Abstract
Aims
Adherence to evidence-based treatments and its consequences after acute myocardial infarction (MI) are poorly defined. We examined the extent to which clopidogrel treatment initiated in hospital is continued in primary care; the factors predictive of clopidogrel discontinuation and the hazard of death or recurrent MI.
Methods and results
We linked the Myocardial Ischaemia National Audit Project registry and the General Practice Research Database to examine adherence to clopidogrel in primary care among patients discharged from hospital after MI (2003–2009). Hospital Episode Statistics and national mortality data were linked, documenting all-cause mortality and non-fatal MI. Of the 7543 linked patients, 4650 were prescribed clopidogrel in primary care within 3 months of discharge. The adjusted odds of still being prescribed clopidogrel at 12 months were similar following non-ST-elevation myocardial infarction (NSTEMI) 53% (95% CI, 51–55) and ST-elevation myocardial infarction (STEMI) 54% (95% CI, 52–56), but contrast with statins: NSTEMI 84% (95% CI, 82–85) and STEMI 89% (95% CI, 87–90). Discontinuation within 12 months was more frequent in older patients [>80 vs. 40–49 years, adjusted hazard ratio (HR) 1.50 (95% CI, 1.15–1.94)] and with bleeding events [HR 1.34 (95% CI, 1.03–1.73)]. 18.15 patients per 100 person-years (95% CI, 16.83–19.58) died or experienced non-fatal MI in the first year following discharge. In patients who discontinued clopidogrel within 12 months, the adjusted HR for death or non-fatal MI was 1.45 (95% CI, 1.22–1.73) compared with untreated patients, and 2.62 (95% CI, 2.17–3.17) compared with patients persisting with clopidogrel treatment.
Conclusion
This is the first study to use linked registries to determine persistence of clopidogrel treatment after MI in primary care. It demonstrates that discontinuation is common and associated with adverse outcomes.
Keywords: ACS, Myocardial infarction, Clopidogrel, Anti-platelet, Observational
Introduction
Dual anti-platelet therapy with aspirin and clopidogrel reduces the risk of thrombotic events and is recommended in the management of acute coronary syndromes.1–4 National Institute for Health and Clinical Excellence (NICE) guideline recommendations are for treatment with clopidogrel to continue for 12 months after non-ST-elevation myocardial infarction (NSTEMI),1 but for only 30 days or 3 months after ST-elevation MI (STEMI), increasing to 12 months if a drug-eluting stent has been deployed.1,2 However, the optimal duration of clopidogrel treatment after coronary stenting is still in doubt5 and late stent thrombosis may occur even with discontinuation beyond 12 months.6 Of greater concern is the hazard of premature discontinuation of clopidogrel and recent data have shown that in patients who do not fill their prescriptions early after hospital discharge the risk of MI or death is significantly increased.7 This takes no account of the potential risk associated with discontinuation of treatment later after discharge, although there is little information available about patient adherence to clopidogrel therapy after MI. Data for statin therapy, however, suggest that discontinuation of evidence-based therapy is not uncommon during the first 12 months and may have important clinical consequences.8
The Myocardial Ischaemia National Audit Project (MINAP) registry in the UK records data, including discharge medications, for patients with MI in all 230 acute hospital trusts in England and Wales.9 Information on continuing treatment in primary care is available from prescription records within the General Practice Research Database (GPRD) that contains the complete anonymized primary care medical records for general practices that are registered with a panel of general practices and use Vision software (8% of the UK population).10 In order to document relations between clopidogrel discontinuation and coronary events in the 12 months after NSTEMI and STEMI, we have linked the MINAP and GPRD registries, and documented outcomes through further linkage with the Hospital Episodes Statistics register and the Office of National Statistics Mortality Data. Our specific objectives were (i) to define the extent to which clopidogrel treatment (initiated in hospital) is continued in primary care, (ii) to identify factors predictive of clopidogrel discontinuation, and (iii) to analyse the hazard of death or recurrent MI associated with clopidogrel discontinuation.
Methods
This is a retrospective observational cohort study using linked data from the MINAP, GPRD, Hospital Episode Statistics (HES), and death certificates.9,10 The protocol was reviewed by independent scientific committees with experience in GPRD and MINAP.
Linkage
In England, for each hospitalized patient, HES records dates of admission and discharge and main diagnoses. For the linkage, a Trusted Third Party used patient identifiers, including the patient's NHS number, date of birth, and postcode to link GPRD to MINAP, HES, and death certificates. After this linkage, all the patient identifiers were removed and researchers had access only to anonymized information. The data were linked for 224 of 592 (38%) GPRD practices, as the linkage was restricted to those practices in England that had already agreed to participate in linkage at the time of the study. However, among participating practices, all patients were included in the linkage.
Study population
The study population includes patients identified in the MINAP dataset, aged ≥40, who were hospitalized for acute coronary syndrome (ACS) from 2003 to 2009, with a discharge diagnosis of STEMI, NSTEMI, it excludes ACS without troponin elevation (unstable angina). Patients who were discharged alive from the hospital to home were followed from the date of hospital discharge up to the patient's death, transfer out of the general practice, or the last GPRD data collection date available for this study.
