Abstract
Background
To investigate links between inhibitory control (IC) and behavior problems in early childhood, as well as genetic and environmental covariance between these two constructs.
Methods
Parent and laboratory ratings of IC and parent ratings of externalizing and ADHD problem behaviors were administered at 24 months of age on a sample of 291 same-sex twin pairs (131 MZ, 160 DZ).
Results
There were significant phenotypic associations between both IC assessments and the two areas of behavioral maladjustment (correlations ranged from −.13 to −.57). Multivariate analyses revealed that phenotypic covariance between IC and behavior problems could be substantially explained by common genetic influences (genetic correlations ranged from −.30 to −.74). Parent ratings of IC showed higher phenotypic and genetic correlations with behavior problems than lab ratings of IC.
Conclusions
This study is the first to examine the etiology of the covariance between IC and related behavioral difficulties in toddlerhood. Findings suggest that low levels of IC can be considered a genetic risk factor for the development of early emerging behavior problems.
Keywords: Inhibitory control, behavior problems, early childhood, twins, genetics
Inhibitory control (IC) is a dimension of child temperament involving the self-regulation of behavioral responses under some form of instruction or expectation (Goldsmith, 1996). Children who develop typical levels of IC are able to successfully inhibit behavior when necessary. IC emerges in the second year, and develops further in the toddler and preschool years (Kochanska, Murray, Jacques, Koenig, & Vandegeest, 1996; Rothbart, 1989a). IC is a component of the effortful control (EC) factor, a self-regulatory aspect of temperament that includes the ability to inhibit and activate appropriate responses (Derryberry & Rothbart, 1997; Rothbart, 1989a, 1989b; Rothbart & Ahadi, 1994; Rothbart & Bates, 2006). The significance of IC is largely attributed to links to child behavior problems and psychopathology, and a role as a potential endophenotype for related behavioral disorders (Goos, Crosbie, Payne, & Schachar, 2009; Nigg, 2010).
Low IC is associated with higher levels of attention and externalizing behavior problems in childhood and adolescence (Nigg, Quamma, Greenberg, & Kusche 1999; Olson, Schilling, & Bates, 1999; Polderman et al., 2009). The broad EC factor (often assessed in terms of IC) is also related to sub-clinical behavior problems in toddlers, preschoolers, and elementary school age children (Eisenberg et al., 2003). Specifically, children with low levels of EC have more total behavior problems than children with moderate levels of EC (Murray & Kochanska, 2002), and lower EC predicts externalizing behaviors (Eisenberg et al., 2001, 2005; Lemery-Chalfant, Doelger & Goldsmith, 2008; Valiente et al., 2003).
The psychiatric diagnosis that is most reliably related to IC in childhood is Attention Deficit Hyperactivity Disorder (ADHD). Cognitive impairments associated with ADHD in the neuroscience and psychopathology literature include several abilities and behaviors that are conceptually related to the temperament dimension of IC. Errors of commission on the go/no go task (Uebel et al., 2010) and poor choice impulsivity and delay aversion (Marco et al., 2009; Paloyelis et al., 2009) are two prominent examples. A dual pathway theory of ADHD (Sonuga-Barke, 2002a; 2002b) proposes two independent pathways for ADHD: (1) delay aversion, the theory that children with ADHD symptoms exhibit impulsivity in circumstances where this behavior leads to a shorter overall delay in receiving a reward; and (2) deficits in response inhibition that reflect poor performance on tasks that require the child to inhibit responses to a prepotent stimulus (Johnson, Wiersema, & Kuntsi, 2009). The theory proposes that each pathway has differing underlying neurophysiology and etiology.
Children with ADHD are a substantially heterogenous group and IC deficits are not universal (Castellanos, Sonuga-Burke, Milham, & Tannock, 2006; Polderman et al., 2009; van Mourik, Oosterlan, & Sergeant, 2005), however, it is clear that IC has relevance to the development of behavior problems and ADHD. Previous investigations have typically used school-aged samples because standard reaction time and stop-signal tasks are too complex for young children. However, basic inhibition and choice impulsivity tasks can be used with toddlers as young as 24 months of age. One recent study showed that toddlers in an ADHD risk group had lower levels of parent-assessed IC than matched controls (Auerbach, Berger, Atzaba-Poria, Arbelle, Cypin, Friedman, & Landau, 2008). Unfortunately, a lack of multi-method research exploring links between IC and behavior problems in early childhood in normative samples represents a gap in our knowledge. Toddlerhood may be a critical stage for the development of behavioral maladjustment (Campbell, 1985; Keenan & Wakschlag, 2000), and temperament is viewed as a possible risk factor (Campbell, 1995; Lavigne et al., 1996). Toddlers with behavior problems are at-risk for a multitude of non-optimal developmental outcomes and given this developmental significance, it is important to understand factors that influence problem behaviors in early childhood (Saudino, Carter, Purper-Ouakil, & Gorwood, 2008). It has also been posited that early temperament contributes to the liability for child psychopathology, and that behavior problems represent the extreme of normally distributed temperament (Goldsmith, Lemery, & Essex, 2004). Early IC has been proposed as contributing to the emergence of behavioral problems (Goldsmith, Pollak & Davidson, 2008), although few studies have examined early associations.
