Abstract
Desmosine crosslinks are responsible for the elastic properties of connective tissues in lungs and cardiovascular system and are often compromised in disease states. We developed a new, fast, and simple cation exchange HPLC assay for the analysis of desmosine and isodesmosine in animal elastin. The method was validated by determining linearity, accuracy, precision, and desmosines stability and was applied to measure levels of desmosines in porcine and murine organs. The detection and quantification limits were 2 and 4 pmol, respectively. The run-time was 8 min. Our cation exchange column does not separate desmosine and isodesmosine, but their level can be quantified from absorbance at different wavelengths. Using this assay, we found that desmosines levels were significantly lower in elastin isolated from various organs of immunodeficient severe combined immunodeficiency mice compared with wild-type animals. We also found that desmosines levels were lower in lung elastin isolated from hyperhomocysteinemic Pcft −/− mice deficient in intestinal folate transport compared with wild-type Pcft +/+ animals.
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s00216-011-5346-z and is accessible for authorized users.
Keywords: Cation exchange HPLC, Desmosine, Isodesmosine, Elastin, Homocysteine, Pcft mouse, SCID mouse
Introduction
Desmosine (Des) and its isomer isodesmosine (isoDes), major crosslinks in elastin, play an important role in the physiological function of elastic fibers. They are formed from four lysine residues, which after oxidative deamination catalyzed by lysyl oxidase, condense to a pyridinum ring-containing structure. Des and isoDes, owning to their uniqueness in mammalian elastin, are widely used as biomarkers for a range of human diseases, such as chronic obstructive pulmonary disease, acute lung injury, aneurysm, cystic fibrosis, pseudoxanthoma elasticum, bronchiectasis, and atherosclerosis [1]. During the last 30 years, a number of desmosines assays have been developed for tissues and biological fluids. Commonly used approaches include amino acid analysis, immunological methods (radioimmunoassay [2–4], ELISA [5–8]), column chromatography [9], and capillary electrophoresis [10–14]. A comprehensive summary of strategies for the detection of desmosines was published in 2007 by Viglio et al. [15]. Since that time, further progress has been made to lower limits of detection and increase robustness. Boutin et al. [16] developed a high-sensitivity method based on a nanoflow liquid chromatography tandem mass spectrometry with multiple reaction monitoring. Detection limit achieved by this approach is 0.1 ng free desmosine/mL. Most recently, Shiraishi et al. [9] employed ultra-performance liquid chromatography coupled to tandem mass spectrometry that allows quantification of human urinary desmosines from 1 to 250 pmol/mL.
The most widely used assay employs HPLC on a reversed phase C18 column [1, 16–19], originally developed in 1981 by Faris et al. [17] for desmosines quantification in animal tissues. To increase the sensitivity of detection, desmosines are derivatized with trinitrophenyl [20], napthalenedialdehyde/cyanide [21], dansylchloride [22], or phenylisothiocyanate [23, 24].
In the present study, we describe a new, simple, and fast HPLC method for the determination of desmosines in animal tissues. The assay utilizes a cation exchange polysulfoethyl aspartamide column. Because their charges are identical, Des and isoDes did not separate from each other and eluted at the same time. The differences in their ultraviolet absorption spectra were used to quantify Des and isoDes levels. The method is validated by determining linearity, accuracy, precision, and Des stability and is applied to measure levels of desmosines in pig and mice organs.
Experimental section
Reagents and proteins
Des and isoDes were from Elastin Products Company and LGC Standards, respectively. Human aorta elastin (soluble), NaCl, and trifluoroacetic acid (TFA) were from Sigma-Aldrich.
Animals
The following animals were used for the preparation of elastin: five male C57BL6/J mice (1 year old; The Jackson Laboratory, Bar Harbor, ME) [25], three male CBySmn.CB17-Prkdc scid/J mice (severe combined immunodeficiency (SCID); The Jackson Laboratory; one 6 months old and two 10 months old) [26], five proton-coupled foliate transporter-deficient (Pcft −/−) and six Pcft +/+ mice (6 and 8 weeks old) [27], and one male pig (7 days old; Złotnicka White from Rolniczy Zakład Doświadczalny Złotniki, Poland).
Preparation of elastin from animal tissues
Elastin was prepared by the hot alkali method [28] from lung, heart, kidney, and liver of five male C57BL 6/J mice [25]; heart, lung, liver, kidney, spleen, testicle, and brain of three male CBySmn.CB17-Prkdc scid/J mice [26]; lung of five Pcft −/− and six Pcft +/+ mice [27]; and from lung, heart, aorta, kidney, liver, and skin of one male pig. Briefly, 1.9–3.7 g of frozen pig tissue was pulverized in liquid nitrogen using a mortar and pestle pre-chilled to −80 °C. The pulverized tissue was extracted by sonication in 10 volumes of phosphate-buffered saline (PBS) on ice (six strokes of 10 s at 70 W power). After centrifugation at 26,000×g for 20 min, pellet was washed with PBS, defatted with 2:1 chloroform/methanol (v/v), and hydrolyzed with 10 volumes of 1 N NaOH on a boiling water bath for 45 min. The NaOH-resistant pellet of elastin was washed once with 10 volumes of 1 N NaOH and twice with 10 volumes of water, suspended in water, aliquoted, and dried by centrifugation under vacuum.
