Abstract
Background
Remission is the established goal in rheumatoid arthritis (RA) treatment. Although originally defined by a disease activity score in 28 joints (DAS28) <2.6, more stringent criteria may imply the absence of disease activity. The 2011 ACR/EULAR remission criteria provide the newest and most stringent definition of remission.
Objectives
To evaluate post hoc the remission by ACR/EULAR criteria and compare the criteria with the conventional DAS28 in TAMARA, an open-label phase IIIb tocilizumab (TCZ) trial including patients with active RA receiving inadequate disease-modifying antirheumatic drugs (DMARDs) or tumour necrosis factor α (TNFα) inhibitor treatment.
Results
286 patients were enrolled, 99.7% of patients were receiving a conventional DMARD and 41.6% had TNFα inhibitor pretreatment. Baseline mean DAS28 of 6.0 ± 1.0 fell to 2.6 ± 1.5 at week 24. DAS28 <2.6 was achieved by 47.6% at week 24. Remission rates with the new ACR/EULAR Boolean-based criteria for clinical studies were 15.0% after 12 weeks and 20.3% after 24 weeks. Of note, 13.5% of patients with previous TNFα blocker inadequate response still achieved remission according to the new ACR/EULAR criteria after 24 weeks. Clinical Disease Activity Index and Simplified Disease Activity Index remission rates were 24.1% and 25.2%, respectively.
Conclusions
Under the definition of the new stringent 2011 ACR/EULAR remission criteria, patients with active RA despite DMARD treatment and even after inadequate response to TNFα inhibitors, receiving TCZ showed significant rates of remission. Similar remission rates were achieved, when clinical practice criteria, not inclusive of acute phase reactants, were used.
Introduction
Remission as the primary therapeutic goal of rheumatoid arthritis (RA) treatment, was commonly defined by the disease activity score in 28 joints (DAS28), with a DAS28 <2.6 indicating remission.1–5 Clear limitations of the DAS28 have been recognised, as the DAS28 theoretically allows more than 10 swollen joints (SJs) for the definition of remission. Additionally, the erythrocyte sedimentation rate (ESR) at low levels is overestimated by DAS28, and an increased ESR can also be caused by inflammation processes independent of RA. Furthermore, DAS28 application in daily clinical practice may be hampered by the immediate need for the actual ESR result. The DAS28 cut-off point for RA remission of <2.6 has therefore been considered controversial.6–11
Likewise based on 28-joint counts, both the Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI) are more stringent for the definition of remission, because remission is limited to the appearance of a maximum of three and two SJs or tender joints (TJs), respectively. Recently, the ACR and EULAR in a joint effort presented new and even more stringent criteria for RA remission, suggesting criteria for clinical trials and for clinical practice. At any time point, a patient in a clinical trial must achieve a tender joint count (TJC) ≤1, a swollen joint count (SJC) ≤1, C-reactive protein (CRP) ≤1 mg/dl, a patient global assessment (PGA) ≤1, or, as an index- based score, an SDAI ≤3.3, to be considered in remission. For clinical practice, CRP was omitted and a CDAI ≤2.8 replaced the SDAI.12
Recently, the results of the phase IIIb study TAMARA were published.13 The purpose of our analysis was to compare the high percentage of patients achieving DAS28 remission (47.6%) while receiving tocilizumab (TCZ) with the more stringent 2011 ACR/EULAR criteria. This may be particularly important when using a therapeutic agent with a significant influence on the acute phase response, such as the interleukin 6-receptor-inhibiting antibody TCZ.
