Abstract
Prolonged exposure of mice to diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) results in hepatobiliary injury, atypical ductular proliferation, oval cell appearance and limited fibrosis. Previously, we reported that short-term ingestion of DDC diet by hepatocyte-specific β-catenin conditional knockout (KO) mice, led to fewer A6-positive oval cells than wild-type (WT) littermates. To examine the role of β-catenin in chronic hepatic injury and repair, we exposed WT and KO mice to DDC for 80 and 150 days. Paradoxically, long-term DDC exposure led to significantly more A6-positive cells indicating greater atypical ductular proliferation in KO, which coincided with increased fibrosis and cholestasis. Surprisingly, at 80 and 150 days in KO, we observed a significant amelioration of hepatocyte injury. This coincided with extensive repopulation of β-catenin null livers with β-catenin-positive hepatocytes at 150 days, which was preceded by appearance of β-catenin-positive hepatocyte clusters at 80 days and a few β-catenin-positive hepatocytes at earlier times. Intriguingly, occasional β-catenin-positive hepatocytes that were negative for progenitor markers were also observed at baseline in the KO livers suggesting spontaneous escape from cre-mediated recombination. These cells with hepatocyte morphology expressed mature hepatocyte markers but lacked markers of hepatic progenitors. The gradual repopulation of KO livers with β-catenin-positive hepatocytes occurred only following DDC injury and coincided with a progressive loss of hepatic cre-recombinase expression. A few β-catenin-positive cholangiocytes were observed albeit only after long-term DDC-exposure and trailed the appearance of β-catenin-positive hepatocytes. In conclusion, in a chronic liver injury model, β-catenin-positive hepatocytes exhibit growth and survival advantages and repopulate KO livers eventually limiting hepatic injury and dysfunction despite increased fibrosis and intrahepatic cholestasis.
Keywords: Wnt, liver, progenitors, injury, regeneration, development
Introductory Statement
Expansion of hepatic progenitors in the liver has been observed in chronic liver injury and is believed to me a mode of repair. One model currently used in mouse that induces chronic liver injury and oval cell activation is the exposure to a diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC). This induces atypical ductular proliferation along with periportal inflammation and plugging of the bile ducts with porphyrin crystallization (1). Ultimately, injury to the biliary epithelium and clogging of the ducts induces biliary stasis and a subsequent rise in serum bilirubin. It is believed that hepatic oval cells arise as a response to the hepatobiliary injury in this model. A more recent study presented long-term feeding of DDC as a model of xenobiotic-induced cholangiopathy representative of sclerosing cholangitis and biliary cirrhosis (2).
Various molecular pathways have been implicated in the oval cell response (3). Wnt pathway, whose role in various stem and progenitor cells is well accepted, has also been shown to be involved in liver development, growth, and regeneration (4, 5). Role of β-catenin in hepatic progenitors has been recently identified. Significant co-localization of the oval cell and ductular marker A6 and β-catenin is observed after DDC feeding in mice (6). In β-catenin null liver, atypical ductular proliferation is significantly blunted indicating a role of β-catenin in induction and proliferation of ductular cells after DDC-injury. Likewise, another study reported expression of β-catenin and multiple Wnt ligands in oval cells after DDC-injury (7).
Here, we investigate the role of β-catenin in chronic hepato-biliary injury in mice in response to long-term DDC-exposure, which resembles PSC phenotype and leads to chronic atypical ductular proliferation and oval cell response (2, 8). Wild-type (WT) and β-catenin conditional liver knock-out (KO) mice were exposed to the DDC diet for 80-150 days and examined histologically and biochemically for the ductular response and liver injury. Interestingly, we found that in the absence of β-catenin, a sustained atypical ductular proliferation occurs in the long-term, along with the development of significant hepatic fibrosis, which results in significant intrahepatic cholestasis. Further evaluation identified a small subset of hepatocytes in baseline KO livers that escape albumin-cre-mediated β-catenin deletion that have a growth advantage after the DDC-injury and eventually repopulate the KO livers by β-catenin-positive hepatocytes, which results in modest but significant alleviation of hepatocyte injury. While both WT and KO livers after chronic DDC-exposure displayed enlargement of the livers, KO livers also showed nodular overgrowth due to increased hepatocyte proliferation, although there was no evidence of tumorigenesis.
Experimental Procedures
Animals
β-catenin conditional knockout in liver was created as previously described (9). The genotype for these animals is Ctnnb1loxp/loxp; Alb-Cre+/− and are referred to as knockout (KO) mice throughout. As reported previously, all other genotypes were unequivocally devoid of any phenotype and referred henceforth as wild-type (WT) controls. Only male mice were used for all experiments. At the time of sacrifice, retro-orbital bleed was performed for serum biochemistry. Portions of lobes from excised liver were fixed in 10% neutral buffered formalin and processed for paraffin embedding. Part of liver was frozen in Tissue-Tek OTC compound for cryosections. The remaining liver was snap frozen in liquid nitrogen and stored at −80°C. All animal studies were performed in strict accordance with the Institutional Animal Use and Care Committee at the University of Pittsburgh and NIH guidelines.
DDC diet feeding
Mice were fed a special diet containing 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC, Bioserve, Frenchtown, NJ) for periods of time ranging from 3 to 150 days to induce atypical ductular proliferation that has been described previously (1).
Serum biochemistry
The University of Pittsburgh, Department of Pathology Lab Support Services, performed serum biochemical measurements. Total bilirubin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured on serum from KO and WT livers fed with DDC for different times.