The elastin preparation procedure for mouse organs was modified due to the small amount of material (0.02–0.10 g from Pcft-deficient mice [27] and 0.04–0.54 g from other mice). Mouse tissues were homogenized and washed with 10 volumes of PBS. The defatting step was omitted and tissue was boiled with shaking in 1 mL NaOH. After centrifugation (18,000×g, 30 min), pellet was washed once with 1 mL NaOH and twice with 1 mL water.
Preparation of desmosines from animal elastin
Elastin from animal tissues was hydrolyzed with 6 M HCl in 1-mL Wheaton Gold Band glass ampoules (sealed under vacuum) for 16–22 h. The hydrolysates were dried, dissolved in 60-μL water, clarified by microcentrifugation, and 20-μL aliquots were subjected to HPLC.
HPLC analysis, detection, and quantification
HPLC analyses were carried out on an Agilent 1,100 series system, equipped with binary pump, autosampler, column thermostat, and photodiode array UV detector. Chromatograms were analyzed with Agilent ChemStation software. Samples (20 μL) were injected into a cation exchange polysulfoethyl aspartamide column (35 × 2 mm, 5 μm, 300 Å; PolyLC, Wilmington, DE) at 24 °C. The flow rate was 0.36 mL/min. The column was eluted with a linear gradient from 0% to 52.5% 1 M NaCl, 0.1% TFA for 6.1 min, followed by 52.5% 1 M NaCl, 0.1% TFA (from 6.1 to 7.1 min), and re-equilibration with 0.1% TFA (from 7.1 to 8 or 12 min). A run-time for each sample was 8 min. The detection was by monitoring UV absorption at λ = 240 nm and λ = 268 nm.
Areas of the peaks recorded at 240 and 268 nm was used for the quantification of Des and Des + isoDes, respectively. Each analyte possesses a molar absorption coefficient which can be deduced from its calibration line and Lambert–Beer law. By using the coefficient, absorbance A can be expressed as:
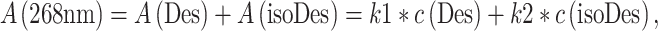 |
where c is concentration, k1 and k2 are molar absorption coefficients of Des and isoDes, respectively.
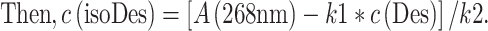 |
With the known k1, k2, and c(Des) deduced at 240 nm, the c(isoDes) can thus be calculated.
In other words, after the c(Des) is obtained from the calibration curve at 240 nm, the absorbance of the Des at 268 nm can be calculated and the value subtracted from the total absorbance of both components at 268 nm. As a result, concentration of isoDes can be estimated in samples.
Calibration and linearity
Authentic Des or isoDes, dissolved in water (10 mM) and stored in aliquots at −20 °C, were used as standards. A calibration line was generated for each series of assays. Three to 11 dilutions of Des and isoDes from 2 pmol to 5 nmol were prepared to generate a calibration line. Each concentration was run in duplicate or triplicate. The corresponding peak areas at 240 and 268 nm were plotted against the amount of Des and isoDes (in pmol). A linear regression was used to calculate levels of Des and Des + isoDes in analyzed samples.
Accuracy
Accuracy of the method was determined by recovery experiments. Human elastin samples were spiked with five different amounts of Des (from 125 to 2,000 pmol) prior to acid hydrolysis. Three human elastin samples without Des as well as standard Des solutions were processed in parallel. Each sample was analyzed in duplicate. From the data, percentage recovery (% recovery = [(a − b)/c] × 100, where, a is elastin spiked with Des, b is elastin, c is the amount of Des standard added), and the relative standard deviation (RSD) of recovery was calculated.
Precision
Precision of the method was determined from within-run and between-run variation studies. For the within-run variation, two to three injections of standard and sample solutions were made and RSD values were calculated. For the between-run variation, four to ten injections of standard sample solutions were made and the RSDs calculated.
Stability of desmosines
Freeze–thaw stability and short-term temperature stability tests were performed on standard Des and isoDes. For freeze and thaw stability, two aliquots of 125, 500, and 2,000 pmol standards were stored at −20 °C for 24 h and thawed unassisted at room temperature. When completely thawed, the samples were kept frozen for at least 24 h under the same conditions. The freeze–thaw cycle was repeated two more times, and HPLC measurement was performed on the third cycle. For short-term stability, two aliquots of each of the above concentrations were kept at room temperature for 20 h then kept at −20 °C until analysis.
