Abstract
The dramatic rise in worldwide prevalence of obesity has necessitated the search for more efficacious anti-obesity strategies to counter the increased cancer risks in overweight and obese individuals. The mechanistic pathways linking obesity status with adult chronic diseases such as cancer remain incompletely understood. A growing body of evidence suggests that novel approaches and interventional agents to disrupt the feed-forward cycle of maternal to offspring obesity transfer that is initiated in utero, will be important for stemming both the obesity pandemic and the associated increase in cancer incidence. The convergence of multiple research areas including those encompassing the insulin and insulin-like growth factor (IGF) systems, epigenetics, and stem cell biology is providing insights into the potential for cancer prevention in adult offspring previously exposed to the intrauterine environment of overweight/obese mothers. Here, we review the current state of this nascent research field, with a focus on three major cancers namely breast, colorectal and liver, and suggest some possible future directions to optimize its impact for the health of future generations.
Keywords: fetus, obesity, cancers, programming, interventions
Obesity pandemic and cancer risks
Obesity has become a global problem (http://apps.nccd.cdc.gov/dcpcglobalatlas/DietNutrition.aspx#WorldMap). The last three decades have seen a huge rise in the number of individuals who are overweight (body-mass index [BMI] 25-29.9 kg/m2) or obese (BMI ≥30 kg/m2). In the United States alone, the age-adjusted prevalence of adult obesity during 2007-2008 reached 33.8% while that for obesity and overweight combined rose to 68.0% of the population (1). Importantly, nearly 50% of American women of childbearing age are overweight or obese. Among children and adolescents, 31.7% were at or above the 85th percentile of BMI for age within the same period (2). These statistics are alarming, as children who are overweight or obese tend to remain so later in life. The presumed foremost cause of the obesity pandemic is the imbalance between caloric intake and physical activity, although environmental, developmental (fetal, postnatal and trans-generational), and genetic factors are contributors as well. The excess burden of bodyweight in both the young and adult brings with it significant health complications including increased rates of: cancer, cardiovascular disease, type 2 diabetes, hypertension, metabolic syndrome, and non-alcoholic fatty liver disease.
Numerous studies have documented the positive associations of cancer incidence with high BMI. In one recent report (3), increased risks of multiple cancers were found to be commensurate with a 5 kg/m2 increase in BMI; this corresponds to weight gains of only 15 kg and 13 kg, respectively in men and women with an average BMI of 23 kg/m2. In men, these cancers included those of the esophagus, thyroid gland, colon, kidney, and rectum as well as malignant melanoma, multiple myeloma, leukemia and non-Hodgkin's lymphoma. In women, cancers whose risk is positively associated with obesity were those of the endometrium, gallbladder, kidney, esophagus, thyroid gland, (post-menopausal) breast, pancreas, and colon in addition to leukemia and non-Hodgkin's lymphoma (3, 4). The magnitude of association of high BMI with colon and rectal cancers was stronger for men than women, whereas that for renal cancer was stronger for women than men, reflecting the complex interactions of obesity and gender with cancer risk (3).
Several recent trends in cancer incidence noted for the US population suggest associations with obesity. For example, whereas the age-adjusted colorectal cancer incidence rates for 1997-2006 declined among both men and women of age 50 years or greater, these rates increased among those younger than 50 years of age (5) and for which obesity rates have also greatly increased. In men, incidence of kidney, liver and esophageal cancers as well as leukemia, myeloma and melanoma have increased between 2002 and 2006 (5). An upward trend in incidence rates of lung, thyroid, pancreas, bladder and kidney cancers and non-Hodgkin's lymphoma, melanoma and leukemia were similarly noted for women of the (5).
Birth weight and birth length as surrogates of fetal growth and nutrition: links with later obesity and cancer incidence
Birth weight and length for infants of normal gestation period has important predictive value for later adult BMI as well as propensity for chronic diseases. High birth weight has been associated with an increased tendency for obesity in later life (6). Low birth weight is associated with increased risk for heart disease, hypertension, type 2 diabetes and glucose intolerance in adulthood (7). Importantly, birth weight and birth length are positively associated with risk for certain cancers (8, 9). Among childhood cancers, birth weight is positively associated with increased risk for neuroblastoma (10) and leukemia (11-13). Further, the risk of prostate (14, 15) and testicular (16, 17) cancers in men is positively associated with birth weight. In contrast, increased risk for colorectal cancers is linked to lower birth length for men, but not women (18).
The influence of birth weight on breast cancer risk is not as straightforward, although several recent reports show a modest positive correlation of birth weight (and length) with occurrence of this cancer in adulthood (19-22). Women who had lower birth weight but had increased adipose tissue deposition while young had lower breast cancer risk (23). In this regard, the apparent positive associations of birth weight and length with a pre-menopausal woman's circulating estradiol levels (considered a major risk factor for breast cancer) are intriguing (24). However, in another study, pre-menopausal women with lower than average birth weights who then gained excess weight as adults tended to have elevated serum estrogen concentrations than did women with higher birth weights and excess weight gain as adults (25). While it is apparent that birth indices can have long lasting effects on circulating estradiol levels, the relative contributions of the ovaries, adrenals and adipose depots to these programmed changes in estradiol synthesis and secretion, with potential impact on breast cancer risk, remain unexplored.
Maternal BMI and progeny's adiposity
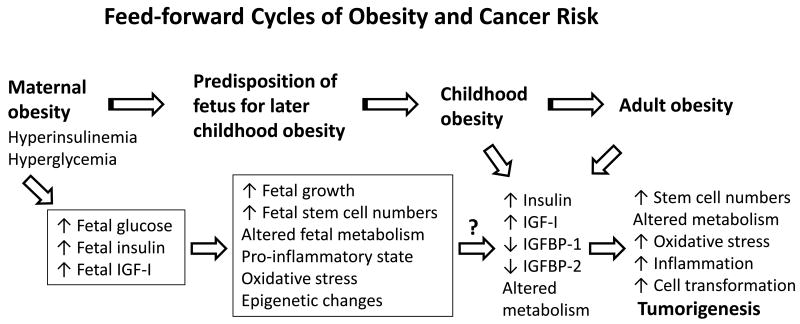
Maternal BMI is positively associated with increased birth weight and neonatal adiposity (26). A high pre-pregnancy BMI and/or excessive weight gain during pregnancy confer a propensity for greater adiposity of children born from these mothers (27-30). Children with high BMI often become obese adults (31-33). The effect of pre-pregnancy obesity on adiposity status of progeny may be amplified by concurrent gestational diabetes (and insulin resistance) in pregnant mothers (30, 34). Thus, a positive feed-forward cycle of adiposity transferred from mother to child will in all likelihood, increase relative risk for cancers in the latter during adulthood and maybe even earlier (Figure 1).
