Abstract
Receipt of a living donor liver transplant (LDLT) has been associated with improved survival compared with waiting for a deceased donor liver transplant (DDLT). However, the survival benefit of liver transplant has been questioned for candidates with model for end-stage liver disease (MELD) scores< 15, and the survival advantage of LDLT has not been demonstrated during the MELD allocation era, especially for low MELD patients. Transplant candidates enrolled in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study after 02/28/02 were followed for a median of 4.6 years. Starting at the time of presentation of the first potential living donor, mortality for LDLT recipients was compared to mortality for patients who remained on the waiting list or received DDLT (no LDLT group) according to categories of MELD score (<15 or 15+) and diagnosis of hepatocellular carcinoma (HCC). Of 868 potential LDLT recipients (453 with MELD<15; 415 with MELD 15+ at entry), 712 underwent transplantation (406 LDLT; 306 DDLT), 83 died without transplant, and 73 were alive without transplant at last follow-up. Overall, LDLT recipients had 56% lower mortality (hazard ratio (HR)=0.44, 95% confidence interval [CI] 0.32–0.60; p<0.0001). Among candidates without HCC, mortality benefit was seen both with MELD<15 (HR=0.39;p=0.0003) and MELD 15+ (HR=0.42;p=0.0006). Among candidates with HCC, a benefit of LDLT was not seen for MELD<15 (HR=0.82, p =0.65) but was seen for MELD 15+ (HR=0.29, p=0.043).
Conclusions
Across the range of MELD scores, patients without HCC derived a significant survival benefit when undergoing LDLT rather than waiting for DDLT in the MELD liver allocation era. Low MELD candidates with HCC may not benefit from LDLT.
Keywords: Living donor liver transplantation, cirrhosis, end stage liver disease, mortality, outcomes
Following the introduction of adult-to-adult living donor liver transplantation (LDLT) in the U.S. in the late 1990’s the procedure gained in popularity, and in 2001 represented approximately 8% of all adult liver transplants performed in the U.S. Subsequently, use of the procedure declined from 412 cases in 2001 to 168 LDLT in the U.S in 2009 (www.optn.transplant.hrsa.gov accessed 08/13/10). Previous retrospective reports by the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) identified a survival benefit for patients who received LDLT as compared to waiting for, or receiving, a deceased donor liver transplant (DDLT).(1)That report employed data accrued over the early years of LDLT in nine active liver transplant centers in the U.S. More than 70% of the potential liver transplant recipients enrolled in that retrospective cohort study were evaluated in the era before the Model for End-Stage Liver Disease (MELD) score was employed for deceased donor liver allocation in the U.S. (February 28, 2002), and thus the benefits of pursuing LDLT as compared to waiting for deceased donor liver transplant (DDLT) in the MELD allocation era are not well understood.
Moreover, there is considerable uncertainty regarding which populations of adult liver transplant candidates benefit most from receipt of LDLT in comparison to awaiting DDLT. Markov modeling suggested benefit associated with receipt of LDLT in patients with hepatocellular carcinoma (HCC) (2,3) compared with waiting for DDLT. Experience from A2ALL, however, demonstrated higher rates of post-transplant recurrence in liver transplant candidates with HCC who underwent LDLT(4). Similarly, there is uncertainty regarding whether there is a survival benefit associated with receipt of LDLT among transplant candidates with MELD<15. Two recent large database analyses of the U.S liver transplant population have suggested that a survival benefit is obtained by transplant candidates undergoing transplantation with MELD scores in excess of 15(5) or 12(6) in comparison to remaining on the waiting list. Analyses from the retrospective cohort study reported by A2ALL suggested a survival benefit for transplant candidates enrolled in A2ALL with laboratory (non-exception) MELD scores less than 15, although the majority of those patients were actually transplanted in the pre-MELD allocation era (1).
To resolve some of the uncertainties delineated above, and better inform liver transplant candidates regarding transplant outcomes in the current allocation paradigm, we examined data from A2ALL for liver transplant candidates who entered into the study following the introduction of the MELD-based liver allocation system on February 28, 2002 through August 31, 2009. Outcomes for these patients who presented to A2ALL transplant centers with their first potential living donor during this period were analyzed in order to assess the potential benefit of receipt of LDLT based on transplant candidate MELD score and presence or absence of HCC. The study design, which examined patient outcomes from the time of first living donor evaluation, was created to allow clinicians to counsel transplant candidates and their donors when the opportunity for LDLT presented itself in the transplant clinic setting.