Patients and methods
Details of the study have been described in detail elsewhere.13 Briefly, in this multicentre, open-label, non-controlled, single-arm study 286 patients with active RA (DAS28 >3.2) despite a stable dose of conventional DMARD (cDMARD) or biological DMARDs were treated with 8 mg/kg TCZ (RoActemra) at 4-weekly intervals for 24 weeks in addition to their cDMARD. One hundred and nineteen patients (41.6%) with mean disease duration of 10.5±7.5 years (median 8.8) had been pretreated with tumour necrosis α factor (TNFα) antagonists. Patients pretreated only with cDMARDs had a shorter disease duration (mean 5.9±5.9 years (median 4.3)). Two hundred and thirty-nine patients (83.6%) completed the full 24 weeks of the trial. The primary objective was to determine the proportion of patients reaching lowDAS <3.2 after 24 weeks, secondary end points comprised the proportion of patients showing a DAS28 remission (<2.6). For this analysis, we assessed remission with the novel ACR/EULAR criteria,12 comparing them with DAS28 remission. The wording of the PGA Visual Analogue Scale (VAS) followed the more open DAS28 wording, in some contrast to the ACR/EULAR definition, which asks for specific arthritis complaints. Thus, the DAS PGA value had to be used as an estimate for the correct values, which might lead to underestimating Boolean rates of ACR/EULAR remission. The last observation carried forward method was used to impute missing values for continuous core variables. For categorical variables missing values were assessed as patients not reaching remission.
Results
A total of 53.4% of patients with disease-modifying antirheumatic drug-inadequate response (DMARD-IR) RA and 41.2% of patients with TNFα-IR RA achieved DAS28 remission (details are published in Burmester et al13).
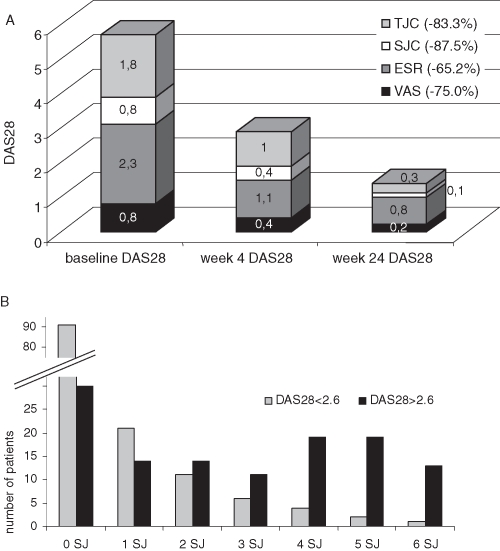
In addition to its pronounced effect on acute phase reactants (APRs), the SJC and the TJC both decreased considerably. Compared with the reduction of the APR the effect on the SJC and the TJC was even more pronounced (see figure 1A and online supplementary table 1). While most patients in DAS remission had no SJs, approximately one in five patients still had at least one, and up to six SJs (figure 1B).
Figure 1.
(A) Twenty-eight joint count Disease Activity Score (DAS28) values of the patients reaching DAS remission at week 24. Each stacked bar represents the DAS28 value at the respective visit. Each segment of a single bar represents the proportion of DAS28 that is due to a specific core variable. In the figure it is, for example, shown that 2.3 points of a total DAS28 of 5.7 at baseline is due to erythrocyte sedimentation rate (ESR) or 0.1 points of a total DAS28 of 1.5 is due to swollen joints (SJs) after 24 weeks. The relative reduction between baseline and week 24 is highest for swollen joint count (SJC) and tender joint count (TJC) (87.5% and 83.5%) compared with reduction of the ESR (65.2%). It is obvious that the TJC and the ESR are weighted higher in the DAS composite than the SJC and the Visual Analogue Scale (VAS). (Please note: numbers in the figure were rounded incorrectly for ESR at week 4 and 24, thus resulting in slightly different numbers.) (B) Number of patients, who are in DAS28 remission (grey) or and with a DAS >2.6 (black) after 24 weeks and their number of SJs. Though most patients in DAS28 remission had no SJs, some patients still had a significant number of SJs.
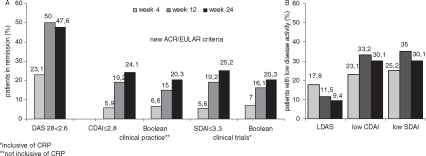
Based on that observation, we assessed remission with the new ACR/EULAR Boolean based remission criteria of patients in clinical trials, defined as described above, allowing only a maximum of one TJ and one SJ. Applying these stringent criteria, 16.1% of patients were in remission after 12 weeks and 20.3% after 24 weeks (Figure 2a). Of note, when clinical practice definitions of remission (non-inclusive of APR) were employed, TCZ achieved remission in 15.0% of patients after 12 weeks and 20.3% after 24 weeks. While remission was achieved in over a quarter of patients (27.6%) pretreated with DMARDs, 13.5% of patients with previous TNFα blocker IR still attained remission according to the new ACR/EULAR criteria after 24 weeks. The CDAI and SDAI were slightly higher in the overall group (24.1% and 25.2%).
