevalence of DAH compared to wild type B6 mice (Fig.5A). The role of T cells in pristane-induced DAH is complex. The prevalence of DAH in T-cell deficient B6 mice was similar to that of wild type B6 mice. Additionally when B6 Rag1-/- mice were reconstituted with total B6 splenic T-cells the prevalence of DAH was reduced, yet when CD4+ T cells were transferred to B6 Rag1-/- mice the prevalence of hemorrhage did not decrease.
B-cells can act protective or pathogenic in inflammation and autoimmunity (34-36). A pathologic role of B cells in pristane-induced DAH appeared dominant based on the rare occurrence of DAH in B6 Igμ-/- mice (Fig.5A-B). The increased susceptibility of B6 Igμ-/- mice reconstituted with wild type CD19+ splenocytes prior to injecting them with pristane further confirmed the pathologic role of B-cells in this model. The accumulation of inflammatory cells and hemorrhage starts within a week of pristane injection, when an increase of serum IgG is not apparent and is too early for an adaptive B-cell response. In the absence of B cells, very few immune cells were found in the lungs of pristane-treated B6 mice (data not shown). Also, no immune complexes or complement deposition was detected in the lungs of pristane-treated B6 mice (data not shown). Although this aspect was not extensively examined, transfer of serum from mice with DAH to naïve mice did not induce DAH. All these data suggest that the B cells contribute to the DAH via non-antibody mediated functions such as production of proinflammatory cytokines and chemokines to recruit monocytes and neutrophils or via antigen-presentation function and costimulation of other immune cells (34, 37). It remains to be clarified whether B cells play a direct or indirect role in the recruitment of the inflammatory cells to the lungs and initiation of hemorrhage in this model.
The pro-inflammatory role of B-cells in DAH, suggested in the current study is also consistent with recent reports on successful treatment of cases of DAH in SLE by B cell depletion therapy using Rituxan (Rituximab) (38-40). Since the effects of Rituximab are rapid, far before any possible effect on the levels of circulating autoantibodies, it has been suggested that the main mechanism of work is via control of antigen presenting function, B-cell cytokine stimulation, and T-cell activation (40). While targeting B cells seems to be a potentially attractive therapy, they represent a minor population found within the hemorrhagic lungs of both SLE patients and B6 mice. Nevertheless, B-cell depleting therapy may be considered as a potential treatment option based on the pathogenic role of B-cells shown in the current study and a few reports of successful treatment of alveolar hemorrhage in SLE (2, 38-40).
In summary, the present study establishes the pristane-induced DAH in B6 mice as a suitable animal model to study the pathogenesis of alveolar hemorrhage. This model exhibits immunopathologic features that closely resemble what are seen in human SLE patients and will be useful to obtain a better understanding of the pathogenesis of DAH. Nevertheless, it should be noted that most mice survive and recover from DAH in this model, different from DAH in human SLE. This could be a potential limitation when testing new treatment for DAH using this model. Based on our data, we would propose the following model. Intraperitoneal injection of pristane provokes a relatively rapid and potent response by cells of the innate immune system in genetically susceptible B6 or B10 mice, but the pathways stimulating these cells are distinct from those regulating the migration of immature monocytes to the peritoneal cavity. The severity and recovery from this insult by the innate immune system is dependent upon the adaptive immune response, with B cells providing pro-inflammatory APCs that enhance and prolong the response. T cells play a more complicated role, with a normal regulatory T cell response being blunted or delayed by the pro-inflammatory B cells. The basis for the greater susceptibility of B6/B10 mice remains unclear and could be due to cells of hematopoietic or non-hematopoietic origin. Our model leads to a number of testable hypotheses that will be the target of future experiments that should shed light on a lethal and poorly understood complication of SLE.
Supplementary Material
Acknowledgments
We would like to thank Molecular Pathology and Immunology core at the University of Florida as well as Dr. Liyang Liu, Dr. Erbin Dai and Dr. Alexandra Lucas for expert assistance with the histological studies and Mr. Ed Butfiloski for expert assistance regarding the flow cytometry. This work was supported by Research Grants from Lupus Foundation of America, Inc. and R01-AR44731 and T32-007603 from the U.S. Public Health Service and by generous gifts from Lupus Link, Inc. and Mr. Lewis M. Schott to the University of Florida Center for Autoimmune Disease.