PATIENTS AND METHODS
A primary objective of the A2ALL study has been to identify transplant candidates who accrue a survival benefit from adult living donor liver transplant. In order to encompass both pre-transplant events and post-transplant survival, patient entry into the study occurred on the date that each potential LDLT candidate’s first potential living donor presented for initial donor history and physical examination at one of the nine A2ALL transplant centers, as previously described (1).
Data sources
Liver transplant candidate and potential donor data were provided by the participating A2ALL transplant centers based on a common protocol. Chart reviews and prospective data collection were supplemented by additional ascertainment of deaths and transplants through the end of 2008 for patients included in the retrospective study only (n=112) and through February 2010 for the remaining patients (n=756) under a data use agreement with the Scientific Registry of Transplant Recipients (SRTR). The study cohort utilized for this report included 868 adult liver transplant candidates for whom the first living liver donor was evaluated between February 28, 2002 and August 31, 2009. For these candidates, median follow-up was 4.6 years (range 4 days-7.9 years). Data from DDLT recipients not enrolled in A2ALL but transplanted at A2ALL centers were obtained from SRTR for comparison with A2ALL patients who received DDLT during the same period.
Statistical methods
The cumulative incidence function was calculated using SAS macro “comprisk”(7). The MELD scores reported were calculated on laboratory data only(8), and ignored MELD exception scores used in organ allocation.
Survival analyses, starting at the time of evaluation of each subject’s first potential donor, were employed to compare mortality after LDLT to the conventional transplant strategy of waiting for and potentially receiving DDLT. The non-LDLT group thus included those who received DDLT, those who remained on the waitlist without receiving a liver transplant at study end, and those who died prior to receiving a DDLT. LDLT (n=4) or DDLT (n=2) procedures that were aborted intra-operatively due to recipient conditions were considered to be transplants. Domino transplants were classified as DDLTs (n=1).
A Cox regression method employing sequential stratification to compare the effect of receipt of LDLT with not receiving LDLT over the entire period of observation was utilized for the primary analysis(1). The sequentially stratified Cox model was adjusted for baseline covariates of age, HCC, HCV, cholestatic liver disease, and MELD score, all determined at the time of first donor evaluation. Multiplicative interactions (effect modification) between LDLT, HCC, and MELD score were evaluated. An additional Cox regression analysis of post-transplant mortality was performed starting on the day of transplant, and compared LDLT vs. DDLT adjusted for age, HCC, HCV, cholestatic liver disease, and MELD score at transplant.
Survival probabilities in the tables and figures were calculated in the following manner. Survival in the absence of receipt of LDLT was estimated from a Cox regression censored at LDLT. This model was adjusted for age, HCC, HCV, cholestatic disease, and MELD score as above. Depiction of probabilities of survival that encompass both the waiting period for liver transplantation and post-transplant period were estimated by multiplying the waitlist survival probability at the respective LDLT median transplant time by the post-transplant survival probability for LDLT recipients. Transplant and survival experiences are shown over the five years from initial donor evaluation, during which time 88% of all patient follow-up and 93% of all deaths occurred. All analyses were performed utilizing SAS 9.2 software (SAS Publishing, Cary, NC: SAS Institute Inc., 2008).
Human subjects protection
The Institutional Review Boards and Privacy Boards of the Data Coordinating Center and the nine participating transplant centers approved the study.
RESULTS
Characteristics at study entry and at transplant
A total of 868 adult transplant candidates were enrolled in the A2ALL study between February 28, 2002 and August 31, 2009. The clinical characteristics of these candidates, measured closest to the time of the evaluation of the first potential living donor, are presented in Table 1 according to MELD<15 (n=453) or 15+ (n=415) and subsequent receipt of LDLT. Among candidates with MELD<15, LDLT recipients, compared with non-LDLT recipients, were significantly (p<0.05) more likely to be white, have cholestatic liver disease, or biliary atresia, and to have a history of upper abdominal surgery. They were less likely to have a diagnosis of hepatitis C or HCC. Among candidates with MELD 15+, LDLT recipients were more likely to have advanced HCC and diagnosis of “other” liver disease.
Table 1.