The MINAP data were used to describe the in-hospital treatment strategy and diagnosis (based on the discharge diagnosis, cardiac biomarkers, and electrocardiograms). The GPRD data were used to describe age at discharge, gender, alcohol use, history of diabetes mellitus, smoking status, body mass index, socioeconomic deprivation status by quintiles,11 history of angina, MI, and ACS (at least 1 month prior to the discharge date), lipid levels, and blood pressure measurements (in 6 months prior to the discharge date). Counts and percentages and means and standard deviations were calculated as appropriate. These descriptive analyses were presented separately for those patients who did or did not receive a GP prescription for clopidogrel within 3 months of hospital discharge.
Clopidogrel prescribing and discontinuation
Logistic regression was used to determine the characteristics associated with GP prescribing of clopidogrel within 3 months of hospital discharge. Characteristics included the in-hospital presentation (blood pressure and heart rate), treatment strategy (reperfusion strategy and coronary intervention), and diagnosis recorded in MINAP, demographics (age, gender, socioeconomic status, calendar year, and geographical region), medical history (diabetes mellitus and angina/MI), prior GP prescribing (clopidogrel, aspirin, and warfarin), and lifestyle behaviours (alcohol consumption, smoking status, and body mass index) recorded in GPRD, and prior hospitalizations (all cause and cardiovascular related) recorded in HES. If there were no records of body mass index, smoking status, or alcohol consumption, indicators of missingness were used. Odds ratios and 95% confidence intervals were calculated. As statins are routinely prescribed following ACS, information on statin discontinuation was used to compare clopidogrel discontinuation with another evidence-based treatment. Discontinuation of clopidogrel or statin was defined as no prescriptions for the medication within a period defined as the estimated duration of the prescription plus a grace period of 91 days. Cox regression was used to assess the same set of characteristics for association with clopidogrel discontinuation within the first year of discharge, with the additional time-dependent characteristic of any bleeding within the treatment period. Clopidogrel prescription on hospital discharge was included only in the MINAP dataset after 2005, and had no duration information associated with it, so this parameter was not analysed in this study. Bleeding was defined as any bleeding event (major or minor) in the patient's GPRD or HES record. Hazard ratios (HRs) and 95% confidence intervals were calculated.
Outcomes
Patients were followed for up to 1 year for incidences of subsequent MI (as recorded in MINAP, GPRD, or HES) or death (as recorded in the national death certificate data). Descriptive Cox regression was used to identify the characteristics associated with these outcomes. Characteristics included discharge diagnosis, age, gender, socioeconomic status, medical history, calendar year, region, and clopidogrel treatment. Clopidogrel treatment following discharge was entered into the model as a time-dependent covariate, with the following categories: no treatment, 0 to <3 months use, 3 to <11 months use, 11+ months use, and discontinued use. This allowed a patient to move from one categorization of clopidogrel exposure to another over time.
All data were analysed using Stata version 11.1 (Copyright 2009 StataCorp LP, College Station, TX, USA).
Results
Patients
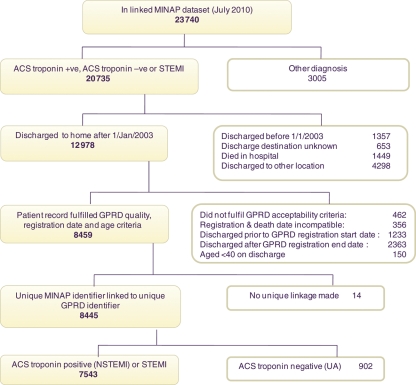
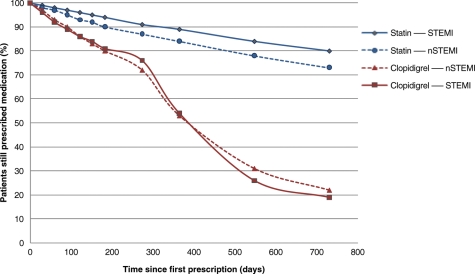
The patient flow diagram (Figure 1) shows the number of patients included in the study, with reasons for exclusion. A total of 23 740 patients in the linked MINAP–GPRD dataset included those with non-ACS diagnoses and those outside the study period, and those not meeting the inclusion criteria (Figure 1): 7543 met inclusion criteria for this study. Specifically, these were patients aged ≥40, discharged from hospital to home from 2003 onwards with a diagnosis of NSTEMI or STEMI. Patient characteristics, stratified by discharge diagnosis and receipt of a primary care prescription for clopidogrel within 3 months of discharge, are shown in Tables 1 and 2. Only 68.6% of patients with NSTEMI, and 62.8% of patients with STEMI, received a primary care clopidogrel prescription in the first 3 months. Clopidogrel discontinuation, after the first prescription, was substantially more frequent compared with statins, both for NSTEMI and for STEMI (Figure 2).
