Abstract
Objective
AMELIA (OsteoArthritis Modifying Effects of Long-term Intra-articular Adant) was designed to compare against placebo the efficacy and safety of repeated injections of hyaluronic acid (HA) and its effect on disease progression over 40 months.
Methods
A multicentre, randomised, patient and evaluator-blinded, controlled study in 306 patients fulfilling American College of Rheumatology criteria for knee osteoarthritis, radiological grades II–III (Kellgren–Lawrence) and joint space width ≥2 mm. Patients received four cycles of five intra-articular HA or placebo injections with a follow-up of 6 months after the first and second cycles, and 1 year after the third and fourth cycles. Osteoarthritis Research Society International (OARSI) 2004 responder criteria were used to assess efficacy. The consumption of rescue medication was a secondary outcome. Adverse events were recorded for safety purposes.
Results
At the 40-month visit significantly more patients responded to HA compared with placebo (OARSI 2004, p=0.004). The number of responders to HA increased through the study, whereas those to placebo did not change. Significant differences were also found in favour of HA for each individual component of the OARSI 2004. No safety problems were recorded.
Conclusions
The results of AMELIA offer pioneer evidence that repeated cycles of intra-articular injections of HA not only improve knee osteoarthritis symptoms during the in-between cycle period but also exert a marked carry-over effect for at least 1 year after the last cycle. In this respect, it is not possible to establish if this carry-over effect reflects true osteoarthritis remission or just a modification of the disease's natural course.
ClinicalTrials.gov number, NCT00669032
Osteoarthritis is a chronic disorder characterised by joint cartilage degeneration as the central feature associated with concomitant changes in synovium and subchondral bone metabolism.1 Osteoarthritis of the knee, the principal large joint to be affected, results in disabling symptoms in 10% of people older than 55 years, a quarter of whom are severely affected.2 Most current pharmacological options are limited to alleviating pain and improving functional activity but effective therapeutic alternatives to slow disease progression are also needed.
Hyaluronic acid (HA) is a key molecule in joint biomechanics. In osteoarthritis and other arthropathies, the reduction in concentration and molecular weight of endogenous HA greatly alters the properties of the synovial fluid, causing cartilage damage and worsening osteoarthritis symptoms.3 Treatment with exogenous HA contributes to restoring the elastic and viscous properties of the synovial fluid, resulting in pain reduction and functional improvement. Besides this mechanical action, different studies have confirmed that HA interacts with mediators of inflammation and matrix turnover in joint cells, reduces the apoptosis of chondrocytes and exerts a biosynthetic chondroprotective effect.4–9
This dual mechanism of action may account for the particular effect of HA on clinical symptoms of osteoarthritis. Unlike traditional analgesics and non-steroidal anti-inflammatory drugs (NSAID), HA is not a rapidly acting agent, but its clinical efficacy on pain and function shows a carry-over effect that extends the results for a long time after the administration is stopped, identifying it as a symptomatic slow-acting class compound (SYSADOA).10 11
Although the use of intra-articular HA injections for the relief of pain in people with knee osteoarthritis is recommended by scientific societies,12 13 the characteristics of the patients who are most likely to benefit from this treatment need to be defined appropriately, and the role of HA in disease progression is still under discussion.12–15 Unfortunately, the lack of long-term studies contributes to this uncertainty. In addition, the variety of HA have different physicochemical characteristics and thus cannot be expected to have the same clinical effect.4 16 17
The current trial AMELIA (OsteoArthritis Modifying Effects of Long-term Intra-articular Adant) was conducted to compare the efficacy and safety of repeated injections of HA compared with placebo over a period of 40 months.
Methods
Study design
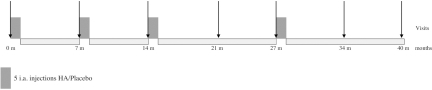
This was a randomised, patient and evaluator blinded, placebo-controlled study with parallel groups. Patients with osteoarthritis of the knee were randomly assigned to receive intra-articular injections of 2.5 ml 1% sodium hyaluronate with a mean molecular weight of 900 000 daltons, obtained through a fermentation process from strains of Streptococcus zoopidemicus (Adant; Tedec-Meiji Farma, Madrid, Spain) or placebo injections (2.5 ml of saline solution). The study consisted of four treatment cycles of five weekly injections each one. The follow-up periods were 6 months long after the first and second cycles and 1 year long after the third and fourth cycles, resulting in a total study duration of 40 months (figure 1). The repeated cycles were administered regardless of whether the patients had symptoms or not.