Characteristics of potential LDLT recipients at enrollment by MELD group at enrollment
| Characteristic at Enrollment * | MELD <15 at Enrollment
|
MELD 15+ at Enrollment
|
||||||
|---|---|---|---|---|---|---|---|---|
| ALL | LDLT | Non-LDLT | LDLT vs. Non-LDLT | ALL | LDLT | Non-LDLT | LDLT vs. Non-LDLT | |
| Mean±SD or Percent | Mean±SD or Percent | Mean±SD or Percent | p-value † | Mean±SD or Percent | Mean±SD or Percent | Mean±SD or Percent | p-value † | |
| Patients (N) | 453 | 224 | 229 | 415 | 182 | 233 | ||
| Age (years) | 52.2 ± 10.1 | 51.8 ± 10.4 | 52.5 ± 9.8 | 0.49 | 51.1 ± 10.7 | 51.3 ± 10.5 | 51.1 ± 10.8 | 0.84 |
| Female | 43.9 | 48.2 | 39.7 | 0.07 | 39.8 | 43.4 | 36.9 | 0.18 |
| Race | 0.028 | 0.68 | ||||||
| White | 87.4 | 91.5 | 83.4 | 87.5 | 89.0 | 86.3 | ||
| African-American | 3.3 | 2.7 | 3.9 | 5.3 | 4.4 | 6.0 | ||
| Other | 9.3 | 5.8 | 12.7 | 7.2 | 6.6 | 7.7 | ||
| Hispanic | 15.2 | 9.8 | 20.5 | 0.002 | 21.0 | 20.3 | 21.5 | 0.78 |
| Body Mass Index (kg/m2) | 26.9 ± 5.0 | 26.5 ± 4.9 | 27.3 ± 5.0 | 0.12 | 27.2 ± 5.3 | 26.9 ± 5.4 | 27.4 ± 5.3 | 0.36 |
| Previous Transplant | 0.7 | 0.4 | 0.9 | 0.575 | 2.2 | 2.2 | 2.1 | 0.97 |
| Diagnosis | ||||||||
| Hepatitis C | 41.7 | 32.6 | 50.7 | <.0001 | 41.2 | 42.3 | 40.3 | 0.69 |
| Hepatocellular Carcinoma (HCC) | 20.5 | 14.3 | 26.6 | 0.001 | 8.9 | 9.3 | 8.6 | 0.79 |
| HCC Stage | 0.09 | 0.01 | ||||||
| T0 | 2.2 | 0.0 | 3.3 | 0.0 | 0.0 | 0.0 | ||
| T1 | 7.5 | 6.3 | 8.2 | 16.2 | 0.0 | 30.0 | ||
| T2 | 43.0 | 34.4 | 47.5 | 45.9 | 41.2 | 50.0 | ||
| T3+ | 44.1 | 56.3 | 37.7 | 35.1 | 52.9 | 20.0 | ||
| Missing | 3.2 | 3.1 | 3.3 | 2.7 | 5.9 | 0.0 | ||
| Alcoholic liver disease | 12.1 | 10.7 | 13.5 | 0.36 | 10.1 | 9.9 | 10.3 | 0.89 |
| Cholestatic liver disease | 21.9 | 31.7 | 12.2 | <.0001 | 23.6 | 22.5 | 24.5 | 0.64 |
| Other non-cholestatic cirrhosis | 17.2 | 18.8 | 15.7 | 0.39 | 20.2 | 18.1 | 21.9 | 0.34 |
| Metabolic disease | 2.4 | 2.2 | 2.6 | 0.79 | 3.4 | 2.7 | 3.9 | 0.53 |
| Biliary atresia | 0.9 | 1.8 | 0.0 | 0.04 | 0.2 | 0.5 | 0.0 | 0.26 |
| Non-HCC malignancy | 4.6 | 6.3 | 3.1 | 0.11 | 0.7 | 1.1 | 0.4 | 0.42 |
| Other | 7.3 | 5.4 | 9.2 | 0.12 | 6.0 | 8.8 | 3.9 | 0.04 |
| Ascites | 57.4 | 54.6 | 60.2 | 0.23 | 73.2 | 71.0 | 75.0 | 0.36 |
| Encephalopathy | 44.5 | 42.1 | 46.9 | 0.31 | 62.4 | 57.6 | 66.1 | 0.08 |
| Variceal Bleed | 28.7 | 30.1 | 27.4 | 0.54 | 27.7 | 27.6 | 27.8 | 0.96 |
| Upper Abdominal Surgery | 30.8 | 35.5 | 25.9 | 0.03 | 31.5 | 32.0 | 31.1 | 0.84 |
| Spontaneous Bacterial Peritonitis | 5.6 | 6.8 | 4.4 | 0.26 | 11.6 | 12.9 | 10.5 | 0.45 |
| TIPSS | 8.5 | 8.1 | 8.9 | 0.78 | 11.9 | 10.4 | 13.0 | 0.42 |
| MELD | 10.9 ± 2.5 | 10.8 ± 2.5 | 10.9 ± 2.5 | 0.66 | 19.8 ± 5.3 | 19.0 ± 5.1 | 20.4 ± 5.5 | 0.01 |
| MELD (categories) | 0.33 | 0.001 | ||||||
| 6–10 | 37.7 | 40.2 | 35.4 | - | - | - | ||
| 11–14 | 62.3 | 59.8 | 64.6 | - | - | - | ||
| 15–20 | - | - | - | 67.0 | 75.8 | 60.1 | ||
| 21–30 | - | - | - | 26.5 | 19.8 | 31.8 | ||
| 31–40 | - | - | - | 6.5 | 4.4 | 8.2 | ||
|
| ||||||||
| MELD at Transplant | 13.3 ± 5.4 | 12.7 ± 4.3 | 14.5 ± 7.0 | 0.004 | 21.6 ± 7.1 | 18.6 ± 5.0 | 24.7 ± 7.6 | <.0001 |
| MELD at Transplant (categories) | 0.06 | <.0001 | ||||||
| 6–10 | 27.7 | 27.7 | 27.6 | 1.4 | 2.2 | 0.5 | ||
| 11–14 | 38.6 | 43.3 | 30.1 | 6.8 | 9.9 | 3.8 | ||
| 15–20 | 19.6 | 20.5 | 17.9 | 41.4 | 56.6 | 26.2 | ||
| 21–30 | 6.3 | 4.0 | 10.6 | 31.5 | 24.7 | 38.3 | ||
| 31–40 | 1.4 | 0.4 | 3.3 | 12.1 | 1.6 | 22.4 | ||
| Missing | 6.3 | 4.0 | 10.6 | 6.8 | 4.9 | 8.7 | ||
Missing <6% for all characteristics except MELD at transplant (as noted in table).