Fig. 1.
Model for maternal to offspring transmission of obesity and cancer risk. IGF-I, insulin-like growth factor-I, (mitogen, cell survival factor); IGFBP-1, insulin-like growth factor-binding protein-1, (an IGF-I binding protein present in sera and tissues and which regulates IGF-I bio-availability); IGFBP-2, insulin-like growth factor-binding protein-2, (an IGF-I binding protein present in sera and tissues and which also regulates IGF-I bio-availability). The experimental data supporting this model are discussed in the text. The model predicts the efficacy of early intervention (i.e., in overweight/obese mothers, prior to or during pregnancy) in lowering risks for both obesity and cancer in their offspring.
Obesity and insulin/IGF systems
The insulin and IGF systems are likely important functional links between obesity and increased cancer risk. Of particular note, obesity leads to enhanced circulating levels of insulin, IGF-I, IGF-II and IGFBP-3, while reducing levels of the low molecular weight IGF-binding proteins (IGFBP-1, IGFBP-2) (Table 1). The net effect of these changes is increased signaling through the insulin and IGF-I receptors, with resultant increased mitogenesis and decreased apoptosis, in many if not all tissue sites. An elevated circulating level of insulin is a major risk factor for colon, breast and liver cancers (Table 2). Similarly, an elevated circulating level of IGF-I and reduced circulating levels of IGFBP-1 and IGFBP-2 are known risk factors for colon cancer (Table 2). The circulating IGF system, as well as pancreatic insulin secretion and tissue insulin sensitivity, are influenced by dietary or obesity effects in utero and/or during lactation (35-38).
Table 1. Associations of obesity or body mass index (BMI) with blood levels of insulin and insulin-like growth factor system components in adults and children.
| Obesity (men) | ↑ insulin | ↓ IGFBP-1 | Ref. 128 |
| ↑ free IGF-I | ↓ IGFBP-2 | ||
|
| |||
| Overweight/obesity (women) | ↑ insulin | ↓ IGFBP-1 | Ref. 129 |
| ↓ IGFBP-2 | |||
|
| |||
| BMI (men) | ↑ IGFBP-3 | ↓ IGFBP-2 | Ref. 130 |
|
| |||
| BMI (men and women) | ↑ IGF-II | ↓ IGFBP-2 | Ref. 131 |
|
| |||
| BMI (women) | ↑ IGF-II | Ref. 132 | |
| ↑ IGFBP-3 | |||
|
| |||
| ↑ bioactive/total | ↓ total IGF-I | Ref. 133 | |
| IGF-I | ↓ IGFBP-1 | ||
|
| |||
| Obesity (children) | ↑ insulin | ↓ IGFBP-1 | Ref. 134 |
| ↑ IGF-II | ↓ IGFBP-2 | ||
| ↑ IGFBP-3 | |||
| ↑ IGFs/IGFBPs | |||
|
| |||
| ↑ insulin | ↓ IGFBP-2 | Ref. 135 | |
|
| |||
| ↑ insulin | Ref. 136 | ||
| ↑ IGF-I | |||
| ↑ IGFBP-3 | |||
|
| |||
| ↑ insulin | Ref. 137 | ||
| ↑ IGFBP-3 | |||
Only those factors that exhibited a statistically significant association with obesity or BMI within a given study are listed. IGF-I, insulin-like growth factor-I; IGFBP-1, insulin-like growth factor-binding protein-1; IGFBP-2, insulin-like growth factor-binding protein-2; IGFBP-3, insulin-like growth factor-binding protein-3; IGF-II, insulin-like growth factor-II.
Table 2. Associations of insulin and insulin-like growth factor system components (in serum or plasma) with risk for three major cancers.
| Elevated insulin | ↑ Colon cancer risk Refs. 138-143 | ↑ Breast cancer risk (pre-menopausal) Ref. 144 | ↑ Breast cancer risk (post-menopausal) Refs. 4, 138, 145 | ↑ Hepatocellular carcinoma risk Refs. 107, 146-148 |
| Elevated IGF-I | ↑ Colon cancer risk Refs. 139, 141, 149, 150 | |||
| IGFBP-1 | ↓ Colon cancer risk Refs. 139, 141, 142 | |||
| IGFBP-2 | ↓ Colon cancer risk Refs. 139, 142 |
Only those endocrine factors that exhibited a statistically significant and consistent (i.e., over multiple studies) association with specific cancer risk are shown. Some studies reported significant associations of serum IGF-I, IGFBP-1, IGFBP-2 and/or IGFBP-3 with pre-menopausal or post-menopausal breast cancer risk, however a comparable number of other studies reported a lack of such associations.
The liver is the major tissue source of circulating IGF-I and IGFBPs. The relationships of serum IGF-I and IGFBPs with hepatocellular carcinoma risk (and status) are complex, due to the dys-regulated expression of these genes in pre-neoplastic, transformed or cancerous liver cells.
Maternal obesity and breast cancer
Pregnancy weight gain may influence risk for breast cancer in mothers and their daughters. For post-menopausal women, excessive weight gain during their prior pregnancies was found to increase risk for development of breast cancer (39). By contrast, a comparable gain in weight may confer short-term protection against pre-menopausal breast cancer (40). Interestingly, BMI, pregnancy weight gain and dietary fat intake do not appear to significantly affect maternal steroid hormone levels during pregnancy (41-43). In animal models of mammary carcinogenesis, consumption of a high fat diet resulted in enhanced pregnancy weight gain and an increase in subsequent mammary cancer incidence (44). Effects of obesity (and of excessive weight gain) during pregnancy on breast cancer risk of daughter(s) has also been examined, albeit only in limited fashion. Pre-pregnancy BMI was not associated with breast cancer risk of daughters, whereas a pregnancy weight gain of 25-34 pounds was associated with a slightly increased risk for breast cancer (OR=1.5; CI 1.1, 2.0) in daughters (45). Paradoxically, women whose mothers gained 35 pounds or more during pregnancy were not at increased risk. While these associations were based on a limited number of subjects (510 case mothers, 436 control mothers), have not been replicated, and the mechanism(s) underlying this association at the molecular level remains unexplored, the potential consequences of these associations if confirmed, are highly relevant to the current global obesity pandemic.