Results and discussion
Cation exchange HPLC of des/isoDes
Our desmosines assay employs a polysulfoethyl aspartamide cation exchange column that is routinely used for the quantification of homocysteine (Hcy), Hcy-thiolactone, and N-Hcy-protein in biological samples [29–31]. Because of the presence of five positive charges in their molecules (four on Lys amino groups and one on the nitrogen in the pyridinum ring), Des and isoDes are bound to the polysulfoethyl aspartamide column much more strongly than any other amino acid and, under conditions used for HPLC separation, elute at the same time. Possible minor elastin crosslinks, e.g., neodesmosine, oxodesmosine, isooxodesmosine, and allodesmosine, contain two to four positive charges [32] are thus expected to be eluted earlier from the polysulfoethyl aspartamide column, which separates according to the number of positive charges in the analyte structure. Desmosines were eluted with a salt gradient and detected by UV absorbance. The elution time for desmosines was approximately 5.6 min, and the run was completed in 8 min (Fig. 1(A, B)). This run-time is shorter than run-times for other methods, for example, 12 (with elution time of 8.95 min) [33] and 15 min (with elution time of 6.5 min) [9]. The identity of desmosines was confirmed by UV spectrum and comigration with Des and isoDes standards. Des has three UV maxima (at 205, 235, and 268 nm), whereas isoDes has two maxima (at 205 and 280 nm) (Fig. 2) [34]. Thus, Des and isoDes can be quantified in mixtures by monitoring UV absorption at λ = 240 nm (Des) and λ = 268 nm (Des + isoDes).
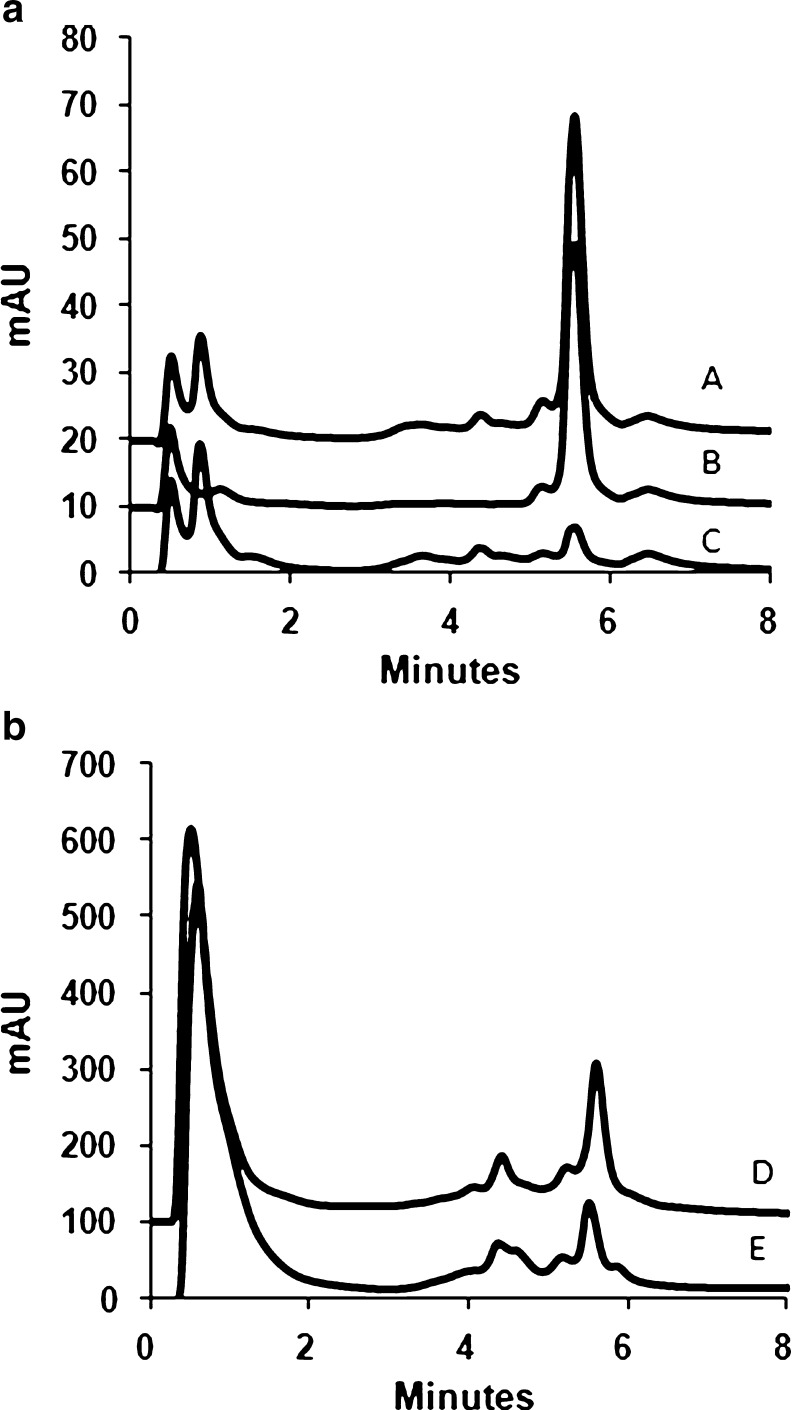
Fig. 1.