Figure 2.
(A)Patients achieving remission according to the different assessment tools DAS28 ((0.56×TJC2) + (0.28×SJC2) + (0.7×ln (ESR)) + (0.014 × PtGA)<2.6) and the new ACR/EULAR criteria for clinical practice (Boolean-criteria SJC, TJC, PtGA all ≤1) and CDAI (SJC + TJC + PhGA + PtGA) ≤2.8) and clinical trials (Boolean-criteria SJC, TJC, PtGA, CRP all ≤1 and SDAI (SJC+ TJC + PhGA + PtGA + CRP (mg/dl)) ≤3.3 ). (B) Patients achieving low disease activity (DAS >2.6 and ≤3.2; CDAI >2.8 and ≤10; SDAI >3.3 and ≤11). (All data shown ‘as observed’). CRP, C-reactive protein; CDAI, Clinical Disease Activity Index; DAS28, 28-joint count Disease Activity Score; ESR, erythrocyte sedimentation rate; PhGA, physician global assessment; PtGA, patient global assessment; SDAI, Simplified Disease Activity Index; SJC, swollen joint count; TJC, tender joint count.
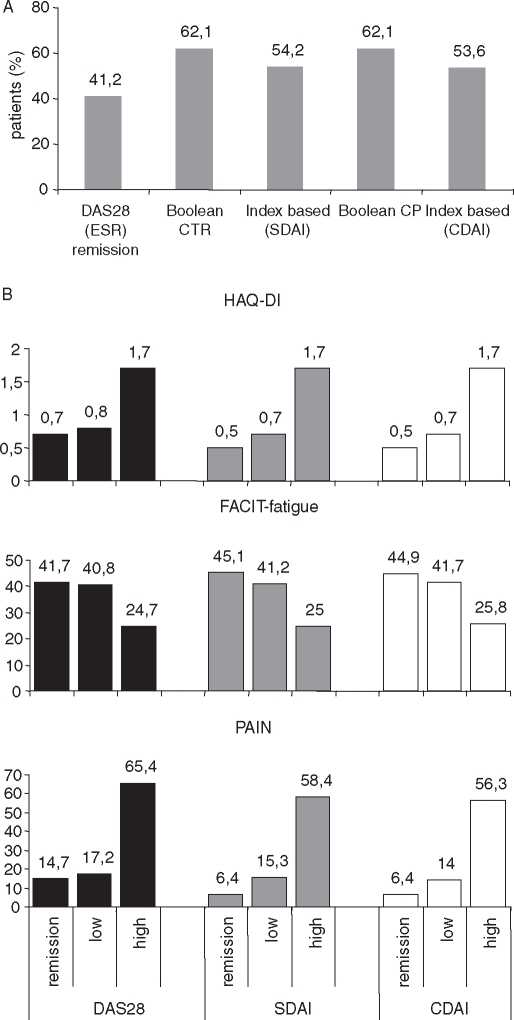
To assess whether the more stringent remission criteria had an impact on patient-related outcomes, we analysed the relationship with the Health Assessment Questionnaire-Disability Index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-fatigue) and pain scores after 24 weeks. The proportion of patients with an HAQ-DI of <0.5 was higher in patients reaching remission according to the new stringent criteria than in patients in DAS28 remission (see figure 3A). Similarly, improvement in other patient-related outcomes was higher in patients in CDAI or SDAI remission than in patients in DAS28 remission. Nevertheless, patients with low disease activity according to CDAI, SDAI and DAS28 still benefited significantly (figure 3B).
Figure 3.
(A)Proportion of patients with HAQ-DI remission (<0.5) according to the five remission criteria (erythrocyte sedimentation rate (ESR); clinical trial (CTR); clinical practice (CP)). (B) Impact of clinical remission according to DAS28, CDAI or SDAI on patient-related outcome HAQ-DI (scaled from 0 to 3, 0=no disability), FACIT-fatigue (higher scores indicate better quality of life) and pain (visual analogue scale 0=no pain, 100=worst pain) after 24 weeks. The more stringent criteria CDAI and SDAI tended to have a better patient-related outcome on the HAQ-DI and pain, but not in the FACIT fatigue score. Noteworthy also patients with low disease activity benefited clearly from tocilizumab treatment. CDAI, Clinical Disease Activity Index; DAS28, 28-joint count Disease Activity Score; FACIT, Functional Assessment of Chronic Illness; HAQ-DI, Health Assessment Questionnaire-Disability Index; SDAI, Simplified Disease Activity Index.