Figure 1.
Study population disposition.
Table 1.
Demographics and medical history of patients by discharge diagnosis and primary care clopidogrel prescription within 3 months
| NSTEMI |
STEMI |
|||
|---|---|---|---|---|
| No clopidogrela (n = 1288) | Clopidogrela (n = 2820) | No clopidogrela (n = 1085) | Clopidogrela (n = 1830) | |
| Age (years), n (%) | ||||
| 40–49 | 42 (3.3) | 158 (5.6) | 91 (8.4) | 209 (11.4) |
| 50–59 | 98 (7.6) | 401 (14.2) | 216 (19.9) | 403 (22.0) |
| 60–69 | 198 (15.4) | 576 (20.4) | 249 (22.9) | 521 (28.5) |
| 70–79 | 417 (32.4) | 794 (28.2) | 298 (27.5) | 416 (22.7) |
| 80+ | 533 (41.4) | 891 (31.6) | 231 (21.3) | 281 (15.4) |
| Gender, n (%) | ||||
| Male | 738 (57.3) | 1777 (63.0) | 765 (70.5) | 1309 (71.5) |
| Female | 550 (42.7) | 1043 (37.0) | 320 (29.5) | 521 (28.5) |
| Smoking status, n (%) | ||||
| Non-smoker | 450 (34.9) | 907 (32.2) | 333 (30.7) | 489 (26.7) |
| Ex smoker | 539 (41.8) | 1220 (43.3) | 329 (30.3) | 659 (36.0) |
| Smoker | 254 (19.7) | 634 (22.5) | 363 (33.5) | 626 (34.2) |
| Unknown smoking status | 45 (3.5) | 59 (2.1) | 60 (5.5) | 56 (3.1) |
| Obesity, n (%) | ||||
| Underweight (BMI <20) | 63 (4.9) | 130 (4.6) | 52 (4.8) | 53 (2.9) |
| Normal (BMI 20 to <25) | 360 (28.0) | 707 (25.1) | 319 (29.4) | 474 (25.9) |
| Overweight (BMI 25 to <30) | 462 (35.9) | 1125 (39.9) | 428 (39.4) | 763 (41.7) |
| Obese (BMI >30) | 297 (23.1) | 717 (25.4) | 225 (20.7) | 446 (24.4) |
| Unknown BMI | 106 (8.2) | 141 (5.0) | 61 (5.6) | 94 (5.1) |
| Socioeconomic deprivation, n (%) | ||||
| 0 (least deprived quintile) | 169 (13.1) | 419 (14.9) | 160 (14.7) | 292 (16.0) |
| 1 | 285 (22.1) | 583 (20.7) | 241 (22.2) | 386 (21.1) |
| 2 | 293 (22.7) | 589 (20.9) | 248 (22.9) | 367 (20.1) |
| 3 | 274 (21.3) | 606 (21.5) | 215 (19.8) | 375 (20.5) |
| 4 (most deprived quintile) | 267 (20.7) | 623 (22.1) | 221 (20.4) | 410 (22.4) |
| History of diabetes | 320 (24.8) | 624 (22.1) | 134 (12.4) | 212 (11.6) |
| Prior medical history, n (%) | ||||
| No history of angina or MI | 724 (56.2) | 1672 (59.3) | 876 (80.7) | 1526 (83.4) |
| History of stable angina only | 417 (32.4) | 836 (29.6) | 129 (11.9) | 180 (9.8) |
| History of unstable angina only | 22 (1.7) | 48 (1.7) | 5 (0.5) | 12 (0.7) |
| History of MI | 125 (9.7) | 264 (9.4) | 75 (6.9) | 112 (6.1) |
| Prior medication in primary care, n (%) | ||||
| Clopidogrel | 94 (7.3) | 339 (12.0) | 20 (1.8) | 84 (4.6) |
| Aspirin | 624 (48.4) | 1339 (47.5) | 270 (24.9) | 442 (24.2) |
| Antiplatelet | 667 (51.8) | 1477 (52.4) | 283 (26.1) | 475 (26.0) |
| Warfarin | 190 (14.8) | 113 (4.0) | 47 (4.3) | 41 (2.2) |
| Test results in 6 months prior to event | ||||
| Total cholesterol measured | ||||
| n (%) | 495 (38.4) | 1206 (42.8) | 356 (32.8) | 634 (34.6) |
| Mean (SD) | 4.7 (1.3) | 4.9 (1.3) | 5.2 (1.3) | 5.2 (1.7) |
| HDL cholesterol measured | ||||
| n (%) | 321 (24.9) | 813 (28.8) | 206 (19.0) | 408 (22.3) |
| Mean (SD) | 1.4 (0.4) | 1.3 (0.4) | 1.3 (0.4) | 1.3 (0.4) |
| LDL cholesterol measured | ||||
| n (%) | 231 (17.9) | 620 (22.0) | 155 (14.3) | 312 (17.0) |
| Mean (SD) | 2.7 (1.0) | 2.8 (1.1) | 3.1 (1.1) | 3.0 (1.1) |
| Triglycerides measured | ||||
| n (%) | 319 (24.8) | 797 (28.3) | 206 (19.0) | 416 (22.7) |
| Mean (SD) | 1.7 (1.2) | 1.7 (1.1) | 1.8 (1.0) | 1.8 (1.2) |
| Systolic BP measured | ||||
| n (%) | 962 (74.7) | 2008 (71.2) | 598 (55.1) | 1014 (55.4) |
| Mean (SD) | 138.9 (21.8) | 139.5 (21.7) | 141.0 (21.0) | 139.3 (20.3) |
| Diastolic BP measured | ||||
| n (%) | 962 (74.7) | 2008 (71.2) | 598 (55.1) | 1014 (55.4) |
| Mean (SD) | 76.0 (12.3) | 77.2 (12.5) | 79.6 (11.2) | 79.7 (12.2) |
aWhether or not a patient was prescribed clopidogrel in primary care within 3 months of ACS event.