The uterine milieu has been shown to affect offspring's risk for adult breast cancer. For example, the condition of preeclampsia has been associated with protection against breast cancer risk in both mothers and their female offspring (19, 46, 47). Pelvic intercristal width in pregnant mothers, which was used as a surrogate for maternal circulating sex steroid hormones, was positively associated with risk of breast cancers in adult offspring (48). Further, a linkage between higher maternal BMI and elevated cord blood C-peptide levels (26), a stable biomarker of insulin secretion, has been reported. The latter provides a possible route by which maternal BMI may adversely influence the fetus.
Exposure of rats or mice during pregnancy and/or lactation to diets rich in energy, fats and/or sugars has detrimental health effects in their offspring. These include: increased adiposity; hyperglycemia; hyperinsulinemia; triglyceridemia; depressed immune function; altered neural and satiety regulatory pathways; hypertension; reduced plasma antioxidant status; and lower bone mineral density (36, 49-56). Pregnant rats fed a high fat diet delivered pups with increased birth weight and their female offspring exhibited shortened mammary tumor latency and increased tumor growth when given a mammary carcinogen as young adults (57). In a similar model of mammary carcinogenesis, offspring of dams fed diets containing a large amount of corn oil as fat source prior to and during pregnancy/lactation, showed increased mammary tumor incidence (58). In contrast, feeding an equivalent amount of olive oil (considered a healthy source of dietary fats) to dams was inhibitory to tumor formation in their progeny. These latter studies highlight the value of dietary lipid composition (good vs. bad fats) in the in utero programming of breast cancer.
Maternal obesity and colo-rectal cancers
Overweight and obesity are positively associated with increased colo-rectal cancer risk in men and to a lesser degree in women (59-63). The positive linkage between colon cancer mortality and obesity is also more evident in men than in women (64). Similar findings were observed in rat (65-67) and mouse (68, 69) models. In obese animals, colon tumor genesis was correlated with elevations in serum insulin, leptin, glucose, triglycerides, and cholesterol. However, proof of causality for any of these factors, individually or together, remains lacking.
Several studies have probed the developmental influences of underweight, overweight and obesity in children on their subsequent risk for colo-rectal cancer. Exposure to energy restriction during childhood and adolescence (Dutch famine years of WW II) was associated with reduced risk of colo-rectal cancer later in life (70), presumably reflecting metabolic programming and/or an epigenetic phenomenon. High BMI (i.e., above the 85th percentile of a US reference population) at adolescence (i.e., 14-19 years of age) was positively associated with increased risk of death (relative risk of 2.1 and 2.0 for males and females, respectively) from colorectal cancers at later life (71). Interestingly, a recent study showed that colon cancer risk in men is highly influenced by less drastic weight gains (63). In their study, Thysegen and colleagues reported that a cumulative mean BMI above 22.5 conferred increased colon cancer risk. Weight gain (1 lb/year) beginning at age 21 was associated with increased cancer risk (63). Short-term (2-4 years prior) weight gain of 10 lbs was positively associated with cancer risk in the proximal half of the colon (63). The rise in propensity for colon cancer with long-term weight gains was demonstrated in a study of African-American women (72). In this case, a ≥30 kg weight gain beginning at age 18 was positively associated with risk for colorectal polyps. Animal studies also support the influence of early postnatal weight gain on colon cancer incidence. Early over-feeding of pre-weanling rats enhanced colon tumorigenic capability later in life (65). In a rat model of intestinal carcinogenesis, switching of pregnant rat dams from a non-obesogenic diet to a more obesogenic diet at parturition accelerated early neonatal body weight accretion, increased colon tumor multiplicity and altered circulating levels of IGF system components in male progeny as later adults (35). However, no studies (using human populations or animals) have yet evaluated the role of maternal obesity on progeny's colon cancer risk at later adulthood.
Maternal obesity and hepatocellular carcinoma
Obesity leads to an increased propensity for liver cancers in humans (73-76). Feeding a ‘Western’ or ‘cafeteria’ diet rich in fats and sugars to pregnant and lactating rats promoted hepatic steatosis and liver oxidative stress response in their progeny (53). Steatosis and oxidative stress are well-known promoters of the liver pathology that precedes hepatocellular carcinoma (77). Thus, it is tempting to speculate that the rising incidence of liver cancer in the US and other western societies (associated with increased rates of hepatitis virus infection) is further fueled, in part, by maternal obesity. However, to the best of our knowledge, no studies have directly examined this possibility.
Maternal obesity and the fetal/neonatal epigenome
Effects of diet and nutrition can be trans-generational. Chronic consumption of high fat diets by young female rats conferred glucose intolerance, hyperinsulinemia, triglyceridemia, and increased adiposity to their male progeny, indicating long-term, programmed and heritable alterations in metabolism, gene expression, and tissue phenotype (78). Feeding a high carbohydrate diet to female rat pups around weaning led to hyperinsulinemia and increased adiposity later in life; effects they transmitted to their progeny (79). Similarly, consumption of a high fat diet for four weeks by dams from pre-pregnancy through to weaning induced a heritable increase in body length and a decrease in insulin sensitivity in first and second-generation rat offspring (80). Most interestingly, these trans-generational effects were propagated via maternal and paternal lineages, indeed supporting an epigenetic basis (80).
The long-term contributions of maternal diet to adult offspring health have been postulated to also involve altered stem cell numbers and/or rates of stem cell renewal (81, 82). To date, only a few known links between nutrition, the insulin and IGF systems, and stem cell renewal have been defined (83-85). While the relevance of insulin as an essential factor for in vitro sphere-forming ability (a measure of cancer stem cell renewal) of multiple cancer stem cell types is compelling, given this factor's mitogenic action, further studies are required to mechanistically explain these connections.
With increased interest in the role that epigenetics plays in disease evolution, studies seeking to understand if, and how, maternal obesity (and maternal diet) affects the epigenome of the fetus and neonate and in so doing, modifies disease susceptibility at later adulthood have grown in numbers within the last several years. Initial studies have focused on rat liver metabolic and metabolism-regulatory genes to model dietary influences involving the epigenome in the impairment of developmental processes in progeny. Dietary protein restriction and folic acid supplementation in pregnant rats were found to elicit specific changes in DNA methylation of liver glucocorticoid receptor and peroxisomal proliferator-activated receptor-α genes in progeny, correlating with respective levels of expression (86-88). Importantly, both of these nuclear receptors are key players in metabolic regulation by the liver. In a study of intrauterine growth-restricted rat fetuses, altered histone methylation status of the liver IGF-I gene was associated with corresponding modifications in hepatic IGF-I expression and deregulated metabolic status (38). Remarkably, a high fat diet fed to male rats programmed pancreas β-cell dysfunction, as well as hypomethylation of a specific pancreatic islet gene in their female progeny (89). Neonatal overfeeding, via litter size restriction, led to increased methylation of the insulin receptor gene promoter in adult rat hypothalamus (90). Such phenomena are not restricted to rodents. A recent study of pregnant women found that maternal folic acid supplementation during pregnancy correlated with increased DNA methylation of the IGF-II gene differentially methylated region (DMR) in their children at age 1½ years (37). Most significantly, an inverse association between extent of IGF-II gene DMR methylation and birth weight was documented. While none of the above studies specifically examined for effects of maternal obesity or overweight on epigenetic phenomena in progeny, they do provide impetus for further investigations in this direction.