p-values for race computed from chi-square test; for HCC stage, MELD at enrollment, and MELD at transplant from 2-sided chi-square test for trend excluding missing category; and for all other characteristics from t-test.
For those transplant candidates with a MELD <15 at the time of study entry, the mean MELD score of those who ultimately received LDLT, was not significantly different from those who received a DDLT or no transplant (Table 1, p=0.66). However, mean MELD at transplant was higher for DDLT recipients than for LDLT recipients (p=0.004). For those transplant candidates with a MELD 15+ at study entry, the mean MELD at entry was lower for those patients who ultimately received an LDLT compared to those who did not (p=0.01). The mean MELD at transplant for recipients of LDLT in this group was much lower than the mean MELD at time of transplant for recipients of DDLT (p<0.0001), an observation reflecting the need for MELD scores to rise in order to receive priority for DDLT.
Outcomes based on MELD score at study entry
Of those transplant candidates with MELD score<15 at enrollment, 224 received LDLT, while 123 received DDLT and 106 did not receive a transplant. Of this latter group, 49 (46%) died on the waitlist without receiving a transplant of any type. Of those transplant candidates with MELD 15+ at enrollment, 182 received LDLT, while 183 received DDLT and 50 did not receive a transplant during the study period. Of this latter group, 34 (68%) died on the waitlist without receiving any transplant.
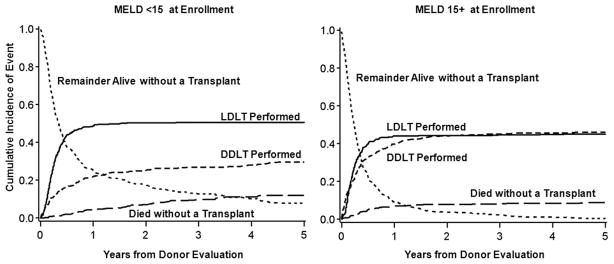
Overall, LDLT recipients had 56% lower mortality (hazard ratio (HR)=0.44, 95% confidence interval [CI] 0.32–0.60; p<0.0001). The probability of receiving an LDLT, receiving a DDLT, or dying on the waitlist, over the five years from the time of initial donor evaluation is shown in Figure 1a for those candidates with MELD<15 at study entry and in Figure 1b for those candidates with MELD 15+ at study entry.
Figure 1.
Outcomes of A2ALL transplant candidates. The probability of LDLT, DDLT, death on the waitlist or remaining alive without transplant over five years after first donor evaluation for living donor candidates with a) MELD<15 at evaluation and b) MELD15+ at evaluation. Estimates are based on the cumulative incidence function.
Mortality in candidates without HCC
Mortality based on MELD score at enrollment
In an adjusted sequential stratification analysis of time from initial donor evaluation to death for transplant candidates with MELD < 15 and no HCC at study entry, patients who underwent LDLT had a mortality hazard ratio [HR] of 0.39 (95% confidence interval [CI] 0.24–0.65; p=0.0003), compared with patients who did not receive LDLT (Figure 2). For these candidates, the median time to receipt of LDLT was 3.0 months after the first potential living liver donor evaluation, while the time to receipt of DDLT was 7.9 months. For patients with MELD 15+ at study entry and no HCC, patients who underwent LDLT had a lower mortality than those who did not receive LDLT (HR=0.42, 95% CI 0.26–0.69; p=0.0006) (Figure 2). For this group of candidates without HCC and a MELD score of 15+ at study entry, the median time to receipt of LDLT was 2.5 months, while the time to receipt of DDLT was 3.0 months, after the first potential living liver donor evaluation. We performed additional analyses to look at smaller subsets of transplant candidates based on MELD score at enrollment to examine consistency of results across categories of MELD scores. For MELD scores of 6–10, 11–14, 15–19, and 20+, there was a nearly constant survival advantage for LDLT across categories, with a range in hazard ratios of 0.38 to 0.44 (Table 2).
