These data provide evidence that quantitative measures of lung structural changes identified by using volumetric CT are associated with the frequency of chronic obstructive pulmonary disease exacerbation, a clinical outcome of public health importance.
Abstract
Purpose:
To test the hypothesis—given the increasing emphasis on quantitative computed tomographic (CT) phenotypes of chronic obstructive pulmonary disease (COPD)—that a relationship exists between COPD exacerbation frequency and quantitative CT measures of emphysema and airway disease.
Materials and Methods:
This research protocol was approved by the institutional review board of each participating institution, and all participants provided written informed consent. One thousand two subjects who were enrolled in the COPDGene Study and met the GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria for COPD with quantitative CT analysis were included. Total lung emphysema percentage was measured by using the attenuation mask technique with a −950-HU threshold. An automated program measured the mean wall thickness and mean wall area percentage in six segmental bronchi. The frequency of COPD exacerbation in the prior year was determined by using a questionnaire. Statistical analysis was performed to examine the relationship of exacerbation frequency with lung function and quantitative CT measurements.
Results:
In a multivariate analysis adjusted for lung function, bronchial wall thickness and total lung emphysema percentage were associated with COPD exacerbation frequency. Each 1-mm increase in bronchial wall thickness was associated with a 1.84-fold increase in annual exacerbation rate (P = .004). For patients with 35% or greater total emphysema, each 5% increase in emphysema was associated with a 1.18-fold increase in this rate (P = .047).
Conclusion:
Greater lung emphysema and airway wall thickness were associated with COPD exacerbations, independent of the severity of airflow obstruction. Quantitative CT can help identify subgroups of patients with COPD who experience exacerbations for targeted research and therapy development for individual phenotypes.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.11110173/-/DC1
Introduction
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are increasingly being recognized as a major and increasing burden to both patients with COPD and society in general. These exacerbations are associated with impaired quality of life (1,2), a more rapid decline in lung function (3,4), and higher mortality (5). The majority of the estimated $49.9 billion spent to address COPD in the United States in 2010 was directed toward the treatment of exacerbations—those necessitating hospitalization, in particular (6). Being able to predict which patients are at the greatest risk for acute COPD exacerbations will enable health care providers to better target these individuals for preventive therapy. COPD traditionally has been clinically staged by using spirometry, which yields a gross measure of overall lung function. While the frequency of COPD exacerbations increases with spirometrically measured airflow obstruction (5), spirometry is inadequate as the sole procedure for risk assessment of COPD exacerbations. For example, there are subsets of patients who have a severely reduced percent predicted forced expiratory volume in the first second of expiration (FEV1) but do not experience frequent exacerbations (7).
Through advances in multidetector computed tomographic (CT) technology that permit whole-lung imaging in a single breath hold and the application of quantitative metrics, CT is becoming an increasingly important tool for evaluating smoking-related lung disease. Quantitative CT metrics of COPD include measures of emphysema severity, airway wall thickness, and airtrapping. These measurements can be performed globally for both lungs together or refined to an individual lung or lung region.
The association between COPD exacerbation frequency and quantitative CT metrics has been preliminarily explored. We previously reported, for a small cohort of 34 patients, that a visual semiquantitative measurement of bronchial thickness was associated with increased COPD exacerbation frequency, after adjustment for FEV1 percent predicted (8). However, it is unclear how these measures perform in a larger more diverse population of patients with COPD. Given the increasing emphasis on quantitative CT COPD phenotypes, we sought to test the hypothesis that a relationship exists between COPD exacerbation frequency and quantitative CT measures of emphysema and airway disease.
Materials and Methods
Patient Selection
This research protocol was approved by the institutional review board at each participating institution, and all participants provided written informed consent. The study population was recruited from among participants in the COPDGene Study (http://www.copdgene.org/), an ongoing National Heart Lung and Blood Institute–funded multicenter observational study designed to identify the genetic factors associated with COPD and involving 21 centers in the United States (clinical trial registration no. NCT00608764) (9). Subjects aged 45–80 years with a 10 or greater pack-year history of cigarette smoking underwent spirometry and whole-lung volumetric multidetector CT. The study group consisted of all subjects from the first 2500 subjects documented in the April 2010 COPDGene data set who (a) were former or current smokers, (b) met the Global Initiative for Chronic Obstructive Lung Disease criteria (10) for stage 1 or higher fixed airflow obstruction with a postbronchodilator FEV1-to–forced vital capacity ratio lower than 0.7, and (c) had complete quantitative CT measurements of emphysema and airway wall thickness. All subjects were required to have experienced the COPD exacerbation 1 month or longer before enrolling in the study or undergoing imaging.
Data Collection and Exacerbation Determination
Demographic data and the smoking and medical histories were collected by using self-administered questionnaires. Self-reported acute exacerbation frequency was quantified with the following question: “Have you had a flare-up of your chest trouble in the last 12 months?” Zero exacerbations were recorded if the answer was no. If the answer was yes, the subject was prompted with the question, “How was the flare-up treated?” If the respondent reported that any of the following had occurred during an episode, this was counted as one exacerbation: “Took additional antibiotic or steroid medication which you keep at home, “Consulted your doctor who prescribed additional antibiotic and/or steroid treatment, but did not admit you to the hospital,” or “Admitted to hospital.” Respondents were allowed to describe between one and six such episodes.