In the present study, we investigate associations between parent- and laboratory-assessed IC and parent-assessed behavior problems in a sample of 24 month-old twins. Because we are interested in early IC, we focus our laboratory assessment on basic inhibition and choice impulsivity tasks. Although most temperament investigations rely on parent report as the sole method of assessment, parent ratings are susceptible to rater biases (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, in press). In addition, parent and lab-based ratings of temperament frequently lack substantial agreement and may represent different aspects of behavior. It has also been reported that there is often overlap between items on questionnaire measures of temperament and behavior problems (Lemery, Essex & Smider, 2002), and the use of observational measures avoids this problem. Therefore, we endorse a multi-method assessment approach that incorporates both parent and observational measures. In addition, we use multivariate genetic analyses to explore the extent to which links between IC and behavioral problems are genetically and environmentally mediated. Previous research finds that both IC and EC are genetically influenced in toddlerhood (Gagne & Saudino, 2010; Goldsmith, Buss & Lemery, 1997), and at least one study indicated that genetic factors contribute to individual differences in behavior problems in toddlers (Saudino et al., 2008). Thus, it is possible that genetic factors mediate associations between the two domains. Indeed, genetic factors have been found to explain the association between the related construct EC and behavior problem symptoms in middle childhood (Lemery-Chalfant et al., 2008). However, given that there is evidence of developmental change in genetic influences in both temperament and behavioral problems across age (e.g., Eley & Stevenson, 1999; Goldsmith, Buss & Lemery, 1997; Nigg & Goldsmith, 1994; Rhee & Waldman, 2002) one can not simply assume that the same pattern will emerge for a younger sample. Any phenotypic stability of IC or behavior problems across age is not equivalent to stability in etiology (Saudino et al., 2008).
We predict that IC will be associated with behavior problems in our toddler sample and share an overlapping genetic etiology. If this hypothesis is supported, toddler IC can be considered a candidate endophenotype for externalizing behavior problems and ADHD. Because endophenotypes can be used as trait markers for disease susceptibility (Goos et al., 2009), the early assessment of IC would be clinically significant and could aid in the identification of children at risk for the development of behavioral maladjustment. Previous investigations indicating stability in early emerging behavior problems (e.g., Briggs-Cowan et al., 2006) suggest that concurrent phenotypic and genetic associations between IC and behavior problems in toddlers could be predictive of later maladjustment, and that early detection and intervention may be beneficial (Saudino et al., 2008). Endophenotypes can also clarify genetic contributions to complex disorders (Goos et al., 2009), and previous candidate gene studies of IC with older children have contributed to molecular investigations of ADHD. The current study can potentially extend behavioral results to future molecular genetic studies in younger children.
Method
Participants
The sample was drawn from the Boston University Twin Project (BUTP), a twin study of child temperament. See Gagne & Saudino (2010) for details on study procedures and recruitment. 291 same-sex twin pairs (131 MZ, 160 DZ) were assessed at 24 months of age (mean age=2.07 years, SD=.05). There were 155 male twin pairs and 136 female pairs in this study, and the racial composition of the sample was 88.2% White, 3.1% Black, 2.1% Asian, and 6.6% mixed, a distribution that is representative of the state of Massachusetts. The average socioeconomic status of the families was predominantly middle class according to the Hollingshead index (mean=51.5, SD=10.4), although there was considerable range (22–66) in the sample.
Observer Ratings of Inhibitory Control
Observed IC was assessed in the laboratory using the Laboratory Temperament Assessment Battery–Preschool Version (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1993) IC episodes, which include “Dinky Toys,” “Snack Delay,” and “Gift.” These tasks are adapted from Kochanska’s lab-based work on IC (Kochanska et al., 1996; Kochanska, Murray, & Harlan, 2000) and require the child to inhibit the impulse to grab multiple rewards (Dinky Toys) or to wait in order to receive a reward (Snack Delay and Gift). The episodes elicit choice impulsivity and inability to wait and are similar to inhibition and choice impulsivity/delay aversion tasks used with older children (see Paloyelis et al., 2009). Coders were trained by master coders and required to meet a 90% inter-rater reliability criterion before they were permitted to code independently. Ten percent of the sample was rated by a second observer and the interrater correlation for the Lab-TAB IC composite was .89.
During Dinky Toys, the child was asked to select one of six attractive trinkets, inhibiting the urge to pick multiple toys or hoard all of them. There were two trials in this episode. In the Snack Delay episode, the child was offered a snack (a cracker or a candy), but was required to wait for the experimenter to ring a bell before it was permissible to eat the snack. There was one practice trial with no waiting time, and six test trials with different pause lengths (5s, 10s, 0s, 20s, 0s, 30s) before the experimenter rang the bell. The Gift episode consisted of the presentation of a small wrapped gift to the child who was required to wait for two minutes before being permitted to open it. Coding guidelines and composite formation for the Lab-TAB IC episodes in the BUTP are explained in Gagne and Saudino (2010). The variables used in the summary scores for each episode were selected on the basis of principal component analyses following guidelines from Goldsmith et al. (1993). All summary scores were significantly correlated, and a composite of observed IC was computed from the mean of these summary scores.