Discussion
The new remission criteria proposed by ACR and EULAR will probably replace the well-established DAS28-based criteria, which have always been controversial. It now remains to be established, if the novel criteria are indeed more useful in trials, which try to model routine clinical practice. The TAMARA phase IIIb study appears to be suitable for analysing this question. This trial had demonstrated very high rates of DAS28 remission of 47.6% with TCZ, while the ESR fell from 28 mm/h to 6 mm/h. Cytokine-blocking biological agents, and TCZ in particular, led to a normal APR in almost all patients. While this constitutes a positive treatment effect, such improvement in ESR might lead to an overestimation of remission rates achieved with the DAS28.14 The novel ACR/EULAR remission criteria are not prone to lead to such bias. However, given that they will not allow for more than one TJ or a VAS >1, remission defined by these criteria might be very difficult to achieve.
When now analysing the same patient population with these new remission criteria, it came as no surprise that the more stringent remission criteria gave significantly lower rates. Nevertheless, TCZ still achieved ACR/EULAR remission in more than a quarter of those patients who had not had an adequate response to cDMARDs, and in 13.5% of patients with active RA despite TNFα inhibitor failure. Moreover, 7% were in ACR/EULAR remission as early as 4 weeks after starting TCZ.
The difference between the numbers for DAS28 and for ACR/EULAR remission was to be expected, given the narrow limits of one clinically SJ and TJ each and the ≤1 cm limit in patient self-assessment. However, these numbers indicate that ACR/EULAR remission is a meaningful concept and an attainable goal for clinical trials and clinical practice. Indeed, the new criteria showed better discrimination between RA refractory to cDMARDs and also not responding to TNF blockers. On the other hand, it was also reassuring to see that modern agents like TCZ now regularly achieve a more stringently defined remission despite inadequate response to DMARDs, and even TNFα blockers—that is, in a subset of patients for whom very few options would have existed 15 years ago.
CDAI and SDAI remission rates, the proposed indexed-based remission criteria,12 were closely linked in our study population, despite the influence of the CRP on SDAI, in view of the absence of APRs in CDAI. This is well in line with CDAI derivation data based on other DMARDs15 and with findings from the TCZ pivotal trials.14 The fact that scores without ESR or CRP worked in the TAMARA trial, as well as the moderate weight of ESR changes between active disease and DAS28 remission (figure 1) suggest that the reduction of the APR is not the decisive factor in leading to remission with TCZ. Our results suggest that the CDAI (without the APR) is sufficient for the follow-up of patients receiving TCZ, because virtually all patients treated with TCZ have a normal CRP and/or ESR.
In comparison with the novel Boolean criteria, CDAI and SDAI are, however, somewhat more flexible in their attribution of points. A patient with a global self-assessment of disease activity of 2.5 cm on the 10 cm scale may still be in CDAI remission, when SJC and TJC are zero. Most probably, the somewhat higher remission rates with these scores are explained by such higher flexibility.
‘True’ remission may not have been achieved in many of the patients in DAS28 remission, and those who do not achieve remission according to the new ACR/EULAR criteria, in particular. Nevertheless, it is not entirely clear that these latter patients will not likewise have an excellent outcome. Although DAS28 remission may not always be sufficient to protect patients receiving methotrexate from structural damage,12 the direct effects of biological response modifiers both on the APR and on joint destruction may offer additional protection. Still, the improvement in the patient-related outcomes HAQ-DI, pain and fatigue was more pronounced in patients in CDAI or SDAI remission than in those in DAS28 remission, while reaching low disease activity was already associated with obvious benefit (figure 3A, B).
Even low disease activity may be an acceptable result for some patients (especially those with longstanding RA), and, on a conciliatory note, the overall percentages of patients achieving at least low disease activity are virtually identical between DAS28, SDAI, and CDAI (see figure 2B). This will cover approximately two out of three patients, while one-third of the patients still need a better individual treatment strategy.
