Abstract
Based on the recently established role for the master coregulator MTA1 and MTA1-containing nuclear remodeling complexes, in oncogenesis and inflammation, we explored the links between parasitism by the carcinogenic liver fluke Opisthorchis viverrini and this coregulator using both an Mta1−/− mouse model of infection and a tissue microarray of liver fluke induced human cholangiocarcinomas. Intense foci of inflammation and periductal fibrosis in the liver and kidney of wild type, Mta1+/+ mice were evident at 23 days post-infection with O. viverrini. In contrast, little inflammatory response was observed in the same organs of infected Mta1−/− mice. Livers of infected Mta1+/+ mice revealed strong upregulation of fibrosis-associated markers such as cytokeratins 18, 19, and annexin-2, as determined by both by immunostaining and by reverse transcription PCR compared to infected Mta1−/− mice. CD4 expression was upregulated by infection in the liver of both experimental groups; however, its levels were several folds higher in the Mta1+/+ mice than in infected Mta1−/− mice. Mta1−/− infected mice also exhibited significantly higher systemic and hepatic levels of host cytokines such as IL-12p70, IL-10 and IFN-γ compared to the levels of these cytokines in the Mta1+/+ mice, suggesting an essential role of MTA1 in the cross-regulation of the Th1 and Th2 responses, presumably due to chromatin remodeling of the target chromatin genes. Immunohistochemical analysis of ~300 liver tissue cores from confirmed cases of O. viverrini induced cholangiocarcinoma showed that MTA1 expression was elevated in more than 80% of the specimens. These findings suggest that MTA1 status plays an important role in conferring an optimal cytokine response in mice following infection with O. viverrini and is a major player in parasite-induced cholangiocarcinoma in humans.
Keywords: Liver and bile duct cancer, Liver Fluke, Tumor Biology, Cancer-causing pathogens, Metastasis associated gene-1 product, host factor for pathogens
Introduction
Infection as a cause of cancer is an evolving concept that is receiving greater recognition because it represents a direct and measurable predisposing factor for a frequently fatal disease (1–5). Other predisposing factors, such as diet, endocrine disorders and genetic constitution have also been characterized as contributing factors in the development of cancer (3, 4). However, most infectious agents involved in carcinogenesis have not received the attention and as such deserve further examination (6). For example, the Asian liver fluke Opisthorchis viverrini causes opisthorchiasis, which involves hepatobiliary abnormalities, including pathology to the liver, extra-hepatic bile ducts, and the gall bladder (7–13). There is a long established link between opisthorchiasis and cholangiocarcinoma (CCA), a malignant tumor arising from the epithelium of the bile duct (5, 12–14). Yet, the nature of molecular carcinogenesis in liver fluke induced CCA has not been characterized.
CCA is the second most common primary cancer in the liver with the highest incidence in Southeast Asian countries, which also have the highest prevalence of O. viverrini infection (10–14). Recent studies have demonstrated that O. viverrini infection represents the major risk factor for CCA in Thailand and is classified by the International Agency for research on Cancer (IARC) as a group 1 carcinogen (5, 14, 15). Humans represent the major definitive host for O. viverrini. Eggs shed by the adult worms can remain in the biliary tree of the liver or enter the intestine and pass in the feces (8, 13). Upon reaching water, eggs are ingested by snails, which represent the first intermediate host (8, 13). Within the snail, the miracidium is released from the egg, penetrates the hepato-pancreas of the snail, and metamorphoses into the sporocyst stage, which reproduces asexually to produce cercariae that are shed into the water. Cercariae seek out and encyst as metacercariae on fresh water fish of the cyprinoid family that serve as the second intermediate host. Humans become infected by ingesting raw or inadequately cooked, cyprinoid fish. In the human host metacercariae excyst in the duodenum, pass through the ampulla of Vater to enter the bile duct, and ascend into the biliary tree to mature. The adult worms can survive in the human body for decades, frequently leading to periductal inflammation and periductal fibrosis, which can culminate in O. viverrini-induced CCA (8, 13).
Little is known about the host-parasite interactions that support successful chronic infection and maintenance of the adult O. viverrini liver fluke in the human biliary tree. Despite the anti-fluke immunological responses (16), it is clear that O. viverrini, like other parasitic helminths, has evolved the means to establish, survive and reproduce in the host for extended periods of time. We speculate that this is possible only if the liver fluke exploits permissive host factors for a productive infection. Although several liver specific markers are upregulated due to liver fluke infection, little information is available on the host factors that are utilized by these parasites (17–22). Employing infection of the Mta1−/− mouse (23) as a model system, we have now identified a distinct contribution of MTA1 in establishing a positive mammalian host/parasite interaction. Moreover, we found that MTA1 plays a significant role in driving periductal fibrosis in the liver and is an essential host factor for parasite survival. Earlier studies have established a central role of MTA1 in tumorigenesis and inflammatory responses (24–29). Based on these findings, we hypothesize that helminth parasites such as O. viverrini utilize the Mta1 host factor for a successful long-term infection. The Mta1 gene product is a chromatin bound coregulator involved in transcriptional regulation of genes associated with multiple cellular pathways (29–31). We now propose that host MTA1 represents a common regulatory factor that is utilized by many parasites for a successful infection. To test this hypothesis, we investigated the role of MTA1 in Opisthorchis viverrini mediated infection using Mta1-null (Mta1−/−) and Mta1-wildtype (Mta1+/+) mice as a model system with the expression of MTA1 in liver fluke-induced cholangiocarcinoma.