Figure 1.
Study diagram. HA, hyaluronic acid; i.a., intra-articular.
Both treatments were packaged identically in order to maintain the blinding conditions. A computer-generated randomised list was used to provide balanced blocks of four patients each. Allocation to treatment as well as efficacy and safety evaluation was performed by a blinded physician, while an unblinded physician was responsible for treatment administration. The injections were non-ultrasound guided and three approaches were permitted: medial/lateral (extended knee) and infrapatellar (flexed knee).
At the screening visit the patients were assessed by the blinded physician for fulfilment of the entry criteria, demographic characteristics and medical history. Knee radiographs were also obtained. Eligible patients were randomly assigned 1:1 to receive intra-articular injections of HA or placebo. In the case of bilateral affectation only the more symptomatic knee at baseline was considered for the study, although the other knee could be treated with the same assigned treatment. Patients with effusion had the joint aspirated before the administration of treatment. Concomitant medications for chronic pathologies other than osteoarthritis were also recorded. Acetylsalicylic acid (maximum 300 mg/day) for vascular prevention, paracetamol up to 4 g/day as rescue medication as well as short cycles of NSAID were permitted. However, for 24 h and 1 week before efficacy evaluation, patients were required to abstain from any paracetamol or NSAID, respectively. During the whole study period corticosteroid injections were not permitted in the target knee. Only two injections were allowed in the contralateral knee if necessary and no more than two injections per year in any other joint than the knee.
Patients' inclusion and exclusion criteria
Eligible patients were men and women of at least 45 years of age with knee osteoarthritis in the medial tibiofemoral compartment according to the American College of Rheumatology18 with grade II to III radiographic stage osteoarthritis19 and minimum medial femorotibial joint space width of the target knee of 2 mm or greater. Patients were required to have pain of 55 mm or greater on a visual analogue scale (VAS) at any time during the week before inclusion.
Main exclusion criteria were body mass index greater than 32 kg/m2, a history of trauma or surgery in the target knee, arthroscopy surgery during the year before inclusion, joint inflammatory diseases and/or microcrystalline arthropathies, coagulation/platelet disorders or any concomitant disease that could interfere with the evaluation. The administration of intra-articular steroids in the previous 3 months, HA injections during the past year or NSAID treatment during 2 weeks before inclusion were also reasons for exclusion.
Evaluation of efficacy
The primary efficacy outcome was the percentage of subjects with a clinical response according to Osteoarthritis Research Society International (OARSI) 2004 criteria20 at the end of follow-up. Patients were classified as responders if the pain or physical function score decreased at least 50% and at least 20 mm on the VAS, or if two of the following three findings were recorded: a decrease in pain of at least 20% or at least 10 mm on the VAS, a decrease in physical function of at least 20% and at least 10 mm on the VAS, or an increase in the score of the patient's global assessment by at least 20% and at least 10 mm on the VAS.
Secondary outcomes included the percentage of subjects with clinical response according to OMERACT–OARSI criteria at each follow-up visit; each component of OMERACT–OARSI (reduction in pain, improvement in function using the Western Ontario and McMaster Universities Osteoarthritis Index function subscale and in patients' global assessment (all of them measured using VAS) and consumption of rescue medication for osteoarthritis (paracetamol and NSAID) throughout the study).
Evaluation of safety
Treatment safety and tolerability was evaluated based on the incidence and type of adverse events (with special attention to allergic reactions such as skin rash, urticaria, pruritus, swelling and/or erythema) and the results of blood laboratory tests and physical examinations throughout the duration of the study. Safety analyses were performed in those patients who received at least one intra-articular injection (safety population).