Figure 2.
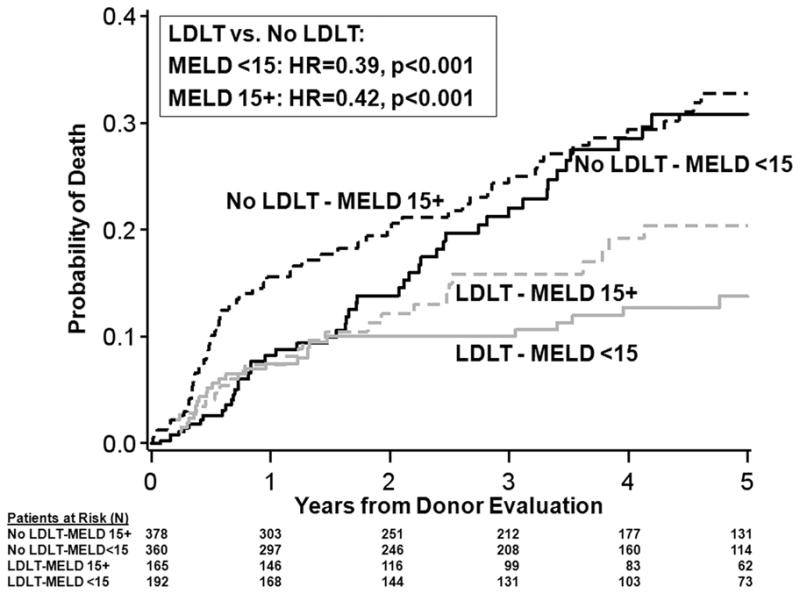
Mortality for transplant candidates. Mortality following initial potential donor evaluation for candidates without HCC according to MELD score at evaluation and whether LDLT was performed or not. For graphical purposes, mortality while awaiting LDLT is assumed to be the same as mortality for candidates for whom LDLT was not available up until the median time for LDLT (3.0 months for MELD<15 and 2.5 months for MELD15+). Shown for patient age=50, no HCC, with HCV, no other cholestatic disease, and MELD=10 at enrollment for MELD<15 group and MELD=20 at enrollment for MELD 15+ group.
Table 2.
Five-year mortality (%)* and relative risk of mortality (HR)** according to MELD score at first potential living donor evaluation for transplant candidates without HCC
| MELD at Entry | LDLT 5-yr mortality (%) | Non-LDLT 5-yr mortality (%) | HR LDLT v. No LDLT | 95% CI | p-value |
|---|---|---|---|---|---|
| MELD 6–10 | 5.5% | 32.1% | 0.43 | (0.17, 1.05) | 0.063 |
| MELD 11–14 | 24.0% | 36.0% | 0.38 | (0.21, 0.70) | 0.0021 |
| MELD 15–19 | 14.3% | 27.6% | 0.42 | (0.23, 0.77) | 0.005 |
| MELD 20+ | 20.1% | 33.4% | 0.44 | (0.19, 1.02) | 0.056 |
Mortality shown for patient age=50, with HCV, no other cholestatic disease, and MELD=8, 12, 16, and 25 for the MELD<10, 11–14, 15–19, and 20+ groups respectively.
Based on sequential stratification model (see text)
Survival from the time of transplantation
While not the primary focus of the A2ALL study, analyses of survival were also performed beginning at the time of transplant (rather than at the time of first donor evaluation) to compare mortality following LDLT and DDLT. Post-transplant mortality risk was similar following LDLT and DDLT. Specifically, the mortality hazard ratio for LDLT compared to DDLT was 0.88 (p=0.78) for non-HCC candidates with MELD<15 at evaluation and 0.83 (p=0.60) for non-HCC candidates with MELD 15+ at evaluation, adjusted for MELD at transplant, age, and diagnoses of hepatitis C, and cholestatic liver disease.
