Abstract
Background
Perfluorinated chemicals (PFCs) have been used widely in consumer products since the 1950s and are currently found at detectable levels in the blood of humans and animals across the globe. In stark contrast to this widespread exposure to PFCs, there is relatively little research on potential adverse health effects of exposure to these chemicals.
Objectives
We performed this cross-sectional study to determine if specific blood PFC levels are associated with impaired response inhibition in children.
Methods
Blood levels of 11 PFCs were measured in children (N = 83) and 6 PFCs: perfluorooctane sulfonate (PFOS), perfluorohexane sulfate (PFHxS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorooctanesulfonamide (PFOSA), and perfluorodecanoic acid (PFDA) – were found at detectable levels in most children (87.5% or greater had detectable levels). These levels were analyzed in relation to the differential reinforcement of low rates of responding (DRL) task. This task rewards delays between responses (i.e., longer inter-response times; IRTs) and therefore constitutes a measure of response inhibition.
Results
Higher levels of blood PFOS, PFNA, PFDA, PFHxS, and PFOSA were associated with significantly shorter IRTs during the DRL task. The magnitude of these associations was such that IRTs during the task decreased by 29–34% for every 1 SD increase in the corresponding blood PFC.
Conclusions
This study suggests an association between PFC exposure and children’s impulsivity. Although intriguing, there is a need for further investigation and replication with a larger sample of children.
INTRODUCTION
Perfluoroalkyl acids constitute a general class of chemicals that include a number of commonly used perfluorinated chemicals (PFCs), including perfluorooctane sulfonate (PFOS), perfluorohexane sulfate (PFHxS), and perfluorooctanoic acid (PFOA). These chemicals have been widely used in consumer and industrial products. With the widespread use of these chemicals and a marked resistance to metabolic or environmental degradation, detectable levels of PFCs have now been documented in wildlife around the world1,2 as well as in humans with3 and without4,5 occupational exposure. Despite a widespread exposure to this class of chemicals, relatively little is known about potential effects to human health and behavior.
A number of studies have considered the potential effects of prenatal exposure to PFOA and PFOS on birth outcomes in humans. PFOA (but not PFOS) was associated with significantly lower birth weight6 as well as smaller birth length and abdominal circumference7 in the Danish National Birth Cohort. “Significance” refers to statistical significance (not necessary clinical significance) here and throughout this manuscript. In contrast to these significant effects, another study found that PFOS (but not PFOA) were negatively correlated with birth weight, and specifically in female infants.8 In addition, PFOA and PFOS were not significantly associated with birth weight but both PFOA and PFOS were significantly associated with lower ponderal index and head circumference.9 Finally, other research has revealed no significant association between birth outcomes and maternal PFC levels.10,11 Based on these inconsistencies, additional research is necessary to determine if prenatal exposure to certain PFCs are indeed related to birth outcomes12 and, if so, to which outcomes.
In addition to the study of birth outcomes, a number of studies have considered potential neurobehavioral effects of PFC exposure in humans. For example, potential neurobehavioral consequences of prenatal exposure to PFOA and PFOS were considered in the Danish National Birth Cohort.13,14 In this cohort, PFOS was associated with delayed gross motor development in the first two years of life14 but no other significant associations with developmental milestones during the first two years of life or children’s subsequent behavioral or coordination problems at age 7 were found in this cohort.13 Two studies have considered children’s serum PFOA, PFOS, PFNA, and PFHxS in relation to parental reports of ADHD diagnosis (with and without medication). First, a cross-sectional analysis of data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000 and 2003–2004 for children 12–15 years of age (N = 571) found significant adjusted odds ratio (OR) for ADHD in association with a 1-μg/L increase in serum PFOS (OR = 1.03; 95% CI: 1.01–1.05), PFOA (OR = 1.12; 95% CI: 1.01–1.23), and PFHxS (OR = 1.06; 95% CI: 1.02–1.11), but no significantly increased odds of ADHD for children with higher serum perfluorononanoic acid (PFNA15). Second, a cross-sectional study was conducted with children 5–18 years of age (N = 10 546) in the C8 Health Project (a study of community residents in Ohio and West Virginia who lived or worked in areas with PFOA-contaminated water supplies16). In this study, the OR for ADD/ADHD was significantly lower with increasing PFOA (OR = 0.80; 95% CI: 0.67–0.95) but significantly greater with increasing PFHxS (OR = 1.33; 95% CI: 1.11–1.59), but no significant association with PFOS or PFNA. The reason for the inconsistency across these studies is not yet understood.
A number of studies have also considered the potential reproductive and developmental effects of PFC exposure using animal models. Initial studies were performed in the rat; however, rats may not be the ideal species in which to study PFOA exposure effects given rapid elimination of PFOA in female rats.17 Increasing prenatal exposure to PFOA in rats was associated with diminished postnatal weight gain and postweaning survival.18 Similarly, prenatal exposure to PFOS was associated with greater postnatal mortality and, for those that survived, developmental delays.19,20 Subsequent studies with lower prenatal doses of PFOS in rats revealed greater motor activity and diminished capacity to habituate to the testing environment in offspring.21 However, prenatal exposure to PFHxS in rats produced no significant effects on mating, fertility, birth outcomes, and development of offspring.22 In studies of prenatal exposure to PFCs in mice, there was slightly diminished activity in an open-field test with increasing PFOS.23 Similarly, neonatal exposure to PFOS and PFOA was associated with diminished habituation to a novel environment and lack of activity (measured with locomotion, rearing, andtotal activity;.24 Studies that have considered combined maternal stress and prenatal exposure to PFOS have failed to find any consistent interactions; however, increasing PFOS was associated with fetal toxicity,25 decreased corticosterone levels in females,26 and some evidence of delayed neuromotor maturation.27
The biological mechanism for potential PFC-induced neurobehavioral problems has yet to be determined. Perhaps prenatal PFC exposure affects birth outcomes and, in turn, these birth outcomes (e.g., birth weight) produce attentional problems.28 Alternatively, PFCs might have direct effects on the central nervous system. Although PFC exposure does not appear to produce differential accumulation in the brain,29 it is detectable in brain tissue following oral exposure.30 Furthermore, although PFC levels in brain tissue are much lower than corresponding levels in serum, these levels might still be sufficient to affect CNS functioning. For example, PFOS and PFOA have been shown to affect proteins in the brain that may affect neuronal growth and synaptogenesis.31 Additional evidence suggests that exposure to PFOS is associated with an increase in norepinephrine concentrations in the paraventricular nucleus of the hypothalamus,32 suggesting a possible pathway to nucleus accumbens activity33 and impulsivity.34 However, this particular neurobiological pathway has yet to be established in humans.