Statistical methods
The study was designed to have a statistical power of 80% to detect a difference of at least 20% of patients responding to treatment compared with placebo (expected response rate up to 30%), with a two-sided significance level of less than 5%. Given the specified statistical power and assuming up to a 40% drop-out rate, the study was planned to include a total of 300 patients (150 per treatment arm).
For quantitative variables, mean, median, SD, maximum and minimum values were calculated. Student's t test was used to compare independent variables following a normal distribution and the Mann–Whitney U test was used if the applicability conditions were not present. Qualitative variables were expressed as total number and relative frequencies. Pearson's χ2 test was used for comparisons of frequencies between groups.
The main study population included all randomly assigned patients with at least one efficacy assessment after randomisation (the modified intention-to-treat population). A sensitivity analysis was performed to assess the imputation method for handling missing data using the mixed method of repeated measures and the last observation carried forward. The last observation carried forward proved to be the more conservative method and was then applied. All statistical tests were performed using SAS software version 9.2.
Results
Disposition of patients and demographic characteristics
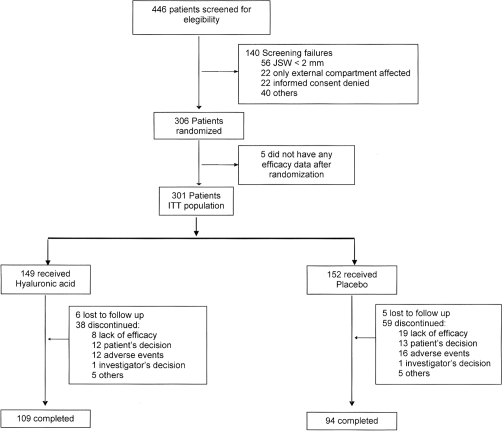
Recruiting started in October 2003 and the last follow-up was performed on July 2009. The 19 participating centres screened a total of 446 patients, of whom 140 (31.4%) were screening failures. Five patients did not provide any efficacy data after randomisation and were not included in the analysis of efficacy, leaving a total of 301 patients in the intention-to-treat population. A total of 109 and 94 patients receiving HA or placebo, respectively, completed the study (figure 2).
Figure 2.
Patients' disposition for clinical assessment. JSW, joint space width.
The clinical and demographic characteristics of the patients did not differ at baseline (table 1). Overall, the great majority of the patients were women (83.7%) the mean age was 63.4 years, with a body mass index of 28.6. The mean duration of knee osteoarthritis was 7.5 years and a mean joint space width value of 3.5 mm. A total of 77.85% of HA patients and 82.24% in the placebo group had bilateral osteoarthritis (p=0.341). Of them, 55.17% of HA patients and 56.02% of the placebo group (p=0.7992) were also treated in the contralateral knee. No differences between groups were found related to the administration approach.
Table 1.
Demographic and baseline clinical characteristics of study groups
| HA (n=153) | Placebo (n=153) | |
|---|---|---|
| Women, n (%) | 128 (83.7) | 128 (83.7) |
| Age, years (mean (SD)) | 63 (8.2) | 63.9 (8.9) |
| BMI, kg/m2 (mean (SD)) | 28.4 (2.7) | 28.7 (2.6) |
| Duration knee osteoarthritis, years (mean (SD)) | 6.9 (6.8) | 8.1 (8.4) |
| Osteoarthritis baseline characteristics | ||
| Pain, VAS 0–100 (mean (SD)) | 69.7 (11.1) | 71.2 (11.2) |
| Morning stiffness, <30 min (n (%)) | 142 (92.8) | 133 (86.9) |
| Joint crackles, n (%) | 141 (92.2) | 138 (90.2) |
| Kellgren–Lawrence grade, n (%) | ||
| II | 108 (70.6) | 114 (74.5) |
| III | 45 (29.4) | 39 (25.5) |
| Joint space width, mm (mean (SD)) | 3.5 (0.82) | 3.5 (0.89) |
| WOMAC, VAS 0–100 (mean (SD)) | ||
| Total | 55.7 (15.5) | 58.2 (14.7) |
| Pain | 56.0 (16.7) | 56.7 (15.2) |
| Stiffness | 54.8 (23.0) | 54.8 (22.4) |
| Function | 57.1 (17.0) | 59.1 (15.1) |
BMI, body mass index; HA, hyaluronic acid; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Treatment efficacy
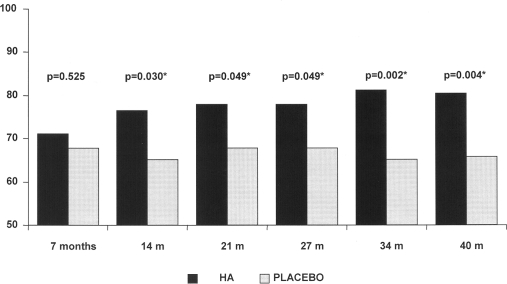
At the end of follow-up (40 months) significantly more patients receiving HA responded to treatment in comparison with placebo according to OARSI 2004 criteria (p=0.004), the number of responders being 22% higher in HA group after the four treatment cycles (RR 1.22, 95% CI 1.07 to 1.41).