Results
Opisthorchis viverrini infection results in severe pathological changes in mice with intact Mta1
To investigate the influence of MTA1 on infection and the establishment of O. viverrini, we isolated liver, small intestine and kidney tissues from infected age-matched Mta1+/+ and Mta1−/− mice. Histopathological analyses using haematoxylin and eosin (H&E) of stained thin sections revealed significant changes in the inflammatory response in Mta1+/+ and age-matched Mta1−/− mice. In particular there was a higher occurrence of periductal fibrosis and infiltrating polymorphonuclear cells in the liver of the wild type mice than in the Mta1−/− mice (Fig. 1A; top panel). An increase in inflammatory response also correlated with a higher percentage (12%) of inflammatory zones in the Mta1+/+ mice. In addition, analysis of H&E-stained sections of the kidney supported the observation that O. viverrini infection resulted in a higher magnitude of inflammatory response in Mta1+/+ mice when compared to age-matched Mta1−/− mice (Fig. 1A; bottom panel).
Figure 1.
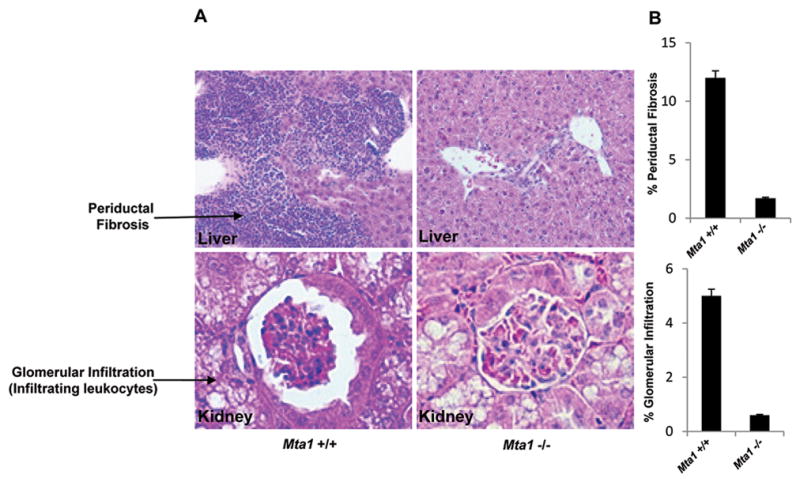
Opisthorchis viverrini infection results in periductal fibrosis and glomerulonephritis in Mta1+/+ mice. (A) Haematoxylin and Eosin staining reveals stark pathological changes and inflammatory responses in the liver and kidney of Mta1+/+ mice. (B) Bar plot showing results from A, data are represented as mean ± SD.
To determine whether the presence or absence of MTA1 had a significant effect on the pathology associated with infection, levels of critical cellular markers known to be upregulated during O. viverrini infection were evaluated using immunohistochemistry and quantitative RT-PCR. We tested expression levels of cytokeratin-19 (CK-19), CK-18, and Annexin-2. Expression of CK-19 has been widely used to study proliferation of biliary epithelium following O. viverrini infection while Annexin-2 appears to be a prognostic marker of O. viverrini infection-induced CCA (19, 31). There were significant increases in expression levels of CK-19, CK-18 and Annexin-2, in the liver tissues from the Mta1+/+ mice when compared to age-matched Mta1 −/− mice using both immunohistochemistry (Fig. 2A–D) and quantitative, real-time PCR (Fig. 2E–G).
Figure 2.
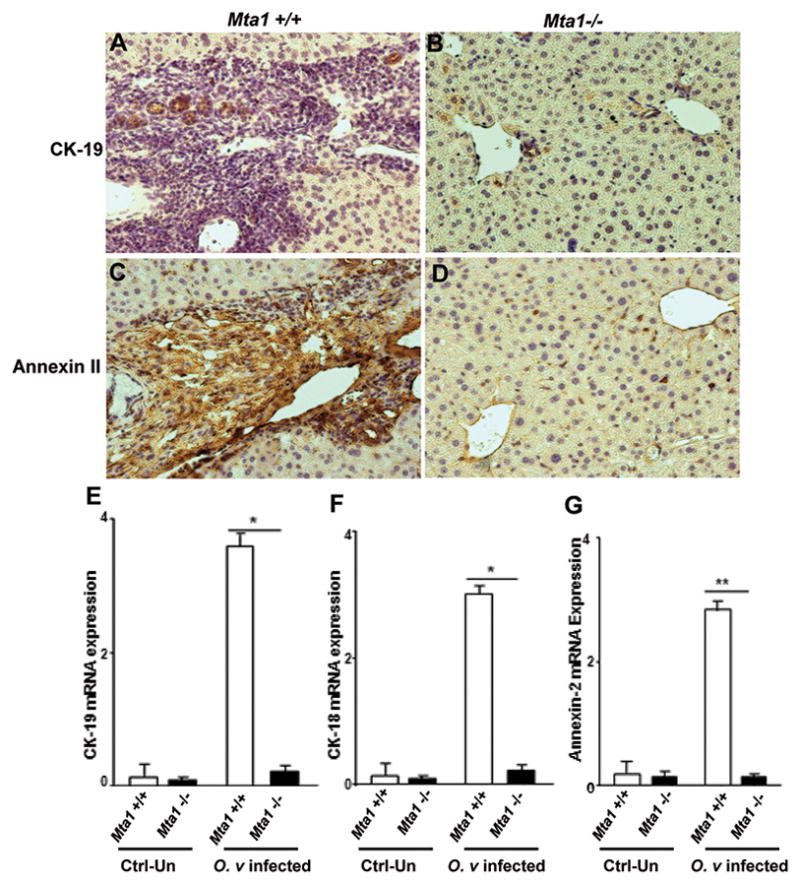
Immunohistochemical analysis reveals biliary hyperplasia of liver in infected Mta1+/+ and Mta1−/− mice. Top panel: Paraffin embedded liver tissue sections were stained for CK-19 (A, B) and Annexin-2 expression (C, D). Bottom panel: Bar plot showing the results of quantitative RT-PCR for CK-19, CK-18 and Annexin-2 using RNA from livers of uninfected and O. viverrini infected Mta1+/+ and Mta1−/− mice (E–G). Data are presented as mean ± SD.