In the present study, we conducted a cross-sectional investigation of potential emotional and behavioral associations with blood PFC levels in children. Children are an important population given the potential for a child’s environmental context to set a trajectory for significant effects in adolescence and adulthood (e.g., ref 35). Specifically, we considered potential PFC effects using a task shown to be sensitive to environmental toxicant exposure, the differential reinforcement of low rates of responding (DRL) task. The DRL task is a well-established behavioral measure of impulsivity36 affected by exposure to a wide range of environmental toxicants.37 Therefore, our study considers the hypothesis that increasing blood PFC levels will be associated with increasing impulsivity in children as measured using the DRL task.
EXPERIMENTAL SECTION
Participants
Participants were recruited as part of an ongoing study designed to address the effects of low level lead (Pb) exposure on cardiovascular responses to acute stress.38 For that study (N = 100), we mailed invitations to homes in Oswego County, NY, containing a child within our target age group (9–11) using a direct mailing list. This recruitment method elicits participation from a sample that closely resembles an eligible population and is cost-effective.39 Further inclusion criteria included (1) reporting no use on the day of testing of medication that might affect cardiovascular functioning (e.g., Ritalin), and (2) having no significant developmental disorders that might affect task performance (a component of our broader study). After acquiring funding for the present study, we began drawing an additional 2 mL of whole blood necessary for the analysis of PFC levels after having already completed blood draws and testing for 12 children. In addition, we were unable to obtain sufficient blood for the additional analysis of PFC levels for five children. For these five children (100% female), their initial venipuncture site closed during the draw and it was our policy to not attempt a second venipuncture if we had sufficient blood for the measurement of nonessential metals, the primary focus of our project. It is unclear why this occurred exclusively with female participants. With the exception of gender, this resulted in a final sample of 83 children (30 females, 53 males) that did not differ significantly from our full sample (N = 100) on any dimensions measured with our covariates (e.g., BMI, race, and family income; p values >0.10). In addition, gender was not significantly associated with the PFCs we measured (p-values > 0.20).
Measurement of Blood PFC Levels
Participants arrived at the blood draw center and signed an assent form while a parent signed a separate consent form approved by the Institutional Review Board of SUNY Oswego. As part of a more extensive blood draw protocol, a whole blood specimen (2-mL) was collected into a Vacutainer tube for the analysis of PFC blood levels. Blood specimens were immediately placed on ice and within 2 h of the blood draw the samples were transferred to 10 mL cryovials and frozen at −80 °C pending shipment to Wadsworth Center, Albany, NY.
Chemical Analysis
PFOS, PFHxS, perfluorobutanesulfonate (PFBS), perfluorodecanesulfonate (PFDS), perfluorooctanesulfonamide (PFOSA), perfluoroheptanoic acid (PFHpA), PFOA, PFNA, perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), and perfluorododecanoic acid (PFDoDA) were analyzed in whole blood samples using an ion-pairing extraction procedure and were determined by use of high-performance liquid chromatography-electrospray tandem mass spectrometry (HPLCESI- MS/MS).40,41 Approximately 0.5 mL of blood sample, 5 ng of internal standards (13C4–PFOS, 13C4–PFOA, 13C2–PFNA, and 13C2–PFDA), 2 mL of 0.25 M sodium carbonate/sodium bicarbonate buffer and 1 mL of 0.8 M tetrabutyl ammonium hydrogen sulfate solution (adjusted to pH 11), were added to a 15 mL polypropylene tube for extraction. After thorough mixing, 5 mL of methyl tert-butyl ether (MTBE) was added, and the mixture was shaken for 40min. The organic and aqueous layers were separated by centrifugation at 3500 rpm for 3 min and an exact volume of MTBE (4 mL) was removed from the solution. The aqueous mixture was rinsed with 3 mL MTBE and separated twice; the rinses were combined in a second polypropylene tube. The solvent was allowed to evaporate gently under nitrogen stream before being reconstituted in 0.5 mL of methanol. The sample was vortexed for 30 s and transferred into an autosampler vial prior to instrumental analysis.
Analyte separation was performed using an Agilent1100 series HPLC. Ten microliter of the extract was injected onto a 100 × 2.1 mm (5 μm; Thermo Electron Corporation, Bellefonte, PA) Betasil C18 column with 2mMammonium acetate/methanol as the mobile phase starting at 10% methanol. At a flow rate of 300 μL/min, the gradient increased to 100% methanol at 10 min before reverting to original conditions at 12 min. For quantitative determination, the HPLC system was interfaced to an API 2000 triple-quadruple tandem mass spectrometer (Applied Biosystems, Foster City, CA) operated in the electrospray negative ionization mode. Instrumental parameters were optimized to transmit the [M-K]− ion before fragmentation to one or more product ions. Declustering potential and collision energies were optimized for each analyte and ranged from 35 to 90 V and 10 to 35 eV, respectively. Data were acquired by tandem mass spectrometry using multiple reaction monitoring (MRM) at transitions, 499 > 99 for PFOS, 503 > 99 for 13C4–PFOS, 497.7 > 77.7 for PFOSA, 398.7 > 79.7 for PFHxS, 298.7 > 79.7 for PFBS, 599 > 99 for PFDS, 363 > 319 for PFHpA, 413 > 369 for PFOA, 417 > 372 for 13C4–PFOA, 463 > 419 for PFNA, 465 > 420 for 13C2–PFNA, 513 > 469 for PFDA, 515 > 470 for 13C2–PFDA, 563 > 519 for PFUnDA, and 613 > 169 for PFDoDA. When possible, multiple daughter ions were monitored for confirmation, but quantitation was based on a single product ion. In all cases, the capillary was held at −4 kV and the desolvation temperature was kept at 400 °C.