The number of responders to HA injections progressively increased after each treatment cycle (from 71.1% to 80.5%), whereas responses to placebo remained fairly stable (from 67.8% to 65.8%).
This progression gave results with strong statistical significance and differences between the two groups from the second until the last evaluation at 40 months (figure 3). Among those non-responders after the first cycle, up to 54% of HA and 38% of placebo patients evolved positively over the study. At the 40-month visit the number of responders in this subgroup was 54% with HA versus 31% in the placebo group, (p=0.026).
Figure 3.
Evolution of responders Osteoarthritis Research Society International, 2004. HA, hyaluronic acid.
All of the OARSI components (pain, function and patient global assessment) were analysed at the end of the study, showing that the degree of improvement in the HA group was significantly higher compared with placebo (p values 0.025, 0.023 and 0.002, respectively) (table 2).
Table 2.
Summary of results from primary and secondary outcomes assessment (intention-to-treat population)
| HA (n=149) | Placebo (n=152) | p Value | |
|---|---|---|---|
| Primary outcome | |||
| Responders OARSI 2004 at end of follow-up, n (%) | 120 (80.5) | 100 (65.8) | 0.004 |
| Secondary outcomes | |||
| Responders OARSI 2004 at each cycle assessments, n (%) | |||
| 7 months | 106 (71.1) | 103 (67.8) | 0.525 |
| 14 months | 114 (76.5) | 99 (65.1) | 0.030 |
| 21 months | 116 (77.9) | 103 (67.8) | 0.049 |
| 27 months | 116 (77.9) | 103 (67.8) | 0.049 |
| 34 months | 121 (81.2) | 99 (65.1) | 0.002 |
| Pain or function reduction 50% (20 mm), n (%) | 97 (65.1) | 79 (52.0) | 0.021 |
| Overall pain reduction 20% (10 mm), n (%) | 118 (79.2) | 103 (67.8) | 0.025 |
| Function improvement 20% (10 mm), n (%) | 105 (70.5) | 88 (57.9) | 0.023 |
| Patient's global assessment reduction 20% (10 mm), n (%) | 111 (74.5) | 88 (57.9) | 0.002 |
| Mean consumption of paracetamol, mg/day (SD) | 408.8 (644.2) | 451.4 (925.8) | NS |
HA, hyaluronic acid; OARSI, Osteoarthritis Research Society International.
A total of 26.8% of patients receiving HA did not complete the study compared with 38.2% in the placebo group. It is noteworthy that the number of losses due to lack of efficacy were significantly higher in the placebo group (p=0.027). The demographic and baseline characteristics of completers and dropouts were analysed, and no differences were found with the exception of age in the placebo group, with the completers being younger than the dropouts (p=0.047). Aspiration in the target knee was performed in 22.82% of patients in the HA group and 21.05% of the placebo group (p=0.712), with a median of two aspirations per patient in both groups during the overall study period.
Overall, rescue medication (paracetamol/NSAID) was consumed during the study by 71.1% and 71.7% of the HA and placebo patients, respectively. Paracetamol was consumed by 48% of the patients and the mean daily dose during the study experienced a 27% reduction in the HA group compared with baseline versus only a 4% reduction in the placebo group (table 2). A logistic regression analysis was performed with no differences between the HA and placebo (p=0.9129) groups, concluding that rescue medication did not interfere with the clinical assessment of patients.