MTA1 required for optimal cytokine responses following infection
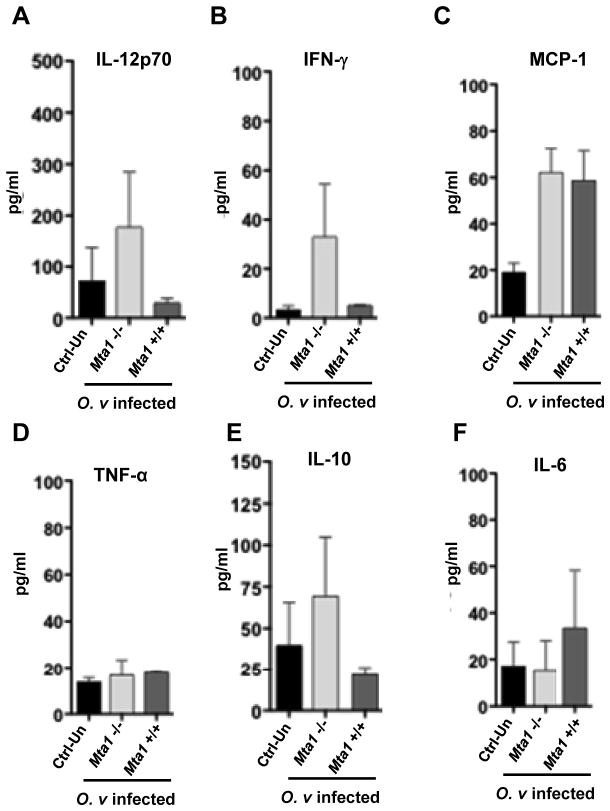
The T-cell repertoire and secreted cytokines play an important role in determining the outcome of parasitic infections. The cross-regulation of Th1 and Th2 responses are important for parasite survival and a successful infection. The cytokine responses to helminth parasitic infections are well established in both laboratory models and human infections; downregulation of the Th1 response and the upregulation of Th2 responses are hallmarks of successful infection (32–34). Here, we demonstrated that Th1-inducing cytokine responses are immuno-protective for the host and prevent a successful infection. We investigated systemic levels of cytokine expression in the uninfected and infected Mta1 +/+ and age-matched Mta1−/− mice. We also measured levels of IgG in control and infected mice against a crude antigen extract of adult O. viverrini. Antibody responses to O. viverrini were similar in both genotypes, indicating that Mta1+/+ and age-matched Mta1−/− mice were similarly infected by metacercariae at the onset of the experiment (Fig. 3A,B). Among the Th1 cytokines examined, elevated levels of interleukin-12 (IL-12) and IFN-γ were observed in Mta1−/− mice compared to infected wildtype mice (Fig. 4A, B). The levels of other Th1 cytokines studied remained similar between both genotypes. Comparative analysis of systemic levels of other cytokines in response to O. viverrini revealed curious profiles. Mta1−/− mice expressed higher levels of the immunomodulator, IL-10 (Fig. 4E). Of the other cytokines assayed, there was a significant increase in proinflammatory cytokine IL-6 in Mta1+/+ compared to Mta1−/− mice (Fig. 4F). Parasite induced IL-6 expression has been reported to be critical for advanced periductal fibrosis during chronic opisthorchiasis and hepatic abnormalities (18). Levels of TNF-α remained unaffected between both genotypes (Fig. 4D). Together these results suggest that MTA1 is a host determinant for optimum cytokine response and immune evasion following O. viverrini infection.
Figure 3.
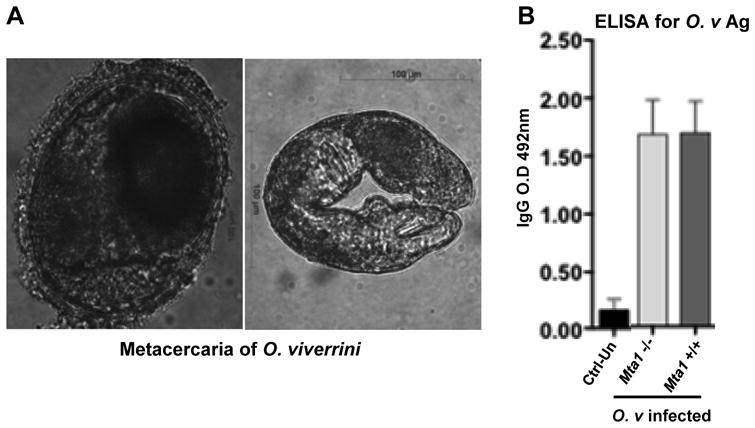
ELISA for O. viverrini antigens reflects equivalent initial parasite burdens in both Mta1+/+ and Mta1−/− mice (A) Photomicrograph to illustrate representative metacercariae of O. viverrini used for infections; left, encysted metacercariae from fish flesh; right, excysted metacercaria. (B) Bar plot showing the results from ELISA against O. viverrini antigen using sera collected from Mta1+/+ and Mta1−/− at 23 days post infection. Data are presented as mean ± SD.
Figure 4.
Analysis of systemic cytokine responses following O. viverrini infection reveals critical role of immunomodulator IL-10 and IL-12p70. Cytokine responses in age matched wildtype Mta1+/+ and Mta1−/− mice at 23 days post-infection: panel (A) IL-12p70, (B) INF-γ (C), MCP-1 (D), TNF-α (E), IL-10 (F), IL-6. Serum samples were used to determine the levels of inflammatory and Th1/Th2 cytokines by cytokine-bead assays. Data are presented as mean ± SD.