Quality Assurance and Quality Control
13C4–PFOS, 13C4–PFOA (99% purity, Wellington Laboratories, Guelph, ON, Canada), 13C2–PFNA and 13C2–PFDA (3 M Company, St. Paul, MN) were spiked as internal standards into each blood sample prior to the addition of reagents for extraction. PFC concentrations were calculated by isotopic dilution method, and further confirmed by matrix matched calibration standards. Recoveries of 13C4–PFOS, 13C4–PFOA, 13C2–PFNA, and 13C2–PFDA were 98±5%, 124±7%, 109±6%, and 141±13%, respectively. Matrix spike recoveries were tested by spiking native standards of all 11 target compounds into 12 randomly selected samples, at levels of 10 ng and 20 ng for each of the target compounds. All the matrix spike samples were analyzed in duplicate. Recoveries of native standards spiked in blood matrix were 96 ± 6%, 101 ± 6%, 114 ± 7%, and 105 ± 5%, for PFOS, PFHxS, PFOA, and PFNA, respectively. The average recoveries for other perfluorochemicals ranged from70% to 125%. The relative standard deviations (RSD) of duplicate analyses were less than 5% for PFOS, PFHxS, PFBS, PFDS, PFOA, PFNA, PFDA, PFHpA, and less than 10% for PFOSA, PFUnDA, and PFDoDA. Milli-Q water (18 MΩ) was analyzed through the entire procedure as a blank, for every batch of 20 samples. Procedure blanks were also spiked with native standards. The recoveries of native standards spiked in water blanks were 96%, 85%, 108%, 109%, and 113%for PFOS, PFHxS, PFOA, PFNA, and PFDA, respectively. Solvents and blood collection tubes were checked for the presence of the perfluorochemicals analyzed in this study. Concentrations in blanks are below limit of detection for all eleven perfluorochemicals. The limit of quantitation (LOQ) was determined based on the linear range of the calibration curve prepared at a concentration range of 0.2–100 ng/mL. Concentrations in samples which were at least 3-fold greater than the lowest acceptable standard concentration were considered to be valid. A curve point was deemed acceptable if (1) it was back-calculated to be within 30% of the theoretical value when evaluated versus the 1/x weighted curve, and (2) the peak area of the standard was at least 3 times greater than that in the blank. Concentration/dilution factors were included in the calculation of the LOQ. The LOQ for PFOS and PFOA was 0.2 and 1.0 ng/mL, and for other perfluorochemicals, it ranged from 0.2 to 1.0 ng/mL. Three of the perfluorochemicals (PFBS, PFDS, and PFUnDA) had levels below the LOQ for all samples and are therefore not discussed further. Two of the perfluorochemicals (PFHpA and PFDoDA) had more than 50% of the samples below the LOQ and therefore results from the analysis of these two PFCs are not reported here (but are included in our online Supporting Information (SI) Table S1). Total PFC levels were computed by summing the remaining six PFCs, and for those PFCs having some levels below the LOQ, ½ the value of the LOQ for the particular PFC was used (cf., ref 42). For those 6 PFCs having levels above the LOQ in 50% of the children, separate analyses were conducting for each PFC.
Procedure
All blood draws occurred on Friday and a laboratory session was scheduled for the following week. The laboratory session commenced with the measurement of height and weight. Unrelated to the present paper, all children underwent an initial cardiovascular acute stress reactivity protocol for 1 h (see ref 38 for details). These cardiovascular responses to acute stress were analyzed in relation to DRL performance (a 20 min task that followed this cardiovascular stress testing) and PFC levels and are reported in SI Table S2. The number of significant associations (5) was approximately what you would expect from chance and the analysis of 80 associations. In addition, the pattern of association (with early and late DRL performance) does not coincide with PFC association (with middle DRL performance).
Differential Reinforcement of Low Rates of Responding (DRL) Task
As employed in a previous study that demonstrated behavioral inhibition deficits as a function of toxicant exposure in children,37 we adopted a relatively short (20 min) DRL task as an assessment of learning rather than acquisition, which can take hours37 or days.43 The present DRL task was presented using an E-Prime (Psychology Software Tools, Inc.) program. Participants were only told:
“You are now going to play a computer game in which you can earn additional money. During this game, I will not be able to speak to you. It is fine if you speak to me while you are playing, but I can’t answer any questions. I will tell you when the game starts, and when it’s over. I can’t tell you the rules to this game—you will need to figure that out on your own. All I can tell you is that pressing the button at the correct time is all that is involved in this game. If played correctly, you could win as much as $15.00!”
The task was then begun and participants earned $.25 for the first three responses and this was followed by a DRL20 schedule. According to the DRL20 schedule, if the space bar was pressed before the completion of 20 s, the time was reset to 20 s and no money was earned. If a response (bar press) occurred 20 s or longer following a prior response, $.25 was earned and the computer screen showed the following message: “CORRECT! You have earned another $.25”. The program sequentially recorded the inter-response time (IRT) of each bar press within a millisecond of precision. IRTs represent the time between responses (i.e., bar presses) and therefore rapid responding will be reflected by short IRTs and delayed responding will be reflected by longer IRTs. The IRTs were stored for subsequent analysis.
Potential Confounds
To strengthen our inferences regarding the effects of children’s blood PFC levels, we considered a number of potential confounds, including ages (child, mother, and father), family income, parent’s education, parent’s occupational class, BMIs (child, mother, and father), child’s gender, child’s race, family history of various chronic illnesses (e.g., diabetes), blood Pb levels, and blood Hg levels (see Table 1 for a partial listing of these variables). Details regarding the methods for measuring these variables are included in the SI as well as in a prior publication.38
Table 1.