Safety
The number of patients who experienced at least one adverse event was the same in both treatment groups, with an overall frequency of 83.0%. Twenty-two patients (11 in each group) experienced a total of 29 related adverse events. Most of them were related to the study intervention, such as local bleeding, pain of mild intensity or allergic reaction, none of them was serious. The frequency and types of related adverse events are summarised in table 3. During the overall study period, there were no abnormalities in vital signs, clinical findings or laboratory parameters that could be considered as being related to the treatment. At the end of the study, 513 and 487 cycles were administered in the HA and placebo groups, respectively, providing a rate of 0.029 related adverse events per cycle in both groups.
Table 3.
Related adverse events
| HA (n=153) | Placebo (n=153) | |||
|---|---|---|---|---|
| Related adverse events (n (%)) | 15 (9.8) | 14 (9.1) | ||
| Mild | 7 (4.6) | 12 (7.8) | ||
| Moderate | 8 (5.2) | 2 (1.3) | ||
| HA (n=153) | Placebo (n=153) | |||
| Mild | Moderate | Mild | Moderate | |
| Allergic reaction | 2 (1.3) (1 rash, 1 swelling) | 1 (0.7) (1 rash) | 3 (1.9) (3 rash) | 0 |
| Pain at injection site | 2 (1.3) | 4 (2.6) | 2 (1.3) | 0 |
| Bleeding at injection site | 2 (1.3) | 0 | 6 (3.9) | 0 |
| Arthralgia | 0 | 2 (1.3) | 1 (0.7) | 1 (0.7) |
| Others | 1 (0.7) | 1 (0.7) | 0 | 1 (0.7) |
HA, hyaluronic acid.
Discussion
This study has assessed the efficacy and safety of repeated intra-articular injections of HA in knee osteoarthritis patients, showing a symptomatic relief of pain and an improvement in function and patient global assessment during a follow-up period of 40 months. At the end of the study, the percentage of responders according to OARSI 2004 criteria was 22% higher in the HA group than in the placebo group (p=0.004).
In recent years, consistent systematic reviews and meta-analyses10 21–26 have concluded that HA is superior to placebo in controlling knee osteoarthritis symptoms.24–26 Although a number of controlled studies have shown that this treatment is comparable in efficacy to systemic forms of active intervention such as steroids or NSAID,22 23 some other studies have questioned the efficacy of the treatment with HA.21 27 The study design weaknesses and the enormous placebo effect in osteoarthritis clinical trials as well as the specific characteristics of the HA used can account for these contradictory results. There are two crucial aspects that have to be strongly considered in the design of clinical trials aimed to demonstrate a SYSADOA effect in osteoarthritis: the time when the analgesia starts and the duration of the carry-over effect. Furthermore, the various HA derivatives that are available on the market are synthesised using different methods and formulated in different physical forms, and thus a uniform efficacy and safety profile should not be expected.4 16 17 28
The starting time of the analgesic effect could not be well captured in some short-term clinical trials, and our study was not designed to address it. In fact, despite some patients going into remission at the first assessment performed 7 months after the first cycle, this effect could not be demonstrated, probably masked by the high efficacy rates found in the placebo group at that time. However, the efficacy of placebo remained quite stable throughout the study period, whereas the responder rate in the HA group increased, the differences being truly significant from 1 year onwards (p<0.05). Several randomised controlled trials in osteoarthritis failed to demonstrate the superiority of active treatment over placebo,27 29–32 reaching efficacy rates up to 60% even with orally administered placebos,30 making it difficult for the other treatments to surpass this level.10 Moreover, the efficacy of placebo has been confirmed in osteoarthritis interventions, and increases with the expectations of the patient with respect to the treatment and the use of invasive administration routes.32 In AMELIA, however, the success of the study was in fact accentuated by the high placebo efficacy detected, making the results found (80.5% of responders in the HA group at the end of follow-up compared with 65.8% for placebo patients), even clinically meaningful and remarkable.30 The RR used to quantify the effect size established a probability of success of HA of 22% higher than placebo 1 year after the last cycle was administered.