MTA1 regulates liver cytokine levels for permissive O. viverrini infection and pathogenesis
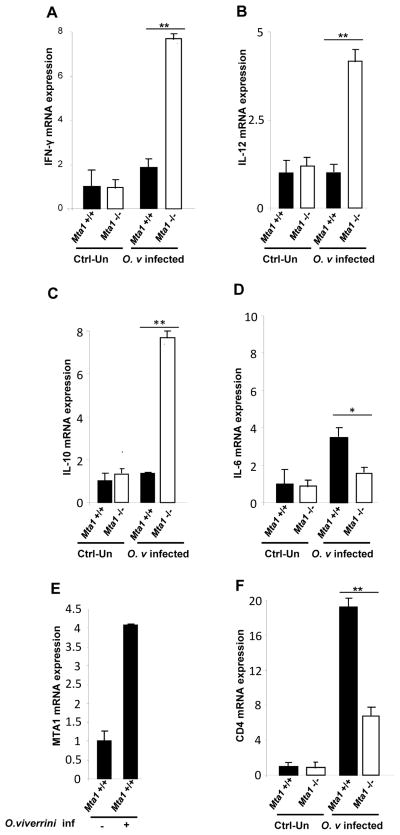
The immune response during opisthorchiasis remains, in general, poorly understood. We next evaluated if systemic changes in cytokine profiles observed between the Mta1+/+ and Mta1−/− mice was also observed in O. viverrini target tissues such as the liver. We utilized quantitative RT-PCR to ascertain local levels of cytokines using RNA isolated from infected Mta1+/+ and Mta1−/− mice. The Th1 cytokine IL-12, was significantly upregulated in the Mta1−/− mice compared to age-matched Mta1+/+ mice. Levels of immunomodulatory IL-10 and the pro-inflammatory cytokines paralleled the systemic expression profile observed between both genotypes (Fig. 5A–D). Since Mta1+/+ mice exhibited cytokine profiles that we hypothesize favor parasite infection, we next evaluated if MTA1 mRNA levels were modulated following O. viverrini infection. We found that there was a robust increase in MTA1 mRNA levels in livers of Mta1+/+ mice following infection (Fig. 5E), indicating that infectious agents such as parasitic helminths, such as O. viverrini, utilize common host-regulatory factors for successful infection and modulation of the host response for immune evasion.
Figure 5.
Liver cytokine expression reveals loss of cytokine interdependence in Mta1−/− mice. Analysis of liver cytokine mRNA levels in Mta1+/+ and Mta1−/− mice. 50 mg of liver tissue was used for RNA isolation and cDNA synthesis. Expression levels of Th1/Th2 cytokines, MTA1, CD4, were assessed by quantitative, real time PCR; (A) IFN-γ, (B) IL-12, (C) IL-10, (D) IL-6, (E) MTA1, and (F)CD4. * P<0.01, **P<0.001.
Upregulation of MTA1 in liver tissues of cholangiocarcinomas in human
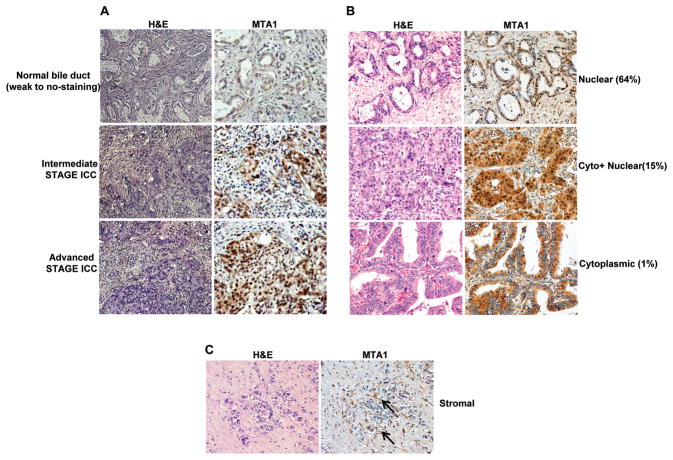
Infection with O. viverrini leads to pathological changes in the liver as well as chronic inflammation, which can eventually result in cholangiocarcinoma, an aggressive form of liver cancer. To date, a few cellular markers have been identified that correlate well with the pathology of this disease and serve as good prognostic markers (16). Our results indicate that MTA1 is a permissive host factor for O. viverrini infection and pathological changes in the liver prompted us to investigate if MTA1 could be a potential diagnostic marker for liver fluke-induced CCA. To address this notion, we used a tissue microarray approach involving immunohistochemical analysis of MTA1. The tissue microarray was comprised of (n = 305) liver tissue cores from confirmed O. viverrini-induced CCA cases (16, 17). In these samples, MTA1 expression was found to be high in hyperplastic bile ducts ( p ≤0.01) when compared to levels in normal bile ducts (Fig. 6A). Overall 80% of tissue cores stained positive for MTA1 (Table 1). In general, MTA1 was predominantly localized (~64%) in the nucleus of most tissue cores (Fig. 6B;top panel). However, it was not uncommon to observe MTA1’s localization in the nucleus and cytoplasm of about ~15% of samples (Fig 6B;middle panel). Interestingly, we also found evidence of the cytoplasmic localization of MTA1 in a small number (~1%) of samples (Fig. 6B;bottom panel). Furthermore, active stromal fibroblasts in the tumor tissue also showed MTA1 expression (Fig. 6C), raising the possibility of MTA1 involvement in stroma-tumor interactions.
Figure 6.