Characteristics (Mean and SD or %) of Participants (N = 83)
| measure | mean (SD)/% |
|---|---|
| child age (yrs) | 10.13 (0.56) |
| mother’s age (yrs) | 39.80 (5.85) |
| father’s age (yrs) | 43.28 (9.34) |
| child’s BMI (kg/m2) | 20.04 (3.92) |
| child’s Body Fat (%) | 24.94 (5.63) |
| mother’s BMI (kg/m2) | 27.50 (5.60) |
| father’s BMI (kg/m2) | 29.49 (4.50) |
| child’s height (inches) | 55.53 (2.81) |
| gender (0 = male; 1 = female) | 36.14% female |
| race (0 = White; 1 = Other) | 84.81% White |
| family History (0 = no; 1 = yes) | |
| diabetes | 37.35% yes |
| heart disease | 43.37% yes |
| cholesterol | 51.81% yes |
| high blood pressure | 68.67% yes |
| stroke | 19.28% yes |
| asthma | 30.12% yes |
| blood Pb (mg/dL | 1.01 (0.44) |
| blood Hg (mg/dL) | 0.80 (1.39) |
Emotional Response to the DRL
Participants were administered the 16 item Brief Mood Introspection Scale (BMIS44). This questionnaire includes a list of adjectives and requests participants to rate themselves on each item using a 4 point scale (1 = definitely do not feel, 2 = do not feel, 3 = slightly feel, and 4 = definitely feel). The BMIS was developed based on the assumption of a pair of orthogonal mood dimensions (pleasant-unpleasant and arousal-calm) with a second set of dimensions (positivetired and negative-relaxed) at a 45° rotation from the first set.45 Items were appropriately reverse scored and averaged to create scores on these four mood dimensions.
Data Analysis
DRL Performance Data
Median IRTs were calculated from 5 min bins across the 20 min task. Median IRTs were positively skewed and therefore a natural log transformation was used to further normalize the data. In addition, the money a child earned at this task was used as an index of overall performance. Based on nonresponses during the task, four children were classified as refusing. One child never pressed the bar/pad and three children stopped pressing after the first 5 min.
Analytic Models
The distribution of blood PFC levels was not normally distributed and was therefore log-transformed before analysis. As an alternative approach to illustrate the data, the online Supporting Information file contains the results of nonparametric regressions on nontransformed data using the locally weighted scatterplot smoothing method46 provided by SAS PROC LOWESS. Furthermore, details regarding measurement of all potential confound and results of bivariate analyses are reported in the online SI. Using SAS PROC REG, DRL outcome measures were regressed on PFC blood levels, controlling for all potential confounds found to have a bivariate relationship to outcome at p < 0.20. A similar approach to covariate selection has been adopted in prior research37 and is considered a conservative approach when controlling for potential confounds.47 As an additional protection against results being a unique product of our particular covariate selection method, the SI includes methods and results for a reanalysis of IRT data using a change-in-estimate approach to covariate selection. As reported in the SI, results were very similar using this alternative approach to covariate selection. To avoid inflating our family wise error, analyses of the four emotional dimensions assessed during the DRL task were only conducted for total PFC concentrations.
RESULTS AND DISCUSSION
Sample Characteristics
Table 1 shows characteristics of the children and their mothers and fathers in this sample. By design, children in our sample were 9, 10, or 11 years old (M = 10.13). Associations between PFC levels and baseline characteristics are reported in SI Table S4. Table 2 reports the PFC levels for the children in the current sample. As shown in Table 3, levels of different PFCs in the blood for this population were intercorrelated. Because PFC levels are not normally distributed, Spearman’s rank correlation coefficients are reported in Table 3. As shown in Table 3, levels of total PFC primarily reflect levels of PFOS, PFOA, and PFHxS. Although not the focus of the present study, serum lipids were measured in this cohort. The partial correlations between PFCs and these levels (controlling for gender, a family history of high cholesterol, and family income) are reported in SI Table S5. Although PFDA was significantly correlated with total cholesterol and LDL, these significant associations might be a result of a conducting numerous correlations and a resulting Type I error.
Table 2.
PFC Content (ng/mL) in Blood Samples (Mean, Standard Deviation, Median, Minimum, Maximum, % of Samples with Levels Above LOQ) of Participants (N = 83)
| mean | SD | median | minimum | maximum | % above LOQ | |
|---|---|---|---|---|---|---|
| total PFC | 21.6 | 14.0 | 19.1 | 5.29 | 91.9 | 100.00 |
| PFOS | 9.90 | 6.09 | 8.79 | 1.13 | 32.6 | 100.00 |
| PFOA | 3.23 | 1.30 | 3.28 | 0.43 | 5.87 | 100.00 |
| PFNA | 0.82 | 0.55 | 0.72 | 0.10 (LOQ) | 4.14 | 98.80 |
| PFDA | 0.26 | 0.13 | 0.26 | 0.10 (LOQ) | 0.82 | 87.95 |
| PFHxS | 6.06 | 8.03 | 3.67 | 0.29 | 53.5 | 100.00 |
| PFOSA | 0.75 | 0.57 | 0.61 | 0.10 (LOQ) | 4.03 | 98.80 |
Table 3.
Spearman’s Rank Correlation Coefficients (rho) among Different PFCs in Blood Samples (N = 83)
| total PFC | PFOS | PFOA | PFNA | PFDA | PFHxS | PFOSA | |
|---|---|---|---|---|---|---|---|
| PFOS | 0.95d | 1.00 | |||||
| PFOA | 0.73d | 0.71d | 1.00 | ||||
| PFNA | 0.16 | 0.14 | 0.31c | 1.00 | |||
| PFDA | 0.38d | 0.32c | 0.48d | 0.66d | 1.00 | ||
| PFHxS | 0.77d | 0.63d | 0.37d | −0.07 | 0.16 | 1.00 | |
| PFOSA | 0.35c | 0.33c | 0.18 | 0.15 | 0.12 | 0.25b | 1.00 |
p < 0.10.
p < 0.05.
p < 0.01.
p < 0.001.