The AMELIA design enables us to gain a better understanding of the clinical response of symptomatic knee osteoarthritis patients subject to HA injections by looking for the carry-over analgesic effect duration. AMELIA was able to detect symptomatic effects even 1 year after the last HA administration cycle, giving us unprecedented information about the HA therapeutic profile. These results are in line with those reported previously, granting HA greater efficacy than NSAID23 and than steroids after 5–8 weeks post-treatment,22 33 even though previous studies do not provide long-term data. We cannot ascertain whether the pre-established therapeutic schedule, consisting of administering HA even to patients without symptoms, could contribute to the carry-over effect, but it could be a plausible explanation. In this regard, it is not possible to establish whether this carry-over effect reflects disease remission or just a modification of the natural course of the disease.
Overall, the use of rescue medication during the study was low. Although the mean paracetamol consumption was 23% lower in the HA group, the differences were not statistically significant, probably due to the high data dispersion. Certainly, the way of controlling rescue medication use is a study limitation because patients were asked at every assessment visit about the frequency and doses of consumption, but it was not delivered in hand, leading to insufficient recording and an inherent inaccuracy. In any case, however, the logistic regression analysis concluded that the use of rescue medication did not interfere with the assessment of the clinical outcome.
Adverse reactions were scarce and were related to the administration procedure. All of them were short lasting and of mild or moderate intensity.
The results of AMELIA reveal that repeated cycles of intra-articular injections of HA not only improve knee osteoarthritis symptoms during the in-between cycle period, but also exert a marked carry-over effect for at least 1 year after the last injections. In this regard, it is not possible to establish whether this carry-over effect reflects a true disease remission or just a modification of the natural course of the disease.
Acknowledgments
The authors would like to thank J Suarez for assisting them with the English translation. They also thank JJ García and M Amor (PIVOTAL SL) for the statistical analysis of the results.
Footnotes
AMELIA Study Group J Toyos, Rheumatology Department, H Virgen Macarena, Sevilla; B Hernández-Cruz, Rheumatology Department, H Virgen Macarena, Sevilla; J Belzunegui, Rheumatology Department, Hospital Ntra Sra de Aránzazu, San Sebastián; S García, Rheumatology, Department, Hospital Puerta del Mar, Cádiz; M E Brito, Rheumatology Department, Hospital Ramón y Cajal, Madrid; J C Acebes, Rheumatology Department, Fundación Jiménez Díaz, Madrid; J L Guerra, Rheumatology Department, Hospital Arquitecto Marcide, El Ferrol (La Coruña); J Pujol, Rheumatology Department, Hospital Sant Pau i Santa Tecla, Tarragona; F Blanco, Rheumatology Department, Hospital Juan Canalejo, La Coruña; P Benito, Rheumatology Department, Hospital del Mar, Barcelona; E Chamizo, Rheumatology Department, Hospital de Mérida, Mérida (Badajoz); F A Martínez, Rheumatology Department, Hospital Virgen de la Arrixaca, Murcia; R García, Rheumatology Department, Hospital Clínico Universitario Virgen de la Victoria, Málaga; M Guzmán, Rheumatology Department, Hospital Virgen de las Nieves, Granada; M García, Traumatology Department, Hospital Vall D'Hebrón, Barcelona; J Mulero, Rheumatology Department, Hospital Puerta de Hierro, Madrid.
Funding This study was supported by Tedec Meiji Farma SA.
Competing interests PC and MG work at Tedec Meiji Farma SA. The other authors received research funds from Tedec Meiji Farma SA as study investigators.
Patient consent Obtained.
