Abstract
Focal Adhesion Kinase plays a major role in cell adhesion, motility, survival, proliferation, metastasis, angiogenesis and lymphangiogenesis. In 2004, we have cloned the promoter sequence of FAK and found that p53 inhibits its activity (BBA, v. 1678, 2004). In 2005, we were the first group to show that FAK and p53 proteins directly interact in the cells (JBC, v. 280, 2005). We have shown that FAK and p53 proteins interact in the cytoplasm and in the nucleus by immunoprecipitation, pull-down and confocal microscopy assays. We have shown that FAK inhibited activity of p53 with the transcriptional targets: p21, Bax and Mdm-2 through protein-protein interactions. We identified the 7 amino-acid site in p53 that is involved in interaction with FAK protein. The present review will discuss the interaction of FAK and p53 proteins and discuss the mechanism of FAK-p53 loop regulation: inhibition of FAK promoter activity by p53 protein and also inhibition of p53 transcriptional activity by FAK protein.
Keywords: Focal Adhesion Kinase, p53, metastasis, tumor, protein interaction
INTRODUCTION
Focal Adhesion Kinase was discovered almost 20 years ago, as a protein that plays a major role in different cellular functions such as adhesion, motility, survival, proliferation and cell cycle. The FAK gene encodes a non receptor tyrosine kinase that localizes at focal adhesions: contact points of cells with extracellular matrix, and is activated by integrin (cell surface receptor) signaling or by growth factor receptor (c-Met, EGFR, PDGFR) or by angiogenesis receptors. The FAK gene was first isolated from chicken embryo fibroblasts transformed by v-src [1]. Our laboratory was the first to isolate FAK gene from human osteosarcoma tumors and to demonstrate that FAK mRNA was up-regulated in invasive and metastatic human tumor samples [1]. This was the first evidence that FAK can be regulated at the level of gene transcription, as well as by other mechanisms (gene amplification), reported by other groups. Subsequently, we have demonstrated up-regulation of FAK protein by immunohistochemical staining in different types of human tumors, including colon, breast, thyroid, ovarian, melanoma, and sarcoma [1,2–4,5,6]. In addition, we have found novel interaction of FAK with several binding partners, such as: RIP [7], and p53 [8], linking FAK with the apoptotic/survival nuclear pathways [9,10]. In addition, we have cloned the promoter region of the FAK gene [11]. We have found that FAK promoter contains p53 binding sites, and that p53 inhibits FAK transcription both in vitro [11] and in vivo [12]. Thus, this review will be focused on FAK intracellular signaling in cancer, linking signaling from extracellular matrix to the nucleus. We found the p53 and FAK interaction as an example focal adhesion protein functioning in the signaling between extracellular matrix and nucleus. The report of Frame et al. discussed that many FERM domain proteins have nuclear export and nuclear localization signals, suggesting of other proteins involvement in the shuttling of proteins from extracellular matrix to the nucleus and exchange of this signaling [13].
FAK has several binding partners in the N-terminal, Central and C-terminal domains. The N-terminal domain of FAK contains one proline-rich domain, and the C-terminal domain of FAK contains another two proline-rich domains that are sites of binding proteins, containing SH3 domains. The C-terminal part of C-terminal domain of FAK (853–1012 a.a) called FAT (Focal adhesion targeting domain) domain that is necessary for targeting of FAK to focal adhesion complexes through binding with different proteins (paxillin, talin, Rho, etc).