Cation exchange HPLC determination of desmosines. Traces obtained with the following samples are shown: (A) acid hydrolysate of human aorta soluble elastin spiked with Des standard (100 pmol), (B) Des standard (100 pmol), (C) acid hydrolysate of human aorta soluble elastin, (D) acid hydrolysate of lung elastin from wild-type C57BL 6/J mice, and (E) acid hydrolysate of mice lung elastin spiked with Des + isoDes 1:1 mixture (100 pmol each). Detection was at λ = 268 nm and λ = 240 nm. For clarity, only traces recorded at λ = 268 nm are shown and the traces are shifted by 10 or 100 mAU. Des and isoDes elute at 5.6 min
Fig. 2.
UV spectra of Des and isoDes. Dotted line, Des; broken line, isoDes; dotted/broken line, spectrum of the 5.6 min peak from the HPLC analysis of pig lung elastin
This method was used to measure the level of desmosines in hydrolysate of soluble elastin from human aorta (Sigma). Figure 1(A) shows chromatograms of the elastin hydrolysate (trace C), the hydrolysate spiked with Des standard (trace A), and Des standard alone (trace B). The average molar ratio of desmosines to elastin for soluble elastin from human aorta calculated from three independent experiments was 1.80 ± 0.18 (corresponsding to 2.7 desmosines/1,000 amino acid residues). This value is within the range of Des + isoDes content in elastin reported in the literature, e.g., levels of Des + isoDes elastin from human aorta presented by John and Thomas were 2.1–3.3/1,000 amino acid residues [35].
The level of desmosines was also measured in hydrolysates of elastin isolated from animal tissues. Figure 1(B) shows chromatograms of mouse lung elastin hydrolysate spiked with Des + isoDes 1:1 mixture (trace D) and elastin hydrolysate without addition of standards (trace E).
Calibration, linearity, accuracy, and precision
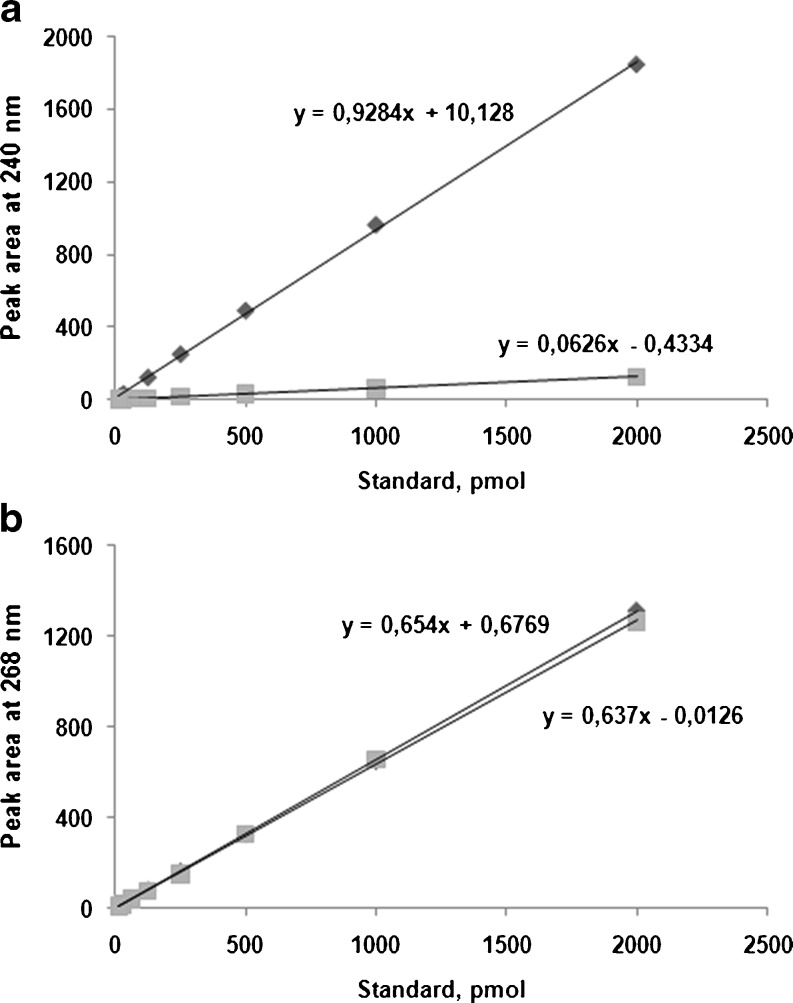
Des and isoDes standards were dissolved in water and subjected to cation exchange HPLC. As shown in Fig. 3, at λ = 240 nm, absorption from Des is much greater than from isoDes, so this wavelength was used to calculate the level of Des. At λ = 268 nm, absorption of Des and isoDes are identical. The calibration plots, obtained by plotting peak areas at 240 (Fig. 3A) and 268 nm (Fig. 3B) vs. the quantity of Des and isoDes were linear from 4 to 2,000 pmol Des (r 2 = 1.0000 and 0.9995, respectively). The limit of detection and quantification was 2 and 4 pmol, respectively. Percentage recovery was 108.8 ± 1.2. The within-run and between-run variations were 5.4% and 15.6%, respectively.