Physiologic Testing
Patients underwent spirometry, which was performed by using the EasyOne spirometry system (NDD, Zurich, Switzerland) before and after the administration of albuterol, a short-acting bronchodilating medication (9). Quality control was performed for all spirometric tests by using both an automated system and manual review.
CT and Quantitative Analysis
Whole-lung volumetric multidetector CT was performed at full inspiration by using a standardized protocol (9), and the lower-spatial-resolution smooth reconstruction algorithm was used for quantitative analysis. Complete details of the CT protocol are outlined in Tables E1–E5 (online). Quantitative analysis of emphysema severity was performed on segmented lung images by using the Slicer software package (http://www.slicer.org/). By using a region-growing approach, the tracheobronchial tree was extracted from a seed point automatically placed in the lumen of the trachea. After segmentation of the central airways, the left and right lungs were automatically identified and segmented from the chest (11). The total emphysema percentage was defined as all lung voxels with a CT attenuation value of less than −950 HU. Automated airway analysis was performed by using the VIDA Pulmonary Workstation, version 2.0 (Vida Diagnostics, Coralville, Iowa, http://www.vidadiagnostics.com/), with use of segmentation methods that have been validated with manual techniques (11). Morphologic measurements were obtained along the center line of the lumen, in the middle third of the airway segment. Airway wall thickness and wall area percentage (100 × [wall area/total bronchial area]) were used as the quantitative CT measures of airway wall thickness (12). Each parameter was measured in one segmental airway of each lung lobe, with the lingula included as a separate lobe: apical segment, right upper lobe; lateral segment, right middle lobe; posterior basal segment, right lower lobe; apicoposterior segment, left upper lobe; superior lingula segment; and posterior basal segment, left lower lobe (13). The mean value across all six lobes was used for analysis. The CT images used in this study were anonymized in compliance with the Health Insurance Portability and Accountability Act.
Statistical Analyses
The three-dimensional smoothed surface plot of the raw data was created with SAS, version 9.2, statistical software (SAS Institute, Cary, NC) by using the PROC G3GRID procedure, with which spline interpolation is used as the smoothing algorithm. Visual inspection of the surface plot was used to explore cutoff points for emphysema percentage and wall thickness. Multivariate analysis was used to evaluate the relationship between quantitative CT metrics and number of exacerbations in the prior year, with a zero-inflated Poisson distribution used to account for excess zeros. All models were adjusted for the scanner model by using dummy variable representation for scanner type. This adjustment was performed with recognition of the comparable but not identical nature of the CT images acquired by using different brands of scanners. We also adjusted for patient age, sex, smoking status, and FEV1 percent predicted. The forward selection regression technique was used to determine the significance of emphysema percentage, wall area percentage, and wall thickness in the multivariate model. Student t tests for continuous variables and χ2 tests for categorical variables were performed with SAS, version 9.1, statistical software (SAS Institute) to compare groups; the results are given in Tables 1 and 2. Zero-inflated Poisson regression analyses were performed by using the R 2.8.1 statistical computation system (http://www.r-project.org/).
Table 1.
Demographic Data and Spirometric and Radiologic Measurements Stratified by COPD Exacerbation Frequency in Year before Study Enrollment
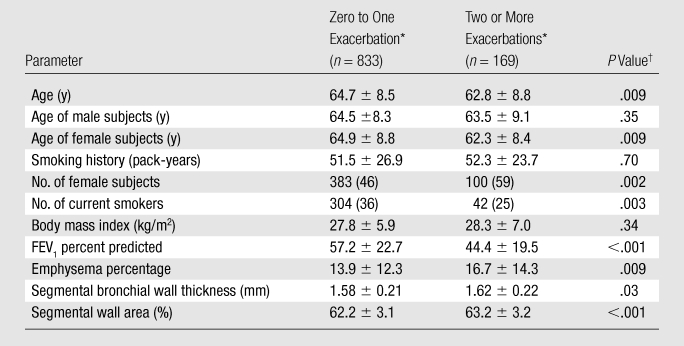
Unless otherwise noted, data are means ± standard deviations of the mean. Numbers in parentheses are percentages of subjects.
P values from Student t test (for continuous variables) or χ2 test (for categorical variables) comparisons between the exacerbation groups.
Table 2.
Characteristics Compared between Emphysema-Predominant and Airway-Predominant COPD Subject Groups
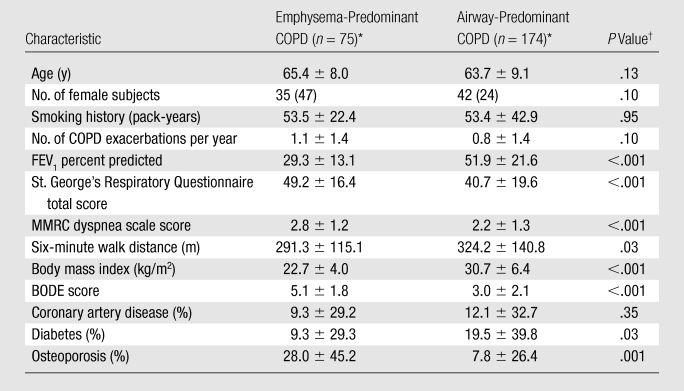
Note.—Emphysema-predominant COPD = 35% or greater emphysema and bronchial wall thickness of less than 1.75 mm. Airway-predominant COPD = less than 35% emphysema and bronchial wall thickness of 1.75 mm or greater. BODE = body-mass index, degree of airflow obstruction, dyspnea, and exercise capacity (as measured in a 6-minute walk test). MMRC = Modified Medical Research Council.