In order to comprehensively address the contribution of the maternal environment, fetal stem cells and the fetal epigenome to the etiology of breast cancer, the epigenetic program of mammary gland development and functional differentiation was recently elucidated (91). This information will provide an initial roadmap into understanding how the in utero and immediate postnatal environments (and interactions with maternal obesity phenotype) modify the mammary gland epigenome as well as this organ's predisposition to or protection from, tumorigenesis in adult progeny. Similar strategies will invariably prove useful for other tissues subject to fetal programming and high cancer incidence such as the liver, colon and uterus. The ultimate goal of such studies is to enable reversal of in utero-instigated epigenetic events (i.e., silencing of tumor suppressive genes and pathways) that contribute to tumor initiation and progression (92-95).
Need for new metabolism-based screening paradigms for cancer pre-disposition
It is now recognized that a greater than average weight gain during the first years of postnatal life is positively associated with obesity and insulin resistance later in life (6, 27, 31, 96), thereby also leading to increased cancer risk. Weight gains from 0 to 3 months of age were negatively associated with serum ghrelin and adiponectin when corrected for body fat at age 17 years (96). Children born from mothers with type 1 diabetes exhibit increased frequency of overweight/obesity and the BMI of their children were found to be positively correlated with cord blood leptin, albeit not with insulin levels (97). Moreover, daughters born from mothers who had gestational diabetes mellitus and impaired glucose tolerance during pregnancy had increased waist circumference and increased insulin resistance at 15 years of age (98). These findings point to the utility of screening paradigms for children born from overweight, obese, or gestational diabetic mothers to estimate pre-disposition for adiposity and associated co-morbidities. Such screens could include indices of rates of weight gain, adiposity, adipose-related serum hormones, and insulin sensitivity/resistance during early childhood. Screening could be coupled with nutritional or other preventive interventions such as changes in lifestyle and increased physical activity.
Interventions prior to and during pregnancy
Dietary interventions prior to and during pregnancy may confer some degree of protection against the programming effects of maternal obesity status (99). Indeed, an increased understanding of the maternal influence on health status of progeny has led to current goals to reduce weight gain prior to and during pregnancy for obese/overweight women and to control hyperglycemia in mother and fetus. The newly revised Institute of Medicine guidelines (http://www.iom.edu/Reports/2009/Weight-Gain-During-Pregnancy-Reexamining-the-Guidelines.aspx) call for less overall body weight gain during gestation for those women who begin pregnancy already overweight or obese. Nonetheless, there is a dearth of understanding, borne from a lack of science, for how children's cancer risk is influenced by the mother's BMI at peri-conception and pregnancy. Additionally, the relative contribution, if any, of paternal overweight and obesity status during the peri-conceptual period to an offspring's cancer risk is unknown. These are important questions, ripe for elucidation using new high-throughput methodologies.
Several hypoglycemic agents that potentially are useful (in combination with diet) during preconception and pregnancy to mitigate the negative effects of maternal obesity include metformin, glyburides and glucagon-like peptide (GLP-1) analogs (100-105). Metformin, in particular, has recently been associated with reduced cancer incidence of multiple tissue sites (including breast, colon and liver) in diabetic individuals (106-111). While actively investigated as a treatment for gestational diabetes, to our knowledge, no studies have examined the efficacy of metformin during pregnancy to prevent cancers in progeny (either animal or human studies). The ‘promise’ of metformin in prevention of cancer programming is highlighted by its ability to traverse the placenta and its lack of teratogenic activity for the human fetus (112).
Maternal obesity and high fat/high calorie maternal diets impose a pro-inflammatory state on the fetus (113, 114). In a study of pregnant rat dams, supplementation of a high-fat diet with the anti-oxidant (and dietary factor) quercetin partially reversed the metabolic syndrome phenotype in progeny (51). This remarkable result raises the possibility that anti-oxidant-enriched maternal diets could be used to favorably affect an offspring's cancer risk. While there are only few pre-clinical studies that directly address this potential, there are many dietary phytochemicals with known anti-oxidant properties and which also exhibit in vivo bioavailability and in vitro or in vivo cancer-inhibiting actions; examples include quercetin, lycopene, resveratrol, anthocyanin(s), curcumin, silymarin, and catechins (115). Diets enriched for these factors, and perhaps used in combination with metformin, may provide benefits to expectant mothers who are obese. Since some of these same bioactive factors appear to be modifiers of the epigenome, the elucidation of their influence on the expression and activity of chromatin-modifying enzymes may provide insights into their potential cancer-inhibitory actions in offspring (116, 117). The feasibility of this approach for minimizing susceptibility to cancer and other adult-onset chronic diseases clearly necessitates pre-clinical studies in rodent models of maternal obesity.
Cancer risk, both in the immediate and longer term, is potentially modifiable by nutritional means (118). A striking example of this is the observation that soyfood consumption during childhood and adolescence lowers breast cancer risk in females during later adulthood (119, 120). Indeed, a soy protein-based diet fed only during gestation delayed the first appearance of tumor, decreased tumor multiplicity, and inhibited tumor grade in female rat progeny given a mammary carcinogen, when compared to control animals (121). Similarly, a maternal (gestation/lactation) diet containing a soy protein isolate resulted in significant reductions in body weight, mammary terminal end bud number, and abdominal fat pad weight, and enhanced mammary gland differentiation in female weanling rats, compared to a control maternal diet (122). A maternal (gestation plus lactation periods) diet containing blueberry powder (a rich source of anthocyanins and polyphenols with demonstrated anti-oxidant activities) enhanced mammary epithelial differentiation in weanling rats, an effect that may indicate tumor-protective actions in later life (123). Lastly, a recent report demonstrated significant improvement in glucose tolerance for 6-month-old mice, whose dams received a soy protein-based diet from preconception and throughout pregnancy (124). These studies provide strong rationale for further development of specific dietary formulations for pregnant obese/overweight mothers with the goal of reducing the potential for cancers in offspring as adults (125). Lastly, given the reports that breast-feeding may confer a lower risk of overweight/obesity for children borne of obese mothers (126), the question of whether the risk of adult disease in progeny may differ with maternal BMI status during pregnancy and lactation in breast-feeding mothers warrants further scrutiny.