The first indirect link of FAK and p53 was provided by [14]. The authors showed that extracellular matrix survival signals mediated by FAK suppressed p53-directed apoptosis [14]. We were the first group to discover the direct binding of FAK and p53 proteins in different cancer cells [8]. The N-terminal domain of p53 (1–92 a.a.) binds the N-terminal domain of FAK [8]. We have shown that p53 can bind FAK promoter and inhibit its luciferase activity [8]. Moreover, FAK can also block p53 transcriptional activity of p21, BAX and Mdm-2. Thus, there is a feedback loop mechanism of regulation of these two proteins [10]. The recent report confirmed direct binding of the N-terminal domain of FAK with p53 and also found interaction of FAK and Mdm-2 providing a novel mechanism of FAK-Mmd-2-mediated ubiquitination of p53 in the nucleus [15]. These data link FAK with the p53 tumor suppressor signaling that we will discuss below. Thus, we will discuss the novel FAK-p53 cross-talk pathways in apoptotic and survival pathways. Then we will pay attention to novel therapeutics approaches to target the FAK-p53 interaction in cancer.
p53 REPRESSES FAK PROMOTER
Our group was first to clone human FAK promoter and to find two p53 binding sites in the FAK promoter [11]. We have shown that p53 can bind FAK promoter and inhibit its transcriptional activity in vitro by EMSA [11] and in vivo by ChIP (chromatin immunoprecipitation) assay [12]. In addition, several other transcription factors, such as SP-1, AP-2, TCF-1 and NF-kappa B were shown to be present in the FAK promoter. NF-kappa B protein has been shown to be linked to p53 pathway [16]. For example, activation of Cox-2 transcription required co-operation of NF-kappa B and p53 [16]. Thus, regulation of FAK promoter can also include association of these two transcription factors, thus providing additional indirect p53-regulated FAK expression mechanism. Recently, one group demonstrated that bortezomib can down-regulate FAK promoter activity through NF-kappa B-dependent inhibitory, but not through p53-dependent mechanism [17].
The global analysis of p53 transcription factor binding sites demonstrated that induction of HCT116 colon cancer cells with 5-fluorouracil transcriptionally down-regulated FAK [18]. Thus, the authors suggested that p53 can suppress metastasis through down-regulation of metastasis-related genes, such as FAK. We have shown recently that p53 can down-regulate FAK expression in human cancer cells [12]. FAK mRNA and protein was increased in primary colon and breast