Fig. 3.
Calibration lines for Des (diamonds) and isoDes (squares) standards at λ = 240 nm (a) and λ = 268 nm (b)
Stability of desmosine
Short-term stability at room temperature and the stability following three freeze–thaw cycles were assessed using two aliquots of three concentrations of standard Des and isoDes. Each aliquot was run in duplicate. No significant reduction in the amount of Des and isoDes was observed following 20 h storage at room temperature (102.7 ± 5.2% and 104.0 ± 2.6% recovery, respectively) or after three freeze–thaw cycles (98.0 ± 1.9% and 102.9 ± 4.9% recovery, respectively).
Desmosines levels in elastin from animal organs
Insoluble elastin was prepared from animal tissues using a hot alkali method that affords a product with an amino acid composition matching the composition expected for elastin. The yield, quantified by weighing the dry insoluble material obtained after the hot alkali procedure, was from 3.6 to 41.9 mg elastin/g tissue. Desmosines were liberated from elastin by hydrolysis with 6 N HCl and quantified by cation exchange HPLC on a polysulfoethyl aspartamide column. A major retained peak eluting at approximately 5.6 min had a UV spectrum characteristic of a mixture of Des and isoDes (Fig. 2).
Desmosines levels in mouse elastin, expressed as pmol per mg tissue, are shown in Table 1. In the wild-type C57BL 6/J mice, the highest levels of Des and Des + isoDes were found in lung and heart elastin and the lowest in liver elastin (Table 1).
Table 1.
Desmosines levels in elastin isolated from different mouse organs
| Organ (weight, g) | Desmosine (pmol/mg tissue) | Desmosine + isodesmosine (pmol/mg tissue) | ||
|---|---|---|---|---|
| C57BL 6/J mice | CBySmn.CB17-Prkdc scid/J mice | C57BL 6/J mice | CBySmn.CB17-Prkdc scid/J mice | |
| Lung (0.15 ± 0.02) | 67.8 ± 9.5 | 49.3 ± 8.3* | 108.9 ± 24.0 | 62.7 ± 9.1* |
| Heart (0.16 ± 0.06) | 7.5 ± 2.7 | 2.1 ± 1.7* | 13.9 ± 5.4 | 4.9 ± 3.3* |
| Kidney (0.41 ± 0.05) | 1.7 ± 0.3 | 1.0 ± 0.2 | 3.1 ± 0.5 | 2.2 ± 0.5* |
| Liver (0.42 ± 0.07) | 1.2 ± 0.3 | 1.6 ± 2.2 | 1.8 ± 0.2 | 0.56 ± 0.09* |
| Spleen (0.05 ± 0.01) | 14.6 ± 0.01 | 21.1 ± 4.5 | ||
| Testicle (0.14 ± 0.01) | 6.5 ± 3.5 | 7.4 ± 2.4 | ||
| Brain (0.24 ± 0.01) | 1.0 ± 1.8 | 1.2 ± 1.9 | ||
Each value is an average of assays with two to five animals
*P = 0.0007–0.035, significantly different from control mice
In the pig, the highest levels of Des were found in aorta (76.4 pmol/mg tissue) and lung (17.3 pmol/mg tissue) elastin and the lowest in liver elastin (0.8 pmol/mg tissue). Pig skin, kidney, and heart contained intermediate levels of desmosines, 8.3, 8.2, and 3.6 pmol/mg tissue, respectively. Des + isoDes had similar distribution in swine tissues. The highest levels of Des + isoDes were found in aorta (128.0 pmol/mg tissue) and lung (32.0 pmol/mg tissue) elastin and the lowest in liver elastin (0.9 pmol/mg tissue). Pig skin, kidney, and heart contained intermediate levels of desmosines, 16.2, 15.2, and 6.6 pmol/mg tissue, respectively. These levels are consistent with levels of desmosines previously reported for animal elastin [36].