Perspectives
Emerging data predict a positive impact of anti-obesity strategies for children on decreasing their long-term cancer risk (127). However, most current strategies do not consider targeting obesity risk beginning in the womb for the dual-prevention of obesity and cancer. Data summarized above demonstrate the maternal contributions to determining an offspring's relative risks for obesity and attendant cancer risk (Figure 1). Intervening in the feed-forward cycle of maternal to offspring adiposity/obesity with new strategies and approaches will be required to stem the obesity pandemic. Given the increasingly acknowledged link between obesity and many cancers, such approaches may also counter the expected rise in occurrence of cancers in children and adults borne from overweight or obese mothers. The fields of epigenetics and stem cells will undoubtedly be important in addressing the large gaps in our understanding of the mechanistic aspects of fetal programming of adult cancers and specifically as influenced by maternal obesity and/or maternal diet. Mature fields such as epidemiology, the study of bioactive dietary factors, and the molecular endocrinology of insulin and IGFs also will find application to the above challenges. Since many aspects of embryo-maternal interaction, placentation, fetal organ system development, and pregnancy are species-specific, it will be important to study multiple animal models, as well as primates and the human where appropriate, to elucidate the generalities of maternal obesity effects on a fetus/neonate's later cancer risk and how this may be countered by targeting the pregnant uterus.
Acknowledgments
We thank members of our research groups for their important contributions to ongoing studies and discussions that helped shape this review.
Grant Support: Work in our laboratories was supported by grants from the National Institutes of Health (RO1 CA136493, RO1 HD21961), the Department of Defense Breast Cancer Research Program (W81XWH-08-0548), and the US Department of Agriculture (USDA-CRIS-6251-5100002-06S). Intramural funding from the UAMS College of Medicine Research Council, Children's University Medical Group Award Program, the Arkansas Biosciences Institute, and the Sturgis Foundation also provided support to our laboratories' research.
Footnotes
Disclosure of Potential Conflicts of Interest; Authors disclose no potential conflicts of interest.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–58. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- 8.Andersson SW, Bengtsson C, Hallberg L, et al. Cancer risk in Swedish women: the relation to size at birth. Br J Cancer. 2001;84:1193–8. doi: 10.1054/bjoc.2000.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlgren M, Wohlfahrt J, Olsen LW, Sørensen TI, Melbye M. Birth weight and risk of cancer. Cancer. 2007;110:412–9. doi: 10.1002/cncr.22773. [DOI] [PubMed] [Google Scholar]
- 10.Harder T, Plagemann A, Harder A. Birth weight and risk of neuroblastoma: a meta-analysis. Int J Epidemiol. 2010;39:746–56. doi: 10.1093/ije/dyq040. [DOI] [PubMed] [Google Scholar]
- 11.Koifman S, Pombo-de-Oliveira MS Brazilian Collaborative Study Group of Infant Acute Leukemia. High birth weight as an important risk factor for infant leukemia. Br J Cancer. 2008;98:664–7. doi: 10.1038/sj.bjc.6604202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–70. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 13.Rangel M, Cypriano M, de Martino Lee ML, et al. Leukemia, non-Hodgkin's lymphoma, and Wilms tumor in childhood: the role of birth weight. Eur J Pediatr. 2010;169:875–81. doi: 10.1007/s00431-010-1139-1. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson M, Wedel H, Wallander MA, et al. The impact of birth weight on prostate cancer incidence and mortality in a population-based study of men born in 1913 and followed up from 50 to 85 years of age. Prostate. 2007;67:1247–54. doi: 10.1002/pros.20428. [DOI] [PubMed] [Google Scholar]
- 15.Cnattingius S, Lundberg F, Sandin S, Grönberg H, Iliadou A. Birth characteristics and risk of prostate cancer: the contribution of genetic factors. Cancer Epidemiol Biomarkers Prev. 2009;18:2422–6. doi: 10.1158/1055-9965.EPI-09-0366. [DOI] [PubMed] [Google Scholar]
- 16.Michos A, Xue F, Michels KB. Birth weight and the risk of testicular cancer: a meta-analysis. Int J Cancer. 2007;121:1123–31. doi: 10.1002/ijc.22771. [DOI] [PubMed] [Google Scholar]
- 17.Ramlau-Hansen CH, Olesen AV, Parner ET, Sørensen HT, Olsen J. Perinatal markers of estrogen exposure and risk of testicular cancer: follow-up of 1,333,873 Danish males born between 1950 and 2002. Cancer Causes Control. 2009;20:1587–92. doi: 10.1007/s10552-009-9403-2. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen TI, Romundstad PR, Troisi R, Potischman N, Vatten LJ. Birth size and colorectal cancer risk: a prospective population based study. Gut. 2005;54:1728–32. doi: 10.1136/gut.2004.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 20.Park SK, Kang D, McGlynn KA, et al. Intrauterine environments and breast cancer risk: meta-analysis and systematic review. Breast Cancer Res. 2008;10:R8. doi: 10.1186/bcr1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva Idos S, De Stavola B, McCormack V Collaborative Group on Pre-Natal Risk Factors and Subsequent Risk of Breast Cancer. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008;5:e193. doi: 10.1371/journal.pmed.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maehle BO, Vatten LJ, Tretli S. Birth length and weight as predictors of breast cancer prognosis. BMC Cancer. 2010;10:115. doi: 10.1186/1471-2407-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–94. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jasienska G, Ziomkiewicz A, Lipson SF, Thune I, Ellison PT. High ponderal index at birth predicts high estradiol levels in adult women. Am J Hum Biol. 2006;18:133–40. doi: 10.1002/ajhb.20462. [DOI] [PubMed] [Google Scholar]
- 25.Finstad SE, Emaus A, Potischman N, et al. Influence of birth weight and adult body composition on 17 beta-estradiol levels in young women. Cancer Causes Control. 2009;20:233–42. doi: 10.1007/s10552-008-9238-2. [DOI] [PubMed] [Google Scholar]
- 26.HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117:575–84. doi: 10.1111/j.1471-0528.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 27.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring) 2008;16:1651–6. doi: 10.1038/oby.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crozier SR, Inskip HM, Godfrey KM, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91:1745–51. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–64. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirkola J, Pouta A, Bloigu A, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010;33:1115–21. doi: 10.2337/dc09-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13:163–9. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124 1:S23–34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- 33.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–7. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 34.Carmody JS, Wan P, Accili D, Zeltser LM, Leibel RL. Respective contributions of maternal insulin resistance and diet to metabolic and hypothalamic phenotypes of progeny. Obesity (Silver Spring) 2011;19:492–9. doi: 10.1038/oby.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao R, Hennings LJ, Badger TM, Simmen FA. Fetal programming of colon cancer in adult rats: correlations with altered neonatal growth trajectory, circulating IGF-I and IGF binding proteins, and testosterone. J Endocrinol. 2007;195:79–87. doi: 10.1677/JOE-07-0256. [DOI] [PubMed] [Google Scholar]