Parent Ratings of Inhibitory Control
Parents rated IC using the Toddler Behavior Assessment Questionnaire-Revised (TBAQ-R; Goldsmith, 1996). 94% of the TBAQ-R ratings were completed by the mothers (the remainder was completed by the fathers). The TBAQ-R requires parents to rate child behaviors in specific situations observed within the past month. Reliability as indexed by Cronbach’s alpha was .83 for the IC subscale in the present sample.
Parent Ratings of Problem Behaviors
Behavior problems were assessed using the Child Behavior Checklist for Ages 1 1/2–5 (CBCL; Achenbach & Rescorla, 2000). The CBCL requires the parent to indicate whether certain problem behaviors have occurred within the last two months. The ADHD-DSM behavior problem and broadband externalizing problem subscales were selected for our analyses due to previous findings indicating associations between these areas of behavioral maladjustment and IC. CBCL alphas were .78 for ADHD-DSM and .90 for externalizing behavior problems, respectively.
Statistical Approach
To account for the nested nature of twin data, dyad-level phenotypic correlations were calculated following procedures outlined by Griffin & Gonzalez (Griffin & Gonzalez, 1995; O’Connor, 2004). A square root transformation was used to correct positive skewness for the CBCL externalizing subscale. Twin intraclass correlations, indexing twin similarity, were computed using a double entry procedure. MZ twin correlations that exceed DZ twin correlations suggest that genetic factors contribute to individual differences for that trait. The foundation of multivariate behavioral genetic methods is the cross-trait, cross-twin correlation whereby twin A’s score on one trait (e.g., observed IC) is correlated with twin B’s score on another trait (e.g., externalizing behavior problems), and vice versa. When cross-trait cross-twin correlations for MZ twins exceed those of DZ twins, it suggests genetic contributions to the covariance between traits.
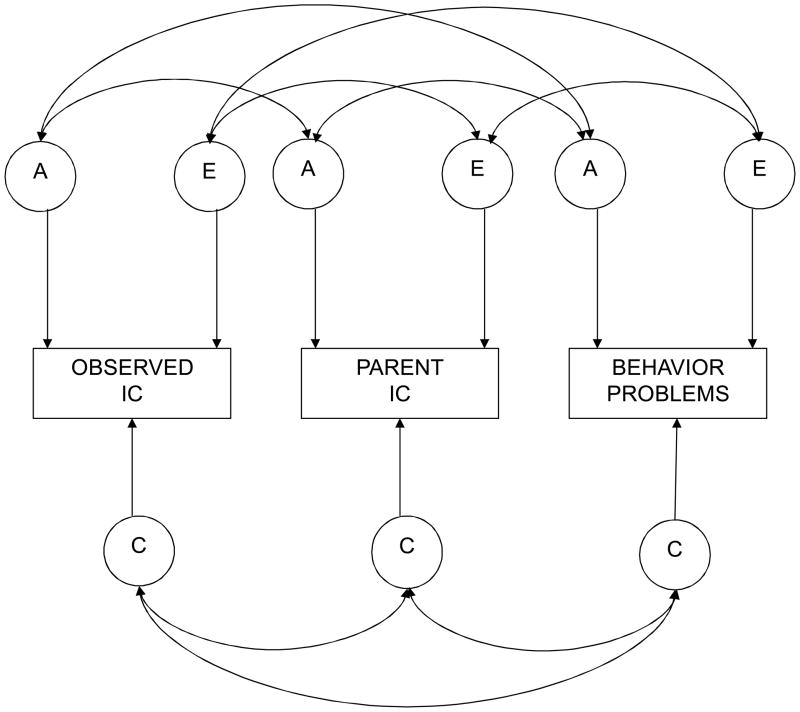
To further explore genetic and environmental sources of covariance between IC and behavior problems, trivariate correlated factors models with both measures of IC and behavior problems (either externalizing or ADHD) were fit to raw twin data using Mx maximum-likelihood model-fitting procedures (Neale, 2003). The basic model is depicted in Figure 1. The observed phenotypic variance of the measured variables is represented by the rectangles. The circles signify latent genetic and environmental variables. A1, C1, and E1 represent genetic, shared environmental, and nonshared environmental factors specific to lab-assessed IC; A2, C2, and E2 represent genetic, shared environmental, and nonshared environmental factors unique to parent-assessed IC; A3, C3, and E3 are factors specific to behavior problems; and the double-headed arrows refer to genetic and environmental correlations between variables.
Figure 1.
Multivariate Correlated Factors Model
In addition to estimating the magnitude of genetic and environmental effects influencing each variable, this model estimates genetic and environmental correlations (i.e., rg, rc, re) between the variables; and the genetic and environmental contributions to phenotypic correlations between phenotypes. For each behavior problem variable, reduced models were tested and compared to the full model using the χ2 difference test. Specifically, the additive genetic and shared environmental variances for each variable, and additive genetic, shared and nonshared environmental covariances between variables were eliminated to test if genetic and/or environmental variances and covariances were significant. A significant difference in χ2 indicates a poorer fit, and that the parameter dropped from the model is significant. Heritability estimates, environmental variances, genetic correlations, environmental correlations, and their 95% confidence intervals were estimated using the best-fitting models.