Immunodeficiency prevents desmosine crosslinks formation in mouse elastin
We assayed desmosines levels in elastin isolated from various organs of immunodeficient SCID mice [26]. Similar to wild-type mice, CBySmn.CB17-Prkdc scid/J mice had the highest levels of desmosines in lung elastin, and the lowest in liver elastin (Table 1). However, Des levels were significantly lower in lung and heart but not in kidney and liver of CBySmn.CB17-Prkdc scid/J mice compared with wild-type C57BL 6/J mice. Des + isoDes levels were significantly lower in elastin isolated from lung, heart, kidney, and liver elastin of mutant mice compared with control animals (Table 1). These findings indicate that deficiency in elastin crosslinking is a novel phenotype contributing to the pathology of immunodeficient SCID mice.
Hyperhomocysteinemia prevents desmosine crosslink formation in mouse lung elastin
We also assayed desmosines levels in lung elastin isolated from Pcft-deficient mice that have 20-fold higher plasma Hcy compared with Pcft +/+ animals [27]. We found that Des and Des + isoDes levels in lung elastin from Pcft −/− mice tended to be lower (1.5- and 1.6-fold, respectively) compared with wild-type Pcft +/+ animals, although the difference did not reach statistical significance (P = 0.12 and 0.08, respectively, Table 2).
Table 2.
Desmosines levels in elastin isolated from lungs of Pcft-deficient and wild-type Pcft +/+ mice
| Genotype (n) | Desmosine (pmol/mg lung) | Desmosine + isodesmosine (pmol/mg lung) |
|---|---|---|
| Pcft −/− (4) | 36.0 ± 18.6* | 35.8 ± 21.0** |
| Pcft +/+ (5) | 52.7 ± 20.4 | 57.8 ± 21.3 |
*P = 0.12; **P = 0.08 vs. control mice
Desmosines from Pcft −/− and Pcft +/+ had essentially identical UV spectra and elastin levels were similar in Pcft −/− and Pcft +/+ mice. Taken together, these results suggest that hyperhomocysteinemia causes deficiency in elastin crosslinking in the mouse lung. These findings are reminiscent of previous findings in rat, where vitamin B6 deficiency [37] or intraperitoneal injections of Hcy + Met [38] elevated plasma Hcy levels and decreased elastin desmosines levels.
Conclusions
We have developed a new cation exchange HPLC assay for the determination of desmosines in animal elastin. Our assay is highly specific (desmosines elute as a major UV-absorbing peak on chromatograms), fast (separation is achieved in 8 min, a much shorter time than previously reported for other methods), inexpensive (requires only two reagents for separation), and reliable. The utility of this new assay was demonstrated for the analysis of human, mouse, and pig elastin. We could measure desmosine crosslinks in elastin hydrolysates from as little as 20 mg of mice lung. Our new assay’s sensitivity of 4 pmol is sufficient for analyses of animal tissues. For analyses of limited amounts of biological material, other techniques, such as, HPLC or UPLC coupled to mass spectrometry are available [9, 16, 33]. Using our new assay, we found that desmosines levels in mouse lung elastin were decreased in immunodeficient SCID and hyperhomocysteinemic Pcft −/− mice compared with wild-type animals. This method should help to elucidate the role of desmosine crosslinks in health and disease.
Electronic supplementary material
Supplementary material, approximately 362 KB.
Acknowledgments
This work was supported in part by grants from the Ministry of Science and Higher Education, Poland (NN 401 230634) and the American Heart Association (0855919D). We thank Ewa Pruszyńska-Oszmałek (Department of Animal Physiology and Biochemistry, Poznań University of Life Sciences) and Ryszard Słomski (Institute of Human Genetics, Polish Academy of Sciences, Poznań, Poland) for kindly providing mouse and pig organs. We thank Jarosław Zimny for assistance in HPLC analyses in the initial stage of the method development. We also thank anonymous reviewers for their constructive comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Des
Desmosine
- Hcy
Homocysteine
- isoDes
Isodesmosine
- Pcft
Proton-coupled folate transporter
- PBS
Phosphate-buffered saline
- SCID
Severe combined immunodeficiency
- TFA
Trifluoroacetic acid
Contributor Information
Joanna Perła-Kaján, Phone: +48-61-8487208, FAX: +48-61-8487211, Email: kajan@up.poznan.pl.
Hieronim Jakubowski, Email: jakubows@umdnj.edu.