Unless otherwise noted, data are means ± standard deviations of the mean. Numbers in parentheses are percentages of subjects.
P values from Student t test (for continuous variables) or χ2 test (for categorical variables) comparisons between the COPD groups.
Results
Baseline demographic data on the 1002 participating subjects from the COPDGene Study are presented in Table 1. These data are stratified by exacerbation frequency (zero to one vs two or more exacerbations), as data from the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) Study suggest that a history of two or more annual exacerbations represents a stable frequent exacerbation phenotype (14). The mean overall exacerbation frequency was 0.68 exacerbation per year. One hundred ninety-two subjects reported experiencing one exacerbation; 84 subjects, two exacerbations; and 85 subjects, three or more exacerbations. Two or more exacerbations in the prior year were reported by 169 (17%) of the 1002 subjects. Compared with the 833 subjects who experienced zero to one exacerbation in the prior year, these 169 subjects were younger (mean age, 62.8 vs 64.7 years; P = .009), more frequently women (59% vs 46%, P = .002), and less frequently current smokers (25% vs 36%, P = .003), and they had a lower FEV1 percent predicted (mean, 44.4% vs 57.2%; P < .001). In addition, the subjects with two or more exacerbations had a higher total lung emphysema percentage (mean, 16.7% vs 13.9%; P = .009), thicker airway walls (mean thickness, 1.62 vs 1.58 mm; P = .03), and a greater segmental wall area percentage (mean, 63.2% vs 62.2%; P < .001). There were no significant differences in smoking history (in number of pack-years) (mean, 52.3 vs 51.5 pack-years, P = .70) or body mass index (mean, 28.3 vs 27.8 kg/m2; P = .34) between the groups.
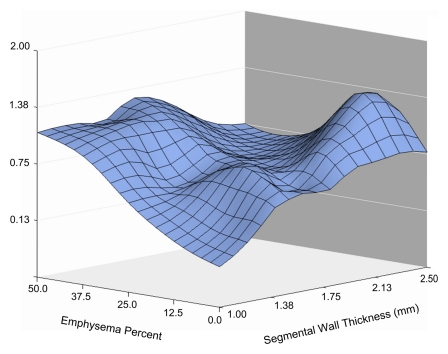
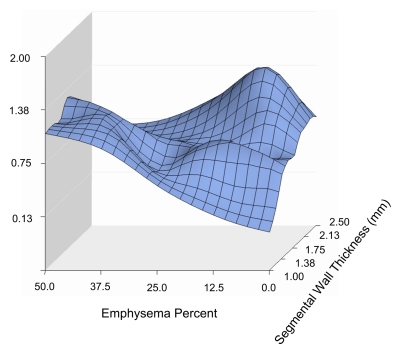
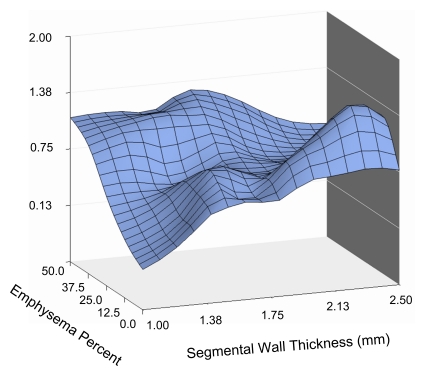
The three-dimensional surface plot in Fig 1a illustrates the relationship between bronchial wall thickness, emphysema percentage, and COPD exacerbation frequency. As Figure 1a shows, the relationships are not completely linear, and both emphysema and bronchial wall thickness appear to influence exacerbation frequency. Rotating the same plot (Fig 1b) reveals that high levels of emphysema are associated with greater exacerbation frequency and that wall thickness is more important at lower levels of emphysema. Further rotation of the plot (Fig 1c) reveals that exacerbation frequency is generally higher with greater wall thickness and that the severity of emphysema becomes important at low bronchial wall thickness levels. These data suggest that the factors driving exacerbations in patients with severe emphysema may be different from those driving exacerbations in patients with severe airway disease.
Figure 1a:
Three-dimensional surface plots demonstrate relationships between emphysema percentage, wall thickness, and COPD exacerbation frequency (vertical axis). Different panels show the plot rotated to allow appreciation of different aspects. (a) Surface view of entire relationship shows the largely independent effects of emphysema percentage and segmental wall thickness. (b) Horizontal view emphasizes the increased exacerbation frequency at greater levels of emphysema. Also note the very strong effect of bronchial wall thickness at low levels of emphysema. (c) Depth view emphasizes relationship between increasing wall thickness and exacerbation frequency at low levels of emphysema.
Figure 1b:
Three-dimensional surface plots demonstrate relationships between emphysema percentage, wall thickness, and COPD exacerbation frequency (vertical axis). Different panels show the plot rotated to allow appreciation of different aspects. (a) Surface view of entire relationship shows the largely independent effects of emphysema percentage and segmental wall thickness. (b) Horizontal view emphasizes the increased exacerbation frequency at greater levels of emphysema. Also note the very strong effect of bronchial wall thickness at low levels of emphysema. (c) Depth view emphasizes relationship between increasing wall thickness and exacerbation frequency at low levels of emphysema.