Transplant candidate morbidities
We used data from SRTR and A2ALL to explore the possibility that candidates for whom LDLT was considered were inherently more ill than candidates not considered for LDLT at the A2ALL centers. The presence of these complications was determined based on SRTR data alone, regardless of whether the patient was enrolled in A2ALL or not. Three comparisons were made between patients enrolled in A2ALL and listed liver transplant candidates at the nine A2ALL centers who were not enrolled in A2ALL: liver disease complications at time of listing (hepatic encephalopathy, ascites, variceal hemorrhage, upper abdominal surgery, spontaneous bacterial peritonitis, hyponatremia (Na < 135 mEq/L) and transjugular intrahepatic portosystemic shunt (TIPSS)), donor risk index at transplantation(9), and post-transplant survival. The frequency of complications was similar between patients enrolled in A2ALL and listed liver transplant candidates at the nine A2ALL centers who were not enrolled in A2ALL. Although significantly more of those not enrolled in A2ALL had TIPSS in the MELD<15 group (5.1% non-A2ALL vs. 2.6% in A2ALL, p<0.01) and more had ascites in the MELD 15+ group (89% non-A2ALL vs. 85% in A2ALL, p=0.03). There were no significant differences in frequency of the remaining complications (for MELD<15, p>0.12 for all except TIPSS; for MELD 15+, p>0.13 for all except ascites).
DDLT donor risk index
We compared donor risk index (DRI)(9) for DDLT recipients enrolled in A2ALL and DDLT recipients from the same centers but not enrolled in A2ALL. Median DRI for non-HCC DDLT recipients with MELD<15 at listing enrolled in A2ALL was 1.35 and was 1.40 for 1458 DDLT recipients not enrolled in A2ALL with MELD<15 at listing who were transplanted at the nine participating centers (p=0.94). For non-HCC DDLT recipients with MELD15+ at listing, the median DRI was 1.33 for A2ALL patients and 1.34 for 2999 non-A2ALLenrolled DDLT recipients (p=0.45).
Post-transplant outcomes
Finally, we compared post-DDLT mortality for non-HCC DDLT recipients. For non-HCC patients with MELD<15 at listing, post-DDLT mortality HR was 0.79 (p=0.23) for A2ALL patients compared with non-A2ALL-enrolled patients. For non-HCC patients with MELD15+ at listing, post-DDLT mortality HR was 1.00 (p=0.98) for A2ALL patients compared to non-A2ALL-enrolled patients. These analyses were adjusted for recipient age, MELD at transplant, and DRI.
Mortality in candidates with HCC
One hundred thirty of 868 (15.0%) of the A2ALL transplant candidates carried a diagnosis of HCC at the time of enrollment. Of these, 93 had a laboratory (non-exception) MELD<15 at study entry and 37 had MELD15+ at study entry. Tumor stages at the time of study entry are presented in Table 1 for these two groups of transplant candidates.
Among the 93 transplant candidates in the MELD<15 group, 32 HCC patients received LDLT at a median of 1.6 months after initial living liver donor evaluation, 49 received DDLT at a median of 2.2 months after study entry, and 12 had not undergone any transplant by last follow-up, including seven who died on the waitlist. Among the 37 transplant candidates in the MELD15+ group, 17 HCC patients went on to receive LDLT at a median of 1.8 months after initial living donor evaluation, 16 received DDLT at a median of 3.1 months after first living donor evaluation, and four had not undergone any transplant at last follow-up, three of whom died on the waitlist.
In an adjusted sequential stratification analysis of time from initial donor evaluation to death for transplant candidates with MELD<15 and HCC at study entry, we were unable to detect a significant survival benefit for LDLT recipients compared to patients who did not receive LDLT (HR=0.82, 95% CI 0.36–1.89; p=0.65). In a similar analysis for patients with MELD15+ at study entry and HCC, patients who underwent LDLT had significantly lower mortality risk than those who did not receive LDLT (HR=0.29, 95% CI 0.09–0.96; p=0.043). When analysis was restricted to those candidates with HCC (adjusted for MELD at transplant, age at transplant, HCV infection and cholestatic liver disease) who actually received either DDLT or LDLT, post-transplant survival did not differ between recipients of LDLT or DDLT. For candidates with MELD less than 15 at enrollment and HCC the HR was 2.17 (vs. DDLT), p=0.19. For candidates with MELD 15+ at enrollment and HCC, the HR was 1.10 (vs. DDLT), p=0.91.