- 36.Nivoit P, Morens C, Van Assche FA, et al. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia. 2009;52:1133–42. doi: 10.1007/s00125-009-1316-9. [DOI] [PubMed] [Google Scholar]
- 37.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tosh DN, Fu Q, Callaway CW, et al. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1023–9. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinnunen TI, Luoto R, Gissler M, Hemminki E, Hilakivi-Clarke L. Pregnancy weight gain and breast cancer risk. BMC Womens Health. 2004;4:7. doi: 10.1186/1472-6874-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilakivi-Clarke L, Luoto R, Huttunen T, Koskenvuo M. Pregnancy weight gain and premenopausal breast cancer risk. J Reprod Med. 2005;50:811–6. [PubMed] [Google Scholar]
- 41.Kaijser M, Jacobsen G, Granath F, Cnattingius S, Ekbom A. Maternal age, anthropometrics and pregnancy oestriol. Paediatr Perinat Epidemiol. 2002;16:149–53. doi: 10.1046/j.1365-3016.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- 42.Faupel-Badger JM, Hoover RN, Potischman N, Roberts JM, Troisi R. Pregnancy weight gain is not associated with maternal or mixed umbilical cord estrogen and androgen concentrations. Cancer Causes Control. 2009;20:263–7. doi: 10.1007/s10552-008-9235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lof M, Hilakivi-Clarke L, Sandin SS, de Assis S, Yu W, Weiderpass E. Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health. 2009;9:10. doi: 10.1186/1472-6874-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Assis S, Wang M, Goel S, Foxworth A, Helferich W, Hilakivi-Clarke L. Excessive weight gain during pregnancy increases carcinogen-induced mammary tumorigenesis in Sprague-Dawley and lean and obese Zucker rats. J Nutr. 2006;136:998–1004. doi: 10.1093/jn/136.4.998. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson M, Williams MA, Daling JR, et al. Maternal factors and breast cancer risk among young women. Paediatr Perinat Epidemiol. 1998;12:397–407. doi: 10.1046/j.1365-3016.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 46.Innes KE, Weitzel L, Laudenslager M. Altered metabolic profiles among older mothers with a history of preeclampsia. Gynecol Obstet Invest. 2005;59:192–201. doi: 10.1159/000084146. [DOI] [PubMed] [Google Scholar]
- 47.Vatten LJ, Forman MR, Nilsen TI, Barrett JC, Romundstad PR. The negative association between pre-eclampsia and breast cancer risk may depend on the offspring's gender. Br J Cancer. 2007;96:1436–8. doi: 10.1038/sj.bjc.6603688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker DJ, Osmond C, Thornburg KL, Kajantie E, Forsen TJ, Eriksson JG. A possible link between the pubertal growth of girls and breast cancer in their daughters. Am J Hum Biol. 2008;20:127–31. doi: 10.1002/ajhb.20688. [DOI] [PubMed] [Google Scholar]
- 49.Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008;586:3219–30. doi: 10.1113/jphysiol.2008.153817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol. 2009;200:293–300. doi: 10.1677/JOE-08-0429. [DOI] [PubMed] [Google Scholar]
- 51.Liang C, Oest ME, Prater MR. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res B Dev Reprod Toxicol. 2009;86:377–84. doi: 10.1002/bdrb.20206. [DOI] [PubMed] [Google Scholar]
- 52.Alzamendi A, Castrogiovanni D, Gaillard RC, Spinedi E, Giovambattista A. Increased male offspring's risk of metabolic-neuroendocrine dysfunction and overweight after fructose-rich diet intake by the lactating mother. Endocrinology. 2010;151:4214–23. doi: 10.1210/en.2009-1353. [DOI] [PubMed] [Google Scholar]
- 53.Bayol SA, Simbi BH, Fowkes RC, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic fatty liver disease in rat offspring. Endocrinology. 2010;151:1451–61. doi: 10.1210/en.2009-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maurer AD, Reimer RA. Maternal consumption of high-prebiotic fibre or -protein diets during pregnancy and lactation differentially influences satiety hormones and expression of genes involved in glucose and lipid metabolism in offspring in rats. Br J Nutr. 2011;105:329–38. doi: 10.1017/S0007114510003533. [DOI] [PubMed] [Google Scholar]
- 55.Odaka Y, Nakano M, Tanaka T, et al. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity (Silver Spring) 2010;18:1688–94. doi: 10.1038/oby.2009.513. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan EL, Smith MS, Grove KL. Perinatal Exposure to High-Fat Diet Programs Energy Balance, Metabolism and Behavior in Adulthood. Neuroendocrinology. 2011;93:1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Assis S, Khan G, Hilakivi-Clarke L. High birth weight increases mammary tumorigenesis in rats. Int J Cancer. 2006;119:1537–46. doi: 10.1002/ijc.21936. [DOI] [PubMed] [Google Scholar]
- 58.Stark AH, Kossoy G, Zusman I, Yarden G, Madar Z. Olive oil consumption during pregnancy and lactation in rats influences mammary cancer development in female offspring. Nutr Cancer. 2003;46:59–65. doi: 10.1207/S15327914NC4601_08. [DOI] [PubMed] [Google Scholar]
- 59.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–91. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2007;13:4199–206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SE, Shim KN, Jung SA, Yoo K, Moon IH. An association between obesity and the prevalence of colonic adenoma according to age and gender. J Gastroenterol. 2007;42:616–23. doi: 10.1007/s00535-007-2074-4. [DOI] [PubMed] [Google Scholar]
- 62.Nock NL, Thompson CL, Tucker TC, Berger NA, Li L. Associations between obesity and changes in adult BMI over time and colon cancer risk. Obesity (Silver Spring) 2008;16:1099–104. doi: 10.1038/oby.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thygesen LC, Grønbaek M, Johansen C, Fuchs CS, Willett WC, Giovannucci E. Prospective weight change and colon cancer risk in male US health professionals. Int J Cancer. 2008;123:1160–5. doi: 10.1002/ijc.23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000;152:847–54. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 65.Newberne PM, Bueche D, Suphiphat V, Schrager TF, Sahaphong S. The influence of pre- and postnatal caloric intake on colon carcinogenesis. Nutr Cancer. 1990;13:165–73. doi: 10.1080/01635589009514057. [DOI] [PubMed] [Google Scholar]
- 66.Weber RV, Stein DE, Scholes J, Kral JG. Obesity potentiates AOM-induced colon cancer. Dig Dis Sci. 2000;45:890–5. doi: 10.1023/a:1005560621722. [DOI] [PubMed] [Google Scholar]
- 67.Koch TC, Briviba K, Watzl B, Bub A, Barth SW. Obesity-related promotion of aberrant crypt foci in DMH-treated obese Zucker rats correlates with dyslipidemia rather than hyperinsulinemia. Eur J Nutr. 2008;47:161–70. doi: 10.1007/s00394-008-0711-1. [DOI] [PubMed] [Google Scholar]