Protein extraction and western blot analysis
Whole cell lysates were extracted in radioimmunoprecipitation assay (RIPA) buffer with protease and phosphatase inhibitors (Sigma). Concentration of proteins was determined by bicinchoninic acid protein assay. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed with 20-100μg of protein resolved on Bio-Rad gels (7.5% or 4-15% gradient gels) under reducing conditions using Mini-Protean electrophoresis module assembly (Bio-Rad, Hercules, CA). This was followed by an hour transfer at constant voltage (100V) in transfer buffer (25 mmol/L Tris [pH 8.3], 192 mmol/L glycine, 20% methanol, and 0.025% sodium dodecyl sulfate) to polyvinylidene difluoride membranes (PVDF, Millipore, Bedford, MA) using the Mini Trans-Blot electrophoretic transfer cell (Bio-Rad).
For western blot analysis, membranes were blocked in 5% milk or BSA for 30 minutes at room temperature (RT) or overnight at 4°C. Membranes were incubated with primary antibody in 5% milk or BSA for 1 hour at RT followed by 2 washes in 1% milk or BSA. Primary antibodies used are listed in online supplementary Table 1. Next, membranes were incubated with appropriate HRP-conjugated secondary antibody (Chemicon, Temecula, CA) at concentrations of 1:10,000-50,000 in 1% milk or BSA, washed and visualized with Western Lightning™ chemiluminescence kit (PerkinElmer Life Sciences, Boston, MA). Autoradiographs were scanned and analyzed for densitometry using the Image J software.
Histology, immunohistochemistry, and special stains
Tissues fixed in 10% formalin were embedded in paraffin and 4μm sections were used for Hematoxylin & Eosin (H&E) staining and immunohistochemistry (IHC). For IHC, sections were rehydrated by passing through xylene, graded alcohol, and distilled water. After antigen retrieval, endogenous peroxide inactivation and blocking, sections were incubated with primary antibody (online supplementary Table 1) for 1 hour at RT, washed and incubated with appropriate biotin-conjugated secondary antibody for 30 minutes. Sections were washed, incubated with ABC reagent, washed and incubated with DAB. Sections were next counterstained with Shandon hematoxylin solution (Sigma) and cover slipped using Cytoseal XYL (Richard Allen Scientific, Kalamazoo, MI). For negative control, the primary antibody was omitted in the protocol. Slides were viewed under an Axioskop 40 (Zeiss) upright research microscope and digital images obtained. Collages were prepared using Adobe Photoshop CS3 software (Adobe, San Jose, CA). For quantification of PCNA positive cells, 5 high power field images were taken for each animal and counted utilizing Zeiss Axiovision software.
For measurement of fibrosis, Masson’s trichrome staining was performed. Photomicrographs were taken at 50x magnification and percentage of the area of fibrosis measured using Adobe Photoshop as previously described (10).
Immunofluorescence
Please refer to online supplement for details on methods for this section.
Real-Time PCR
Please refer to online supplement for details on methods for this section.
Statistical analysis
All experiments were performed three or more times or with three or more animals and representative data are presented. Quantification of positive cells (A6, PCNA), serum biochemistry measurements, and fibrosis measurements were compared for statistical analysis by Student T test (Excel) and P value of less than 0.05 was considered significant.
Results
Greater atypical ductular response in β-catenin KO after long-term DDC feeding
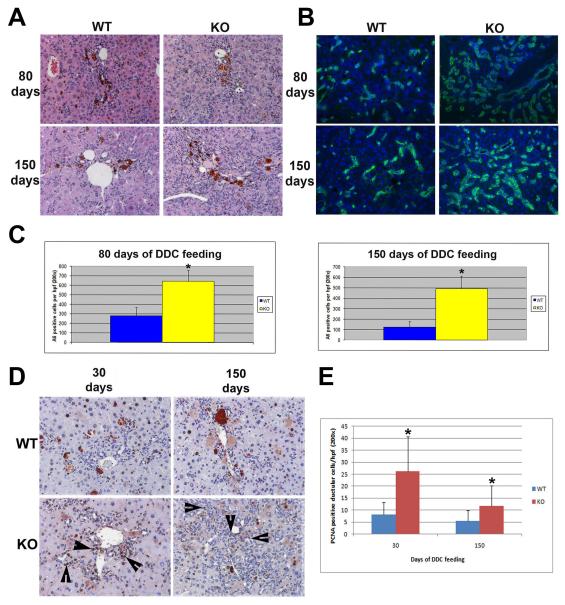
We previously reported a blunted atypical ductular proliferation in β-catenin KO after DDC feeding for 2 and 3.5 weeks (6). To further examine the impact of β-catenin loss on chronic hepatic injury, we placed KO and littermate wild-type (WT) mice on long-term DDC diet for time periods ranging from 30 to 150 days. H&E staining, along with immunofluorescence was on liver samples from these time-points. Interestingly, H&E staining showed a clear increase in atypical ductular reaction in the KO liver when compared to WT at 80 and 150 days of DDC feeding (Fig. 1a). To verify and quantify, atypical ductular response, immunofluorescence for A6, a marker that recognizes atypical ductules and oval cells, was performed. Indeed, significantly more A6 positive cells are found in the KO liver at 80 and 150 days of DDC feeding (Fig. 1b, c). We also observe an increase in PCNA positive ductular cells in the KO livers at 30, 80 and 150 days of DDC feeding when compared to the WT (shown 30 and 150 days, Fig. 1d, e). CD45-positive inflammatory cells were comparably present in KO and WT livers at all stages (data not shown). These findings suggest that a greater atypical ductular reaction due to enhanced proliferation occurs after an initial delay in the KO mice after chronic DDC-injury.
Figure 1. Increased atypical ductular proliferation in KO after long-term DDC.