Results
Descriptive Statistics and Phenotypic Correlations
Descriptive statistics for all study variables are presented in Table 1. IC was negatively associated with behavior problems, such that 2-year-olds with low levels of IC had higher levels of maladjustment (Table 2). Associations between parent-rated IC and the CBCL subscales were larger than those between observed IC and behavior problems.
Table 1.
Sample Sizes, Means (and Standard Deviations): IC and Behavior Problems.
| n | Mean (SD) | |
|---|---|---|
| Observed IC | 590 | 0 (.65) |
| Parent IC | 590 | 38.88 (8.86) |
| Externalizing problems | 590 | 3.13 (1.21) |
| ADHD-DSM problems | 590 | 4.32 (2.67) |
Table 2.
Phenotypic Correlations: IC and Behavior Problems.
| Parent IC | Externalizing problems | ADHD-DSM problems | |
|---|---|---|---|
| Observed IC | .21 | −.13 | −.20 |
| Parent IC | - | −.54 | −.57 |
| Externalizing problems | - | - | .82 |
Note. All correlations are significant at the p < .01 level.
Twin Correlations
For all variables, MZ twin correlations exceeded DZ correlations (Table 3), signifying genetic influences. Cross-trait, cross-twin correlations between parent- and lab-assessed IC and related behavior problems for MZ twins exceeded DZ cross correlations, suggesting that genetic factors contribute to the covariance between IC and externalizing and ADHD. The MZ cross correlations were greater in magnitude in the negative direction than the DZ cross correlations, indicating that overlapping genetic influences operated in different directions across IC and behavior problems (e.g., genetic factors that influence low IC also influence high ADHD).
Table 3.
Twin Intraclass Correlations, Cross-twin Cross-trait Correlations: IC and Related Behavior Problems.
| Twin Intraclass Correlations
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Observed IC
|
Parent IC
|
Externalizing problems
|
ADHD-DSM problems
|
|||||
| MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | |
| .41** | .15** | .85** | .55** | .81** | .59** | .72** | .35** | |
| Cross-twin Cross-trait Correlations
| ||||
|---|---|---|---|---|
| Observed IC
|
Parent IC
|
|||
| MZ | DZ | MZ | DZ | |
| Externalizing problems | −.16** | −.06 | −.51** | −.27** |
| ADHD-DSM problems | −.20** | −.11* | −.47** | −.27** |
Note.
p < .05,
p < .01.
MZ=monozygotic twins, DZ=dizygotic twins.
Model-Fitting
IC and Externalizing
Table 4 presents the fit statistics for the multivariate models. It was not possible to fit reduced models without genetic (model 2) or nonshared environmental (model 4) covariance for IC and externalizing behavior problems. However, shared environmental covariance could be dropped with no significant change in χ2 (model 3). Because earlier analyses indicated no shared environmental influences on observed IC (Gagne & Saudino, 2010), we fit a reduced model dropping shared environmental influences on observed IC as well shared environmental covariance (model 5) with no decrement in fit. Thus, there was significant genetic covariance between observer- and parent-rated IC and externalizing problems (genetic correlations ranged from −.75 to .41), and significant nonshared environmental covariance between parent-assessed IC and externalizing problems (re = −.41). Genetic influences accounted for approximately 39–67% of the variance in IC and externalizing problems (Table 5), and the shared environment explained 16% and 27% of the variance on parent-rated IC and externalizing, respectively. The remaining variance for all three variables was due to non-shared environmental influences.
Table 4.
Fit Statistics for Trivariate Models of Inhibitory Control and Behavior Problems.
| Overall Fit of the Model
|
Relative Fit of the Model
|
|||||
|---|---|---|---|---|---|---|
| −2LL | df | AIC | χ2diff | dfdiff | p | |
| Observed IC, Parent-rated IC and Externalizing Behavior Problems | ||||||
| 1. Full Model | 6765.53 | 1763 | 3239.53 | - | - | - |
| 2. No A Covariance | 6795.02 | 1766 | 3263.02 | 29.49 | 3 | .00 |
| 3. No C Covariance | 6766.90 | 1766 | 3234.90 | 1.37 | 3 | .71 |
| 4. No E Covariance | 6792.69 | 1766 | 3260.69 | 27.16 | 3 | .00 |
| 5. No C on Observed IC and No C Covariance | 6766.90 | 1767 | 3232.90 | 1.37 | 4 | .85 |
| Observed IC, Parent-rated IC and ADHD Behavior Problems | ||||||
| 1. Full Model | 7924.77 | 1798 | 4328.77 | - | - | - |
| 2. No A Covariance | 7965.26 | 1801 | 4363.26 | 40.49 | 3 | .00 |
| 3. No C Covariance | 7925.77 | 1801 | 4323.77 | 1.0 | 3 | .80 |
| 4. No E Covariance | 7943.11 | 1801 | 4341.11 | 18.34 | 3 | .00 |
| 5. No C on Observed IC, ADHD and No C Covariance | 7925.77 | 1803 | 4319.77 | 1.0 | 5 | .96 |
Note. −2LL= Likelihood Statistic; df= degrees of freedom; AIC= Akaike’s Information Criterion; χ2diff= Chi-square difference between full ACE/ACE model and reduced model; dfdiff= df difference between full ACE/ACE model and reduced model; A=additive genetic effects; C=shared environmental effects; E=nonshared environmental effects. Boldface denotes best fitting model.