Our study has some limitations: this assessment with the new remission criteria is a post hoc analysis. The predefined outcome of the study was low disease activity and remission according to DAS28. In consequence, the wording of the patient VAS was more open, while the ACR/EULAR criteria suggest concentration on arthritis complaints. This may underestimate the Boolean rate of remission. TAMARA was an open-label, one-arm trial, which could increase both investigator bias and placebo effect. This might lead to an overestimate of remission rates. Moreover, the trial had a duration of 6 months only, and radiographic change was not assessed.
Nevertheless, to the best of our knowledge, this is the first report using the new 2011 ACR/EULAR remission criteria for patients from a phase IIIb study receiving TCZ. The new criteria have been validated in clinical trials with TNFα blockers16–18 showing remission rates in 22% of patients with early RA (data in Felson et al 12). Such clinical trials, where patients meet stringent inclusion criteria, differ from real-life medical care.19 Our data from the TAMARA study, which was designed to reflect daily clinical routine, show even higher remission rates of up to 27.6% of patients whose RA had not adequately responded to cDMARDs before. Achieving stringent and sustained remission in patients with RA remains an ambitious aim, even with the treatment options we now have available. However, the new ACR/EULAR criteria for assessing remission define a realistic, but very ambitious level of disease control, which will push us further toward reaching ‘true remission’. The fact this may still be more difficult to achieve for patients with longlasting disease once again underlines the importance of early and effective treatment in daily clinical practice.
Conclusions
Measurement of RA remission with DAS28 has been shown to be of limited usefulness for treatment with biological response modifiers. The new 2011 ACR/EULAR criteria for remission provide a new tool to stringently define remission of RA in trials and in routine clinical practice. Despite the stringency of these criteria and the highly active disease of the patients in the TAMARA study with DMARD pretreatment, TCZ induced remission rates in over a quarter of patients. Of note, significant remission rates with TCZ were even achieved after inadequate response to TNFα inhibitors and also with disease activity measures not inclusive of APRs such as the CDAI and the new ACR/EULAR criteria for clinical practice. Cytokine blocking agents, such as TCZ, can rapidly achieve stringent remission after DMARD (and TNFα blocker) failure. Thus, the new ACR/EULAR remission criteria are feasible and produce meaningful numbers in settings close to everyday clinical routine.
Acknowledgments
The authors thank all participating centres and patients as well as Dr Uta Düesberg for supportive medical writing and Dr Thomas Fischer, senior biometrician at Winicker Norimed, Nürnberg, Germany for his help.
Footnotes
Principal Investigators Dr. Christopher Amberger, Bad Neuenahr; Dr. Peer-Malte Aries, Hamburg; Prof. Dr. Christoph Baerwald, Leipzig; Dr. Walter Behringer, Fulda; Dr. Sylvia Berger, Naunhof; Dr. Raoul Bergner, Ludwigshafen; Dr. Martin Bohl-Bühler, Potsdam; Dr. Jan Brandt-Jürgens, Berlin; Prof. Dr. Jürgen Braun, Herne; Dr. Matthias Braun, Cuxhaven; Prof. Dr. Harald Burkhardt, Frankfurt; Dr. Edmund Edelmann, Bad Aibling; Dr. Andreas Engel, Stuttgart; Prof. Dr. Christoph Fiehn, Baden-Baden; Prof. Dr. Markus Gaubitz, Münster; Dr. Georg Gauler, Osnabrück; Prof. Dr. Angela Gause, Hamburg; Dr. Joachim Georgi, Damp; Dr. Mathias Grünke, München; Dr. Irmgard Gürtler, Neuss; Prof. Dr. Michael Hammer, Sendenhorst; PD Dr. Bernhard Heilig, Heidelberg; Dr. Maria Höhle, Hamburg; Dr. Andreas Kapelle, Hoyerswerda; Dr. Kirsten Karberg, Berlin; Dr. Thomas Karger, Köln; Dr. sc. Peter Kästner, Erfurt; Dr. Jörg Kaufmann, Ludwigsfelde; Prof. Dr. Jörn Kekow, Gommern; Prof. Dr. Herbert Kellner, München; Prof. Dr. Gernot