Abstract
Objective
This study examined subjective and other behavioral effects of methylphenidate (MPH) among adolescents.
Methods
Standard abuse liability assessment methods that have been used in adult populations were modified for attention-deficit/hyperactivity disorder (ADHD) adolescents. MPH effects (0, 0.25 mg/kg) were evaluated under randomized, double-blind conditions in two 5-hour laboratory sessions in 24 (13 female) 11–15 year olds diagnosed with ADHD.
Results
Repeated measures analysis of covariance indicated significant dose and dose by time interactions on subjective ratings on the modified amphetamine (A) [F (1, 20) = 5.98; p < 0.05; η2 = 0.36], morphine–benzedrine group (MBG) [F (1, 21) = 8.93 p < 0.01; η2 = 0.38] and benzedrine group scale (BG) [F (1, 21) + 13.10 p < 0.01; η2 = 0.37] scales of the Addiction Research Center Inventory; “Hungry” and “How sure are you that you got the medication today?” from the Visual Analogue Scale, the Profile of Mood States Depression scale, performance on the Continuous Performance Task, heart rate and blood pressure, and level of activity.
Conclusions
This is the first study to document subjective effects of stimulants in adolescents with ADHD that have been associated with drug abuse potential in adults. There are increasing concerns about nontherapeutic stimulant use in adolescents and young adults. Assessing subjective effects of pharmacotherapies for ADHD along with other measures of abuse potential such as drug self-administration may aid in assessing the therapeutic effects and/or risk of medications used in the treatment of ADHD.
INTRODUCTION
Stimulants, Particularly Methylphenidate (MPH), are the most frequently prescribed and thoroughly investigated medications for the management of attention-deficit/hyperactivity disorder (ADHD). The therapeutic effects of these medications have been well established (e.g., Rushton et al. 2004).
There is controversy regarding whether use of stimulant drugs with clearly established abuse potential in ADHD adolescents puts them at risk for subsequent drug use and abuse. There is evidence that drug abuse rates are lower in ADHD adolescents who are treated with stimulants as compared to ADHD adolescents who are not treated (Biederman et al. 1999; Wilens et al. 2003). Furthermore, there is evidence that ADHD symptoms contribute to risk for drug abuse. For example, the interval of time between initial use and development of dependence is significantly shorter for adolescents with ADHD compared to controls (Biederman et al. 1997), even with controlling for co-morbid conduct disorder (CD; Wilens et al. 1997). Contradictory evidence suggests that therapeutic stimulant use may be associated with increased tobacco and cocaine use (Lambert and Hartsough 1998). Issues of nonprescription stimulant use by students and diversion of prescription stimulants are receiving increased attention (Johnston et al. 2003; McCabe et al. 2004; Upadhyaya et al. 2005; McCabe et al. 2006).
Despite this controversy about drug abuse risks, there is little research in adolescents with ADHD in the area of the subjective effects of stimulants that have been associated with drug abuse potential. This is in stark contrast to the extensive research on the subjective effects of stimulants in adults (e.g., Martin et al. 1971; Jasinski et al. 1984; Henningfield et al. 1987; Folton and Fischman 1991; Heil et al. 2002). Drugs with abuse potential engender subjective effects that have been characterized as euphorigenic (Jasinski et al. 1984; Henningfield et al. 1987). Measures that have been used to assess the subjective effects of stimulants and other drugs with abuse potential in adults include the Addiction Research Center Inventory (ARCI; Martin et al. 1971), the Profile of Mood States (POMS; McNair et al. 1971), and Visual Analogue Scales (VAS; Foltin and Fischman, 1991).
Specifically drug “liking” measured using the VAS and “euphoria” measured by the ARCI morphine–benzedrine group (MBG) scale have been associated with reinforcing effects of drugs (Jasinski et al. 1984; Foltin and Fischman 1991). It is not clear whether stimulant medications that are used for the treatment of ADHD engender subjective effects in adolescents that are similar to subjective effects seen in adults. Measurement of the behavioral effects of stimulant medication in children and adolescents usually involves global clinical responses in various settings, such as home and school, and relying on family and teacher responses on structured questionnaires such as the Conners’ Rating Scales (Conners and Barkley 1985). Other behavioral measures have included performance on cognitive and psychomotor tasks (Barkley et al. 1991; Swanson et al. 2002). More recently, these measures (e.g., teacher ratings, completion of math problems, computerized measures of reaction time) have also been used to assess the time course of therapeutic effects of stimulants (Swanson et al. 2002). Only a handful of studies have examined subjective stimulant effects in children and adolescents. In one of the early studies of benzedrine, the racemic form of amphetamine, hospitalized children reported positive subjective effects, such as euphoria, following drug (mean 17 mg) administration (Bradley 1937). Rapaport et al. (1980) found that prepubertal normal and ADHD children reported feeling “funny” on the van Kammen–Murphy Moods Scale (Van Kammen and Murphy 1975) in response to dextroamphetamine administration (mean 16 mg) but not euphoria, as was reported by adults in both the high (mean 34 mg) and low (mean 17 mg) dose conditions. MacKay et al. (1973) found that among 10 adolescents ages 13–17 years old, only the 17 year old reported an increase in positive mood following methylphenidate (mean 30 mg). POMS and VAS items were used to measure subjective effects in ADHD children; however, only minimal changes have been observed following stimulant administration (Walker et al. 1988; Kollins et al. 1998). For example, Kollins et al. (1998) reported that standard therapeutic doses (5–20 mg) of MPH decreased reports of “I am hungry today” and increased reports of “My heart is beating fast today,” but only two of 22 items were affected by the drug.