(A) Representative photomicrographs of H&E staining of WT and KO livers after 80 and 150 days of DDC diet. (B) Representative photomicrographs of immunofluorescence for A6 (green) in WT and KO livers after 80 and 150 days of DDC diet. Nuclei were counterstained with DAPI. (C) Quantification of A6 positive cells in WT and KO livers at 80 and 150 days of DDC diet shows an increase in KO versus WT at 80 and 150 days of DDC diet. (D) Representative photomicrographs for PCNA IHC in WT and KO liver after 30 and 150 days of DDC diet feeding showing several PCNA positive ductular cells (arrowheads) especially in KO. (E) Quantification of PCNA positive ductular cells shows an increase in KO vs. WT at 30 and 150 days of DDC diet. All images are shown at 200x magnification. *p<0.05
Increased serum bilirubin, fibrosis, and hepatic nodule formation in KO after long-term DDC
To monitor hepatic injury after long-term DDC feeding, serum analysis was performed for AST, ALT, bilirubin and ALP. Levels of AST were modestly higher at 150 days of DDC feeding in WT compared to KO, whereas ALT levels were higher in WT at 80 and 150 days (Table 1). This finding was surprising given that we expected KO to be more susceptible to hepatocyte injury. Conversely, serum bilirubin levels were significantly higher in the KO at 80 days with a trend towards being higher in KO at 150 days. Additionally, levels of ALP were higher in KO at 150 days of DDC feeding. These findings indicate that biliary injury and intrahepatic cholestasis after long-term DDC feeding was greater in the KO than the WT; however, the hepatocyte injury was only modest in the KO.
TABLE 1.
Liver function tests in wild-type (WT) and β-catenin knockout (KO) mice at 80 and 150 days after DDC-diet ingestion.
| Serum Biochemistry at 80 days | Serum Biochemistry at 150 days | |||
|---|---|---|---|---|
| Total Bilirubin | WT | KO | WT | KO |
| 1 | 0.4 | 11.8 | 0.3 | 0.7 |
| 2 | 0.3 | 11.1 | 0.3 | 1.6 |
| 3 | 0.8 | 1.9 | 0.4 | 1.2 |
| 4 | 5.6 | 5.4 | 0.4 | 11.8 |
| 5 | 0.4 | 0.4 | 1.1 | |
| 6 | 0.1 | 0.2 | 1.1 | |
| 7 | 0.1 | 1.0 | 0.8 | |
| 8 | 0.2 | |||
| Average | 1.07 | 6.133 | 0.45 | 2.933 |
|
Alkaline
Phosphatase |
WT | KO | WT | KO |
| 1 | 254 | 647 | 173 | 524 |
| 2 | 315 | 518 | 106 | 367 |
| 3 | 435 | 319 | 380 | 456 |
| 4 | 493 | 209 | 197 | 549 |
| 5 | 395 | 272 | 168 | 416 |
| 6 | 298 | |||
| 7 | 264 | |||
| 8 | 199 | |||
| Average | 331.625 | 393 | 204.8 | 462.4* |
| AST | WT | KO | WT | KO |
| 1 | 268 | 486 | 556 | 426 |
| 2 | 331 | 349 | 343 | 334 |
| 3 | 604 | 173 | 952 | 462 |
| 4 | 398 | 334 | 732 | 751 |
| 5 | 231 | 261 | 727 | 495 |
| 6 | 298 | 996 | 263 | |
| 7 | 443 | 1186 | ||
| 8 | 287 | |||
| Average | 355 | 320.6 | 717.6667 | 455.167* |
| ALT | WT | KO | WT | KO |
| 1 | 634 | 408 | 827 | 509 |
| 2 | 713 | 251 | 434 | 367 |
| 3 | 914 | 243 | 1215 | 574 |
| 4 | 797 | 366 | 908 | 480 |
| 5 | 526 | 428 | 882 | 497 |
| 6 | 509 | 1476 | 411 | |
| 7 | 1033 | 1566 | ||
| 8 | 617 | |||
| Average | 717.875 | 339.2* | 1044 | 473* |
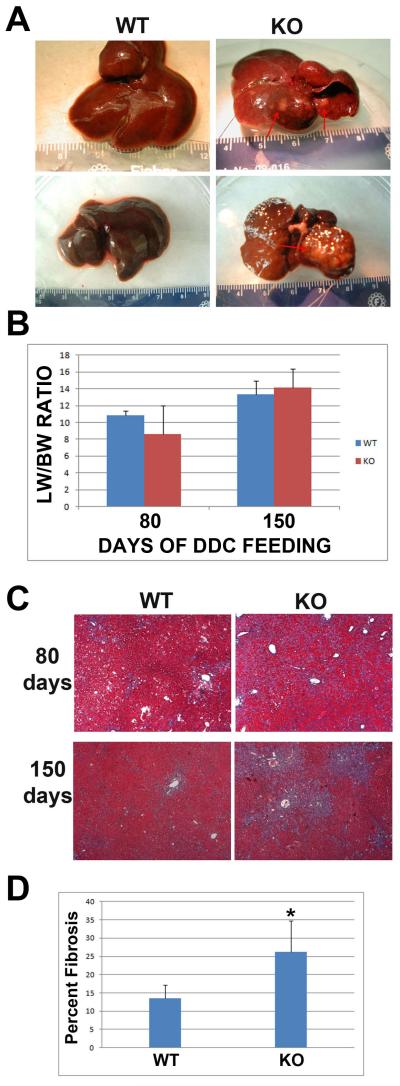
Upon gross examination, we observed significant enlargement and darkening of the liver after 150 days of DDC feeding in both KO and WT livers (Fig. 2a). We, however, noted the formation of hepatic nodules in 7 out of 7 KOs after 150 days of DDC diet feeding, however no nodules were observed in the WT (Fig. 2a). We previously reported that our β-catenin KO mice have smaller livers than WT (9). However, after 80 days of DDC feeding LW/BW ratio has equalized with a modest increase in the KO liver at 150 days of DDC feeding (Fig. 2b).
Figure 2. Hepatic nodule formation associated with an increase in hepatic fibrosis in KO after long-term DDC feeding.