Figure 1c:
Three-dimensional surface plots demonstrate relationships between emphysema percentage, wall thickness, and COPD exacerbation frequency (vertical axis). Different panels show the plot rotated to allow appreciation of different aspects. (a) Surface view of entire relationship shows the largely independent effects of emphysema percentage and segmental wall thickness. (b) Horizontal view emphasizes the increased exacerbation frequency at greater levels of emphysema. Also note the very strong effect of bronchial wall thickness at low levels of emphysema. (c) Depth view emphasizes relationship between increasing wall thickness and exacerbation frequency at low levels of emphysema.
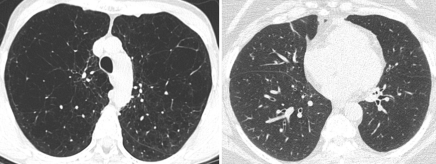
The appearances of the surface graphs were used to define two CT phenotypes of subjects who experienced increased exacerbations: an emphysema-predominant group (≥35% emphysema, <1.75-mm bronchial wall thickness) and an airway-predominant group (<35% emphysema, ≥1.75-mm bronchial wall thickness). A segmental wall thickness of 1.75 mm was chosen on the basis of the inspection of the surface graph. CT scans obtained in subjects representative of the two CT phenotype groups are shown in Figure 2, and the characteristics of these two groups are presented in Table 2. Both groups demonstrated a higher COPD exacerbation frequency than did the subjects with less than 35% emphysema and a wall thickness of less than 1.75 mm, who reported experiencing a mean of 0.62 exacerbation per year compared with means of 1.1 exacerbations per year reported by the emphysema-predominant group (P < .0001) and 0.8 exacerbation per year reported by the airway-predominant group (P = .07). The BODE (body-mass index, degree of airflow obstruction, dyspnea, and exercise capacity as measured in a 6-minute walk test) index is a mortality index, with higher scores indicating increased risk of death (15). The emphysema-predominant group had much lower percent predicted FEV1 values and significantly higher BODE indexes (15) than did the airway-predominant group owing to significant differences in all BODE components. It is interesting that there were also differences in comorbidities between the two groups, with a lower prevalence of diabetes but higher prevalence of osteoporosis in the emphysema-predominant group and equivalent prevalences of self-reported histories of coronary artery disease.
Figure 2:
Axial CT scans in two subjects with different COPD phenotypes (Table 2): emphysema-predominant COPD (≥35% emphysema, <1.75-mm segmental bronchial wall thickness) (left) and airway-predominant COPD (<35% emphysema, ≥1.75-mm segmental bronchial wall thickness) (right).
A multivariate model for the total number of exacerbations in the prior year was constructed (Table 3) to explore several cutoff points for emphysema percentage and wall thickness based on visual inspection of the surface plot in Figure 1 and adjusted for FEV1 percent predicted. Increasing wall thickness was significantly associated with increased exacerbation frequency, with a 1.84-fold change per 1-mm increase. For subjects with an emphysema percentage of greater than or equal to 35%, increasing emphysema was also associated with a significant increase in exacerbation frequency, with a 1.18-fold change for each 5% increase in emphysema. The influence of emphysema on exacerbation frequency at lower levels of emphysema, when adjusted for lung function and wall thickness, was less clear. For patients with less than 10% emphysema, no significant relationship between emphysema and exacerbation frequency was observed. Decreased exacerbation frequency was seen for subjects with 10%–35% emphysema. These results echo the patterns noted in the three-dimensional surface plot. In addition, female sex, younger age, and current nonsmoking status were significantly associated with increased exacerbation frequency. Wall area percentage was tested in this model and found not to be significantly associated with exacerbation frequency (P = .91). With FEV1 removed from the model, however, a trend of significance for wall area percentage was observed (P = .12).
Table 3.
Multivariate Model for Annual Reported COPD Exacerbation Frequency
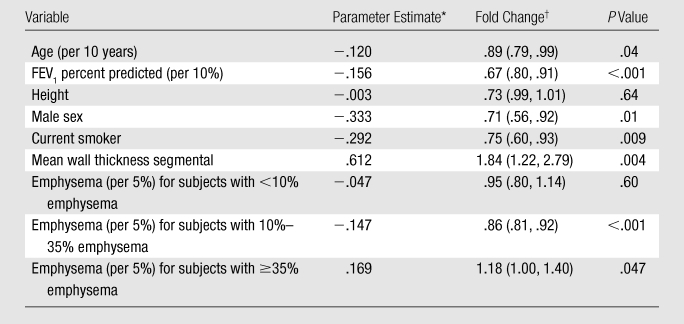
Regression analysis estimates from multivariate model.† Numbers in parentheses are 95% confidence intervals.