Abbreviations
- DAH
Diffuse Alveolar Hemorrhage
- MyD88
myeloid differentiation factor 88
- SLE
Systemic Lupus Erythematosus
- TLR
toll like receptor
- RNP
ribonucleoprotein
References
- 1.Kamen DL, Strange C. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med. 2010;31:479–488. doi: 10.1016/j.ccm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Todd DJ, Costenbader KH. Dyspnoea in a young woman with active systemic lupus erythematosus. Lupus. 2009;18:777–784. doi: 10.1177/0961203309104860. [DOI] [PubMed] [Google Scholar]
- 3.Santos-Ocampo AS, Mandell BF, Fessler BJ. Alveolar hemorrhage in systemic lupus erythematosus: presentation and management. Chest. 2000;118:1083–1090. doi: 10.1378/chest.118.4.1083. [DOI] [PubMed] [Google Scholar]
- 4.Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997;76:192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Badsha H, Teh CL, Kong KO, Lian TY, Chng HH. Pulmonary hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum. 2004;33:414–421. doi: 10.1016/j.semarthrit.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Schwab EP, Schumacher HR, Jr, Freundlich B, Callegari PE. Pulmonary alveolar hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum. 1993;23:8–15. doi: 10.1016/s0049-0172(05)80022-8. [DOI] [PubMed] [Google Scholar]
- 7.Desnoyers MR, Bernstein S, Cooper AG, Kopelman RI. Pulmonary Hemorrhage in Lupus Erythematosus Without Evidence of an Immunologic Cause. Arch Intern Med. 1984;144:1398–1400. [PubMed] [Google Scholar]
- 8.Castaneda S, Herrero-Beaumont G, Aguado JM, Vidal J. Pulmonary Hemorrhage in Lupus Erythematosus Without Evidence of an Immunologic Cause. Arch Intern Med. 1985;145:2128-b–2129. doi: 10.1001/archinte.1985.00360110204046. [DOI] [PubMed] [Google Scholar]
- 9.Satoh M, Kumar A, Kanwar YS, Reeves WH. Anti-nuclear antibody production and immune-complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci U S A. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. the immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- 12.Lee PY, Kumagai Y, Li Y, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PY, Li Y, Kumagai Y, et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175:2023–2033. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PY, Weinstein JS, Nacionales DC, et al. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nacionales DC, Kelly KM, Lee PY, et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane) Am J Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh M, Weintraub JP, Yoshida H, et al. Fas and Fas ligand mutations inhibit autoantibody production in pristane-induced lupus. J Immunol. 2000;165:1036–1043. doi: 10.4049/jimmunol.165.2.1036. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhary VR, Grande JP, Luthra HS, David CS. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology (Oxford) 2007;46:1405–1410. doi: 10.1093/rheumatology/kem117. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 19.Satoh M, Hamilton K, Ajmani A, et al. Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J Immunol. 1996;157:3200–3206. [PubMed] [Google Scholar]
- 20.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang H, Narain S, Sobel E, et al. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005;117:238–250. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 23.Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Swigris JJ, Fischer A, Gillis J, Meehan RT, Brown KK. Pulmonary and thrombotic manifestations of systemic lupus erythematosus. Chest. 2008;133:271–280. doi: 10.1378/chest.07-0079. [DOI] [PubMed] [Google Scholar]
- 26.Churg A, Franklin W, Chan KL, Kopp E, Carrington CB. Pulmonary hemorrhage and immune-complex deposition in the lung. Complications in a patient with systemic lupus erythematosus. Arch Pathol Lab Med. 1980;104:388–391. [PubMed] [Google Scholar]
- 27.Mintz G, Galindo LF, Fernandez-Diez J, Jimenez FJ, Robles-Saavedra E, Enriquez-Casillas RD. Acute massive pulmonary hemorrhage in systemic lupus erythematosus. J Rheumatol. 1978;5:39–50. [PubMed] [Google Scholar]
- 28.Myers JL, Katzenstein AA. Microangiitis in lupus-induced pulmonary hemorrhage. Am J Clin Pathol. 1986;85:552–556. doi: 10.1093/ajcp/85.5.552. [DOI] [PubMed] [Google Scholar]
- 29.Satoh M, Richards HB, Shaheen VM, et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol. 2000;121:399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vollmer J, Tluk S, Schmitz C, et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami M, Yoshioka H, Shirai T, Tsubata T, Honjo T. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int Immunol. 1995;7:877–882. doi: 10.1093/intimm/7.5.877. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton KJ, Satoh M, Swartz J, Richards HB, Reeves WH. Influence of microbial stimulation on hypergammaglobulinemia and autoantibody production in pristane-induced lupus. Clin Immunol Immunopathol. 1998;86:271–279. doi: 10.1006/clin.1997.4481. [DOI] [PubMed] [Google Scholar]
- 33.Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2005;17:518–522. doi: 10.1097/01.bor.0000170479.01451.ab. [DOI] [PubMed] [Google Scholar]
- 34.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderton SM, Fillatreau S. Activated B cells in autoimmune diseases: the case for a regulatory role. Nat Clin Pract Rheumatol. 2008;4:657–666. doi: 10.1038/ncprheum0950. [DOI] [PubMed] [Google Scholar]
- 36.Haas KM, Watanabe R, Matsushita T, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5:564–576. doi: 10.1038/nrd2085. [DOI] [PubMed] [Google Scholar]
- 38.Nellessen CM, Poge U, Brensing KA, Sauerbruch T, Klehr HU, Rabe C. Diffuse alveolar haemorrhage in a systemic lupus erythematosus patient successfully treated with rituximab: a case report. Nephrol Dial Transplant. 2008;23:385–386. doi: 10.1093/ndt/gfm701. [DOI] [PubMed] [Google Scholar]
- 39.Pinto LF, Candia L, Garcia P, et al. Effective treatment of refractory pulmonary hemorrhage with monoclonal anti-CD20 antibody (rituximab) Respiration. 2009;78:106–109. doi: 10.1159/000156965. [DOI] [PubMed] [Google Scholar]
- 40.Narshi CB, Haider S, Ford CM, Isenberg DA, Giles IP. Rituximab as early therapy for pulmonary haemorrhage in systemic lupus erythematosus. Rheumatology (Oxford) 2010;49:392–394. doi: 10.1093/rheumatology/kep356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