(A) Examination of gross liver shows an increase in size in both WT and KO liver after 150 days of DDC feeding. Hepatic nodules are present only in KO liver as indicated by red arrows. (B) Liver weight/body weight ratio for WT and KO liver after 80 and 150 days of DDC diet. (C) Representative photomicrographs for trichrome staining that stains collagen blue, in livers from WT and KO after 80 and 150 days of feeding with DDC diet, shows increased fibrosis in KO. Images are taken at 50x magnification. (D) Quantification of the percentage of the area of sections covered by fibrosis at 150 days of feeding with DDC diet shows an increase in KO liver compared to WT. *p<0.05
It has been previously reported that DDC feeding induces activation of stellate cells, which results in fibrosis in the mouse liver, as a function of atypical ductular proliferation (2). We performed trichrome staining to analyze the amount of fibrosis in our study. KO livers showed greater fibrosis after 80 and 150 days of DDC feeding than the WT controls at the same stages of DDC exposure (Fig. 2c). Overall, percentage of area of fibrosis was twice as much in the KOs when compared to WT at 150 days and the difference was statistically significant (Fig. 2d). At 150 days, spread of fibrosis between portal triads was evident in the KO liver suggestive of significant progression of disease in these animals.
Progressive repopulation of KO liver hepatocyte population with β-catenin positive cells after long-term DDC feeding
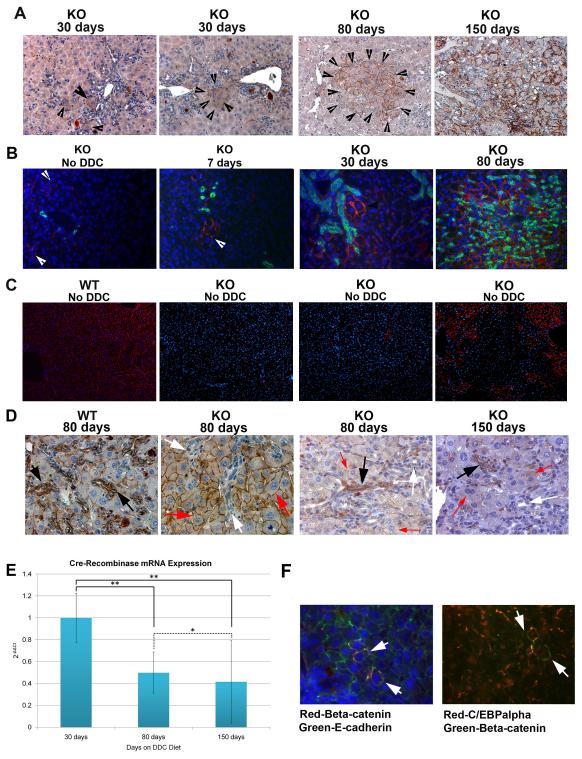
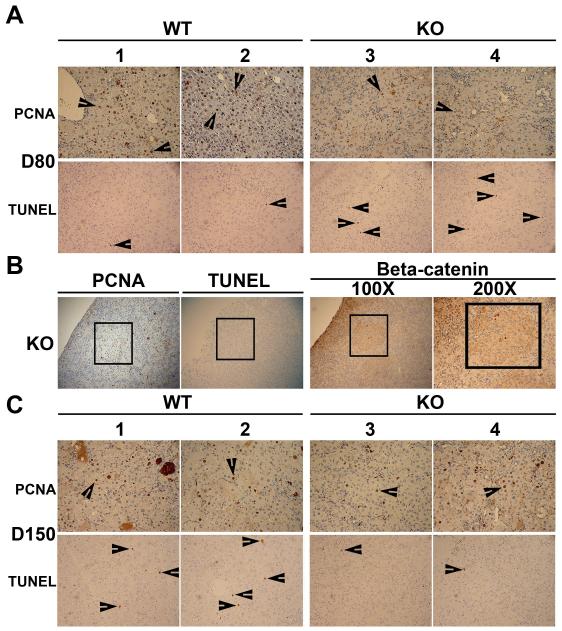
Given the paradoxical decrease in hepatocyte injury spontaneously in the KO mice after long-term DDC, we sought the mechanism of such improvement. We initiated the analysis by examining β-catenin expression in the KO livers at 150 days where unequivocal differences in AST and ALT were evident when compared to WT. Immunohistochemical analysis for β-catenin performed at 150 days, identified extensive β-catenin-positive hepatocytes throughout the liver in the KO livers (Fig. 3a). This was also evident in the western analysis that revealed a dramatic recovery in β-catenin expression levels in the KO liver at 150 days of DDC feeding (Fig. 6). To determine the chronology of appearance of β-catenin-positive hepatocytes in the KO livers, we next examined earlier time-points after the DDC-exposure. At 80 days of DDC feeding, small clusters of β-catenin expressing hepatocytes were observed, surrounded by β-catenin-negative parenchyma by IHC and immunofluorescence (Fig. 3a,b). At 30 days after being on DDC diet, even fewer β-catenin-positive hepatocytes were observed especially in the periportal region by these two imaging modalities (Fig. 3a,b). Analysis of KO livers after 7-days of DDC exposure also revealed a few β-catenin-positive hepatocytes in the periportal areas (Fig. 3b). This led us to analyze baseline livers at 3 months in chow-fed KO mice. Surprisingly around 1-2 hepatocytes per 200X magnification were β-catenin-positive in the KO livers as detected by immunofluorescence (Fig. 3b). As an additional control, livers from 5-8 months old mice were assessed for degree of spontaneous repopulation with β-catenin-positive hepatocytes without any DDC-exposure by IF (n=6) and IHC (n=5). By IF, four KO livers showed less than 1% β-catenin-positive hepatocytes, while two KO livers had around 20-30% spontaneous repopulation (Fig. 3c). Five additional KO livers showed les than 1% hepatocytes to be β-catenin-positive in the KO by IHC as well (data not shown). Thus majority of the KO livers did not show many β-catenin-positive hepatocytes at baseline suggesting only a DDC-injury dependent spontaneous repopulation with β-catenin-positive hepatocytes, which may have initially escaped albumin-cre driven β-catenin deletion. However, a very small subset of KO livers did show presence of greater numbers of β-catenin-positive cells due to unknown reasons, albeit it was nowhere to the extent observed after DDC-exposure.