Discussion
In a large prospective cohort of current and prior smokers with COPD based on GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria, our study results, obtained by using quantitative CT metrics, demonstrated that the frequency of COPD exacerbations is related to both emphysema severity and airway disease. In a multivariate model adjusted for FEV1 percent predicted, increased segmental bronchial wall thickness was associated with greater exacerbation frequency. Similarly, in the same model, incremental increases in emphysema were associated with greater exacerbation frequency in the subjects with 35% or greater emphysema. Both the multivariate model results and the surface plot data suggest that at lower levels of emphysema, the influence of bronchial wall thickness is greater than the influence of the quantity of emphysema.
The mean number of annual exacerbations in our patient population was 0.68, which is lower than the means of 0.78–0.85 exacerbation reported in two large studies (16,17). The lower frequency in our study is probably due to the higher mean FEV1 percent predicted that was observed in our patient population, 56%, compared with the emphysema percentages of 48% and 44% reported in the other studies (16,17). While our findings support and extend the findings of others in establishing a clear link between disease severity defined according to FEV1 percent predicted and COPD exacerbation frequency (18,19), patients with even very low FEV1 percent predicted may not experience exacerbations, whereas others with a higher FEV1 percent predicted may have frequent exacerbations (7). Given the strong effect of frequent exacerbations on mortality, health status, and health care costs (1–6), there is considerable interest in further refining COPD severity staging by using models that incorporate variables other than spirometric values to better predict health outcomes. Several multidimensional models have been proposed and include the BODE index (15).
Our findings support the utility of radiologic phenotyping with use of whole-lung volumetric high-spatial-resolution CT. While the data in Table 1 indicate that the absolute differences in wall thickness and emphysema percentage between subjects experiencing zero to one exacerbation and those experiencing two or more exacerbations in the prior year are small, when substantial emphysema and wall thickening are present, a clear association with exacerbation frequency exists and defines unique disease phenotypes. These radiologically defined subgroups of patients who experience more frequent exacerbations have clinically distinct symptoms and physiologic and comorbidity profiles that ultimately may lead to a better understanding of the heterogeneity inherent to COPD (20).
The multivariate model demonstrates a correlation between exacerbation frequency and bronchial wall thickness. Our study results corroborate our published preliminary findings and the findings of others (8,21). Airway inflammation, as measured by using wall thickness at the segmental level, offers additional potentially important prognostic information beyond that assessed with spirometry alone. Wall thickness may be the macroscopic correlate of mucus gland hypertrophy and airway inflammation, which plausibly relate to infection, the principal cause of COPD exacerbations. These data, however, cannot be used to determine whether bronchial wall thickness is simply the effect of the previous exacerbations or predates them and thus possibly has a causal role. Prospective studies are needed to address this question and determine whether bronchial wall thickness measurement is superior to a history of prior exacerbations for the prediction of future exacerbations.
In our analysis, wall thickness—not wall area percentage—was predictive of exacerbation frequency in the multivariate model adjusted for FEV1 percent predicted. We believe that this is because use of the wall area percentage may mask the presence of airway wall thickening if the bronchus is both dilated and thick walled. Hence, a bronchus that is dilated and has thicker walls may have the same wall area percentage as a normal, nondilated airway with normal wall thickness. This observation confirms our findings from a prior study, in which a visual measure of wall thickness, not wall area percentage, was associated with exacerbation frequency (8).
An important—and in our opinion, novel—finding was the nonlinear relationship (adjusted for FEV1 percent predicted) that we observed between emphysema percentage and exacerbation frequency. This finding expands on the data reported by the ECLIPSE investigators, who also observed a relationship between increasing emphysema severity and exacerbation frequency but did not study airway wall thickness (14). Our analysis results demonstrate that the relationship between emphysema severity and exacerbation frequency depends on both emphysema severity and airway wall thickness. The reason that emphysema is independently associated with increased exacerbation frequency at higher levels after adjustment for FEV1 is unknown, although other physiologic changes associated with severe emphysema, such as hyperinflation and airtrapping, might play a role. The possibility that significant emphysema results from recurrent exacerbations is less plausible, given the available data on the rate of change of emphysema extent seen on serial CT scans (22). The three-dimensional surface plot data suggest that the relative importance of emphysema to exacerbation frequency at lower levels of emphysema is relatively lower than the relative importance of wall thickness. It is interesting that the surface plot shows a slight dip toward the far corner, indicating a possible decrease in exacerbation frequency in subjects who have both high levels of emphysema and airway disease. However, our study cohort included very few subjects with both 35% or greater emphysema and a segmental bronchial wall thickness of 1.75 mm or greater. While it is possible that these two characteristics represent a unique phenotype, we do not believe that any conclusions can be drawn, given the small numbers of subjects.
Our finding that exacerbations are more common in women is consistent with findings in several previous studies (17,23,24). Whether the sex-based differences in COPD symptoms reflect currently undefined biologic, physiologic, or psychological factors is unknown (25). While the current analysis was focused on segmental airways, histologic examination of more distal airways (<2 mm in diameter) has revealed that women have smaller lumina and disproportionately thicker bronchial walls than do men (26), suggesting that biologic differences may explain the varying clinical presentation. Given the increased tendency of women to report more severe dyspnea, cough, and sputum production than men (23), they might also be more likely to report exacerbations.