- 68.Yakar S, Nunez NP, Pennisi P, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147:5826–34. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 69.Gravaghi C, Bo J, Laperle KM, et al. Obesity enhances gastrointestinal tumorigenesis in Apc-mutant mice. Int J Obes (Lond) 2008;32:1716–9. doi: 10.1038/ijo.2008.149. [DOI] [PubMed] [Google Scholar]
- 70.Hughes LA, van den Brandt PA, de Bruïne AP, et al. Early life exposure to famine and colorectal cancer risk: a role for epigenetic mechanisms. PLoS One. 2009;4:e7951. doi: 10.1371/journal.pone.0007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–7. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 72.Wise LA, Rosenberg L, Palmer JR, Adams-Campbell LL. Anthropometric risk factors for colorectal polyps in African-American women. Obesity (Silver Spring) 2008;16:859–68. doi: 10.1038/oby.2007.139. [DOI] [PubMed] [Google Scholar]
- 73.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–54. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 74.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 75.Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938–45. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 76.Donadon V, Balbi M, Casarin P, Vario A, Alberti A. Association between hepatocellular carcinoma and type 2 diabetes mellitus in Italy: potential role of insulin. World J Gastroenterol. 2008;14:5695–700. doi: 10.3748/wjg.14.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16:6035–43. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291:E792–9. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- 79.Patel MS, Srinivasan M. Metabolic programming due to alterations in nutrition in the immediate postnatal period. J Nutr. 2010;140:658–61. doi: 10.3945/jn.109.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eden JA. Breast cancer, stem cells and sex hormones: part 1. The impact of fetal life and infancy. Maturitas. 2010;67:117–20. doi: 10.1016/j.maturitas.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Capittini C, Bergamaschi P, De Silvestri A, et al. Birth-weight as a risk factor for cancer in adulthood: The stem cell perspective. Maturitas. 2011;69:91–3. doi: 10.1016/j.maturitas.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–78. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 84.Baik I, Devito WJ, Ballen K, et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. 2005;65:358–63. [PubMed] [Google Scholar]
- 85.Kakarala M, Brenner DE, Korkaya H, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–85. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–6. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 87.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–73. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr. 2008;100:278–82. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–6. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 90.Plagemann A, Roepke K, Harder T, et al. Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J Perinat Med. 2010;38:393–400. doi: 10.1515/jpm.2010.051. [DOI] [PubMed] [Google Scholar]
- 91.Rijnkels M, Kabotyanski E, Montazer-Torbati MB, et al. The epigenetic landscape of mammary gland development and functional differentiation. J Mammary Gland Biol Neoplasia. 2010;15:85–100. doi: 10.1007/s10911-010-9170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–8. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Delage B, Dashwood RH. Dietary manipulation of histone structure and function. Annu Rev Nutr. 2008;28:347–66. doi: 10.1146/annurev.nutr.28.061807.155354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Godman CA, Joshi R, Tierney BR, et al. HDAC3 impacts multiple oncogenic pathways in colon cancer cells with effects on Wnt and vitamin D signaling. Cancer Biol Ther. 2008;7:1570–80. doi: 10.4161/cbt.7.10.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davidson LA, Wang N, Shah MS, Lupton JR, Ivanov I, Chapkin RS. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–84. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Larnkjaer A, Schack-Nielsen L, Mølgaard C, Ingstrup HK, Holst JJ, Michaelsen KF. Effect of growth in infancy on body composition, insulin resistance, and concentration of appetite hormones in adolescence. Am J Clin Nutr. 2010;91:1675–83. doi: 10.3945/ajcn.2009.27956. [DOI] [PubMed] [Google Scholar]
- 97.Lindsay RS, Nelson SM, Walker JD, et al. Programming of adiposity in offspring of mothers with type 1 diabetes at age 7 years. Diabetes Care. 2010;33:1080–5. doi: 10.2337/dc09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Egeland GM, Meltzer SJ. Following in mother's footsteps? Mother-daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet Med. 2010;27:257–65. doi: 10.1111/j.1464-5491.2010.02944.x. [DOI] [PubMed] [Google Scholar]
- 99.Gallou-Kabani C, Vigé A, Gross MS, et al. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am J Physiol Endocrinol Metab. 2007;292:E1095–100. doi: 10.1152/ajpendo.00390.2006. [DOI] [PubMed] [Google Scholar]
- 100.Rowan JA, Hague WM, Gao W, et al. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 101.Dhulkotia JS, Ola B, Fraser R, Farrell T. Oral hypoglycemic agents vs insulin in management of gestational diabetes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:457.e1–9. doi: 10.1016/j.ajog.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 102.Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. 2010;33:9–16. doi: 10.2337/dc09-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shyangdan DS, Royle PL, Clar C, Sharma P, Waugh NR. Glucagon-like peptide analogues for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Endocr Disord. 2010;10:20. doi: 10.1186/1472-6823-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waugh N, Royle P, Clar C, et al. Screening for hyperglycaemia in pregnancy: a rapid update for the National Screening Committee. Health Technol Assess. 2010;14:1–183. doi: 10.3310/hta14450. [DOI] [PubMed] [Google Scholar]
- 105.Simmons D. Metformin treatment for Type 2 diabetes in pregnancy? Best Pract Res Clin Endocrinol Metab. 2010;24:625–34. doi: 10.1016/j.beem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 107.Donadon V, Balbi M, Ghersetti M, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–11. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–8. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–83. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 110.Monami M, Colombi C, Balzi D, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34:129–31. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–5. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38:833–40. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bouanane S, Benkalfat NB, Baba Ahmed FZ, et al. Time course of changes in serum oxidant/antioxidant status in overfed obese rats and their offspring. Clin Sci (Lond) 2009;116:669–80. doi: 10.1042/CS20080413. [DOI] [PubMed] [Google Scholar]