Figure 3. Progressive repopulation of KO liver with β-catenin positive hepatocytes over the course of long-term feeding of DDC diet.
(A) Representative photomicrographs for IHC for β-catenin in KO liver at 30, 80, and 150 days of DDC diet shows progressive repopulation of KO liver with β-catenin positive hepatocytes (arrowheads). Images are shown at 200x magnification. (B) Immunofluorescence showing lack of colocalization of A6 (green) and β-catenin (red) (white arrowhead) in the KO liver at baseline or at 7, 30 and 80 days of feeding the DDC diet. Nuclear counterstain is for DAPI (blue). Images are shown at 200x magnification. (C) Immunofluorescence showing β-catenin-positive hepatocytes in WT liver, and three KO livers at baseline. While two livers represent a common phenotype of a few β-catenin-positive hepatocytes in KO, one liver was an exception and showed greater repopulation (right most panel). Images are shown at 100X magnification (D) Representative IHC images at 400x magnification for β-catenin after 80 days of DDC diet feeding in WT and KO showing strong expression of β-catenin in atypical ductular cells in WT (black arrows) and lack thereof in KO liver (white arrows) even within newly developed clusters of β-catenin positive hepatocytes (red arrow). However, some β-catenin positive duct cells (black arrow) do appear while others remain negative (white arrow) amidst β-catenin-positive hepatocytes (red arrow) at 80 and 150 days after DDC feeding. (E) Cre-Recombinase mRNA expression in KO mice at 30, 80, and 150 days after DDC diet shows a progressive and significant decrease (*p<0.1; **p<0.001). mRNA expression values were normalized to cyclophilin-A as a reference gene. Bars show standard deviation values of triplicate readings. (F) Representative immunofluorescence photomicrographs demonstrate colocalization (arrows) of β-catenin (red) and E-cadherin (green) in KO liver at baseline (left panel). Only occasional (arrow) β-catenin-positive hepatocyte (green) is observed in KO liver, which always shows nuclear CEBPα (red). Images shown are at 400x magnification.
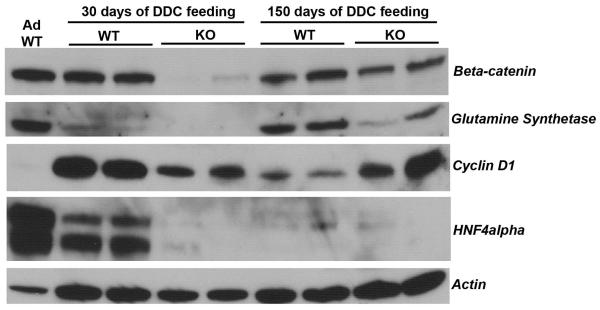
Figure 6. Western blot analysis for β-catenin and its targets along with markers of hepatic differentiation.
Representative immunoblots demonstrate protein expression of β-catenin, glutamine synthetase, cyclin D1, HNF4α, and E-cadherin in livers from normal adults; WT and KO after 30-days of DDC ingestion; and WT and KO after 150 days of DDC feeding. Actin is shown as a loading control.
Origin of β-catenin-positive hepatocytes in the KO liver is not biliary epithelial cells or oval cells
Previously, we have reported that β-Catenin is strongly positive in bile duct epithelia in normal liver, whereas it is lacking in the KO despite the fact that conditional deletion of the floxed gene was brought about by the albumin-cre, perhaps due to embryonal expression of albumin in a common progenitor of hepatocytes and cholangiocytes (9). Next we address if β-catenin-positive biliary epithelial cells, which have also been shown to be the precursors of oval cells, may be the source of β-catenin-positive hepatocytes in the KO liver (11). A comprehensive analysis showed that none of the bile ducts in the KO livers were β-catenin-positive at baseline and 7 or 30 days of DDC-exposure, as indicated by a lack of colocalization of β-catenin and A6, which is known to be a marker of biliary ductular epithelia and oval cells in the liver (Fig. 3b) (1, 12). Most bile ducts continued to be β-catenin-negative in the KO livers after 80 days of DDC-exposure, whereas in WT livers, all bile ducts (atypical and atypical) were strongly β-catenin-positive (Fig. 3d). At this stage only an occasional bile duct was lined by β-catenin-positive epithelia, whereas at 150 days, a few additional ducts were β-catenin-positive (Fig. 3d). Thus, appearance of β-catenin-positive hepatocytes precedes the appearance of β-catenin-positive bile ducts in the KO liver at baseline as well as after chronic DDC-injury and hence cannot be the source of repopulation.
Progressive loss of cre-recombinase in the KO liver after long-term DDC feeding
To examine whether the increase in the number of β-catenin positive hepatocytes correlates with a decrease in expression of the transgene, we performed real-time PCR analysis for Cre-recombinase at 30, 80, and 150 days after DDC feeding. Real-time PCR analysis was performed for Cre-recombinase in three separate mouse livers using three different reference genes. The analysis showed that there was no significant difference in mRNA expression of Cre-recombinase between untreated age-and sex-matched KO mouse livers and the 30 days DDC-fed KO livers (data not shown). However, between 30 and 80 days after being on the DDC diet, there was a significant decrease in Cre-recombinase expression (p = 0.00043) (Fig. 3e). This expression continued to significantly decrease with ongoing DDC-exposure (30 to 150 days on DDC, p = 0.00005; 80 to 150 days on DDC, p = 0.091). Thus, this analysis suggests repopulation of the KO liver after long-term DDC feeding is by hepatocytes, which have escaped expression of the Cre-recombinase transgene.