The limitations of this study include potential recall bias associated with the retrospective collection of exacerbation frequency data. However, a recent analysis (14) revealed no significant difference when patients’ recall of exacerbations in the year prior to recruitment was compared with the number of exacerbations detected by using diary cards prospectively completed during the subsequent year, suggesting that the retrospective recall used was not as important as possibly suspected, if it was important at all. Furthermore, 1 year after recruitment there was no significant difference between the number of exacerbations remembered by patients at the end of that year and the number of exacerbations recorded on diary cards during the same period. In addition, the ECLIPSE Study investigators also reported that the best predictor of exacerbations that were prospectively recorded was the patient’s self-reported history of exacerbations in the year prior to study entry (14). These data support the robustness of patient exacerbation frequency recall as an outcome measure. Furthermore, the frequency of acute COPD exacerbations in our study cohort was very similar to previously published data. A final limitation of our investigation was that the number of exacerbations that a subject was allowed to list in the prior year was truncated to six, and this might have blunted the association between radiologic parameters and exacerbation frequency.
In summary, these data provide evidence that the quantitative measures of lung structural changes identified with volumetric CT are associated with COPD exacerbation frequency, a clinical outcome of public health importance. When present, both emphysema and bronchial wall thickness are used to define subgroups of patients who experience exacerbations and thereby help health care providers better understand the heterogeneity inherent to COPD among those who experience exacerbations. Because these changes can be detected noninvasively with CT and their relationship with exacerbation frequency is independent of spirometrically measured lung function, our results suggest that radiologic characterization has prognostic value in the selection of more homogeneous subgroups of patients for clinical trials and possibly in clinical practice to help identify patients at risk for more frequent exacerbations with more targeted medical therapy.
Advance in Knowledge.
Independent of the severity of airflow obstruction, a 5% increase in total lung emphysema in those with 35% or greater emphysema is associated with a 1.18-fold increase in chronic obstructive pulmonary disease (COPD) exacerbation frequency; a 1-mm increase in segmental airway wall thickness is associated with a 1.84-fold increase in COPD exacerbation frequency.
Implications for Patient Care.
CT might be able to help identify subgroups of the population at greatest risk for acute COPD exacerbations.
Subgroups identified by using quantitative CT may enable targeted research and development of individualized preventive therapies.
Disclosures of Potential Conflicts of Interest: M.K.H. Financial activities related to the present article: received a consulting fee or honorarium from Novartis, CSL Behring, GlaxoSmithKline, Pfizer, and Boehringer-Ingelheim; received financial support for travel to meetings from Astra-Zeneca. Financial activities not related to the present article: is a paid consultant for Genentech; received grants or has grants pending with Chicago Community Trust; received royalties from UpToDate. Other relationships: none to disclose. E.A.K. No potential conflicts of interest to disclose. D.A.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a member of the board of Actelion; is a paid consultant for Actelion, Intermune, Gilead, Perceptive Imaging, Novartis, and Centocor; received grants or has grants pending with Siemens. L.X.L. No potential conflicts of interest to disclose. S.M. No potential conflicts of interest to disclose. J.L.C. No potential conflicts of interest to disclose. G.J.C. No potential conflicts of interest to disclose. V.K. No potential conflicts of interest to disclose. R.P.B. No potential conflicts of interest to disclose. N.A.H. No potential conflicts of interest to disclose. A.R.A. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a member of the boards of GSK, Dey Pharma, Pfizer, BI, Bayer-Schering Pharma, and Schering-Pleough; is a paid consultant for GSK, Dey Pharma, Pfizer, BI, Bayer-Schering Pharma, and Schering-Pleough; received honoraria from GSK, Dey Pharma, Pfizer, BI, Bayer-Schering Pharma, and Schering-Pleough; received payment for the development of educational presentations from Dey Pharma, Pfizer, BI, Bayer-Schering Pharma, and Schering-Pleough. Other relationships: none to disclose. B.J.M. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is on the advisory boards of Forest, Astra-Zeneca, Novaritis, Dey Pharma, Nycomed, Respironics, Schering, Sequal, Embryon, Boehringer-Ingelheim, Pfizer, and GlaxoSmithKline; is a paid consultant for Astellas, Talecris, and Chiesi; received grants or has grants pending with Astra-Zeneca, GlaxoSmithKline, Pfizer, NABI, Boehringer-Ingelheim, and Sunovian; received payment for lectures from GlaxoSmithKline, Boehringer-Ingelheim, and Pfizer; received payment for manuscript preparation from Astra-Zeneca; received payment for review of documents related to a clinical trial from Spiration; received payment for video presentation from Boehringer-Ingelheim and Pfizer. Other relationships: none to disclose. J.E.H. No potential conflicts of interest to disclose. J.D.C. No potential conflicts of interest to disclose. E.K.S. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for GlaxoSmithKline and Astra-Zeneca; received grants or has grants pending with GlaxoSmithKline; received payment for lectures from GlaxoSmithKline and Astra-Zeneca. Other relationships: none to disclose. F.J.M. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a member of the boards of GSK, MedImmune/Astra-Zeneca, Merck, Pearl, Novartis, UBC, MPex, and Forest/Almirall; is a paid consultant for Boehringer-Ingelheim, Nycomed/Forest, Roche, Bayer, Schering, HLS, Talecris, Comgenix, fb Communications, BoomComm, Actelion, and Pfizer; received grants or has grants pending with Boehringer-Ingelheim, Actelion, and Nycomed; received payment for lectures from GSK, NACE, MedEd, Potomac, Pfizer, Boehringer-Ingelheim, Schering, Vox Medic, WebMD, ePocrates, Astra-Zeneca, and Altana/Nycomed; received royalties from Associates in Medical Marketing and Castle Connolly; received payment for the development of educational presentations from HIT Global and UpToDate. Other relationships: none to disclose. G.R.W. No potential conflicts of interest to disclose.