Evaluation of MTA1 expression in O. viverrini-induced human cholangiocarcinomas. (A) Immunohistochemical analysis of MTA1 in bile duct hyperplasia, early stage cholangiocarcinoma, and advanced-stage cholangiocarcinoma. (B) Representative examples of subcellular localization of MTA1 in the nuclear, cytolasmic, and both compartments (Cyto + Nuclear). (C) Representative examples of MTA1 immunoreactivity in the stromal compartment; arrowed indicate examples of MTA1-positive, stromal fibroblasts. For each situation, consecutive paraffin sections were stained with Haematoxylin and Eosin (H&E) (left panels) and with anti MTA1 antibody (right panels).
Table 1.
MTA1 phenotype of tumors on a tissue microarray prepared from ~300 cholangiocarcinomas (CCAs). The CCAs arose in persons with long term infection with, and or exposure to, the liver fluke Opisthorchis viverrini - see Materials and Methods for details.
| MTA1 phenotype | No. of CCAs | Percentage |
|---|---|---|
| Negative | 55 | 18.3% |
| Positive | 240 | 80.0% |
| Strongly positive | 5 | 1.7% |
Discussion
Epidemiological findings have long associated infection with the liver fluke O. viverrini and cholangiocarcinoma, an aggressive tumor arising in the biliary epithelium of the bile duct (14). Infection with O. viverrini leads to pathological changes in the biliary tree and the liver (12). Despite the significance of host-parasite interactions, little is known about the nature of host factors that support successful infection and maintenance of the liver fluke. However, it can be expected that O. viverrini worms exploit host factors for establishment, development, and successful parasitism at large. Based on the recently established role for the Metastasis associated gene-1 product (MTA1) in oncogenesis and inflammation (24–29), we explored previously unknown links between parasitism by O. viverrini and this coregulator using an Mta1-null mouse model of infection (23) and a tissue microarray of liver fluke induced human tumor specimens (16). Metastasis associated protein 1, the gene product of MTA1, is a master coregulator of putative target genes with roles in several cellular processes (28, 36). Over-expression of MTA1 has been associated with a variety of cancers and previous investigations have established a distinct role for MTA1 in mediating inflammatory responses (24–28).
We hypothesized that parasitic helminths utilize similar host-regulatory factors like MTA1 for successful infection. We tested this hypothesis by infecting age-matched Mta1+/+ and Mta1−/−mice with metacercariae, the infective stage of O. viverrini. Whereas humans are the usual definitive host for this liver fluke, the parasite will infect and reproduce in several piscivorous mammals including fishing cats (Prionailurus viverrinus, from whom the liver fluke takes its name), and the domesticated cat and dog. In addition, it will develop to maturity in laboratory rodents including the golden hamster and the gerbil. The closely related liver fluke C. sinensis will also develop in these hosts and the laboratory rat (41). While (at least some strains of) the laboratory mouse is not as permissive a host as the gerbil or hamster, mice can be infected by stomach intubation with metacercariae. Given our interest here to investigate the relationship between liver fluke infection and cancer, and the availability of Mta1 knockout mice (but not similar mutants of gerbils, hamsters or rats), we were constrained in the choice of model rodent. Nonetheless, the findings with O. viverrini infection of these mice strongly indicated that MTA1 is an integral factor for mediating liver fluke infection and infected related inflammation. Infected MTA1 wildtype mice exhibited many of symptoms of O. viverrini infection observed in permissive laboratory animal models (hamsters) and even the human infection, including periductal fibrosis, hepatic infiltration of inflammatory cells, and marked inflammatory responses. By contrast, similar pathological changes were not apparent in the Mta1−/− mice. These findings strongly implicate MTA1 as a host-mediator of this parasitic infection.
CD4 T cells are comprised of two distinct subsets: Th1 cells and Th2 cells, which are characterized based on phenotype of cytokine secretions. Each T cell subset produces a cytokine that inhibits effector functions of the reciprocal subset (34). Since T cell repertoire plays a critical role in mediating parasitic infections (36–39), we evaluated CD4 expression in the liver of Mta1+/+ and Mta1−/− mice. Uninfected mice of both genotypes exhibited equivalent CD4 expression. Intriguingly, in the mice infected with O. viverrini, CD4 expression was upregulated in the liver of wildtype mice and was several folds higher in the age matched Mta1−/− mice (Fig. 5F). These results could indicate that MTA1 regulates distinct CD4 positive subsets of T cells to maintain optimum cytokine expression following infection. This interpretation was strengthened by the finding that MTA1 is an early responsive gene for O. viverrini infection. Evaluation of central players in the immune response in both genotypes provided supporting evidence for this observation. Thus, we observed a loss of cytokine cross-regulation and inter-dependence in Mta1−/−mice in response to infection. Mta1−/− mice exhibited high systemic and local levels of IL-12 and IL-10. Furthermore, levels of INF-γ were significantly upregulated in the Mta1−/− mice compared to age-matched Mta1+/+ mice. IL-12 is a Th1 cytokine and generally results in a strong immune response to infection; indeed, IL-12 and INF-γ constitute part of the host-defense against pathogens (17). We speculate that the loss of MTA1 expression as seen in Mta1−/− results in an over-expression of key Th1 cytokines and early parasite clearance. Levels of IL-10 were significantly higher in Mta1−/− mice following infection with O. viverrini. IL-10 is an immunomodulator that induces a shift between Th1 and Th2 responses. This outcome suggests that MTA1 is a host regulator of T cell repertoire and cytokine expression. Loss of MTA1 results in aberrant cytokine expression and we now speculate that, following helminth parasite infection, aberrant cytokine expression is disadvantageous for the establishment of infection and/or a productive parasitism.