Table 2.
Interventions performed during admission for index myocardial infarction: non-ST-elevation myocardial infarction or ST-elevation myocardial infarction discharge diagnosis and primary care clopidogrel prescription within 3 months
| Characteristics | NSTEMI, n (%) |
STEMI, n (%) |
||
|---|---|---|---|---|
| No clopidogrel (n = 1288) | Clopidogrel (n = 2820) | No clopidogrel (n = 1085) | Clopidogrel (n = 1830) | |
| No reperfusion treatment | 1234 (95.8) | 2699 (95.7) | 172 (15.9) | 374 (20.4) |
| In-hospital thrombolytic treatment | 27 (2.1) | 44 (1.6) | 779 (71.8) | 865 (47.3) |
| Primary PCI | 0 (0.0) | 9 (0.3) | 30 (2.8) | 352 (19.2) |
| Other, blank, or unknown | 27 (2.1) | 68 (2.4) | 106 (9.6) | 239 (13.1) |
| No coronary intervention performed | 1119 (86.9) | 1966 (69.7) | 949 (87.5) | 855 (46.7) |
| PCI | 53 (4.1%) | 574 (20.4%) | 63 (5.8%) | 740 (40.4%) |
| CABG | 31 (2.4%) | 10 (0.4%) | 7 (0.6%) | 9 (0.5%) |
| Other, blank, or unknown | 85 (6.6%) | 270 (9.6%) | 66 (6.1%) | 226 (12.3%) |
Figure 2.
Discontinuation of clopidogrel and statin prescribing in primary care following non-ST-elevation myocardial infarction or ST-elevation myocardial infarction.
Clopidogrel prescribing
Clopidogrel prescription within first 3 months: temporal trends
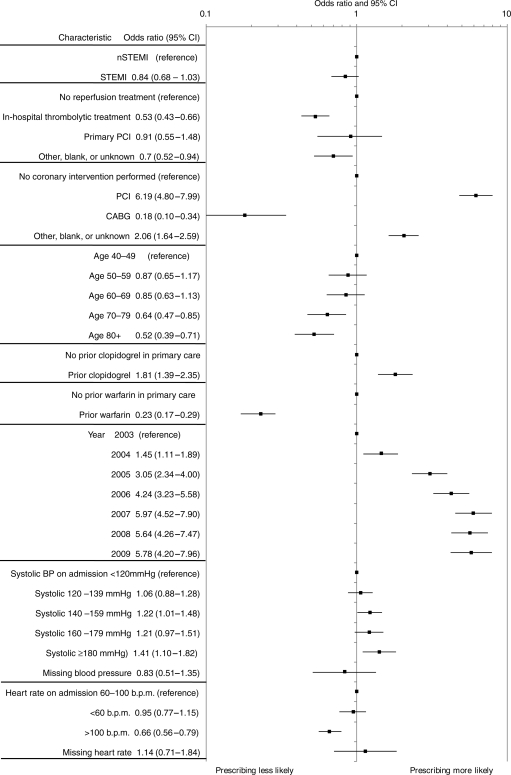
The adjusted odds of receiving a primary care clopidogrel prescription (Figure 3) in the first 3 months increased from 1.45 (95% CI, 1.11–1.89) in 2004 to 5.78 (95% CI, 4.20–7.96) in 2009. The odds were higher in younger age groups, and decreased progressively with advancing age: age 70–79 years, 0.64 (95% CI, 0.47–0.85); 80+ years, 0.52 (95% CI, 0.39–0.71) compared with age 40–49 years. In patients managed with percutaneous intervention during the hospital admission, the odds of receiving a clopidogrel prescription were substantially higher, 6.19 (4.80–7.99), compared with patients in whom no coronary intervention was performed. Clopidogrel prescribing increased over time from 35% in 2003 to 79% in 2009.
Figure 3.
Characteristics associated with primary care clopidogrel prescribing within 3 months of hospital discharge.