β-Catenin-positive hepatocytes in the KO liver are indistinguishable from normal hepatocytes and do not express markers for hepatic progenitors
While the morphology of the β-catenin-positive hepatocytes was indistinguishable from β-catenin-negative within the KO liver other than occasional size heterogeneity such that β-catenin-positive hepatocytes were sometimes larger than β-catenin-negative cells, we next wanted to further address their differentiation status. As mentioned previously, these cells were A6-negative and hence did not show any biliary or oval cell phenotype (Fig. 3b). Immunofluorescence for E-cadherin was done next and revealed that β-catenin-positive cells were E-cadherin-positive even at a single cell stage and thus epithelial (Fig. 3f). We next evaluated these cells in the baseline KO liver for expression of CCAAT enhancer binding protein-α (CEBPα), a hepatocyte-enriched transcription factor. β-Catenin-positive hepatocytes were also positive for nuclear CEBPα (Fig. 3f).
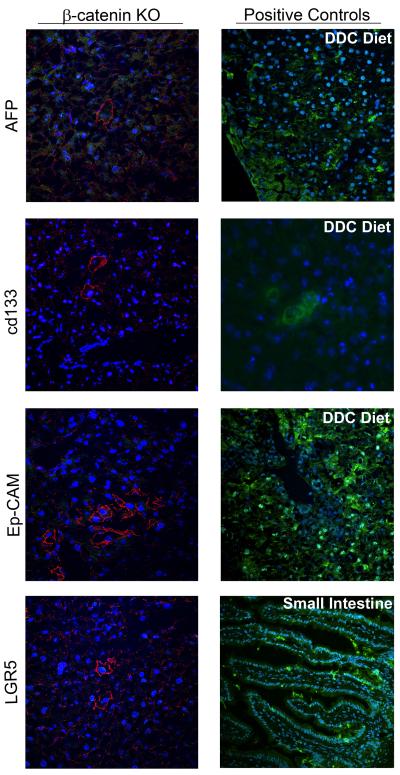
We also examine the status of β-catenin positive for some known markers of hepatic progenitors. In KO livers at baseline, none of the β-catenin-positive cells were positive for commonly used markers of oval cells such as EpCAM, CD133 or LGR5 (Fig. 5). Interestingly, in the KO livers, occasional β-catenin-positive hepatocytes were α-fetoprotein-positive as were a few non-β-catenin-positive hepatocytes (Fig.5).
Figure 5. Proliferative and survival advantages of β-catenin-positive hepatocytes in the β-catenin KO livers.
(A) Several PCNA-positive and only occasional TUNEL-positive hepatocytes (arrowheads) are observed in representative liver sections from 2 WT mice on DDC-diet for 30 days. Only few PCNA-positive and many TUNEL-positive hepatocytes (arrowheads) are seen in KO livers after 30 days of DDC-ingestion. All images are at 200X magnification. (B) A small foci (boxed) of β-catenin-positive hepatocytes in KO liver after 30-days of DDC-exposure shows many PCNA-positive and TUNEL-negative hepatocytes in adjacent sections (100X or as shown). (C) Several PCNA-positive and also a modest number of TUNEL-positive hepatocytes (arrowheads) are observed in livers of WT mice on DDC diet for 150 days as shown in two representative animals. At the same stage in KO mice, as shown in two representative hepatic sections, comparable numbers of PCNA-positive hepatocytes but far fewer TUNEL-positive hepatocytes (arrowheads) as WT were observed. All photomicrographs are at 200X magnification.
Thus, this analysis suggests that β-catenin-positive cells in the KO livers are mostly mature hepatocytes, which amidst chronic insult due to chronic DDC-exposure, may display growth and survival advantage to gradually repopulate the KO liver.
Repopulating β-catenin-positive hepatocytes in the KO livers show increased proliferation and decreased apoptosis after chronic DDC exposure
Next, we examined the functional implications of gradually increasing β-catenin-positive hepatocytes in the KO livers after DDC exposure, by comparing hepatocyte proliferation and survival at 80 days, when repopulation is observed in small clusters, versus 150 days, when β-catenin-positive hepatocytes comprise most of the hepatic parenchyma. The analysis was aimed at identifying any proliferative or survival advantages of β-catenin-positive hepatocytes within the KO livers following DDC-induced injury. By PCNA and TUNEL IHC, we observed high numbers of hepatocytes in S-phase of cell cycle and very few hepatocytes displaying apoptotic nuclei, respectively in the WT on DDC diet for 80 days (Fig. 5a). In the KO liver, only few hepatocytes were positive for PCNA, although more were TUNEL-positive when compared to the WT (Fig. 5a). Interestingly, when foci of β-catenin-positive hepatocytes within the KO livers at 80 days of DDC-exposure were examined for PCNA and TUNEL, these showed many PCNA-positive and virtually no TUNEL-positive hepatocyte (Fig. 5b). At 150 days of DDC exposure comparable numbers of PCNA-positive hepatocytes were evident in WT and KO livers (Fig. 5c). While TUNEL-positive hepatocytes were present in modest numbers in the WT livers at this stage, only occasional TUNEL-positive hepatocyte was observed in the KO livers (Fig. 5c). This analysis demonstrates greater resilience of β-catenin-positive hepatocytes in the KO livers enabling them to proliferate and survive in the DDC-induced adverse environment when compared to β-catenin-deficient cells.
β-Catenin signaling in WT livers and in KO livers following repopulation, after chronic DDC exposure
Lastly, we wanted to address any changes in the β-catenin signaling itself after long-term DDC injury in a WT animal. While we did not observe any dramatic changes in total β-catenin protein in WT livers at 30 and 150 days after DDC-exposure, we observed a decrease in the protein expression of its target glutamine synthetase (GS) at 30 days followed by its regain at 150 days (Fig. 6). Interestingly, its proliferation target cyclin-D1 was dramatically increased at 30 days while its levels decreased considerably at 150 days. At the same time, we examined some differentiation markers of hepatocytes and identify a noteworthy decrease in C/EBPα (not shown) and HNF4α in the WT livers at 30 and 150 days of DDC-exposure (Fig. 6). These observations suggest that in response to DDC, the liver cells lose terminal differentiation and undergo proliferation and temporal changes in β-catenin signaling may be an adaptive response to chronic DDC-injury.