O. viverrini-induced CCA is an aggressive form of liver cancer (16). At present, there are no markers for early detection and/or evaluation of CCA progression. We found that MTA1 is an early host responsive gene following infection and that MTA1 is an essential host component in mediating the positive inflammatory response for an optimum parasite survival. This notion is also supported by the finding that MTA1 is a marker of liver fluke-induced CCA. Our observation of readily detectable MTA1 expressed in stromal fibroblasts proximal to the CCA is also significant, as it conforms with previous reports of an association between advanced periductal fibrosis and CCA (10, 18).
In conclusion, more than 340 species of helminths are known to infect people, and among them, about 30 are widespread, important agents of human disease (40). In addition, three of them, Schistosoma haematobium (blood fluke), C. sinensis (Chinese liver fluke) and O. viverrini (Asian liver fluke), have established links with cancer (40). Carcinogenic liver flukes such as O. viverrini and C. sinensis can reside in the infected person for years, even decades, where the fluke modulates the host immune response for immune evasion and successful parasitism of the host (15, 18, 19, 21, and 40). These present findings implicate MTA1 as a key host factor and critical mediator of O. viverrini infection and inflammatory response in target organs, particularly the liver and kidneys. Results presented here also raised possibility to develop strategies to target MTA1 host factor to reduce the global burden of diseases caused by parasite-induced inflammation. Further, MTA1 deserves close scrutiny as a marker for infection, inflammation and carcinogenesis in liver fluke infected populations.
Material and methods
Opisthorchis viverrini; infection of mice
Metacercariae (MC) of O. viverrini were obtained from naturally infected cyprinoid fish by pepsin digestion, as detailed (42). The MC were shipped form Khon Kaen University, Thailand to the George Washington University (GWU) in Washington DC at 4° C, with permission from the Centers for Disease Control, permit no. PHS 2010-05-103. Mice were infected with O. viverrini by feeding 50 intact, viable MC to each mouse by orogastric tube. Mta1−/− and Mta1+/+ mice were bred in our laboratory, as described (23, 28). Seven mice of each genotype, Mta1−/− and Mta1+/+, age and sex matched per group were infected and included in the investigation. Infected mice, and control non-infected mice, were euthanized at 23 days after infection by an overdose of pentobarbital sodium plus phenytoin sodium (Euthasol, Virbac, Fort Worth, TX). At necropsy, blood for serum was removed by cardiac puncture, after which the liver, spleen, kidneys, lungs and bladder were removed from the mouse. About half of each of the solid organs were stored by snap freezing them in liquid N2, and the remainder fixed and stored in 4% formalin in PBS. The investigation of O. viverrini infection of these mice was undertaken with the approval of the Institutional Animal Use and Care committee of the George Washington University.
Indirect ELISA
An indirect ELISA was used to measure levels of Immunoglobulin G (IgG) to an O. viverrini soluble adult worm preparation produced as described. A pool of positive control sera was derived from equal portions of sera from each genotype at 23 days after infection. A pool of negative control sera was sourced from the age and sex matched mice without any other apparent infection. PolySorp™ (Nalge, Nunc International, Rochester, NY) 96-well microtiter plates were coated with 100 μl/well of 5 μg/ml of soluble adult worm antigen, prepared from adult O. viverrini worms (15) in carbonate-bicarbonate buffer, pH 9.6, sealed, and incubated overnight at 4°C. Plates were washed three times with PBS (pH 7.2) and blocked with 200 100 μl/well of 3% bovine serum albumin (BSA) (Sigma, St. Louis, MO) diluted in PBS (pH 7.2). Control and experimental serum samples were diluted 1:4,000 in PBS (pH 7.2) and 100 μl was added to each well of the microtiter plate in duplicates. The plates were sealed and incubated overnight at 4°C and then washed three times with PBS with 0.05% Tween 20 (PBST) at pH 7.2. A biotinylated goat anti-mouse IgG antibody (Vector Laboratories Inc., Burlingame, CA) was used at a 1:5,000 dilution in 3% BSA and PBS and applied 100 μl/well and then incubated for 90 minutes at room temperature (RT). After incubation, the plates were washed with PBST and incubated with a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated streptavidin (GE Healthcare, Buckinghamshire, UK) in 3% BSA and PBS for 60 minutes at RT in the dark. The plates were incubated in the dark at RT for 30 minutes with o-phenylenediamine dihydrochloride. Sulfuric acid (50 μl) was added to each well to stop the reaction, after which Optical Density (OD) at 492 nm measured (SpectraMax 340 PC reader, Molecular Devices, Sunnyvale, CA) with data capture and analysis performed by SOFTmax Pro software (Molecular Devices).
Cytokine profilies
The BD™ Cytometric Bead Array (CBA) Mouse Inflammation Kit and Mouse Th1/Th2 Cytokine Kit (BD Biosciences, San Diego, CA) were used. In brief, to detect concentrations of Interleukin (IL)-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, Monocyte Chemoattractant Protein (MCP)-1, Interferon (INF)-γ and Tumor Necrosis Factor (TNF)-α in the serum of O. viverrini-infected mice and positive and negative serum controls, a standard reference curve (Mouse Inflammation Standard or Mouse Th1/Th2 Cytokine Standards) provided in the CBA Kit was used to interpolate picograms per microliter levels of each cytokine from the sera. Nine-fold serial dilutions were performed with the standard from each kit in order to obtain a standard curve within a range between 20 and 5000 pg/ml. Each serum sample was diluted 1:2 in RMPI for a final volume of 25 μl. In parallel, RPMI alone was also used as a negative control. A cocktail of the beads from each measured cytokine was made using 3 μl of each bead per sample. Fifteen μl cytokine capture bead cocktail was added to all samples, standards and controls. After vortexing for 10 seconds, 18 μl of the Mouse Inflammation PE Detection Reagent or Mouse Th1/Th2 PE Detection Reagent was added to each sample, standard and control. Tubes were incubated at RT in the dark for 2 hours. Samples were washed with 500 μl of washing buffer and centrifuged for 7 min at 1300 rpm and 18–23°C. After aspirating the supernatants until ~200 μl of sample, samples were analyzed using a FACScan™ flow cytometer and the BD CBA Software (BD Biosciences). The findings are presented in pg/ml.