Table 5.
Multivariate Estimates of Genetic and Environmental Variance, and Genetic and Environmental Correlations (and 95% Confidence Intervals) for Inhibitory Control and Behavior Problems using the best-fitting Trivariate Model.
| Variance Components
|
Genetic and Environmental Correlations
|
|||||
|---|---|---|---|---|---|---|
| a2 | c2 | e2 | rg | re | ||
| IC and Externalizing Problems
| ||||||
| Observed IC | .39 (.25;.51) | - | .61 (.49;.75) | Observed IC-Parent IC | .41 (.22;.61) | - |
| Parent IC | .67 (.51;.85) | .16 (.01;.31) | .17 (.13;.22) | Parent IC-Externalizing problems | −.74 (−.88; −.62) | −.41 (−.54; −.26) |
| Externalizing problems | .54 (.38;.70) | .27 (.12: .41) | .19 (.14;.25) | Observed IC-Externalizing problems | −.30 (−.53; −.08) | - |
|
| ||||||
| IC and ADHD-DSM Problems
| ||||||
| Observed IC | .39 (.25;.51) | - | .61 (.49;.75) | Observed IC-Parent IC Parent IC- | .45 (.25;.67) | - |
| Parent IC | .58 (.43;.74) | .25 (.10;.39) | .17 (.13;.22) | ADHD-DSM problems | −.74 (−.88; −.62) | −.34 (−.47; −.19) |
| ADHD-DSM problems | .73 (.65;.79) | - | .27 (.21;.35) | Observed IC-ADHD-DSM problems | −.35 (−.54; −.16) | - |
Note. a2 = genetic variance, c2 = shared environmental variance, e2 = nonshared environmental variance, rg = genetic correlation, re = nonshared environmental correlation.
IC and ADHD
For the IC and ADHD behavior problems models, we were unable to drop all genetic (model 2) or nonshared environmental (model 4) covariance from reduced models, but shared environmental covariance could be dropped with no significant change in fit (model 3). We were also able to drop shared environmental influences associated with observed IC and ADHD behavior problems with no decrement in fit (model 5). Therefore, the most parsimonious model included genetic influences on all variables, shared environmental influences on parent-rated IC, and genetic and nonshared environmental covariance between all variables. Genetic factors accounted for 39–73% of the variance in observed and parent-assessed IC and ADHD problems, and shared environmental factors explained 25% of the variance on parent-rated IC (Table 5). Genetic correlations between all variables ranged from −.74 to .45, and there was a significant nonshared environmental correlation between parent-assessed IC and ADHD problems (re = −.34).
Discussion
This is the first study examining genetic and environmental covariance between IC and behavior problems in early childhood. We used parent and laboratory ratings of IC in order to provide a comprehensive assessment of temperament. IC, externalizing and ADHD behavioral problems were significantly related, replicating findings from previous studies with older children (e.g., Auerbach et al., 2008; Nigg, 1999; Olson et al., 1999). In addition to our novel phenotypic findings, this study adds to prior research by demonstrating that there is significant overlap between the genetic factors that influence IC and problem behaviors.
As predicted, genetic covariance between both IC measures and the CBCL subscales were negative, indicating that genetic factors related to higher levels of behavior problems were also related to low IC. There are two plausible explanations for common genetic effects between IC and behavioral symptoms. Pleiotropic genetic effects may be present, with the same genetic influences affecting multiple phenotypes. Genetic covariance could also be present because IC and behavior problems measures tap the same genetically-influenced behaviors to some extent or that IC mediates genetic effects on the externalizing behaviors. Phenotypic associations between parent-assessed IC and the externalizing and ADHD CBCL subscales were also due to nonshared environmental covariance. The nonshared environmental correlations were negative, mirroring phenotypic and genetic correlations between parent-rated IC and behavioral symptoms. Possible sources of nonshared environment that may play a role in the shared etiology of IC and related behavior problems include differential parental treatment, child-specific experiences, and rater effects.
Parent-assessed IC showed higher phenotypic, genetic, and nonshared environmental correlations than Lab-TAB IC across both externalizing and ADHD behavior problems. This pattern may be due to conceptual differences between the lab-based and parent-rated temperament measures. The Lab-TAB IC episodes are more narrowly focused on delay ability than the broader TBAQ-R subscales, and different genetic and nonshared environmental factors may influence these varied aspects of IC. It is also possible that IC parent rating items are more similar to CBCL behavior problem scale items (e.g., Lemery et al., 2002) than the Lab-TAB IC scores, and therefore tap more similar genetic and environmental influences. In addition, because the same informant (i.e., the mother in most all cases) rated both TBAQ-R IC and the CBCL behavior problem scales, rater effects may also contribute to greater covariances between parent-rated IC and behavior problems. The same rater is more likely to endorse items on the TBAQ-R and the CBCL in a similar manner than two independent raters (e.g., teachers and parents). Overlapping nonshared environmental factors did not contribute to phenotypic associations between Lab-TAB IC and the CBCL subscales, bolstering the view that informant-specific effects may play a role in the presence of higher covariances between the TBAQ-R and CBCL.