DISCUSSION
There is considerable uncertainty regarding the benefit of liver transplantation in adult candidates with low MELD scores. Prior work demonstrated little or no net survival benefit for transplant candidates with low MELD scores (MELD<15) who received deceased donor liver transplant in the U.S.(5). This observation resulted in a major change in deceased donor liver allocation policy in the U.S., termed Share15, in a manner that markedly limited the opportunity for receipt of DDLT for adult candidates with low MELD scores. Subsequent analysis employing SRTR data suggested a positive transplant benefit (incorporating pre-transplant and post-transplant mortality risk measures) for transplant candidates at somewhat lower MELD scores(6). The majority of liver transplant candidates with MELD scores of 12 or greater would benefit by liver transplantation based on that analysis. Timely receipt of DDLT for such liver transplant candidates with MELD scores of 12–15, however, is unlikely in the setting of allocation policies that preferentially offer DDLT to candidates with the highest MELD scores in order to minimize waitlist mortality. For example, in the current analysis, only 42% of candidates with MELD< 15 who did not undergo LDLT received DDLT within 12 months of donor evaluation.
An alternative strategy to achieve timely transplantation for candidates with lower MELD scores is LDLT. The A2ALL consortium enrolled a large cohort of patients with low MELD scores for whom LDLT was an option, and thus analysis of patients enrolled in this study provided an opportunity to ascertain whether LDLT in patients with low MELD offers transplant survival benefit. As detailed above, receipt of LDLT in candidates without HCC whose MELD scores were less than 15 at time of study enrollment was associated with significant survival advantage in comparison to waiting for, or receiving, DDLT. Such benefit could be the result of either diminished waitlist mortality, or improved post-transplant survival. As post-transplant survival was similar in both LDLT and DDLT recipients in the MELD<15 group, the net survival benefit must be attributed largely to reduced waitlist mortality. Although low MELD scores have been associated with relatively low risk of death at 90 days and one year(10–12), 10.8% of low MELD patients died on the waitlist at a median of 9.8 months following entry into this cohort. This number approximates the percentage difference in estimated 3-year mortality between the LDLT recipients and non-LDLT recipients (Figure 2). Avoidance of waitlist deaths as a consequence of timely transplant, as reflected by a median wait for LDLT of 3.0 months after study entry, thus appears to be the major contributor to favorable outcomes in the low MELD group. Additional support for the notion that the primary survival benefit associated with LDLT is avoidance of waitlist mortality is derived from analysis of outcomes in the candidates with HCC with MELD <15. LDLT was not associated with significant survival benefit in this group, for whom waiting time for LDLT (median 1.6 months) was only slightly less than waiting time to DDLT (median 2.2 months).
We considered an alternative explanation for the survival benefit experienced by LDLT recipients in the MELD<15 group and explored the possibility that the quality of the DDLT grafts received by these patients was inferior, and resulted in higher post-transplant mortality following DDLT. Three lines of evidence refute this speculation. First, as mentioned above, post-transplant survival was not different in low MELD patients who received LDLT and those who received DDLT (HR=0.96, p=0.91 for non-HCC recipients). Second, we examined the DRI for the DDLT organs received by the low MELD candidates enrolled in A2ALL, and compared that to the median DRI of high MELD patients receiving DDLT at the participating centers. The median DRI for the DDLT organs received by the MELD<15 candidates without HCC who were enrolled in A2ALL was very similar to the median DRI for DDLT organs transplanted during the post-MELD era into recipients at A2ALL centers with MELD15+ at listing who had not enrolled in A2ALL. Most importantly, recipients of DDLT enrolled in A2ALL did not have higher post-transplant mortality than non-A2ALL-enrolled recipients of DDLT at the same centers.
As has been true throughout the history of LDLT, the survival benefits observed here for LDLT recipients must be balanced by the risks of morbidity and mortality experienced by LDLT donors. It must also be recognized that the A2ALL study does not reflect the outcomes of a randomized trial of LDLT versus those listed for DDLT at the nine A2ALL transplant centers. Rather, the study reports upon the observational outcomes experienced by transplant candidates for whom consideration of living liver donation was felt to be an appropriate option by the treating transplant team, and was possibly available, based on the presence of a donor presenting for evaluation at the participating transplant center. It could be postulated that the candidates with low MELD scores for whom LDLT was seriously entertained by our transplant centers represent a group of individuals with perceived increased risk of mortality beyond that associated with their MELD score. This possibility was explored by examining the frequency of hepatic encephalopathy, ascites, variceal hemorrhage, previous upper abdominal surgery, spontaneous bacterial peritonitis, hyponatremia (Na < 135 mEq/L), and TIPS in patients enrolled in A2ALL at the nine participating centers, as well as in listed transplant candidates at the nine centers who were not enrolled in the A2ALL study. Somewhat unexpectedly, there was no significant difference in the frequency of these complications in those candidates for whom LDLT was seriously contemplated (i.e., for whom a donor was evaluated at their transplant center) and in those who did not have such a donor. Despite this objective finding, there remains the possibility that experienced transplant teams still may have applied some selection bias in the recommendation of pursuit of LDLT in their centers such that the reported survival benefit may not be universally obtained by all candidates. Given this possibility, one should remain cautious about the generalized pursuit of LDLT in all candidates with low MELD scores presenting to transplant centers. Future analyses of very large cohorts of LDLT recipients may permit the further identification of subsets of candidates who receive maximal benefit from this procedure.