We also examined β-catenin signaling in the KO livers after repopulation in response to DDC-injury. In accordance with IHC, β-catenin levels are very low in the KO liver at 30 days after DDC feeding, but are higher at 150 days (Fig 6). Interestingly, levels of GS increased modestly, whereas that of cyclin D1 increased dramatically, suggestive of high proliferative state in the KO liver at 150 days after DDC. Concomitant with increased proliferation in KO livers at 150 days of DDC-exposure, we observed increased β-catenin and cyclin-D1 expression and low expression of markers of hepatocyte differentiation such as GS and HNF4α (Fig. 6); and C/EBPα and Cyp2E1 (not shown).
Discussion
We previously reported that β-catenin KO mice show a blunted atypical ductular proliferation after DDC feeding for 2 weeks when compared to WT due to lack of β-catenin in atypical ductules and oval cells (6). While the KO continued to lag behind the WT in ductular proliferation and oval cell response at 3.5 weeks, there numbers increased over the 2-week time-point. In the current study, we report a continued upward trend in the atypical ductular proliferation in the KO liver such that it surpasses the A6-positive cells than the WT at 80 and 150 days of DDC feeding. These atypical ducts continue to lack β-catenin, and thus appear to have bypassed the need for β-catenin for their proliferation unlike the short-term observations (6). This is analogous to the regeneration after partial-hepatectomy in the KO reported by others and us (9, 13). Peak hepatocyte proliferation in the KO was delayed by 24 hours and appears to have been compensated by alternate molecular mechanisms bypassing the requirement for β-catenin for proliferation. However, despite an increase in atypical ductular proliferation, the KO livers continue to show significantly greater intrahepatic cholestasis and biliary dysfunction as evident by increased alkaline phosphatase and bilirubin. This appears to be due to an increase in hepatic fibrosis that is evident in KO livers at this stage. Indeed it has been independently shown that the proliferating cholangiocytes and atypical ductules are a source of profibrotic cytokines including TNFα, PDGF, TGFβ and Osteopontin and cause activation of hepatic stellate cells and fibrosis (2).
Another noteworthy observation in this study consisted of a spontaneous repopulation of the KO liver with β-catenin positive hepatocytes after chronic DDC-injury. A careful tracking of the β-catenin-positive cells reveals the presence of occasional hepatocytes at baseline in a KO liver that escape albumin-cre-dependent β-catenin deletion, highlighting an imperfect recombination. Indeed suboptimal albumin-cre-driven recombination has also been reported recently for dicer-floxed and β-catenin-floxed mice (14, 15). At baseline, none of the β-catenin-positive cells were positive for any oval cell or biliary markers such as A6, but were positive for hepatocyte-enriched transcription factor such as CEBPα and for epithelial markers such as E-cadherin. In fact all cholangiocytes, which are normally strongly positive for β-catenin, were negative for this marker at the outset in the KO (9). The significance of some hepatocytes escaping cre-deletion is unclear and appears to not contribute to hepatic functions at baseline since these livers continue to lack several β-catenin targets as has been reported by multiple laboratories (9, 16-18). Similarly, these ‘escaped’ hepatocytes don’t undergo expansion during regeneration after partial hepatectomy (9, 13). Also, a counterintuitive increase in hepatic tumorigenesis observed in the KO livers in response to DEN alone or DEN and Phenobarbital, was not due to expansion of β-catenin-positive hepatocytes as predominant subset of tumors were negative for β-catenin and its targets such as GS (19, 20). Interestingly, a recent study reports higher incidence of spontaneous HCC in KO, and these tumors were composed of β-catenin-positive tumor cells (21). However, this has not been observed by two other independent studies (19, 20). β-Catenin-positive hepatocytes do became relevant during sustained hepatic injury such as continuous DDC-diet administration over 150 days. By this time KO livers showed close to 100% repopulation by β-catenin-positive hepatocytes, concomitant with improved resolution of hepatocyte injury observed as an improvement but not normalization in serum AST and ALT in the KO over the WT. This was due to the fact that β-catenin-positive hepatocytes were indeed more apt at expansion and survival in the adverse milieu of chronic DDC exposure exhibited as enhanced atypical ductular reaction and fibrosis. It was interesting to note that hepatic regeneration reflected by hepatocyte proliferation was ongoing in both WT and KO livers at 80 and 150 days when the hepatocytes lacked terminal differentiation as reflected by decreased expression of HNF4α, C/EBPα and others. The lack of maturation may be due to ongoing proliferation of the hepatocytes or additional unknown factors due to chronic DDC injury and will need further evaluation.
Intriguingly, no atypical ductular proliferation, oval cells, or cholangiocytes were positive for β-catenin in the KO livers at baseline or at the time of initiation of their expansion. The first time when β-catenin positive cholangiocytes were observed in KO livers was at 80 and 150 days after being on DDC diet, suggesting that some of these cells may have been derived from β-catenin positive hepatocytes. Transdifferentiation of hepatocytes to biliary epithelial cells has been demonstrated before and might be an attempt at repairing biliary damage brought about by DDC (22). Does lack of β-catenin in cholangiocytes as well as atypical ductules in KO liver after chronic DDC administration, impede optimal bile duct organization thus also contributing to intrahepatic cholestasis? A role of β-catenin in biliary specification of the hepatoblasts is known (23-26). Furthermore, β-catenin is important in oval cell proliferation in rats and mice, and its role has recently been shown in differentiation of oval cells to hepatocytes (6, 7, 27). β-Catenin may also have an important role in bile duct homeostasis. Indeed, in a recent collaborative study, we have shown an important role of β-catenin in regulating bile duct morphology (28).