References
- 1.Yamaguchi Y, Haginaka J, Kunitomo M, Yasuda H, Bando Y. High-performance liquid-chromatographic determination of desmosine and isodesmosine in tissues and its application to studies of alteration of elastin induced by atherosclerosis. J Chromatogr-Biomed Appl. 1987;422:53–59. doi: 10.1016/0378-4347(87)80439-5. [DOI] [PubMed] [Google Scholar]
- 2.Harel S, Janoff A, Yu S, Hurewitz A, Bergofsky E. Desmosine radioimmunoassay for measuring elastin degradation in vivo. Am Rev Respir Dis. 1980;122:769–773. doi: 10.1164/arrd.1980.122.5.769. [DOI] [PubMed] [Google Scholar]
- 3.King GS, Mohan VS, Starcher BC. Radioimmunoassay for desmosine. Connect Tissue Res. 1980;7:263–267. doi: 10.3109/03008208009152362. [DOI] [PubMed] [Google Scholar]
- 4.Starcher B, Scott M. Fractionation of urine to allow desmosine analysis by radioimmunoassay. Ann Clin Biochem. 1992;29(Pt 1):72–78. doi: 10.1177/000456329202900111. [DOI] [PubMed] [Google Scholar]
- 5.Gunja-Smith Z. An enzyme-linked immunosorbent assay to quantitate the elastin crosslink desmosine in tissue and urine samples. Anal Biochem. 1985;147:258–264. doi: 10.1016/0003-2697(85)90036-3. [DOI] [PubMed] [Google Scholar]
- 6.Laurent P, Magne L, De Palmas J, Bignon J, Jaurand M. Quantitation of elastin in human urine and rat pleural mesothelial cell matrix by a sensitive avidin-biotin ELISA for desmosine. J Immunol Methods. 1988;107:1–11. doi: 10.1016/0022-1759(88)90002-6. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, Ishimori K, Verplanke A, Matsuki H, Kasuga H. An enzyme-linked immunosorbent assay (ELISA) for the quantitation of urinary desmosine. Tokai J Exp Clin Med. 1989;14:347–356. [PubMed] [Google Scholar]
- 8.Osakabe T, Seyama Y, Yamashita S. Comparison of ELISA and HPLC for the determination of desmosine or isodesmosine in aortic tissue elastin. J Clin Lab Anal. 1995;9:293–296. doi: 10.1002/jcla.1860090503. [DOI] [PubMed] [Google Scholar]
- 9.Shiraishi K, Matsuzaki K, Matsumoto A, Hashimoto Y, Iba K. Development of a robust LC-MS/MS method for determination of desmosine and isodesmosine in human urine. J Oleo Sci. 2010;59:431–439. doi: 10.5650/jos.59.431. [DOI] [PubMed] [Google Scholar]
- 10.Annovazzi L, Viglio S, Perani E, Luisetti M, Baraniuk J, Casado B, Cetta G, Iadarola P. Capillary electrophoresis with laser-induced fluorescence detection as a novel sensitive approach for the analysis of desmosines in real samples. Electrophoresis. 2004;25:683–691. doi: 10.1002/elps.200305607. [DOI] [PubMed] [Google Scholar]
- 11.Annovazzi L, Viglio S, Gheduzzi D, Pasquali-Ronchetti I, Zanone C, Cetta G, Iadarola P. High levels of desmosines in urine and plasma of patients with pseudoxanthoma elasticum. Eur J Clin Invest. 2004;34:156–164. doi: 10.1111/j.1365-2362.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 12.Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L, Iadarola P, De Rosa E, Fabbri L, Mapp C. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61:1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stolk J, Veldhuisen B, Annovazzi L, Zanone C, Versteeg E, van Kuppevelt T, Berden J, Nieuwenhuizen W, Iadarola P, Luisetti M. Short-term variability of biomarkers of proteinase activity in patients with emphysema associated with type Z alpha-1-antitrypsin deficiency. Respir Res. 2005;6:47. doi: 10.1186/1465-9921-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giummelly P, Botton B, Friot R, Primaputra D, Atkinson J. Measurement of desmosine and isodesmosine by capillary zone electrophoresis. J Chromatogr A. 1995;710:357–360. doi: 10.1016/0021-9673(95)00487-4. [DOI] [Google Scholar]
- 15.Viglio S, Annovazzi L, Luisetti M, Stolk J, Casado B, Iadarola P. Progress in the methodological strategies for the detection in real samples of desmosine and isodesmosine, two biological markers of elastin degradation. J Sep Sci. 2007;30:202–213. doi: 10.1002/jssc.200600260. [DOI] [PubMed] [Google Scholar]
- 16.Boutin M, Berthelette C, Gervais F, Scholand M, Hoidal J, Leppert M, Bateman K, Thibault P. High-sensitivity nanoLC-MS/MS analysis of urinary desmosine and isodesmosine. Anal Chem. 2009;81:1881–1887. doi: 10.1021/ac801745d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faris B, Ferrera R, Glembourtt M, Mogayzel PJ, Crombie G, Franzblau C. Rapid quantitation of desmosine content in tissue hydrolysates by high-performance liquid chromatography. Anal Biochem. 1981;114:71–74. doi: 10.1016/0003-2697(81)90453-X. [DOI] [PubMed] [Google Scholar]
- 18.Covault H, Lubrano T, Dietz A, Rubinstein H. Liquid-chromatographic measurement of elastin. Clin Chem. 1982;28:1465–1468. [PubMed] [Google Scholar]