Keyßer, Halle (Saale); Prof. Dr. Ina Kötter, Tübingen; Prof. Dr. Andreas Krause, Berlin; Prof. Dr. Klaus Krüger, München; Dr. Brigitte Krummel-Lorenz, Frankfurt; Dr. Reiner Kurthen, Aachen; PD Dr. Hans-Eckhard Langer, Düsseldorf; Dr. Michael Leidert, Lüneburg; Dr. Werner Liman, Hagen; Dr. Thomas Linde, Halle; Dr. Lothar Meier, Hofheim; Prof. Dr. Gerhard A. Müller, Göttingen; Prof. Dr. med. Hubert Nüßlein, Nürnberg; Dr. Wolfgang Ochs, Bayreuth; Dr. Blanche Piper, München; Jürgen Rech, Erlangen; Sven Remstedt, Berlin; Dr. Constanze Richter, Stuttgart; Dr. Matthias Richter, Rostock; Dr. Karin Rockwitz, Goslar; PD Dr. Ekkehard Röther, Villingen-Schwenningen; Prof. Dr. Andrea Rubbert-Roth, Köln; Prof. Dr. Reinhold-Ernst Schmidt, Hannover; Prof. Dr. Andreas Schwarting, Mainz; Dr. Holger Schwenke, Dresden; Dr. Helmut Sörensen, Berlin; Prof. Dr. Christof Specker, Essen; Dr. Rainer Sprekeler, Zeven; Dr. Frank Trautmann, Mainz; Dr. Leonore Unger, Dresden; Dr. Andreas Viardot, Ulm; Dr. Ulrich von Hinüber, Hildesheim; Dr. Jan Voswinkel, Homburg-Saar; Dr. Siegfried Wassenberg, Ratingen; Dr. Jörg Wendler, Erlangen; Dr. Wolf-Dieter Wörth, Wiesbaden; Dr. Silke Zinke, Berlin.
Funding The study ‘TAMARA’ was funded by Roche Pharma AG Grenzach-Wyhlen, Germany and Chugai Pharma Marketing Frankfurt, Germany.
Competing interests GRB, EF, MA, JW and CIK are members of advisory boards, received grant support or honoraria as speakers from Roche/Chugai. ST is an employee of Roche Pharma Germany and TM is an employee of Chugai Pharma Germany.
Ethics approval This study was conducted with the approval of the Charite Berlin, Germany.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, et al. ; T2T Expert Committee Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 1998;41:1845–50 [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde DM, van 't Hof M, van Riel PL, et al. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579–81 [PubMed] [Google Scholar]
- 5.Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8 [DOI] [PubMed] [Google Scholar]
- 6.Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 2005;52:2625–36 [DOI] [PubMed] [Google Scholar]
- 7.Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gülfe A, Aletaha D, Saxne T, et al. Disease activity level, remission and response in established rheumatoid arthritis: performance of various criteria sets in an observational cohort, treated with anti-TNF agents. BMC Musculoskelet Disord 2009;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakker MF, Jacobs JW, Verstappen SM, et al. Tight control in the treatment of rheumatoid arthritis: efficacy and feasibility. Ann Rheum Dis 2007;66(Suppl 3):iii56–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landewé R, van der Heijde D, van der Linden S, et al. Twenty-eight-joint counts invalidate the DAS28 remission definition owing to the omission of the lower extremity joints: a comparison with the original DAS remission. Ann Rheum Dis 2006;65:637–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mäkinen H, Kautiainen H, Hannonen P, et al. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann Rheum Dis 2005;64:1410–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13 [DOI] [PubMed] [Google Scholar]
- 13.Burmester GR, Feist E, Kellner H, et al. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA). Ann Rheum Dis 2011;70:755–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum 2011;63:43–52 [DOI] [PubMed] [Google Scholar]
- 15.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23(5 Suppl 39):S100–8 [PubMed] [Google Scholar]
- 16.Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93 [DOI] [PubMed] [Google Scholar]
- 17.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37 [DOI] [PubMed] [Google Scholar]
- 18.Klareskog L, van der Heijde D, de Jager JP, et al. ; TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81 [DOI] [PubMed] [Google Scholar]
- 19.Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum 2006;54:3399–407 [DOI] [PubMed] [Google Scholar]