Clopidogrel discontinuation during first year
In patients prescribed clopidogrel in the 3 months following discharge (NSTEMI, 2820; STEMI, 1830), the odds of a patient still being prescribed clopidogrel at 12 months were similar for NSTEMI [53% (95% CI, 51–55)] and STEMI [54% (95% CI, 52–56)] patients. However, clopidogrel prescribing, in the same patients, declined more rapidly than that for statins: statin prescribing in NSTEMI was 84% (95% CI, 82–85) and in STEMI was 89% (95% CI, 87–90).
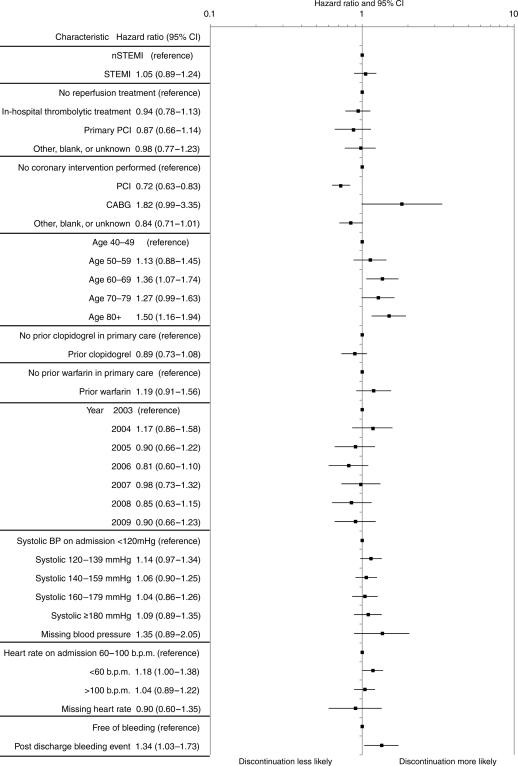
The adjusted HRs for clopidogrel discontinuation showed no significant change during the study period (Figure 4). The hazard for discontinuation was higher in patients aged >80 [HR 1.50 (95% CI, 1.16–1.94)], and those who had a bleeding event after leaving hospital [HR 1.34 (95% CI, 1.03–1.73)]. The adjusted hazard for clopidogrel discontinuation differed in those following in-hospital percutaneous coronary intervention (PCI) [HR 0.72 (95% CI, 0.63–0.83)] and no in-hospital PCI (Figure 4) but discontinuation tended to be more frequent following coronary artery bypass graft (CABG) [HR 1.82 (95% CI, 0.99–3.35)].
Figure 4.
Characteristics associated with discontinuation of primary care clopidogrel prescribing within 1 year of hospital discharge.
Bleeding events
In patients prescribed clopidogrel in primary care within 3 months of discharge, the rates of bleeding per 100 patient-years were similar following NSTEMI 12.03 (95% CI, 10.62–13.64) and STEMI 10.55 (95% CI, 8.96–12.42); overall 11.44 (95% CI, 10.36–12.63).
Death and non-fatal myocardial infarction
In patients prescribed clopidogrel in primary care, the rate of death and non-fatal MI in the first year post discharge (per 100 person-years) was 18.15 (95% CI, 16.83–19.58) overall; 22.42 (95% CI, 20.54–24.48) following NSTEMI, 11.69 (95% CI, 10.07–13.57) following STEMI. In those who did not receive a prescription within 3 months, the rate was 36.69 (95% CI, 34.14–39.41) overall; 48.42 (95% CI, 44.43–52.76) following NSTEMI, and 23.55 (95% CI, 20.67–26.83) following STEMI.
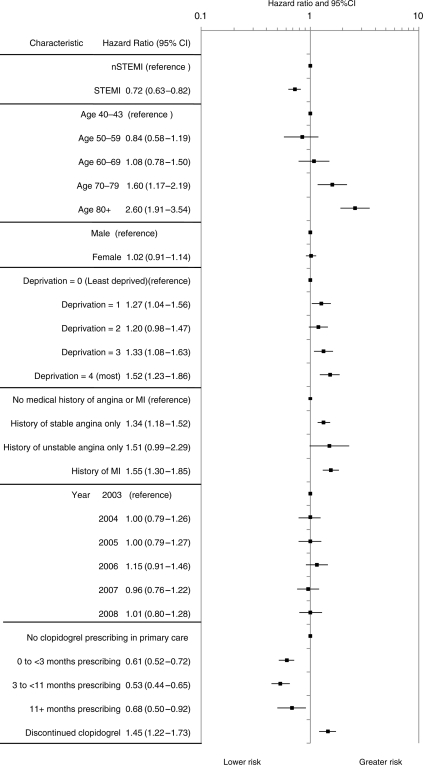
Adjusted HRs for death or non-fatal MI in the first year following discharge are shown in Figure 5. Hazard was higher for NSTEMI compared with STEMI and increased progressively with age and deprivation status. Clopidogrel prescribing was associated with a lower hazard of death or non-fatal MI compared with patients who received no prescriptions [months 0 to <3, HR 0.61 (95% CI, 052–0.72); months 3–11, HR 0.53 (95% CI, 0.44–0.65); 11+ months, HR 0.68 (95% CI, 0.50–0.92)]. This descriptive analysis found that clopidogrel discontinuation within 12 months was independently associated with an increased risk of death or MI both compared with untreated patients [HR 1.45 (1.22–1.73)] and with those patients persisting with clopidogrel treatment [HR 2.62 (2.17–3.17)].