tumors with mutant p53 versus wild type p53 tumors [12]. We demonstrate that adenoviral p53 directly blocked FAK mRNA and FAK protein levels [12]. In addition, we have demonstrated high correlation between FAK overexpression and p53 mutation in 600 breast cancer tumors [12, 14, 19]. Recently, another group also have shown the p53-dependent repression of FAK in breast cancer in response to estradiol [20]. FAKmRNA and promoter were down-regulated by estradiol in estrogen-dependent breast cancer cell lines with wild type p53, but not with mutant p53 [20]. The data show that p53 is an important regulator of FAK in breast cancer cell lines and that that loss of p53 function in breast cancer may enhance metastasis of estrogen-responsive tumors through upregulated FAK expression upon estrogens stimulation.
DIRECT FAK AND p53 PROTEIN INTERACTION
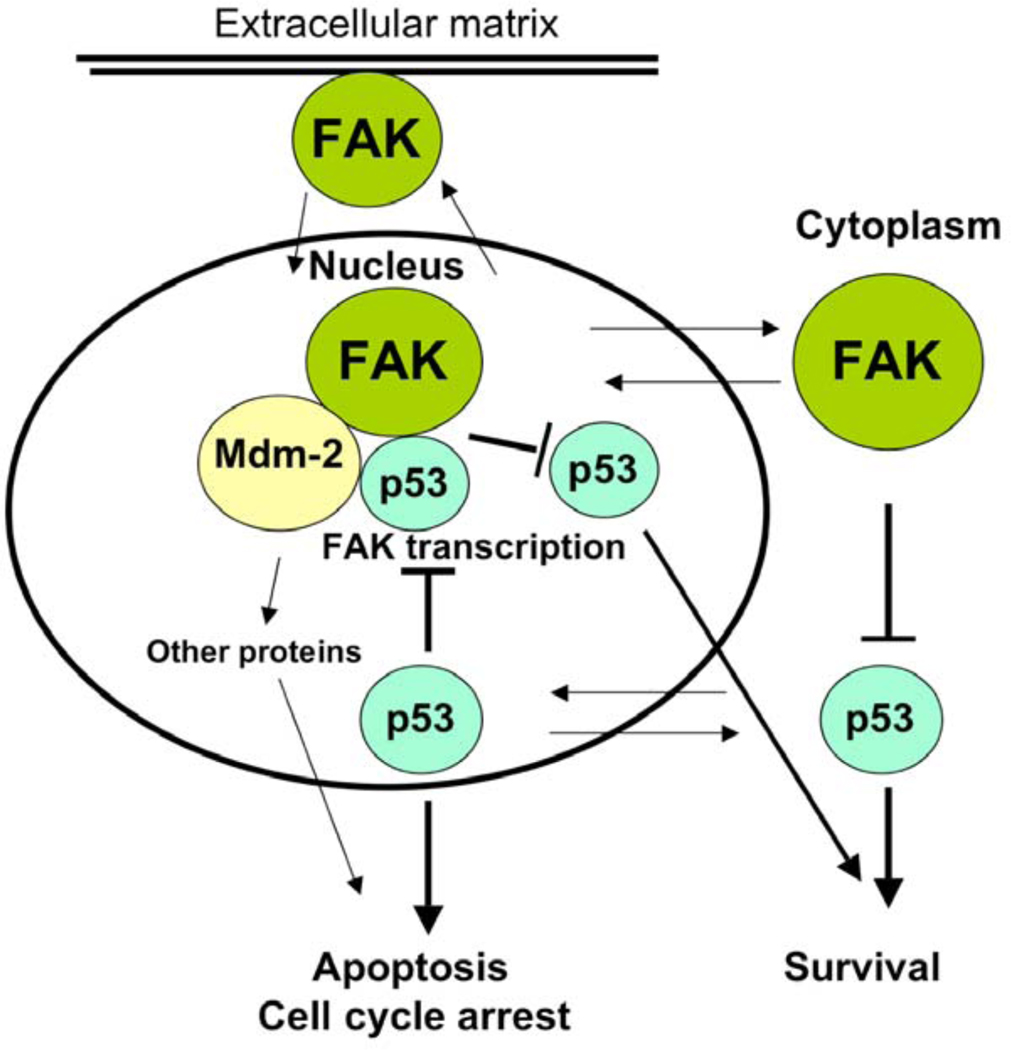
We were the first group to demonstrate the direct interaction of FAK and p53 proteins by immunoprecipitation, pull-down and confocal microscopy methods [21]. We have demonstrated that the N-terminal domain of p53 (1–92 a.a.) physically directly binds the N-terminal domain of FAK [8]. Three years later another group confirmed interaction of FAK and p53 proteins [15], demonstrating also interaction of FAK and Mdm-2 proteins and providing support for the nuclear function of FAK (Fig. 1). The authors demonstrate the regions of FAK that bind Mdm-2 and p53. There have been several reports on the localization of the N-terminal part of FAK in the nucleus [22–25]. Furthermore, the N-terminus of FAK was shown to cause apoptosis in breast cancer cell lines [23] and its nuclear localization was regulated by caspase inhibitors in endothelial cells [25]. In addition, p53 has been reported to be localized in the cytoplasm [26]. P53 directly activated Bax and released pro-apoptotic molecules, activating multidomain proteins in the cytoplasm. This mechanism required 62–91 residues in the proline-rich N-terminal domain of p53 [26]. We detected interaction and co-localization of p53 and FAK in tumor colon cancer samples. Moreover, we have shown that 7 amino-acids (65–71 a.a.) from the proline-rich region of p53 were involved in interaction with FAK [27]. Thus, we have shown direct interaction of FAK and p53 proteins [21] and detected exact region of p53 that is involved in interaction with FAK protein [27]. Thus, understanding the detail mechanism and functions of FAK/p53-interaction may ultimately have important implications for targeted cancer therapy.