Overall, the above findings demonstrate a lack of an optimal reparative response in the absence of β-catenin to DDC-induced chronic liver injury, which is observed as increased atypical ductular proliferation resulting in greater hepatic fibrosis and development of intrahepatic cholestasis. This occurs despite repopulation of the livers with β-catenin-positive hepatocytes, which however does improve hepatocyte function in the KO when compared to the WT. These finding support an important role of Wnt/β-catenin signaling in bile duct homeostasis and reiterate its pro-survival and pro-proliferative role in hepatocyte biology.
Supplementary Material
Figure 4. β-Catenin-positive hepatocytes in KO livers do not express hepatic progenitor markers.
There was no co-localization in the KO livers of β-catenin (red) with CD133, EpCAM or LGR5 (green), while the positive controls showed positive signal in DDC liver or small intestine (right column). In addition, some β-catenin-positive cells (red) were positive for α-fetoprotein (green), while some non-β-catenin-positive cells were also β-catenin-positive. Images shown are at 400x magnification.
Acknowledgments
Grant Support This study was funded by NIH grants 1R01DK62277 and 1R01CA124414 to SPSM and by Rango’s Fund for the Enhancement of Pathology Research and NIH grant 1F30DK083235 to MDT.
Abbreviations
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- WT
wild-type littermate control mice
- KO
β-catenin conditional liver knock-out mice
- ALP
alkaline phoshatase
- ALT
alanine amino transferase
- AST
Aspartate amino transferase
- DAPI
4,6-diamidino-2-phenylindole (DAPI)
- PCNA
proliferating cell nuclear antigen
- CEBPα
CCAAT enhancer binding protein-α
- HNF
hepatocyte nuclear factor
- GS
glutamine synthetase
- RIPA buffer
radioimmunoprecipitation assay buffer
- RT
room temperature
- IHC
immunohistochemistry
Footnotes
Disclosure: No conflict of interest for any of the authors relevant to this manuscript.
REFERENCES
- 1.Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103–109. [PubMed] [Google Scholar]
- 2.Fickert P, Stoger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowes KN, Croager EJ, Olynyk JK, Abraham LJ, Yeoh GC. Oval cell-mediated liver regeneration: Role of cytokines and growth factors. J Gastroenterol Hepatol. 2003;18:4–12. doi: 10.1046/j.1440-1746.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- 4.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nejak-Bowen K, Monga SP. Wnt/beta-catenin signaling in hepatic organogenesis. Organogenesis. 2008;4:92–99. doi: 10.4161/org.4.2.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 7.Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, Sylvester KG. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Dahab GM, Kheriza MM, El-Beltagi HM, Fouda AM, El-Din OA. Digital quantification of fibrosis in liver biopsy sections: description of a new method by Photoshop software. J Gastroenterol Hepatol. 2004;19:78–85. doi: 10.1111/j.1440-1746.2004.03183.x. [DOI] [PubMed] [Google Scholar]
- 11.Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151:905–909. [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177:1812–1822. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 14.Braeuning A, Singh Y, Rignall B, Buchmann A, Hammad S, Othman A, von Recklinghausen I, et al. Phenotype and growth behavior of residual beta-catenin-positive hepatocytes in livers of beta-catenin-deficient mice. Histochem Cell Biol. 2010 doi: 10.1007/s00418-010-0747-1. [DOI] [PubMed] [Google Scholar]
- 15.Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y, Hebrok M. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304–2315. e2301–2304. doi: 10.1053/j.gastro.2009.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braeuning A, Sanna R, Huelsken J, Schwarz M. Inducibility of drug-metabolizing enzymes by xenobiotics in mice with liver-specific knockout of Ctnnb1. Drug Metab Dispos. 2009;37:1138–1145. doi: 10.1124/dmd.108.026179. [DOI] [PubMed] [Google Scholar]
- 17.Burke ZD, Reed KR, Phesse TJ, Sansom OJ, Clarke AR, Tosh D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology. 2009;136:2316–2324. e2311–2313. doi: 10.1053/j.gastro.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 18.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 19.Rignall B, Braeuning A, Buchmann A, Schwarz M. Tumor Formation in Liver of Conditional {beta}-Catenin-Deficient Mice Exposed to a Diethylnitrosamine / Phenobarbital Tumor Promotion Regimen. Carcinogenesis. 2010 doi: 10.1093/carcin/bgq226. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XF, Tan X, Zeng G, Misse A, Singh S, Kim Y, Klaunig JE, et al. Conditional beta-catenin loss in mice promotes chemical hepatocarcinogenesis: role of oxidative stress and platelet-derived growth factor receptor alpha/phosphoinositide 3-kinase signaling. Hepatology. 2010;52:954–965. doi: 10.1002/hep.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekine S, Ogawa R, Kanai Y. Hepatomas with activating Ctnnb1 mutations in ‘Ctnnb1-deficient’ livers: a tricky aspect of a conditional knockout mouse model. Carcinogenesis. 2011;32:622–628. doi: 10.1093/carcin/bgr002. [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, Perret C, et al. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47:247–258. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 24.Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp Cell Res. 2004;292:157–169. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 26.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams JM, Oh SH, Jorgensen M, Steiger N, Darwiche H, Shupe T, Petersen BE. The role of the Wnt family of secreted proteins in rat oval “stem” cell-based liver regeneration: Wnt1 drives differentiation. Am J Pathol. 2010;176:2732–2742. doi: 10.2353/ajpath.2010.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh TH, Krauland L, Singh V, Zou B, Devaraj P, Stolz DB, Franks J, et al. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology. 2010;52:1410–1419. doi: 10.1002/hep.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.