Supplementary Material
Acknowledgments
The members of the COPDGene Study Group (as of June 2010) are as follows: Jeffrey Curtis, MD (principal investigator [PI]), and Ella Kazerooni, MD (radiologist [RAD]), from Ann Arbor VA Health System; Nicola Hanania, MD, MS (PI), Philip Alapat, MD, Venkata Bandi, MD, Kalpalatha Guntupalli, MD, Elizabeth Guy, MD, Antara Mallampalli, MD, Charles Trinh, MD (RAD), and Mustafa Atik, MD, from Baylor College of Medicine, Houston, Tex; Dawn DeMeo, MD, MPH (Co-PI), Craig Hersh, MD, MPH (Co-PI), George Washko, MD, and Francine Jacobson, MD, MPH (RAD), from Brigham and Women’s Hospital, Boston, Mass; R. Graham Barr, MD, DrPH (PI), Byron Thomashow, MD, and John Austin, MD (RAD), from Columbia University, New York, NY; Neil MacIntyre, Jr, MD (PI), Lacey Washington, MD (RAD), and H. Page McAdams MD (RAD), from Duke University Medical Center, Durham, NC; Richard Rosiello, MD (PI), and Timothy Bresnahan, MD (RAD), from Fallon Clinic, Worcester, Mass; Charlene McEvoy, MD, MPH, and Joseph Tashjian, MD (RAD), from Health Partners Research Foundation, Minneapolis, Minn; Robert Wise, MD (PI), Nadia Hansel, MD, MPH, Robert Brown, MD (RAD), and Gregory Diette, MD, from Johns Hopkins University, Baltimore, Md; Richard Casaburi, MD (PI), Janos Porszasz, MD, PhD, Hans Fischer, MD, PhD (RAD), and Matt Budoff, MD, from Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Los Angeles, Calif; Amir Sharafkhaneh, MD (PI), Charles Trinh, MD (RAD), Hirani Kamal, MD, and Roham Darvishi, MD, from Michael E. DeBakey VA Medical Center, Houston, Tex; Dennis Niewoehner, MD (PI), Tadashi Allen, MD (RAD), Quentin Anderson, MD (RAD), and Kathryn Rice, MD, from Minneapolis VA Medical Center, Minneapolis, Minn; Marilyn Foreman, MD, MS (PI), Gloria Westney, MD, MS, and Eugene Berkowitz, MD, PhD (RAD), from Morehouse School of Medicine, Atlanta, Ga; Russell Bowler, MD, PhD (PI), Adam Friedlander, MD, David Lynch, MB (RAD), Joyce Schroeder, MD (RAD), and John Newell, Jr, MD (RAD), from National Jewish Health, Denver, Colo; Gerard Criner, MD (PI), Victor Kim, MD, Nathaniel Marchetti, DO, Aditi Satti, MD, A. James Mamary, MD, Robert Steiner, MD (RAD), and Chandra Dass, MD (RAD), from Temple University, Philadelphia, Pa; William Bailey, MD (PI), Mark Dransfield, MD (Co-PI), and Hrudaya Nath, MD (RAD), from University of Alabama, Birmingham, Ala; Joe Ramsdell, MD (PI), and Paul Friedman, MD (RAD), from University of California, San Diego, Calif; Geoffrey McLennan, MD, PhD (PI), Edwin JR van Beek, MD, PhD (RAD), Brad Thompson, MD (RAD), and Dwight Look, MD, from University of Iowa, Iowa City, Iowa; Fernando Martinez, MD (PI), MeiLan Han, MD, and Ella Kazerooni, MD (RAD), from University of Michigan, Ann Arbor, Mich; Christine Wendt, MD (PI), and Tadashi Allen, MD (RAD), from University of Minnesota, Minneapolis, Minn; Frank Sciurba, MD (PI), Joel Weissfeld, MD, MPH, Carl Fuhrman, MD (RAD), and Jessica Bon, MD, from University of Pittsburgh, Pittsburgh, Pa; Antonio Anzueto, MD (PI), Sandra Adams, MD, Carlos Orozco, MD, and Mario Ruiz, MD (RAD), from University of Texas Health Science Center at San Antonio, San Antonio, Tex; James Crapo, MD (PI), Edwin Silverman, MD, PhD (PI), Barry Make, MD, Elizabeth Regan, MD, Sarah Moyle, MS, and Douglas Stinson, from the Administrative Core; Terri Beaty, PhD, Barbara Klanderman, PhD, Nan Laird, PhD, Christoph Lange, PhD, Michael Cho, MD, Stephanie Santorico, PhD, John Hokanson, MPH, PhD, Dawn DeMeo, MD, MPH, Nadia Hansel, MD, MPH, Craig Hersh, MD, MPH, Jacqueline Hetmanski, MS, and Tanda Murray, from the Genetic Analysis Core; David Lynch, MB, Joyce Schroeder, MD, John Newell, Jr, MD, John Reilly, MD, Harvey Coxson, PhD, Philip Judy, PhD, Eric Hoffman, PhD, George Washko, MD, Raul San Jose Estepar, PhD, James Ross, MSc, Rebecca Leek, Jordan Zach, Alex Kluiber, Jered Sieren, Heather Baumhauer, Verity McArthur, Dzimitry Kazlouski, Andrew Allen, Tanya Mann, and Anastasia Rodionova, from the Imaging Core; Robert Jensen, PhD, from the PFT QA Core, LDS Hospital, Salt Lake City, Utah; Homayoon Farzadegan, PhD, Stacey Meyerer, Shivam Chandan, and Samantha Bragan, from the Biological Repository, Johns Hopkins University; James Murphy, PhD, Douglas Everett, PhD, Carla Wilson, MS, Ruthie Knowles, Amber Powell, Joe Piccoli, Maura Robinson, Margaret Forbes, and Martina Wamboldt, from the Data Coordinating Center and Biostatistics, National Jewish Health; and John Hokanson, MPH, PhD, Marci Sontag, PhD, Jennifer Black-Shinn, MPH, and Gregory Kinney, MPH, from the Epidemiology Core, University of Colorado School of Public Health, Denver, Colo.