Figure 5.
Characteristics associated with death or myocardial infarction in the first year post hospital discharge.
Discussion
This study provides the first analysis using linked national registries to define persistence with clopidogrel therapy among patients discharged from hospital after acute MI. The observations were compared with statin prescribing in the same population. The data show that clopidogrel persistence, as reflected by prescription rates, declines steeply in the first 12 months, particularly in older patients and those who have a bleeding event. An even steeper decline is observed after 12 months (Figure 2), consistent with many guideline recommendations.1–4
The findings contrast with the more gradual decline in statin prescribing in these patients. In this observational analysis, discontinuation of clopidogrel prescription after acute MI is associated with a significant increase in the risk of death or recurrent infarction during the first 12 months, even after adjustment for baseline variables. Nevertheless, the possibility exists that unmeasured variables may have influenced this association and the observation does not establish causality.
Guideline recommendations are for clopidogrel to continue for 12 months in patients with NSTEMI and in those patients with STEMI who receive drug-eluting stents.1–4 Yet, we found that in around half of all patients with NSTEMI, prescription of clopidogrel was discontinued within the first 12 months after discharge from hospital. However, discontinuation was less frequent in NSTEMI patients who underwent PCI intervention [HR 0.72 (95% CI, 0.63–0.83)]. The pattern was similar for STEMI and, regardless of the type of infarction, showed little year-on-year change during the course of the study. The trajectory of decline for clopidogrel prescription in the first 12 months was steeper than that for statins, suggesting that it was driven by factors additional to the patient-related influences associated with under-treatment (for example, general patient compliance issues). Thus, the relation between advanced age and the under-use of evidence-based treatment has widely been reported and was demonstrated for clopidogrel in the present study.12 However, side-effects and complications of treatment also influence prescribing, and our finding that bleeding events were associated with discontinuation of clopidogrel was not unexpected. Rates of major and non-major bleeding (NSTEMI 12 per 100 patient-years) were higher than observed in the original randomized trial (8.5% rate of all bleeds, for a median duration of 9 months), but this observational study reflects the spectrum of patients in clinical practice, rather than the trial population.13
The main finding in the present study was the association between clopidogrel discontinuation in the first 12 months and the outcome following either NSTEMI or STEMI. Clopidogrel prescription during this period was associated with a reduction in the hazard of death or non-fatal MI compared with patients who received no prescription, consistent with trial evidence of clopidogrel's prognostic benefit in patients with acute coronary syndromes.13 Importantly, among those patients prescribed clopidogrel who then had it discontinued, the hazard of death or non-fatal MI was significantly greater compared with patients who continued to receive a clopidogrel prescription. The hazards of death and MI (including the impact of withdrawal of anti-thrombotic therapy) associated with bleeding have been recognized in trial data14 and in the European Guidelines.4 For statin treatment, there is corresponding evidence indicating that the benefits of treatment may only be sustained through continued use of these drugs.8
Our findings of an increased hazard of death and non-fatal MI associated with discontinuation of clopidogrel are consistent with those of Ho et al.7 who reported that any delay in filling clopidogrel prescriptions after hospital discharge in patients treated with drug-eluting stents was associated with nearly twice the rate of coronary events during follow-up for 22 months. Again it was older patients who were more likely, than younger patients, to delay filling their prescriptions. Our findings are also consistent with other observational studies in US and Australian populations where discontinuation of clopidogrel therapy within a year of stent was associated with worse outcomes.15–17 Clopidogrel discontinuation was associated with bleeding. In all these situations, the adverse consequences of clopidogrel withdrawal are presumed mediated by changes in platelet activation triggering thrombotic events. It is plausible that similar mechanisms drove the association between clopidogrel discontinuation and adverse outcomes in the present study although in the absence of platelet function data and cause-specific mortality data the interpretations must be cautious.
A major strength of our study was the use of MINAP, the national registry of acute coronary syndromes, which we linked with GPRD to obtain longitudinal primary healthcare data with which to document outpatient prescription of clopidogrel and its relation to death and non-fatal infarction. An additional strength was the reference to statin prescribing in the same patients (Figure 2). These findings suggest that characteristics of the treatments, rather than the patients, were responsible for the differences observed.