- 114.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299:R711–22. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278:88–100. doi: 10.1016/j.tox.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Tollefsbol TO. Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem. 2010;17:2141–51. doi: 10.2174/092986710791299966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80:1771–92. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ronco AL, De Stéfani E, Stoll M. Hormonal and metabolic modulation through nutrition: towards a primary prevention of breast cancer. Breast. 2010;19:322–32. doi: 10.1016/j.breast.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 119.Shu XO, Jin F, Dai Q, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–8. [PubMed] [Google Scholar]
- 120.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–6. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 121.Su Y, Eason RR, Geng Y, Till SR, Badger TM, Simmen RCM. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28:1046–51. doi: 10.1093/carcin/bgl240. [DOI] [PubMed] [Google Scholar]
- 122.Su Y, Shankar K, Simmen RCM. Early soy exposure via maternal diet regulates rat mammary epithelial differentiation by paracrine signaling from stromal adipocytes. J Nutr. 2009;139:945–51. doi: 10.3945/jn.108.103820. [DOI] [PubMed] [Google Scholar]
- 123.Wu X, Rahal O, Kang J, Till SR, Prior RL, Simmen RCM. In utero and lactational exposure to blueberry via maternal diet promotes mammary epithelial differentiation in prepubescent female rats. Nutr Res. 2009;29:802–11. doi: 10.1016/j.nutres.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 124.Cederroth CR, Nef S. Fetal programming of adult glucose homeostasis in mice. PLoS One. 2009;4:e7281. doi: 10.1371/journal.pone.0007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lagiou P, Lagiou A, Samoli E, Hsieh CC, Adami HO, Trichopoulos D. Diet during pregnancy and levels of maternal pregnancy hormones in relation to the risk of breast cancer in the offspring. Eur J Cancer Prev. 2006;15:20–6. doi: 10.1097/01.cej.0000186639.12249.c7. [DOI] [PubMed] [Google Scholar]
- 126.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13:362–71. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 127.Fuemmeler BF, Pendzich MK, Tercyak KP. Weight, dietary behavior, and physical activity in childhood and adolescence: implications for adult cancer risk. Obes Facts. 2009;2:179–86. doi: 10.1159/000220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nam SY, Lee EJ, Kim KR, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21:355–9. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- 129.Ahmed RL, Thomas W, Schmitz KH. Interactions between insulin, body fat, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2007;16:593–7. doi: 10.1158/1055-9965.EPI-06-0775. [DOI] [PubMed] [Google Scholar]
- 130.Rowlands MA, Holly JM, Gunnell D, et al. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (Prostate testing for cancer and Treatment) study. Cancer Causes Control. 2010;21:1829–42. doi: 10.1007/s10552-010-9610-x. [DOI] [PubMed] [Google Scholar]
- 131.Martin RM, Holly JM, Davey Smith G, Gunnell D. Associations of adiposity from childhood into adulthood with insulin resistance and the insulin-like growth factor system: 65-year follow-up of the Boyd Orr Cohort. J Clin Endocrinol Metab. 2006;91:3287–95. doi: 10.1210/jc.2006-0745. [DOI] [PubMed] [Google Scholar]
- 132.Fowke JH, Matthews CE, Yu H, et al. Racial differences in the association between body mass index and serum IGF1, IGF2, and IGFBP3. Endocr Relat Cancer. 2010;17:51–60. doi: 10.1677/ERC-09-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK. Bioactive insulin-like growth factor-I in obesity. J Clin Endocrinol Metab. 2009;94:3093–7. doi: 10.1210/jc.2009-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Radetti G, Bozzola M, Pasquino B, et al. Growth hormone bioactivity, insulin-like growth factors (IGFs), and IGF binding proteins in obese children. Metabolism. 1998;47:1490–3. doi: 10.1016/s0026-0495(98)90075-0. [DOI] [PubMed] [Google Scholar]
- 135.Ballerini MG, Ropelato MG, Domené HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab. 2004;17:749–57. doi: 10.1515/jpem.2004.17.5.749. [DOI] [PubMed] [Google Scholar]
- 136.Kong AP, Choi KC, Wong GW, et al. Serum concentrations of insulin-like growth factor-I, insulin-like growth factor binding protein-3 and cardiovascular risk factors in adolescents. Ann Clin Biochem. 2011 Apr 8; doi: 10.1258/acb.2011.010267. [DOI] [PubMed] [Google Scholar]
- 137.Reinehr T, Panteliadou A, de Sousa G, Andler W. Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and growth in obese children before and after reduction of overweight. J Pediatr Endocrinol Metab. 2009;22:225–33. doi: 10.1515/jpem.2009.22.3.225. [DOI] [PubMed] [Google Scholar]
- 138.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 139.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 140.Ma J, Giovannucci E, Pollak M, et al. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546–53. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 141.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–5. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 142.Jenab M, Riboli E, Cleveland RJ, et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121:368–76. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 143.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S Japan Public Health Center-based Prospective Study Group. Plasma C-peptide, insulin-like growth factor-I, insulin-like growth factor binding proteins and risk of colorectal cancer in a nested case-control study: the Japan public health center-based prospective study. Int J Cancer. 2007;120:2007–12. doi: 10.1002/ijc.22556. [DOI] [PubMed] [Google Scholar]
- 144.Del Giudice ME, Fantus IG, Ezzat S, et al. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat. 1998;47:111–20. doi: 10.1023/a:1005831013718. [DOI] [PubMed] [Google Scholar]
- 145.Lawlor DA, Smith GD, Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: findings from the British Women's Heart and Health Study. Cancer Causes Control. 2004;15:267–75. doi: 10.1023/B:CACO.0000024225.14618.a8. [DOI] [PubMed] [Google Scholar]
- 146.Hwang DL, Huang SP, Lan WS, Lee PD. Elevated insulin, proinsulin and insulin-like growth factor-binding protein-1 in liver disease. Growth Horm IGF Res. 2003;13:316–21. doi: 10.1016/s1096-6374(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 147.Hung CH, Wang JH, Hu TH, et al. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265–71. doi: 10.3748/wjg.v16.i18.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chao LT, Wu CF, Sung FY, et al. Insulin, glucose, and hepatocellular carcinoma risk in male hepatitis B carriers: results from 17-year follow-up of a population-based cohort. Carcinogenesis. 2011 Apr 3; doi: 10.1093/carcon/bgr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manousos O, Souglakos J, Bosetti C, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999;83:15–7. doi: 10.1002/(sici)1097-0215(19990924)83:1<15::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 150.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–37. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]