Findings of genetic covariance between IC and behavior problems are consistent with a recent investigation of EC in 8-year-olds (Lemery-Chalfant et al., 2008), a phenotype that includes IC. This study found that parent-rated EC was negatively correlated with both internalizing and externalizing symptoms, and that shared additive genetic factors accounted for phenotypic covariation. These findings are fairly broad as they incorporate the general EC temperament factor and both the internalizing and externalizing broadband behavioral maladjustment scales. Our research shows more specificity, as IC is the phenotype of interest, and phenotypic and genetic covariance is restricted to externalizing and ADHD symptoms. In addition, our findings are comparatively robust, as we employed both parent and lab-based assessments of IC, and the pattern of covariance shows similar results across these multiple measures.
The use of both the TBAQ-R and the Lab-TAB IC assessments expands on previous studies that employ parent ratings (Auerbach et al., 2008; Lemery-Chalfant et al., 2008), and may provide a methodological advantage to the use of response inhibition tasks in childhood. Several recent papers have criticized the use of cognitive assessments of inhibitory deficits in ADHD research (Castellanos et al., 2006; van Mourik et al., 2005; Polderman et al., 2009), and conclude that IC deficits are not homogenous. It has been argued that poor IC in ADHD involves multiple cognitive profiles that include low IC as assessed by both standard response inhibition and delay aversion tasks (Castellanos et al., 2006). Conceptualizing IC as a temperament dimension and using delay ability tasks is effective and shows findings similar to our parent ratings. One of the advantages of using a multi-method approach is that findings related to the IC phenotype can be demonstrated as more consistent if patterns of phenotypic and genetic covariance with behavior problems are similar across methodologies. This multi-method temperament assessment perspective on IC should be adopted in studies with older children as well.
Because IC is linked to behavior problems and shares overlapping genetic influences, it can be considered a potential endophenotype. Some endophenotypes are identified as more proximal to the gene (e.g., at the level of a protein) whereas others may be more distal (e.g., a behavioral measure). Although in our study, IC does not meet all of Gottesman and Gould’s (2003) criteria for identifying candidate endophenotypes, it does satisfy several. These criterion include being associated with the trait under study, and individual differences in the endophenotype being at least partly due to genetic factors. The criteria for an endophenotype being state-independent, and appearing in first degree relatives at a rate somewhere between the rate in affected persons and the rate in the general population have been explored in a recent family study with favorable conclusions (Goos et al., 2009). The current study shows that there are overlapping genetic influences between the measures of IC and externalizing and ADHD measures. Low IC in toddlerhood can also be considered the extreme end of normally distributed temperament (Goldsmith et al., 2004), and a genetic risk factor for the development of adjustment difficulties and psychopathology. On the other hand, IC could be viewed itself as a complex trait and there would be value in future multivariate genetic model-fitting studies in older children investigating the contribution of more narrowly defined measures of cognitive function (e.g., go/no go performance, choice impulsivity) to IC.
Links between IC and behavior problems in toddlerhood also suggest that there may be candidate genes associated with both of these phenotypes. Significant associations have been identified for several candidate genes in molecular genetic investigations of ADHD, including the dopamine transporter (DAT1), the dopamine receptor D4 (DRD4), the dopamine receptor D5 (DRD5), the serotonin transporter (5HTT), the serotonin receptor 1B (HTR1B) and the synaptosomal-associated protein 25 (SNAP25) genes (Gizer, Ficks & Waldman, 2009). While these findings are promising, there is substantial heterogeneity across studies indicating that future work should explore potential moderators (Gizer at al., 2009). Preliminary investigations of relations between childhood IC and the DAT1, DRD4 and SNAP25 genes indicated that better IC was associated with DRD4 and poor IC was associated with SNAP25 (Crosbie, Perusse, Barr & Schachar, 2008). In studies of EC, the DAT1 gene has been found to be associated with executive attention in childhood (Rueda, Rothbart, McCandliss, Saccomanno & Posner, 2005). Although the search for candidate genes associated with IC in early childhood has barely begun, some of these markers are potential starting points.
A limitation to the current research is the relatively modest sample size which limits the power to conduct certain twin analyses (e.g., analyses by gender). We sacrifice some power with our sample, but we gain in our ability to utilize more objective, laboratory-based measures of IC that are rarely employed in large-scale twin studies. Investigations of temperament and personality have been criticized for employing single assessments-typically parent ratings in childhood and self-reports in adulthood (Goldsmith, Lemery, Aksan & Buss, 2000; McClelland, 1996). Using multiple sources of information about IC in quantitative genetic analyses allows for more robust conclusions about phenotypic relations, genetic and environmental contributions to individual differences, and genetic and environmental covariance (Saudino, 2005). Our findings should be viewed as providing strong initial support for genetic covariance between IC and behavior problems in toddlerhood, and suggest that it may be profitable to identify genes that are common to early IC and behavior problem symptoms. The results of our study are clinically relevant in that children with low levels of IC in early childhood can be considered at-risk for the development of concurrent and future behavior problems, and earlier detection and intervention efforts can be pursued.
Key Points
IC is related to externalizing behavior problems and ADHD in middle to late childhood, but little is known about links in toddlerhood.
In this study of 2-year-old twins, correlations between parent- and lab-assessed IC and externalizing and ADHD behavior problems ranged from −.13 to −.57.