Immunohistochemistry
Immunohistochemistry (IHC) was performed as described29. Thin sections of 5 μm were cut from paraffin embedded mouse liver and kidney. Paraffin tissue sections were de-paraffinized in xylene and then rehydrated with graded ethanol. Following antigen retrieval and blocking endogenous peroxidase the sections were blocked for 20 min in normal goat serum and incubated with primary antibodies against CK-18, CK-19 or Annexin-II (Abcam, USA) for 3 h. Samples were washed and incubated in secondary antibody for 1 h. Samples were rinsed three times in wash buffer, and incubated in horseradish peroxidase labeled second antibody for 15 min. Samples were rinsed three times in wash buffer after which they were stained with haematoxylin for two minutes. The slides were scored in by three investigators in a coded, blinded fashion. Micrographs of stained sections of mouse tissues were taken using a digital camera (Zeiss AxioCam ICc3) fitted to an inverted microscope (Zeiss Axio Observer A1) or to compound microscope (Nikon).
Tissue microarrays; immunohistochemical staining for MTA1
The tissue microarray (TMA) was developed by the Department of Pathology, Faculty of Medicine, Khon Kaen University, Thailand, with appropriate ethical approval, as described (16, 17). A 2010 version of the array employed here included 305 O. viverrini associated Thai intrahepatic cholangiocarcinoma (ICCs); the findings with the TMA are shown in Fig. 6 and Table 1. Antigens were retrieved from de-paraffinized and re-hydrated tissues by pre-treating the slides in citrate buffer (pH 6.0) for 10 minutes at 108°C by autoclave. Immunohistochemical staining was performed using purified anti-MTA-1 immunoglobulin, prepared as described (24, 29) Scoring was assessed semi-quantitatively as negative (no detectable staining or positive staining in <10% of tumor cells); weakly positive (positive staining between 10% to 25% of tumor cells); positive (positive staining in 25 to 75% of tumor cells), and strongly positive (>75%) by two independent investigators.
Quantitative real-time PCR
Quantitative real-time PCR was performed as described (24, 25–27). Sequences of primers are available on request.
Statistical Analysis
Differences among groups were compared by Analysis of Variance (ANOVA) and Student’s t-test. P values ≤0.05 were considered statistically significant.
Acknowledgments
Support from awards AI065871 (to PJB) from the National Institute of Allergy and Infectious Disease and award CA155297 from the National Cancer Institute (to JMB and PJB) from the National Institutes of Health is gratefully acknowledged. This work was supported in part by National Institutes of Health Grants CA98823 and CA98823-S1 and institutional new program funds to RK.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2010 Dec 30; doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, et al. Cancer and inflammation: Promise for biologic therapy. J Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, et al. A review of human carcinogens--part B: Biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 6.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31(11):686–696. doi: 10.1111/j.1365-3024.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhamarapravati N, Thammavit W, Vajrasthira S. Liver changes in hamsters infected with a liver fluke of man, opisthorchis viverrini. Am J Trop Med Hyg. 1978;27:787–794. doi: 10.4269/ajtmh.1978.27.787. [DOI] [PubMed] [Google Scholar]
- 8.Harinasuta C, Harinasuta T. Opisthorchis viverrini: Life cycle, intermediate hosts, transmission to man and geographical distribution in Thailand. Arzneimittelforschung. 1984;34(9B):1164–1167. [PubMed] [Google Scholar]
- 9.Sripa B. Pathobiology of opisthorchiasis: An update. Acta Trop. 2003;88(3):209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Sripa B, Kaewkes S. Gall bladder and extrahepatic bile duct changes in Opisthorchis viverrini-infected hamsters. Acta Trop. 2002;83(1):29–36. doi: 10.1016/s0001-706x(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 11.Wongratanacheewin S, Sermswan RW, Sirisinha S. Immunology and molecular biology of Opisthorchis viverrini infection. Acta Trop. 2003;88(3):195–207. doi: 10.1016/j.actatropica.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Sripa B, Pairojkul C. Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol. 2008;24(3):349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4(7):e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 15.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 16.Ninlawan K, O’Hara SP, Splinter PL, Yongvanit P, Kaewkes S, Surapaitoon A, LaRusso NF, Sripa B. Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int. 2010 Dec;59(4):616–21. doi: 10.1016/j.parint.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinawath N, Chamgramol Y, Furukawa Y, Obama K, Tsunoda T, Sripa B, Pairojkul C, et al. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology. 2006;44(4):1025–1038. doi: 10.1002/hep.21330. [DOI] [PubMed] [Google Scholar]