Median IRTs
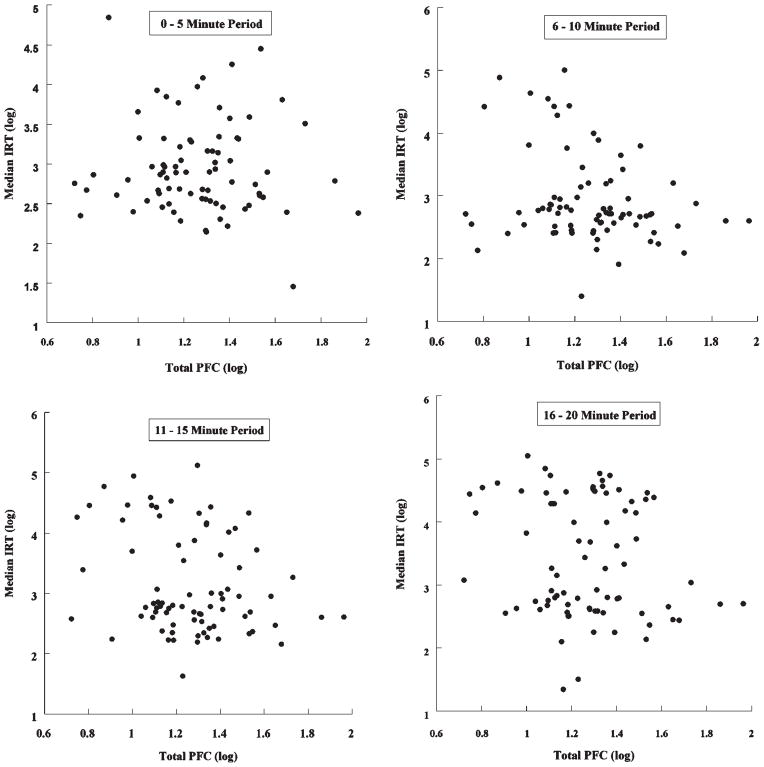
The median IRTs increased for each 5 min “bin” across the 20 min DRL task (2.95, 5.51, 8.90, and 13.40 s, respectively), suggesting learning acquisition. After covariate control for potential confounds, we considered the association of blood PFC levels with median IRT. As shown in Table 4, increasing total PFCs were associated with significantly shorter IRTs at 5–10 min and 11–15 min (β s = −0.30, p-values < 0.01) as well as significantly shorter IRTs at 16–20 min (β = −0.23, p < 0.05). Figure 1 shows the scatterplots for the association between total blood PFC and IRTs. As can be seen in these figures, higher PFC blood levels are associated with both shorter IRTs and smaller between-subject variability. Associations with individual PFCs are also shown in Table 4 and exhibit a similar pattern of associations; namely, the weakest associations were found during the beginning of the task. This pattern is not unusual for the DRL task (cf., ref 37) and reflects the fact that differences in learning (and thereby performance) can only emerge as exposure to the learning task progresses through time.
Table 4.
Relationship of PFC Blood Levels to Median IRTs within Each Time Period of the DRL Task (Standardized β and 95% Confidence Intervals Are Shown; N = 79)a
| PFC | DRL time period
|
|||
|---|---|---|---|---|
| 0–5 minb | 6–10 minc | 11 – 15 mind | 16–20 mine | |
| total | −0.11 [−0.32, 0.10] | −0.30** [−0.50, −0.09] | −0.30** [−0.50, −0.09] | −0.23* [−0.45, −0.01] |
| PFOS | −0.05 [−0.26, 0.16] | −0.18 [−0.40, 0.04] | −0.25* [−0.46, −0.04] | −0.20# [−0.42, 0.02] |
| PFOA | −0.03 [−0.24, 0.18] | −0.11 [−0.34, 0.11] | −0.20# [−0.41, 0.01] | −0.17 [−0.39, 0.05] |
| PFNA | −0.07 [−0.29, 0.14] | −0.24* [−0.46, −0.02] | −0.15 [−0.38, 0.07] | −0.05 [−0.28, 0.18] |
| PFDA | −0.09 [−0.30, 0.13] | −0.24* [−0.45, −0.02] | −0.23* [−0.45, −0.02] | −0.20# [−0.43, 0.02] |
| PFHxS | −0.13 [−0.34, 0.09] | −0.31** [−0.53, −0.10] | −0.20# [−0.42, 0.02] | −0.10 [−0.32, 0.13] |
| PFOSA | −0.13 [−0.35, 0.08] | −0.25* [−0.46, −0.04] | −0.17 [−0.38, 0.05] | −0.19# [−0.42, 0.03] |
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001.
Covariates included a family history of diabetes, gender, race, father and child BMI, and mother’s age.
Covariates included blood Pb, gender, body fat, parent’s education, family history of high blood pressure, and asthma.
Covariates included gender, race, parent’s education, father’s age, family history of diabetes.
Covariates included race, father’s age, parent’s education, father’s BMI, and family history of diabetes, heart disease, stroke, and high cholester.
Figure 1.
Relationship of total PFC concentrations in blood to IRTs during each DRL time period.
In order to illustrate the magnitude of significant effects using the original units, Table 5 reports changes in median IRTs (in seconds) and corresponding number of responses associated with a 1 SD increase in the particular PFC. As shown in Table 5, a 1 SD increase in total PFC was associated with a decrease in median IRT of 1.96, 3.65, and 8.27 s (for the 6–10, 11–15, and 16–20 min DRL periods, respectively) and a corresponding increase in the number of responses to the task of 30.1, 23.4, and 50, during these same DRL periods. Expressed as a percent change for those significant effects shown in Table 5, median IRT decreased by 29–34% for every 1 SD increase in the corresponding blood PFC. The magnitude of effects for specific PFCs (PFOS, PFNA, PFDA, PFHxS, and PFOSA) significantly associated with reduced IRTs are also reported in Table 5.
Table 5.
Magnitude of Significant Associations Reported in Table 4, Expressed As the Change in Median IRTs (in Seconds) and Number of Responses During DRL Period Associated with Each 1 Standard Deviation (SD) Increase in Blood PFC
| PFC DRL period | 1 SD increase in blood PFC
|
|
|---|---|---|
| associated decrease from median IRT (in sec) [95% confidence interval] | associated increase from median number of responses [95% confidence interval] | |
| total PFC (SD = 14.00 ng/mL) | ||
| 6–10 min | 5.51a−1.96 [0.72–2.88] | 54.4b + 30.1 [8.2–59.7] |
| 11–15 min | 8.90 − 3.65 [1.31–5.18] | 33.7 + 23.4 [5.8–46.9] |
| 16–20 min | 13.40 − 8.27 [5.81–9.85] | 22.4 + 50 [17.1–62.1] |
| PFOS (SD = 6.09 ng/mL) | ||
| 11 – 15 min | 8.90 − 2.73 [0.58–4.33] | 33.7 + 14.9 [2.4–31.9] |
| PFNA (SD = 0.56 ng/mL) | ||
| 6–10 min | 5.51 − 1.88 [0.14–2.45] | 54.4 + 28.2 [1.5–43.6] |
| PFDA (SD = 0.13 ng/mL) | ||
| 6–10 min | 5.51 − 1.62 [0.14–2.69] | 54.4 + 22.7 [1.5–52.0] |
| 11–15 min | 8.90−3.01 [0.38–4.82] | 33.7 + 17.2 [1.5–39.8] |
| PFHxS (SD = 8.03 ng/mL) | ||
| 6–10 min | 5.51 − 1.79 [0.61–2.69] | 54.4 + 26.2 [6.8–52.0] |
| PFOSA (SD = 0.57 ng/mL) | ||
| 6–10 min | 5.51 − 1.71 [0.26–2.75] | 54.4 + 24.5 [2.7–54.3] |
These represent the median IRT within the particular DRL period.