Our study has important limitations which are inherent in any large registry analysis. Data on hospital clopidogrel prescribing, both during the admission and on discharge, were not comprehensively recorded and was not included in the study. Although GP prescription information was robust, we do not know whether clopidogrel was collected or taken by individual patients, or was prescribed by a third party. This limited the focus of the study to clopidogrel prescriptions within primary care. Also, we cannot determine whether the association between clopidogrel discontinuation and adverse events was causal or was confounded by the influence of unrecorded co-morbidities or bleeding events.13,16–18 The analysis undertaken was descriptive, rather than hypothesis testing, and further research, incorporating more complex statistical methodologies, is required to fully investigate the association found. Rates of intervention were lower in this study than contemporary studies outside the UK, and lower than the current rates in the UK.19,20 Primary PCI is now the dominant treatment for STEMI.19 At the time of this study, >80% of patients with STEMI received reperfusion treatment, but this was mainly with thrombolysis (Table 2). MINAP was designed to capture all patients with STEMI and NSTEMI, but it did not capture all patients with unstable angina, and such patients were excluded from this analysis.
Healthy user bias is difficult to avoid in studies of this type; however, similar healthy user bias will apply to prescribing of statins which showed a slower decline in prescribing than clopidogrel.18 Although our outcome analysis was adjusted for baseline factors that included prior coronary events and hospitalizations (all cause and cardiovascular), we do not know whether the patients who discontinued clopidogrel were predisposed to adverse outcomes through other mechanisms.
In conclusion, this study has demonstrated the feasibility of linking a nationwide in-hospital registry programme (MINAP) with general practitioner and outcome survival data. This is the first study to use linked registries to determine the prognostic associations of clopidogrel discontinuation in patients discharged from hospital after NSTEMI and STEMI. Our data show that discontinuation of clopidogrel during the first 12 months is common, particularly in older patients, and associated with an increased risk of death or recurrent MI. This study does not establish that the association between discontinuation and death or MI is causal. Nonetheless, discontinued prescribing of clopidogrel ahead of guideline recommendations must be treated with caution, and strategies to promote the appropriate use of secondary prevention treatment in this high-risk group of patients are required.
Authors’ contributions
All authors contributed to study design and interpretation of results. R.B. performed all analyses. K.A.A.F. chaired the steering group. The manuscript was reviewed and approved by all authors prior to submission.
Funding
This study was funded by AstraZeneca UK Ltd. A.T. received funding from AstraZeneca for sharing MINAP data used in this study. H.H. is funded by the UK National Institute for Health Research (RP-PG-0407–10314; http://www.nihr.ac.uk/) and the Wellcome Trust (086091/Z/08/Z) for research linking MINAP to primary care and investigator-led cohorts. Funding to pay the Open Access publication charges for this article was provided by AstraZeneca UK Ltd.
Conflict of interest: K.A.A.F. and members of his group have received grant or honoraria funding from Bayer, J&J, Lilly, and AstraZeneca. K.K.R. has received honoraria for lectures and consulting from Lilly/Daiichi and AstraZeneca. A.B. has received advisory, consultancy, and lecture fees from AstraZeneca and fees or travel grants from are Takeda, Menarini, Servier, MSD, Solvay, Pfizer, and Roche. A.T. is a committee member of the National Institute of Cardiac Outcomes Research (NICOR). H.H. is a committee member of NICOR. C.E. is a full-time employee of AstraZeneca, the manufacturer of ticagrelor. R.B. and T.P.v.S.: GPRD is owned by the UK Department of Health and operates within the Medicines and Healthcare products Regulatory Agency (MHRA). GPRD has received funding from the MHRA, Wellcome Trust, Medical Research Council, NIHR Health Technology Assessment programme, Innovative Medicine Initiative, UK Department of Health, Technology Strategy Board, Seventh Framework Programme EU, various universities, contract research organizations, and pharmaceutical companies. Department of Pharmacoepidemiology & Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, the private–public-funded Top Institute Pharma (www.tipharma.nl, includes co-funding from universities, government, and industry), the Dutch Medicines Evaluation Board, and the Dutch Ministry of Health.