- 19.Chen J, Takahashi M, Kushida K, Suzuki M, Suzuki K, Horiuchi K, Nagano A. Direct detection of crosslinks of collagen and elastin in the hydrolysates of human yellow ligament using single-column high performance liquid chromatography. Anal Biochem. 2000;278:99–105. doi: 10.1006/abio.1999.4412. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto D. Aging and cross-linking in human aorta. Biochem Biophys Res Commun. 1982;109:1264–1269. doi: 10.1016/0006-291X(82)91913-1. [DOI] [PubMed] [Google Scholar]
- 21.Lunte SM, Mohabbat T, Wong OS, Kuwana T. Determination of desmosine, isodesmosine, and other amino-acids by liquid-chromatography with electrochemical detection following precolumn derivatization with naphthalenedialdehyde cyanide. Anal Biochem. 1989;178:202–207. doi: 10.1016/0003-2697(89)90380-1. [DOI] [PubMed] [Google Scholar]
- 22.Guida E, Codini M, Palmerini CA, Fini C, Lucarelli C, Floridi A. Development and validation of a high-performance liquid-chromatographic method for the determination of desmosines in tissues. J Chromatogr. 1990;507:51–57. doi: 10.1016/S0021-9673(01)84180-7. [DOI] [PubMed] [Google Scholar]
- 23.Hanis T, Deyl Z, Struzinsky R, Miksik I. Separation of elastin cross-links as phenylisothiocyanate derivatives. J Chromatogr. 1991;553:93–99. doi: 10.1016/S0021-9673(01)88477-6. [DOI] [PubMed] [Google Scholar]
- 24.Salomoni M, Muda M, Zuccato E, Mussini E. High-performance liquid chromatographic determination of desmosine and isodesmosine after phenylisothiocyanate derivatization. J Chromatogr. 1991;572:312–316. doi: 10.1016/0378-4347(91)80496-y. [DOI] [PubMed] [Google Scholar]
- 25.Black B, Croom J, Eisen E, Petro A, Edwards C, Surwit R. Differential effects of fat and sucrose on body composition in A/J and C57BL/6 mice. Metabolism. 1998;47:1354–1359. doi: 10.1016/S0026-0495(98)90304-3. [DOI] [PubMed] [Google Scholar]
- 26.Bosma M, Schuler W, Bosma G. The scid mouse mutant. Curr Top Microbiol Immunol. 1988;137:197–202. doi: 10.1007/978-3-642-50059-6_29. [DOI] [PubMed] [Google Scholar]
- 27.Jakubowski H, Perla-Kajan J, Finnell RH, Cabrera RM, Wang H, Gupta S, Kruger WD, Kraus JP, Shih DM. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J. 2009;23:1721–1727. doi: 10.1096/fj.08-127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mecham R. Methods in elastic tissue biology: elastin isolation and purification. Methods. 2008;45:32–41. doi: 10.1016/j.ymeth.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakubowski H. The determination of homocysteine-thiolactone in biological samples. Anal Biochem. 2002;308:112–119. doi: 10.1016/S0003-2697(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 30.Chwatko G, Jakubowski H. The determination of homocysteine-thiolactone in human plasma. Anal Biochem. 2005;337:271–277. doi: 10.1016/j.ab.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Jakubowski H. New method for the determination of protein N-linked homocysteine. Anal Biochem. 2008;380:257–261. doi: 10.1016/j.ab.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M, Sawai T. Alteration of cross-linking amino acids of elastin in human aorta in association with dissecting aneurysm: analysis using high performance liquid chromatography. Tohoku J Exp Med. 1999;187:291–303. doi: 10.1620/tjem.187.291. [DOI] [PubMed] [Google Scholar]
- 33.Ma S, Lin Y, Turino G. Measurements of desmosine and isodesmosine by mass spectrometry in COPD. Chest. 2007;131:1363–1371. doi: 10.1378/chest.06-2251. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J, Elsden DF, Partridge SM. Partial structure of two major degradation products from the cross-linkages in elastin. Nature. 1963;200:651–652. doi: 10.1038/200651a0. [DOI] [PubMed] [Google Scholar]
- 35.John R, Thomas J. Chemical compositions of elastins isolated from aortas and pulmonary tissues of humans of different ages. Biochem J. 1972;127:261–269. doi: 10.1042/bj1270261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell JT, Whitney PL. Postnatal development of rat lung. Changes in lung lectin, elastin, acetylcholinesterase and other enzymes. Biochem J. 1980;188:1–8. doi: 10.1042/bj1880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers BA, Dubick MA, Reynolds RD, Rucker RB. Effect of vitamin B-6 (pyridoxine) deficiency on lung elastin cross-linking in perinatal and weanling rat pups. Biochem J. 1985;229:153–160. doi: 10.1042/bj2290153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffiths R, Tudball N, Thomas J. Effect of induced elevated plasma levels of homocystine and methionine in rats on collagen and elastin structures. Connect Tissue Res. 1976;4:101–106. doi: 10.3109/03008207609152205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 362 KB.