Received February 8, 2011; revision requested March 31; revision received May 9; accepted May 18; final version accepted May 20. M.K.H.
supported by funding from NHLBI grant K23 HL093351. G.R.W. supported by funding from NHLBI grant K23 HL089353 and an award from the Parker B. Francis Foundation.
Funding: This research was supported by National Heart Lung and Blood Institute (grants U01HL089897 and U01Hl089856).
Clinical trial registration no. NCT00608764
Abbreviations:
- COPD
- chronic obstructive pulmonary disease
- FEV1
- forced expiratory volume in the first second of expiration
References
- 1. Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics 2005;23(4):345–363 [DOI] [PubMed] [Google Scholar]
- 2. Schmier JK, Halpern MT, Higashi MK, Bakst A. The quality of life impact of acute exacerbations of chronic bronchitis (AECB): a literature review. Qual Life Res 2005;14(2):329–347 [DOI] [PubMed] [Google Scholar]
- 3. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanner RE, Anthonisen NR, Connett JE; Lung Health Study Research Group Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med 2001;164(3):358–364 [DOI] [PubMed] [Google Scholar]
- 5. Donaldson GC, Wedzicha JA. COPD exacerbations. I. Epidemiology. Thorax 2006;61(2):164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Heart Lung and Blood Institute; NHLBI morbidity and mortality chart book; National Heart Lung and Blood Institute. Web site. http://www.nhlbi.nih.gov/resources/docs/cht-book.htm. Accessed January 5, 2011.
- 7. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD 2009;6(6):459–467 [DOI] [PubMed] [Google Scholar]
- 9. Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7(1):32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176(6):532–555 [DOI] [PubMed] [Google Scholar]
- 11. Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric x-ray CT images. IEEE Trans Med Imaging 2001;20(6):490–498 [DOI] [PubMed] [Google Scholar]
- 12. Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med 2000;162(3 Pt 1):1102–1108 [DOI] [PubMed] [Google Scholar]
- 13. Tschirren J, Hoffman EA, McLennan G, Sonka M. Segmentation and quantitative analysis of intrathoracic airway trees from computed tomography images. Proc Am Thorac Soc 2005;2(6):484–487, 503–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363(12):1128–1138 [DOI] [PubMed] [Google Scholar]
- 15. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350(10):1005–1012 [DOI] [PubMed] [Google Scholar]
- 16. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356(8):775–789 [DOI] [PubMed] [Google Scholar]
- 17. Tashkin D, Celli B, Kesten S, Lystig T, Decramer M. Effect of tiotropium in men and women with COPD: results of the 4-year UPLIFT trial. Respir Med 2010;104(10):1495–1504 [DOI] [PubMed] [Google Scholar]
- 18. Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl 2003;41:46s–53s [DOI] [PubMed] [Google Scholar]
- 19. Miravitlles M, Mayordomo C, Artés M, Sánchez-Agudo L, Nicolau F, Segú JL. Treatment of chronic obstructive pulmonary disease and its exacerbations in general practice: EOLO Group—Estudio Observacional de la Limitación Obstructiva al Flujo aEreo. Respir Med 1999;93(3):173–179 [DOI] [PubMed] [Google Scholar]
- 20. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182(5):598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mair G, Maclay J, Miller JJ, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med 2010;104(11):1683–1690 [DOI] [PubMed] [Google Scholar]
- 22. Stolk J, Putter H, Bakker EM, et al. Progression parameters for emphysema: a clinical investigation. Respir Med 2007;101(9):1924–1930 [DOI] [PubMed] [Google Scholar]
- 23. Cote CG, Celli BR. BODE index: a new tool to stage and monitor progression of chronic obstructive pulmonary disease. Pneumonol Alergol Pol 2009;77(3):305–313 [PubMed] [Google Scholar]
- 24. de Torres JP, Casanova C, Hernández C, Abreu J, Aguirre-Jaime A, Celli BR. Gender and COPD in patients attending a pulmonary clinic. Chest 2005;128(4):2012–2016 [DOI] [PubMed] [Google Scholar]
- 25. Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med 2007;176(12):1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 2007;176(3):243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.