The phenotypic covariance between IC and behavior problems could be substantially explained by common genetic influences (genetic correlations ranged from −.30 to −.74).
Findings indicate that low levels of IC can be viewed as a genetic risk factor for the development of early emerging behavior problems.
This investigation has clinical relevance in that detection and intervention efforts can be pursued with younger children who display low levels of IC.
Acknowledgments
This research was supported by National Institute of Mental Health MH076353 and an Elizabeth Munsterberg Koppitz Child Psychology Graduate Fellowship from the American Psychological Foundation awarded to the first author. The BUTP is supported by grant MH062375 from the National Institute of Mental Health (K. Saudino).
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- CBCL
Child Behavior Checklist for Ages 1 1/2–5
- IC
inhibitory control
- Lab-TAB
Laboratory Temperament Assessment Battery
- TBAQ-R
Toddler Behavior Assessment Questionnaire-Revised
Contributor Information
Jeffrey R. Gagne, University of Wisconsin-Madison
Kimberly J. Saudino, Boston University
Philip Asherson, King’s College London.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- Auerbach JG, Berger A, Atzaba-Poria N, Arbelle S, Cypin N, Friedman A, Landau R. Temperament at 7, 12, and 25 months in children at familial risk for ADHD. Infant and Child Development. 2008;17:321–338. [Google Scholar]
- Briggs-Gowan M, Carter AS, Bosson-Heenan J, Guyer AE, Horwitz SM. Are infant-toddler social-emotional and behavioral problems transient? Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1–10. doi: 10.1097/01.chi.0000220849.48650.59. [DOI] [PubMed] [Google Scholar]
- Campbell SB. Hyperactivity in preschoolers: Correlates and prognostic implications. Clinical Psychology Review. 1985;5:405–428. [Google Scholar]
- Campbell SB. Behavior problems in preschool children: A recent review of the research. Journal of Child Psychology. 1995;36:113–149. doi: 10.1111/j.1469-7610.1995.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Burke EJS, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Science. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Perusse D, Barr CL, Schachar RS. Validating psychiatric endophenotypes: Inhibitory control and attention deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2008;32:40–55. doi: 10.1016/j.neubiorev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Valiente C, Murphy SH, Losoya SH, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL, Fabes RA, Losoya SH, Valiente C, Reiser M, Cumberland A, Shepard SA. The relations of problem behavior status to children’s negative emotionality, effortful control, and impulsivity; Concurrent relations and prediction of change. Developmental Psychology. 2005;41:193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Fabes RA, Smith CL, Reiser M, Shepard SA, Losoya SH, Guthrie IK, Murphy BC, Cumberland A. The relations of effortful control and ego control to children’s resiliency and social functioning. Developmental Psychology. 2003;39:761–776. doi: 10.1037/0012-1649.39.4.761. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: A genetic analysis of the effects of age and sex. Journal of Child Psychology & Psychiatry. 1999;40:1273–1282. [PubMed] [Google Scholar]
- Gagne JR, Saudino KJ. Wait for it! The etiology of inhibitory control in early childhood. Behavior Genetics. 2010;40:327–337. doi: 10.1007/s10519-009-9316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, Goldsmith HH. Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment. doi: 10.1037/a0021746. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: expanded content, stronger genetic evidence, new evidence for the importance of environment. Developmental Psychology. 1997;33:891–905. doi: 10.1037//0012-1649.33.6.891. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery K, Aksan N, Buss KA. Temperamental substrates of personality development. In: Molfese D, Molfese V, editors. Temperament and Personality Development across the Lifespan. Mahwah, NJ: Erlbaum; 2000. pp. 1–32. [Google Scholar]
- Goldsmith HH, Lemery KS, Essex MJ. Temperament as a liability factor for childhood behavioral disorders: The concept of liability. In: DiLalla LF, editor. Behavior genetics principles: Perspectives in development, personality, and psychopathology. Washington, DC: American Psychological Association; 2004. pp. 19–39. [Google Scholar]
- Goldsmith HH, Pollak SD, Davidson RJ. Developmental neuroscience perspectives on emotion regulation. Child Development Perspectives. 2008;2:132–140. doi: 10.1111/j.1750-8606.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Technical Report. Department of Psychology, University of Wisconsin-Madison; 1993. Preliminary manual for the Preschool Laboratory Temperament Assessment Battery (version 1.0) [Google Scholar]
- Goos LM, Crosbie J, Payne S, Schachar R. Validation and extension of the endophenotype model in ADHD patterns of inheritance in a family study of inhibitory control. American Journal of Psychiatry. 2009;166:711–717. doi: 10.1176/appi.ajp.2009.08040621. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Griffin D, Gonzalez R. Correlational analysis of dyad-level data in the exchangeable case. Psychological Bulletin. 1995;118:430–439. [Google Scholar]