These represent the median number of responses given the length of the DRL period (5 min) and the median IRT.
Task Reward
As might be expected, poor performance (as represented by short IRTs) resulted in significantly less money being earned by those with increasing PFDA (β = −0.28, p < 0.05) and marginally less money for those with increasing blood PFOS and PFOA (β = −0.19 and −0.21, respectively, p values <0.10). In these analyses, covariates included father’s age, family educational attainment, and a family history of diabetes.
Non-PFC Contaminants
Blood Pb and Hg were measured as potential confounding contaminant exposures.37 Hg was not associated with any outcome at p < 0.20 and therefore was not included as a covariate in any model above. However, given the significant positive association with blood PFCs and prior findings of an association between prenatal MeHg and DRL performance,37 we did repeat the analyses above with blood Hg added as a covariate and, as expected, the results were unchanged. Blood Pb was associated with IRTs during the 6–10 min period and with the number of responses during the 16–20 min time period. However, when entered as covariates in the full model, blood Pb was not significantly associated with these behavioral outcomes (p values >0.10). This study did not have a sample large enough to consider potential interactions between PFC and either Pb or Hg exposure.
Task Emotion
Participants’ mood was assessed using the BMIS following the DRL task. The analysis of the associations between total PFC blood levels and the four mood dimensions revealed no significant associations: pleasant–unpleasant (β = 0.19, p=0.10), negative–relaxed (β=−0.14, p >0.25), arousal–calm (β = 0.01, p > 0.25), and positive–tired (β = −0.12, p > 0.25).
PFCs are ubiquitous environmental contaminants. In fact, three PFCs that we measured (PFOS, PFOA, and PFHxS) were found at detectable levels in all children in our sample. The present study demonstrates that PFOS, PFNA, PFDA, PFHxS, PFOSA levels in blood were all associated with significantly poorer performance on a task requiring behavioral inhibition. The results demonstrate significantly shorter IRTs and more frequent responses, particularly in the middle of the task (between 5 and 15 min). In addition, increasing blood PFDA was associated with children earning significantly less money during this task. In short, increasing blood levels for a number of different PFCs appear to be associated with greater impulsivity in children and this translated into poor performance on the DRL task. Although the pattern of associations did not suggest an isolated effect of a particular PFC, this question is difficult to address empirically in this sample because of the high intercorrelations among the six individual PFCs we measured at detectable levels. In addition, although the concentration of some PFCs (PFOS and PFOA) appear to have declined in the general U.S. population from 1999 to 2000 to 2003–2004,48 we still observed significant associations at levels apparent in 2008–2009. It remains to be determined whether the apparent decline in PFOS and PFOA will continue or plateau and whether associations with neurobehavioral outcomes will decline or continue. It is also unknown whether levels of PFCs other than PFOS and PFOA are declining over time.
The association between PFCs and impulsivity (a defining feature of ADHD) revealed in the present study might explain the recently observed associations between PFC levels and ADHD.15 Two studies have now documented a significant increase in ADHD risk associated with greater PFHxS 15,16 and our study demonstrated a consistent association between PFHxS and impulsivity. However, there remain a number of inconsistencies (e.g., a significant association with ADHD/impulsivity was found for PFOS in our study and the NHANES sample, but not in theC8Health Project) that are not readily explained. Finally, if exposure to particular PFCs represents an increasing risk for impulsivity and a corresponding diagnosis of ADHD, then do PFC levels in the environment “track” ADHD prevalence rates? The increasing prevalence of ADHD49 coupled with a decline in PFC levels48 appears inconsistent with the idea that these two variables might be causally connected. However, not all PFCs have been declining (e.g., PFNA48). Futhermore, it is not known whether our cross-sectional measurement of blood PFC levels in 9–11 year old children serves as an indirect measure of prenatal or perinatal PFC exposure. Therefore, if neurotoxic effects of PFCs occur specifically with prenatal or perinatal PFC exposure, then a corresponding 9–11 year time-lag would be expected between the environmental peak in PFC levels in the 1990s50 and any corresponding peak in ADHD prevalence in children. Finally, a potential link between temporal trends in environmental PFCs and ADHD prevalence would depend on an understanding of the nature of the PFC-ADHD relationship (e.g., linear vs nonlinear). For example, the results of smoothed nonparametric regression (see SI Figure 1S) suggest a potential nonlinear association with no IRTPFC association (flat slope) at higher PFC levels. Therefore, perhaps it is necessary for PFC levels to decline and reach an effect threshold before ADHD prevalence rates are affected.
With respect to the generalizability of our findings, DRL tasks vary in the particular response that is reinforced, the length of the task, and the reinforcement schedule. For example, one DRL task for children used points earned on a computer task and provided reinforcement of withholding responses for 6 s (a DRL-6 schedule51). In another study, the DRL apparatus included a operant panel made to look like a clown’s face with marbles and money used to reward pushing the clown’s nose every 20 s.37 The task used in the present study represents a relatively brief (20 min) assessment of DRL-20 learning. Although most children did not consistently respond every 20 s (i.e., learn the schedule), the increase in IRTs over time demonstrates learning. More importantly, the significant associations emerged relatively quickly (after 5 min; cf., ref 37). Therefore, it is unlikely that the current associations would differ as a function of the particular response, reward employed, or task length. It is possible that different DRL schedules are more (or less) sensitive to toxicant exposures however we have no data with which to address this possibility.