The majority of transplant candidates in the A2ALL retrospective study(1) underwent both listing and transplant prior to the initiation of the MELD-based liver allocation system. In the current MELD era analysis, candidates who enrolled in A2ALL with MELD15+, who did not have HCC, and who received LDLT, had markedly lower mortality compared to those waiting for, or receiving DDLT (HR 0.42, 95% CI 0.26–0.69; p=0.0006). This survival benefit was similar to that previously reported by our group(1) and strongly supports the continued application of LDLT in this group of patients with higher MELD scores. As there were only 27 patients enrolled in A2ALL in the post-MELD era with MELD scores at enrollment of greater than 30, and only eight of these patients received LDLT, we were unable to perform an analysis restricted to transplant candidates with very high MELD scores and cannot comment on the presence or absence of possible futility associated with LDLT in these high MELD candidates.
Fifteen percent of the patients in the current analysis carried a diagnosis of HCC. As detailed in Table 1, patients who ultimately went on to receive LDLT were more likely to have stage T3 or higher tumors than those who received DDLT, most likely as a consequence of standardized (higher) exception MELD scores for those with stage T2 HCC, which permitted relatively expeditious DDLT. It is of note that despite the relatively large percentage of patients with T3 tumors, fairly quick access to DDLT was noted for the HCC patients with lower laboratory MELD scores, such that wait times for DDLT for these patients was far less than that for non-HCC candidates (7.9 months median wait for DDLT for MELD<15 candidates without HCC who received DDLT versus 2.2 months median wait for MELD<15 candidates with HCC). This wait time for DDLT for low MELD HCC patients was similar to the wait time for LDLT in this group (median 1.6 months). In this setting, the lack of a survival advantage associated with receipt of LDLT for low MELD HCC transplant candidates was not surprising. Transplant candidates with MELD15+ with HCC had significantly lower mortality with LDLT.
In summary, results from the A2ALL study in the MELD liver allocation era continued to demonstrate significant survival advantage associated with receipt of LDLT in comparison to continued waiting for DDLT. This survival benefit exists for patients with low laboratory MELD scores and for patients with MELD scores of 15 and higher. These results justify a continued role for LDLT in the U.S., especially in the context of a severe and ongoing limitation in the supply of deceased donor organs and substantial waitlist mortality. The data presented in this study should serve to guide the discussion that occurs between transplant physicians and transplant candidates regarding the survival benefits associated with receipt of a living donor liver transplant. With the identification and quantification of this survival benefit, transplant candidates and centers may be better prepared to advocate for pursuit of living donor liver transplantation in transplant candidates. Future efforts should focus on delineating those transplant candidates that benefit most from receipt of LDLT, and on identifying those patients for whom DDLT serves as the best avenue to successful transplantation.
List of Abbreviations
- A2ALL
Adult to adult living donor liver transplant study
- DDLT
Deceased Donor Liver Transplant
- DRI
Donor risk index
- HCC
hepatocellular carcinoma
- LDLT
Living Donor Liver Transplant
- MELD
Model for End-stage Liver Disease
- SRTR
Scientific Registry for Transplant Recipients
- TIPSS
Transjugular intrahepatic portosystemic shunt
Footnotes
Disclosure: No conflicts of interest exist.
Presented in part at the 60th annual meeting of the American Association for the Study of Liver Diseases, Boston, Massachusetts, November, 2009.
Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
This is publication number xx of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
References
- 1.Berg CL, Gillespie BW, Merion RM, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarasin FP, Majno PE, Llovet JM, et al. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1703–1709. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 3.Cheng SJ, Pratt DS, Freeman RB, Jr, et al. Living-donor versus cadaveric liver transplantation for non-resectable small hepatocellular carcinoma and compensated cirrhosis: A decision analysis. Transplantation. 2001;72:861–868. doi: 10.1097/00007890-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RA, Kulik LM, Freise CE, et al. Recurrence of hepatocellular carcinoma and death following living donor and deceased donor liver transplantation in the A2ALL cohort study. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merion RM, Schaubel DE, Dyskstra DM, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 6.Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim WR, Thernau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 8.Freeman RB, Jr, Wiesner RH, Roberts JP, et al. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4(Suppl 9):114–131. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 9.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry for Transplant Recipients: Transplant Data 1999–2008. U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: 2010. [Google Scholar]
- 11.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]