- Johnson KA, Wiersema JR, Kuntsi J. What would Karl Popper say? Are current psychological theories of ADHD falsifiable? Behavioural and Brain Functions. 2009;5:15. doi: 10.1186/1744-9081-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Wakschlag L. More than the terrible twos: The nature and severity of behavior problems in clinic-referred preschool children. Journal of Abnormal Child Psychology. 2000;28:33–46. doi: 10.1023/a:1005118000977. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. IC in young children and its role in emerging internalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- Lavigne JV, Gibbons RD, Christoffel KK, Arend R, Rosenbaum D, Binns H, Dawson N, Sobel H, Isaacs C. Prevalence rates and correlates of psychiatric disorders among preschool children. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:204–214. doi: 10.1097/00004583-199602000-00014. [DOI] [PubMed] [Google Scholar]
- Lemery KS, Essex MJ, Smider NA. Revealing the relation between temperament and behavior problem symptoms by eliminating measurement confounding: Expert ratings and factor analyses. Child Development. 2002;73:867–882. doi: 10.1111/1467-8624.00444. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Doelger L, Goldsmith HH. Genetic relations between effortful control and attentional control symptoms of psychopathology in middle childhood. Infant and Child Development. 2008;17:365–385. doi: 10.1002/icd.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Müller U, Andreou P, Butler L, Christiansen H, Gabriels I, Medad S, Albrecht B, Uebel H, Asherson P, Banaschewski T, Gill M, Kuntsi J, Mulas F, Oades R, Roeyers H, Steinhausen HC, Rothenberger A, Faraone SV, Sonuga-Barke EJ. Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- McClelland DC. Does the field of psychology have a future? Journal of Research in Personality. 1996;30:429–434. [Google Scholar]
- Murray KT, Kochanska G. Effortful control: Factor structure and relation to externalizing and internalizing behaviors. Journal of Abnormal Child Psychology. 2002;30:503–514. doi: 10.1023/a:1019821031523. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical modeling. 6. Medical College of Virginia: Department of Psychiatry; Richmond, VA: 2003. [Google Scholar]
- Nigg JT. The ADHD response inhibition deficit as measured by the Stop Task: Replication with DSM-IV combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27:391–400. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Attention-Deficit/Hyperactivity Disorder: Endophenotypes, structure, and etiological pathways. Current Directions in Psychological Science. 2010;19:24–29. [Google Scholar]
- Nigg JT, Goldsmith HH. Genetics of personality disorders: Perspectives from personality and psychopathology research. Psychological Bulletin. 1994;115:346–380. doi: 10.1037/0033-2909.115.3.346. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Quamma JP, Greenberg MT, Kusche CA. A two-year longitudinal study of neuropsychological and cognitive performance in relation to behavioral problems and competencies in elementary school children. Journal of Abnormal Child Psychology. 1999;27:51–63. doi: 10.1023/a:1022614407893. [DOI] [PubMed] [Google Scholar]
- O’Connor BP. SPSS and SAS programs for addressing interdependence and basic levels-of-analysis issues in psychological data. Behavior Research Methods, Instruments, & Computers. 2004;36:17–28. doi: 10.3758/bf03195546. [DOI] [PubMed] [Google Scholar]
- Olson SL, Schilling EM, Bates JE. Measurement of impulsivity: Construct coherence, longitudinal stability, and relationship with externalizing problems in middle childhood and adolescence. Journal of Abnormal Child Psychology. 1999;27:151–165. doi: 10.1023/a:1021915615677. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry. 2009;48:837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman TJC, de Geus EJC, Hoekstra RA, Bartels M, van Leeuwen M, Verhulst FC, Posthuma D, Boomsma DI. Attention problems, inhibitory control, and intelligence index overlapping genetic factors: A study in 9-, 12-, and 18-year-old twins. Neuropsychology. 2009;23:381–391. doi: 10.1037/a0014915. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rothbart MK. Temperament and development. In: Kohnstamm GA, Bates JA, Rothbart MK, editors. Temperament in childhood. New York: Wiley; 1989a. pp. 187–247. [Google Scholar]
- Rothbart MK. Biological processes of temperament in childhood. In: Kohnstamm GA, Bates JA, Rothbart MK, editors. Temperament in childhood. New York: Wiley; 1989b. pp. 77–110. [Google Scholar]
- Rothbart MK, Ahadi SA. Temperament and the development of personality. Journal of Abnormal Psychology. 1994;103:55–66. doi: 10.1037//0021-843x.103.1.55. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenberg N, editor. Handbook of child psychology. 6. Vol. 3. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 99–166. Social, emotional, and personality development. [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ. Multiple informants. In: Everitt B, Howell D, editors. Encyclopedia of Statistics in Behavioral Science. Vol. 4. Chichester, UK: John Wiley & Sons; 2005. pp. 1332–1333. [Google Scholar]
- Saudino KJ, Carter AS, Purper-Ouakil D, Gorwood P. The etiology of behavioral problems and competencies in very young twins. Journal of Abnormal Psychology. 2008;117:48–62. doi: 10.1037/0021-843X.117.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res. 2002a;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2002b;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. J Child Psychol Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente C, Eisenberg N, Smith CL, Reiser M, Fabes RA, Losoya S, Guthrie IK, Murphy BC. The relations of effortful control and reactive control to children’s externalizing problems: A longitudinal assessment. Journal of Personality. 2003;71:1171–1196. doi: 10.1111/1467-6494.7106011. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant JA. The Stroop revisited: a meta-analysis of interference control in AD/HD. Journal of Child Psychology and Psychiatry. 2005;46:150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]