References
- 1.NICE Clinical Guideline 94. Unstable angina and NSTEMI. The early management of unstable angina and non-ST-segment-elevation myocardial infarction. 2010. March http://www.nice.org.uk/nicemedia/live/12949/47921/47921.pdf. (1 June 2011) [PubMed]
- 2.NICE Clinical Guideline 48. MI: secondary prevention. 2007. May http://www.nice.org.uk/nicemedia/live/11008/30497/30497.pdf. (1 June 2011)
- 3.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. doi:10.1093/eurheartj/ehn526. [DOI] [PubMed] [Google Scholar]
- 4.Bassand J-P, Hamm CW, Ardissino D, Boersma E, Budaj A, Hasdai D, Fernandez-Aviles F, Fox KAA, Ohman EM, Wallentin L, Wijns W. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. doi:10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 5.Berger PB. Optimal duration of clopidogrel use after implantation of drug-eluting stents—still in doubt. N Engl J Med. 2010;362:1441–1443. doi: 10.1056/NEJMe1002553. doi:10.1056/NEJMe1002553. [DOI] [PubMed] [Google Scholar]
- 6.McFadden EP, Stabile E, Regar E, Cheneua E, Ong ATL, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of anti-platelet therapy. Lancet. 2004;354:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. doi:10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 7.Ho PM, Tsai TT, Maddox TM, Powers JD, Carroll NM, Jackevicius C, Go AS, Margolis KL, DeFor TA, Rumsfeld JS, Magid DJ. Delays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of care. Circ Cardiovasc Qual Outcomes. 2010;3:261–266. doi: 10.1161/CIRCOUTCOMES.109.902031. doi:10.1161/CIRCOUTCOMES.109.902031. [DOI] [PubMed] [Google Scholar]
- 8.Daskalopoulou SS, Delaney JAC, Filion KB, Brophy JM, Nancy E, Mayo NE, Suissa S. Discontinuation of statin therapy following an acute myocardial infarction: a population-based study. Eur Heart J. 2008;29:2083–2091. doi: 10.1093/eurheartj/ehn346. doi:10.1093/eurheartj/ehn346. [DOI] [PubMed] [Google Scholar]
- 9.Herrett E, Smeeth L, Walker L, Weston C on behalf of the MINAP Academic Group. The Myocardial Ischaemia National Audit Project (MINAP) Heart. 2010;96:1264–1267. doi: 10.1136/hrt.2009.192328. doi:10.1136/hrt.2009.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkinson J, Davis S, van Staa TP. The General Practice Research Database (GPRD): now and the future. In: Mann R., editor. Pharmacovigilance. 2007. Chichester: Wiley-Blackwell; [Google Scholar]
- 11.Davey Smith G, Hart CL, Watt G, Hole DJ, Hawthorne VM. Individual social class, area-based deprivation, cardiovascular disease risk factors, and mortality: the Renfrew and Paisley Study. J Epidemiol Commun Health. 1998;52:399–405. doi: 10.1136/jech.52.6.399. ISSN 0143–005X doi:10.1136/jech.52.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen HL, Goldberg RG, Gore JM, Fox KAA, Eagle KA, Gurfinkel EP, Spencer FA, Reed G, Quill A, Anderson FA. Age and sex differences, and changing trends, in the use of evidence-based therapies in acute coronary syndromes: perspectives from a multinational registry. Coron Artery Dis. 2010;21:336–344. doi: 10.1097/MCA.0b013e32833ce07c. http://www.ncbi.nlm.nih.gov/pubmed/20661139. doi:10.1097/MCA.0b013e32833ce07c. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. doi:10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, Mehran R, Clayton TC, Nikolsky E, Parise H, Fahy M, Lansky AJ, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Stone GW. Prognostic modeling of individual patient risk and mortality impact of ischemic and hemorrhagic complications: assessment from the acute catheterization and urgent intervention triage strategy trial. Circulation. 2010;121:43–51. doi: 10.1161/CIRCULATIONAHA.109.878017. doi:10.1161/CIRCULATIONAHA.109.878017. [DOI] [PubMed] [Google Scholar]
- 15.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. J Am Med Assoc. 2007;297:159–168. doi: 10.1001/jama.297.2.joc60179. doi:10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 16.Wiederkehr D, Ogbonnaya A, Casciano R, Makenbaeva D, Mozaffari E, Corbelli J. Clinical impact of early clopidogrel discontinuation following acute myocardial infarction hospitalization or stent implantation: analysis in a nationally representative managed-care population. Curr Med Res Opin. 2009;25:2327–2334. doi: 10.1185/03007990903156087. doi:10.1185/03007990903156087. [DOI] [PubMed] [Google Scholar]
- 17.Butler MJ, Eccleston D, Clark DJ, Ajani AE, Andrianopoulous N, Brennan A, New G, Black A, Yan BP, Shaw JA, Dart AM, Duffy SJ. The effect of intended duration of clopidogrel use on early and late mortality and major adverse cardiac events in patients with drug-eluting stents. Am Heart J. 2009;157:899–907. doi: 10.1016/j.ahj.2009.02.018. doi:10.1016/j.ahj.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Lafleur J, Nelson RE, Sauer BC, Nebeker JR. Overestimation of the effects of adherence on outcomes: a case study in healthy user bias and hypertension. Heart. doi: 10.1136/hrt.2011.223289. doi:10.1136/hrt.2011.223289. Published online ahead of print 17 May 2011. [DOI] [PubMed] [Google Scholar]
- 19.2010. Myocardial Ischaemia National Audit Project (MINAP) Public Report http://www.ucl.ac.uk/nicor/audits/minap/publicreports .
- 20.Gale CP, Manda SOM, Batin PD, Weston CF, Birkhead JS, Hall AS. Predictors of in-hospital mortality for patients admitted with ST-elevation myocardial infarction: a real-world study using the Myocardial Infarction National Audit Project (MINAP) database. Heart. 2008;94:1407–1412. doi: 10.1136/hrt.2007.127068. doi:10.1136/hrt.2007.127068. [DOI] [PubMed] [Google Scholar]