Ethics approval The study was performed in accordance with the principles of good clinical practice guidelines and in compliance with the declaration of Helsinki. The study was conducted with the approval of the ethics committees from each of the centres where the study was performed.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Herrero-Beaumont G, Roman-Blas JA, Castañeda S, et al. Primary osteoarthritis no longer primary: three subsets with distinct etiological, clinical, and therapeutic characteristics. Semin Arthritis Rheum 2009;39:71–80 [DOI] [PubMed] [Google Scholar]
- 2.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fam H, Bryant JT, Kontopoulou M. Rheological properties of synovial fluids. Biorheology 2007;44:59–74 [PubMed] [Google Scholar]
- 4.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum 2002;32:10–37 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg DD, Stoker A, Kane S, et al. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage 2006;14:814–22 [DOI] [PubMed] [Google Scholar]
- 6.Karna E, Miltyk W, Palka JA, et al. Hyaluronic acid counteracts interleukin-1-induced inhibition of collagen biosynthesis in cultured human chondrocytes. Pharmacol Res 2006;54:275–81 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Goomer RS, Harwood F, et al. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1 beta (IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage 1999;7:182–90 [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Hashimoto S, Kubo T, et al. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol 2000;27:1713–20 [PubMed] [Google Scholar]
- 9.Waddell DD, Kolomytkin OV, Dunn S, et al. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res 2007;465:241–8 [DOI] [PubMed] [Google Scholar]
- 10.Bruyère O, Burlet N, Delmas PD, et al. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system. BMC Musculoskelet Disord 2008;9:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougados M. Symptomatic slow-acting drugs for osteoarthritis: what are the facts? Joint Bone Spine 2006;73:606–9 [DOI] [PubMed] [Google Scholar]
- 12.Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905–15 [DOI] [PubMed] [Google Scholar]
- 13.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The National Collaborating Centre for Chronic Conditions Osteoarthritis: National Clinical Guideline for Care and Management in Adults. London: Royal College of Physicians, 2008 [PubMed] [Google Scholar]
- 15.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137–62 [DOI] [PubMed] [Google Scholar]
- 16.Prieto JG, Pulido MM, Zapico J, et al. Comparative study of hyaluronic derivatives: rheological behaviour, mechanical and chemical degradation. Int J Biol Macromol 2005;35:63–9 [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Lázaro J, Díaz-Gállego L. The clinical impact of physical-chemical characteristics of different hyaluronic acids as a treatment for osteoarthritis. Int J Biol Macromol 2006;38:300–1 [DOI] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49 [DOI] [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham T, van der Heijde D, Altman RD, et al. OMERACT–OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 2004;12:389–99 [DOI] [PubMed] [Google Scholar]
- 21.Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. Can Med Assoc J 2005;172:1039–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannuru RR, Natov NS, Obadan IE, et al. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum 2009;61:1704–11 [DOI] [PubMed] [Google Scholar]
- 23.Bellamy N, Campbell J, Welch V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;2:CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo GH, LaValley M, McAlindon T, et al. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA 2003;290:3115–21 [DOI] [PubMed] [Google Scholar]
- 25.Modawal A, Ferrer M, Choi HK, et al. Hyaluronic acid injections relieve knee pain. J Fam Pract 2005;54:758–67 [PubMed] [Google Scholar]
- 26.Wang CT, Lin J, Chang CJ, et al. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am 2004;86-A:538–45 [DOI] [PubMed] [Google Scholar]
- 27.Jørgensen A, Stengaard-Pedersen K, Simonsen O, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis 2010;69:1097–102 [DOI] [PubMed] [Google Scholar]
- 28.Band PA. OARSI update on the evidence for osteoarthritis therapies: comment on the nomenclature used for intra-articular hyaluronan. Osteoarthritis Cartilage 2010;18:1235; author reply 1236 [DOI] [PubMed] [Google Scholar]
- 29.Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol 1998;25:2203–12 [PubMed] [Google Scholar]
- 30.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;354:795–808 [DOI] [PubMed] [Google Scholar]
- 31.Smith MD, Wetherall M, Darby T, et al. A randomized placebo-controlled trial of arthroscopic lavage versus lavage plus intra-articular corticosteroids in the management of symptomatic osteoarthritis of the knee. Rheumatology (Oxford) 2003;42:1477–85 [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Robertson J, Jones AC, et al. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 2008;67:1716–23 [DOI] [PubMed] [Google Scholar]
- 33.Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;2:CD005328 [DOI] [PubMed] [Google Scholar]