- 18.Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, Tessana S, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50(4):1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, Brindley PJ, et al. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5(10):e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvenna J, Sripa B, Brindley PJ, Gorman J, Jones MK, Colgrave ML, Jones A, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10(5):1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sripa J, Laha T, To J, Brindley PJ, Sripa B, Kaewkes S, Dalton JP, et al. Secreted cysteine proteases of the carcinogenic liver fluke, Opisthorchis viverrini: Regulation of cathepsin F activation by autocatalysis and trans-processing by cathepsin B. Cell Microbiol. 2010;12(6):781–795. doi: 10.1111/j.1462-5822.2010.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young ND, Campbell BE, Hall RS, Jex AR, Cantacessi C, Laha T, Sohn WM, et al. Unlocking the transcriptomes of two carcinogenic parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Negl Trop Dis. 2010;4(6):e719. doi: 10.1371/journal.pntd.0000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manavathi B, Peng S, Rayala SK, Talukder AH, Wang MH, Wang RA, Balasenthil S, et al. Repression of Six3 by a corepressor regulates rhodopsin expression. Proc Natl Acad Sci U S A. 2007;104:13128–13133. doi: 10.1073/pnas.0705878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohshiro K, Rayala SK, Wigerup C, Pakala SB, Natha RS, Gururaj AE, Molli PR, et al. Acetylation-dependent oncogenic activity of metastasis-associated protein 1 co-regulator. EMBO Rep. 2010;11(9):691–697. doi: 10.1038/embor.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bui-Nguyen TM, Pakala SB, Sirigiri DR, Martin E, Murad F, Kumar R. Stimulation of inducible nitric oxide by hepatitis B virus transactivator protein HBx requires MTA1 coregulator. J Biol Chem. 2010;285(10):6980–6986. doi: 10.1074/jbc.M109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Bui-Nguyen TM, Pakala SB, Sirigiri RD, Xia W, Hung MC, Sarin SK, Kumar V, et al. NF-kappaB signaling mediates the induction of MTA1 by hepatitis B virus transactivator protein HBx. Oncogene. 2010;29(8):1179–1189. doi: 10.1038/onc.2009.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghanta KS, Pakala SB, Reddy SD, Li DQ, Nair SS, Kumar R. MTA1 coregulation of transglutaminase 2 expression and function during inflammatory response. J Biol Chem. 2010 doi: 10.1074/jbc.M110.199273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pakala SB, Bui-Nguyen TM, Reddy SD, Li DQ, Peng S, Rayala SK, Behringer RR, et al. Regulation of NF-kappaB circuitry by a component of the nucleosome remodeling and deacetylase complex controls inflammatory response homeostasis. J Biol Chem. 2010;285(31):23590–23597. doi: 10.1074/jbc.M110.139469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Pakala SB, Reddy SD, Bui-Nguyen TM, Rangparia SS, Bommana A, Kumar R. MTA1 coregulator regulates LPS response via MyD88-dependent signaling. J Biol Chem. 2010;285(43):32787–32792. doi: 10.1074/jbc.M110.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol. 2001;3(1):30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- 30.Bowen NJ, Fujita N, Kajita M, Wade PA. Mi-2/NuRD: Multiple complexes for many purposes. Biochim Biophys Acta. 2004;1677(1–3):52–57. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Denslow SA, Wade PA. The human mi-2/NuRD complex and gene regulation. Oncogene. 2007;26(37):5433–5438. doi: 10.1038/sj.onc.1210611. [DOI] [PubMed] [Google Scholar]
- 32.Laothong U, Pinlaor P, Hiraku Y, Boonsiri P, Prakobwong S, Khoontawad J, Pinlaor S. Protective effect of melatonin against Opisthorchis viverrini-induced oxidative and nitrosative DNA damage and liver injury in hamsters. J Pineal Res. 2010;49(3):271–282. doi: 10.1111/j.1600-079X.2010.00792.x. [DOI] [PubMed] [Google Scholar]
- 33.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30(6):735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 34.Jittimanee J, Sermswan RW, Puapairoj A, Maleewong W, Wongratanacheewin S. Cytokine expression in hamsters experimentally infected with Opisthorchis viverrini. Parasite Immunol. 2007;29(3):159–167. doi: 10.1111/j.1365-3024.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 35.Manavathi B, Kumar R. Metastasis tumor antigens, an emerging family of multifaceted master coregulators. J Biol Chem. 2007;282(3):1529–1533. doi: 10.1074/jbc.R600029200. [DOI] [PubMed] [Google Scholar]
- 36.Davies SJ, Grogan JL, Blank RB, Lim KC, Locksley RM, McKerrow JH. Modulation of blood fluke development in the liver by hepatic CD4+ lymphocytes. Science. 2001;294(5545):1358–1361. doi: 10.1126/science.1064462. [DOI] [PubMed] [Google Scholar]
- 37.Davies SJ, Lim KC, Blank RB, Kim JH, Lucas KD, Hernandez DC, Sedgwick JD, et al. Involvement of TNF in limiting liver pathology and promoting parasite survival during schistosome infection. Int J Parasitol. 2004;34(1):27–36. doi: 10.1016/j.ijpara.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamb EW, Crow ET, Lim KC, Liang YS, Lewis FA, Davies SJ. Conservation of CD4+ T cell-dependent developmental mechanisms in the blood fluke pathogens of humans. Int J Parasitol. 2007;37(3–4):405–415. doi: 10.1016/j.ijpara.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamb EW, Walls CD, Pesce JT, Riner DK, Maynard SK, Crow ET, Wynn TA, Schaefer BC, Davies SJ. Blood fluke exploitation of non-cognate CD4+ T cell help to facilitate parasite development. PLoS Pathog. 2010 Apr 29;6(4):e1000892. doi: 10.1371/journal.ppat.1000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smout MJ, Sripa B, Laha T, Mulvenna J, Gasser RB, Young ND, Bethony JM, et al. Infection with the carcinogenic human liver fluke, opisthorchis viverrini. Mol Biosyst. 2011;7(5):1367–1375. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boonmars T, Boonjaraspinyo S, Kaewsamut B. Animal models for Opisthorchis viverrini infection. Parasitol Res. 2009 Feb;104(3):701–3. doi: 10.1007/s00436-008-1268-x. Epub 2008 Dec 3. [DOI] [PubMed] [Google Scholar]
- 42.Tesana S, Kaewkes S, Srisawangwong T, Phinlaor S. The distribution and density of Opisthorchis viverrini metacercariae in cyprinoid fish in Khon Kaen Province. J Parasit Trop Med Ass Thai. 1985;8:36–39. [Google Scholar]