There is a remarkable similarity between the PFC-DRL associations in the present study and prior findings of associations between DRL performance and prenatal exposure to other toxicants, namely polychlorinated biphenyls (PCBs) and Hg.37 There are two likely explanations for this similarity across studies. Perhaps the current associations with PFCs emerged due to underlying (and unmeasured) associations with prenatal exposure to PCBs and Hg or, alternatively, those prior associations of PCBs and Hg with DRL performance may have emerged due to underlying (and unmeasured) associations with postnatal PFC exposure (unfortunately, we were unable to measure PCBs due to a lack of sufficient blood for this analysis). As partial support for this idea, blood PFC and Hg levels were significantly correlated in the present sample. PFC and Hg are likely correlated due to a common food source for these toxicants, namely fish.52 In contrast to prior findings for prenatal Hg exposure,37 blood Hg for the present cohort was not found to be significantly associated with DRL performance. Alternatively, some environmental toxicants may exert effects on executive control and response inhibition via multiple and separate pathways, all being sensitive to the final outcome as measured by the DRL task. Additional study is clearly needed to gain an understanding of the mechanism by which blood PFC levels might affect impulsivity, if such a causal pathway exists.
There are notable limitations with the present study. Foremost, we employed a cross-sectional study design making it difficult to establish causality. For example, it is possible that children’s impulsive behavior produced behaviors that increased exposure to PFCs. Similarly, although we controlled for many potential confounds, other variables (e.g., maternal and paternal impulsivity) should be considered in future studies of PFC exposure effects. The rationale would be that greater maternal and paternal impulsivity might be associated with greater impulsivity in their child as well as associated with greater PFC exposure through consumer choices and differential exposure to PFCs. In addition, other toxicants (e.g., PCBs) might be confounds however we did not have a sufficient volume of blood to measure additional toxicant exposures. As a second limitation, our sample was a subset drawn from a larger study of volunteers. In addition, the sample used in the present analyses did not include all children that volunteered, resulting in disproportionately more males. However, gender was included as a covariate when necessary and other potential covariates we measured were not found to differ between our full sample and the subset drawn for the present study. Finally, our DRL task was administered to 9–11 year old children during their second hour of testing. As such, the 20 min we allowed for this task was not sufficiently long to enable all children to achieve learning acquisition. Nevertheless, IRTs did increase during the task suggesting that, on average, children were beginning to learn the reinforcement schedule albeit at differing rates across differing blood PFC levels.
CONCLUSIONS
The present study investigates blood levels of various PFCs in children and associations with behavioral inhibition. The results demonstrated significant associations between blood levels for multiple PFCs and behavioral inhibition deficits in children as measured using the DRL task. As a result, increasing blood PFC levels were associated with earning significantly less money during this task. These results are intriguing and suggest a need for further investigation and replication with a larger sample of children.
Supplementary Material
Acknowledgments
Grant ES015619 from the National Institutes of Health as well as funding from SUNY Oswego and Syracuse University supported this work. We are grateful for the assistance of Drs. Patrick J. Parsons and Christopher D. Palmer (Wadsworth Center and University at Albany) for the measurement of nonessential metals (Pb and Hg) in our samples. In addition, we are grateful to Keri Favreau, Arlen Halstead, Julia Stead, Christie Turenchak, and Jordan Greeno for their assistance in data collection and for the assistance of Ed Hogan (Laboratory Manager), Ed Hale (Chemistry Supervisor), and Barb Samson (phlebotomist) with the Oswego Hospital Laboratory.
Footnotes
Some measurement details and analyses. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kannan K, Koistinen J, Beckman K, Evans T, Gorzelany JF, Hansen KJ, Jones PD, Helle E, Nyman M, Giesy JP. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol. 2001;35:1593–1598. doi: 10.1021/es001873w. [DOI] [PubMed] [Google Scholar]
- 2.Kannan K, Perrotta E, Thomas NJ. Association between perfluorinated compounds and pathological conditions in Southern Sea Otters. Environ Sci Technol. 2006;40:4943–4948. doi: 10.1021/es060932o. [DOI] [PubMed] [Google Scholar]
- 3.Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health. 2007;81:231–246. doi: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- 4.Olsen GW, Church TR, Hansen KJ, Burris JM, Butenhoff JL, Mandel JH, Zobel LR. Quantitative evaluation of perfluorooctanesulfonate (PFOS) and other fluorochemicals in the serum of children. J Child Health. 2004;2:53–76. [Google Scholar]
- 5.Calafat AM, Kuklenyik Z, Cuadill SP, Reidy JA, Needham LL. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ Sci Technol. 2006;40:2128–2134. doi: 10.1021/es0517973. [DOI] [PubMed] [Google Scholar]
- 6.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fei C, McLaughlin JK, Tarone RE, Olsen J. Fetal growth indicators and perfluorinated chemicals: A study in the Danish National Birth Cohort. Am J Epidemiol. 2008;168:66–72. doi: 10.1093/aje/kwn095. [DOI] [PubMed] [Google Scholar]
- 8.Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect. 2009;117:660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115:1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112:1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I. Maternal exposure to perfluorinated acids and fetal growth. J Exposure Sci Environ Epidemiol. 2009;20:589–597. doi: 10.1038/jes.2009.57. [DOI] [PubMed] [Google Scholar]
- 12.Savitz DA. Guest editorial: Biomarkers of perfluorinated chemicals and birth weight. Environ Health Perspect. 2007;115:A528–A529. doi: 10.1289/ehp.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7. Environ Health Perspect. 2010;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ Health Perspect. 2008;116:1391–1395. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit hyperactivity disorder in US children aged 12–15 years. J Environ Health Perspect. 2010;118:1762–1767. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savitz DA, Steenland K, Fletcher KE. Status report: Association of perfluorinated compounds with attention deficit/attention deficit hyperactivity disorder and learning disorder among children aged 5–18 years with elevated community exposure to PFOA. [accesed May 26, 2011];Unpublished manuscript. www.c8sciencepanel.org/pdfs/Status_Report_C8_and_ADD_31Jan2011.pdf.