Fig. (1).
A model of FAK and p53 interaction and functions in cells and signal transduction pathways from extracellular matrix to the cytoplasm and to the nucleus. Numerous binding partners of FAK integrate signals from the extracellular matrix through growth factor receptors and integrins to control motility, survival, proliferation, metastasis, lymphangiogenesis and angiogenesis this signaling. In the nucleus, p53 binds FAK promoter and inhibits its transcription and causes cell cycle arrest, apoptosis or other mechanisms of growth inhibition. FAK binds p53 and sequesters p53 from apoptotic signaling and inhibits it growth inhibition function. FAK also binds Mdm-2 to facilitate p53 proteosomal degradation and enhancing cell survival. There is a feedback loop in FAK-p53 regulation. Thus, FAK and p53 mediate signaling from extracellular matrix to the cytoplasm and nucleus.
FEEDBACK MODEL OF FAK-p53 PROTEIN INTERACTION
We have shown that p53 can suppress FAK transcription [11,12]. The global characterization of 65,572 p53 ChIP DNA fragments was done in HCT116 colorectal cancer cell line, treated with 5-fluorouracil to activate p53 [18]. The authors identified novel targets of p53, that are involved in cell adhesion, migration and metastasis, and PTK2 or FAK was one of these kinases [18]. Interestingly, in HCT116 cells, treated with 5-fluorouracil that increases p53 level, the PTK2 (FAK) expression was also inhibited [18].
CONCLUSIONS
We have also shown that FAK can suppress transcriptional activity of p53 through its interaction, as p53-mediated activation of p53-targets: p21, Mdm-2 and Bax was blocked by overexpression of FAK [8]. Thus, p53 can regulate FAK (by inhibiting transcription, and also FAK can regulate p53 by sequestering it from apoptotic signaling and by ubiquitination that decreases p53 transcriptional functions [15] (Fig. 1). Thus FAK and p53 can be regulated through a feedback mechanism [10]. Mutations of p53 that are frequently found in cancers, can lead to up-regulation and overexpression of FAK. Thus, novel mechanisms of FAK survival function, FAK and wild type or mutant p53 interactions remain to be discovered during carcinogenesis. This novel interaction open avenue for targeting the complex of FAK and p53 and Mdm-2 proteins and developing novel therapeutics.
ACKNOWLEDGEMENTS
The work was supported by NIH grant CA65910 to WGC and by Susan Komen for the Cure grant BCTR0707148 to VMG.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST
Dr. Cance and Dr. Golubovskaya are inventors of patents and Co-Founders and shareholders of CureFAKtor Pharmaceuticals.
REFERENCES
- 1.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Research. 1995;55(13):2752–2755. [PubMed] [Google Scholar]
- 2.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann. Surg. Oncol. 1996;3(1):100–105. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 3.Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 2000;6(6):2417–2423. [PubMed] [Google Scholar]
- 4.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86(8):1551–1556. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance WG. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin. Cancer Res. 2003;9(1):215–222. [PubMed] [Google Scholar]
- 6.Lightfoot HM, Jr, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, Craven RJ, Cance WG. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res. Treat. 2004;88(2):109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 7.Kurenova E, Xu L-H, Yang X, Baldwin AS, Jr, Craven RJ, Hanks SK, Liu Z-g, Cance WG. Focal Adhesion Kinase Suppresses Apoptosis by Binding to the Death Domain of Receptor-Interacting Protein. Mol. Cell. Biol. 2004;24(10):4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J. Biol. Chem. 2005;280(26):25008–25021. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 9.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int. Rev. Cytol. 2007;263:103–153. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 10.Cance WG, Golubovskaya VM. Focal Adhesion Kinase Versus p53: Apoptosis or Survival? Sci. Signal. 2008;1(20) doi: 10.1126/stke.120pe22. pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golubovskaya V, Kaur A, Cance W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: nuclear factor kappa B and p53 binding sites. Biochimica et Biophysica Acta (BBA) - Gene Struc. Exp. 2004;1678(2–3):111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. p53 regulates FAK expression in human tumor cells. Mol. Carcinog. 2008;47(5):373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 11(11):802–814. doi: 10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- 14.Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 1998;143(2):547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell. 2008;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benoit V, de Moraes E, Dar NA, Taranchon E, Bours V, Hautefeuille A, Taniere P, Chariot A, Scoazec JY, de Moura Gallo CV, Merville MP, Hainaut P. Transcriptional activation of cyclooxygenase-2 by tumor suppressor p53 requires nuclear factor-kappaB. Oncogene. 2006;25(42):5708–5718. doi: 10.1038/sj.onc.1209579. [DOI] [PubMed] [Google Scholar]
- 17.Ko BS, Chang TC, Chen CH, Liu CC, Kuo CC, Hsu C, Shen YC, Shen TL, Golubovskaya VM, Chang CC, Shyue SK, Liou JY. Bortezomib suppresses focal adhesion kinase expression via interrupting nuclear factor-kappa B. Life Sci. 86(5–6):199–206. doi: 10.1016/j.lfs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, Liu J, Zhao XD, Chew JL, Lee YL, Kuznetsov VA, Sung WK, Miller LD, Lim B, Liu ET, Yu Q, Ng HH, Ruan Y. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Golubovskaya VM, Cance W. Focal adhesion kinase and p53 signal transduction pathways in cancer. Front. Biosci. 2010;15:901–912. doi: 10.2741/3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anaganti S, Fernandez-Cuesta L, Langerod A, Hainaut P, Olivier M. p53-Dependent repression of focal adhesion kinase in response to estradiol in breast cancer cell-lines. Cancer Lett. 2010;300(2):215–224. doi: 10.1016/j.canlet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Mei L, Xiong WC. FAK interaction with MBD2: A link from cell adhesion to nuclear chromatin remodeling. Cell Adh. Migr. 2010;4(1):77–80. doi: 10.4161/cam.4.1.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart A, Ham C, Zachary I. The focal adhesion kinase amino-terminal domain localises to nuclei and intercellular junctions in HEK 293 and MDCK cells independently of tyrosine 397 and the carboxy-terminal domain. Biochem. Biophys. Res. Commun. 2002;299(1):62–73. doi: 10.1016/s0006-291x(02)02547-0. [DOI] [PubMed] [Google Scholar]
- 23.Beviglia L, Golubovskaya V, Xu L, Yang X, Craven RJ, Cance WG. Focal adhesion kinase N-terminus in breast carcinoma cells induces rounding, detachment and apoptosis. Biochem. J. 2003;373(Pt 1):201–210. doi: 10.1042/BJ20021846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones G, Stewart G. Nuclear import of N-terminal FAK by activation of the FcepsilonRI receptor in RBL-2H3 cells. Biochem. Biophys. Res. Commun. 2004;314(1):39–45. doi: 10.1016/j.bbrc.2003.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Lobo M, Zachary I. Nuclear localization and apoptotic regulation of an amino-terminal domain focal adhesion kinase fragment in endothelial cells. Biochem. Biophys. Res. Commun. 2000;276(3):1068–1074. doi: 10.1006/bbrc.2000.3547. [DOI] [PubMed] [Google Scholar]
- 26.Chipuk JE, Green DR. Cytoplasmic p53: Bax and Forward. Cell Cycle. 2004;3(4):429–431. [PubMed] [Google Scholar]
- 27.Golubovskaya V, Finch R, Zheng M, Kurenova EV, Cance WG. The 7 amino-acid site in the proline-rich region of the N-terminal domain of p53 is involved in interaction with FAK and is critical for p53 functioning. Biochem. J. 2008;411(1):151–160. doi: 10.1042/BJ20071657. [DOI] [PubMed] [Google Scholar]