- 17.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.03.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butenhoff JL, Kennedy GL. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196:95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ, Butenhoff JL. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology. 2005;215:126–148. doi: 10.1016/j.tox.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003;74:382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- 21.Butenhoff JL, Ehresman DJ, Chang SC, Parker GA, Stump DG. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+ PFOS) in rats: Developmental neurotoxicity. Reprod Toxicology. 2009;27:319–330. doi: 10.1016/j.reprotox.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Butenhoff JL, Chang SC, Ehresman DJ, York RG. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod Toxicol. 2009;27:331–341. doi: 10.1016/j.reprotox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes S, Vicens P, Colomina MT, Domingo JL. Behavioral effects in adult mice exposed to perfluorooctane sulfonate (PFOS) Toxicology. 2007;242:123–129. doi: 10.1016/j.tox.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Fuentes S, Colomina MT, Rodriguez J, Vicens P, Domingo JL. Interactions in developmental toxicology: concurrent exposure to perfluorooctane sulfonate (PFOS) and stress in pregnant mice. Toxicol Lett. 2006;164:81–89. doi: 10.1016/j.toxlet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Ribes D, Fuentes S, Torrente M. Combined effects of perfluorooctane sulfonate (PFOS) and maternal restraint stress on hypothalamus adrenal axis (HPA) function in the offspring of mice. Toxicol Appl Pharmacol. 2010;243:13–18. doi: 10.1016/j.taap.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Fuentes S, Colomina MT, Vicens P, Franco-Pons N, Domingo JL. Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: effects on postnatal development and behavior of the offspring. Toxicol Sci. 2007;98:589–598. doi: 10.1093/toxsci/kfm121. [DOI] [PubMed] [Google Scholar]
- 28.Shum D, Neulinger K, O’Callaghan M, Mohay H. Atttentional problems in children born very preterm or with extremely low birth weight at 7–9 years. Arch Clin Neuropsychol. 2008;23:103–112. doi: 10.1016/j.acn.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Lau C, Thibodeaux J, Ehresman D, Tanaka S, Froehlich J, Butenhoff J. Evaluation of perfluorooctanesulfonate in rat brain. The Toxicologist. 2006;90:118. [Google Scholar]
- 30.Bogdanska J, Borg D, Sundstrom MUB, Halldin K, Abedi-Valugerdi M, Bergman A, Nelson B, DePierre J, Nobel S. Tissue distribution of 35S-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology. 2011;284:54–62. doi: 10.1016/j.tox.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Johansson N, Eriksson P, Viberg H. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci. 2009;108:412–418. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- 32.Austin ME, Kasturi BS, Barber M, Kannan K, Mohan- Kumar PS, MohanKumar SMJ. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111:1485–1489. doi: 10.1289/ehp.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajnal A, Mark GP, Rada PV, Lénáard L. Norepinephrine microinjections in the hypothalamic paraventricular nucleus increase extracellular dopamine and decrease acetylcholine in the nucleus accumbens: Relevance to feeding reinforcement. J Neurochem. 1997;68:667–674. doi: 10.1046/j.1471-4159.1997.68020667.x. [DOI] [PubMed] [Google Scholar]
- 34.Basar K, Sesia T, Groenewegen H, Steinbusch WM, Visser-Vanderwalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Moody-Ayers S, Lindquist K, Sen S, Covinsky KE. Childhood social and economic well-being and health in older age. Am J Epidemiol. 2007;166:1059–1067. doi: 10.1093/aje/kwm185. [DOI] [PubMed] [Google Scholar]
- 36.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol, Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Stewart P, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level PCB, MeHg and Pb exposure in children. Environ Health Perspect. 2006;114:1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gump BB, MacKenzie JA, Bendinskas K, Morgan R, Dumas AK, Palmer CD, Parsons PJ. Low-level Pb and cardiovascular responses to acute stress in children: The role of cardiac autonomic regulation. Neurotoxicol Teratol. 2011;33:212–219. doi: 10.1016/j.ntt.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinshaw LB, Jackson SA, Chen MY. Direct mailing was a successful recruitment strategy for a lung-cancer screening trial. J Clin Epidemiol. 2007;60:853–857. doi: 10.1016/j.jclinepi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Kannan K, Corsolini S, Falandysz J, Fillman G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Wouwe NV, Yang JH, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 41.Hansen KJ, Clemen LA, Ellefson ME, Johnson HO. Compound-specific, quantitative characteristics of organic: Fluorochemicals in biological matrices. Environ Sci Technol. 2001;35:766–770. doi: 10.1021/es001489z. [DOI] [PubMed] [Google Scholar]
- 42.Schantz SL, Gasior DM, Polverejan E, McCaffrey RJ, Sweeney AM, Humphrey HEB, Gardiner JC. Impairments of memory and learning in older adults exposed to polychorinated biphenyls via consumption of Great Lakes fish. Environ Health Perspect. 2001;109:605–611. doi: 10.1289/ehp.01109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoffel EC, Cunningham KA. The relationship between the locomotor response to a novel environment and behavioral disinhibition in rats. Drug Alcohol Depend. 2008;92:69–78. doi: 10.1016/j.drugalcdep.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Mayer JD, Gaschke YN. The experience and meta-experience of mood. J Pers Soc Psychol. 1988;55:102–111. doi: 10.1037//0022-3514.55.1.102. [DOI] [PubMed] [Google Scholar]
- 45.Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- 46.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 47.Stewart PW. The critical concept of control in human neurobehavioral toxicology studies: We can, and must, do better. In: Bellinger D, editor. Clinical and Developmental Neurotoxicology. New York, NY: Marcel Dekker, Inc; 2006. pp. 361–378. [Google Scholar]
- 48.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CDC. Increasing prevalence of parent-reported Attention- Deficit/Hyperactivity Disorder among children—United States 2003—2007. Morbidity and Mortality Weekly Report. 2010;59:1439–1443. [PubMed] [Google Scholar]
- 50.Sundström M, Ehresman DJ, Bignert A, Butenhoff JL, Olsen GW, Chang SC, Bergman Å. A temporal trend study (1972–2008) of perfluorooctanesulfonate perfluorohexanesulfonate and perfluorooctanoate in pooled human milk samples from Stockholm Sweden. Environ Int. 2011;37:178–183. doi: 10.1016/j.envint.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Gordon M, Mettelman BB. The assessment of attention: I. Standardization and reliability of a behavior-based measure. J Clin Psychol. 1988;44:682–690. doi: 10.1002/1097-4679(198809)44:5<682::aid-jclp2270440504>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 52.Huang LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen GB, Alexander J, Meltzer KM, Knutsen HK. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ Int. 2010;36:772–778. doi: 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.