Fredericks and Kollins (2004, 2005) have also examined stimulant self-administration in ADHD populations. In a study with ADHD young adults (18–22 years), Fredericks and Kollins (2004) found greater choices of capsules containing MPH (maintenance dose) than either placebo or no-capsule options. Drug choice was more closely associated with MPH-induced reductions in ADHD symptoms than the subjective effects of the drug. MPH choice behavior was also examined in 5 adolescents (10–14 years of age) in a second study (Fredericks and Kollins 2005). Three of the 5 adolescents reliably chose MPH over placebo or the no-capsule option; however, no MPH-induced subjective effects were detected.
It is not clear whether: (1) stimulant drugs do not engender the same subjective effects in adolescents that are commonly reported in adults; (2) insensitive self-report measures have been used to assess subjective effects in adolescents; or (3) adolescents have inadequate training and/or experience to report subjective changes in a reliable manner as is seen with adults following stimulant drug administration.
The present study examined the subjective effects of MPH in 11–15 year olds with ADHD trained to recognize and report changes in subjective cues with commonly used adult self-report measures modified for use with ADHD adolescents. The terms and method of presentation of self-report items were modified to account for the level of comprehension and distractibility of the population. It was hypothesized that MPH would engender subjective effects that would be similar to those reported by adults in previous studies.
METHODS
Participants
Participants between the ages of 11 and 15 (13 ± 1.5) were recruited from a child psychiatry clinic, a general pediatric clinic, and a pediatric clinic for families with more limited resources. Patients with current or past histories of uncomplicated stimulant medication use for ADHD who were not using other psychotropic medications currently and had no significant medical or psychiatric disorders other than ADHD were eligible. Efforts were made to schedule sessions during breaks from school.
Following attempts to recruit an equal number of males and females; data from 13 females and 11 males were analyzed. Four were African American, and one subject was biracial. A Tanner-stage exam revealed the following distribution: 1 in stage 1; 11 in stage 2; 4 in stage 3; 3 in stage 4; and 5 in stage 5. There was variability in current and prior therapeutic stimulant exposure, with 10 reporting use of long-acting dextroamphetamine salts, 1 use of D-amphetamine, 5 use of long-acting MPH, and 8 use of short-acting MPH. Nontherapeutic drug use was minimal. Only 1 subject reported past use of other drugs (nicotine), and drug use (marijuana) was detected in only one urine sample obtained during the prestudy screen (not on either of the laboratory test days) in a second subject. One subject did not complete both test days, and a second completed the study but did not swallow the pill on one test day. Data from these 2 subjects were not included in the analysis.
The Medical Institutional Review Board of the University of Kentucky approved the research protocol, and subject assent and parental consent were obtained prior to the study.
Procedure
Modification of self-report measures
Prior to the initiation of the laboratory study, groups of adolescents who did not participate in the study were asked to review the terminology in the ARCI and the POMS. The adolescents identified statements or phrases that were unclear. The interviewer then defined the statements or phrases, and the adolescents suggested alternative phrases that conveyed roughly equivalent meanings. The edited items for the ARCI MBG subscale are presented in Table 1 and those from the remaining ARCI subscales and the POMS are available on request from the authors.
TABLE 1.
Revised Addiction Research Center Inventory MBG Scale (Original adult Item is the Second Statement in Italics)
| Today I say things easily. |
| Today I say things in the easiest possible way. |
| Things around me seem nicer than usual. |
| Things around me seem more pleasing than usual. |
| I have a good feeling in my stomach. |
| I have a pleasant feeling in my stomach. |
| I feel I will lose the happiness that I have now. |
| I feel I will lose the contentment that I have now. |
| I feel happy with the world and people around me. |
| I feel in complete harmony with the world and those about me. |
| I can completely understand what other people are saying when I feel the way I do now. |
|
I can completely appreciate what others are saying when I am
in this mood. |
| I would be happy all the time if I felt like I feel now. |
| I would be happy all the time if I felt as I feel now. |
| I feel so good that I know other people can tell it. (No change) |
| I feel like something nice has just happened to me. |
| I feel like something pleasant has just happened to me. |
| I feel more clear headed than sleepy or dreamy. |
| I feel more clear headed than dreamy. |
| I feel as if I would be more popular with people today. (No change) |
| I feel a very good empty feeling. |
| I feel a very pleasant emptiness. |
| My thoughts come more easily than usual. (No change) |
| I feel more like trying than usual. |
| I feel less discouraged than usual. |
| I am in the mood to talk about my feelings. |
| I am in the mood to talk about the feelings I have. |
Orientation/training and screening
All subjects completed an orientation/training session designed to familiarize them with the experimental protocol within 10 days of the first laboratory test day. To enhance the ability of adolescents to identify and report subjective cues, a module was presented to demonstrate how ratings on questionnaires can change over time on the basis of changes in subjective states. In this training procedure, subjects were asked to rate subjective states on questionnaires presented in a format similar to that used in the study, and heart rate was measured. They were told to read each item and to answer it according to how they felt at the moment they were completing the questions. Subjects were then instructed to perform 20 jumping jacks. Heart rate was reassessed immediately, and subjects were then asked to answer the same questionnaire. The subjects then compared their heart rate and self-report ratings before and after the jumping jacks and noted the changes. The subjects then completed two practice sessions with the Continuous Performance Task (CPT).
Subjects and their parents were informed that they would receive either placebo or a MPH dose adjusted to body weight and that the dose would not exceed 20 mg. They were told to fast the morning of the study and that they would receive breakfast at the laboratory. They were told to withhold all medication until after the lab session was completed.
Experimental sessions
All subjects arrived at a General Clinical Research Center at 8 a.m. on 2 days separated by 1, 2, or 3 days. On arrival, breath samples were analyzed with a hand-held alcohol sensor (Alco-Sensor, Intoximeters, Inc.). Saliva was collected and analyzed for cotinine, and a urine sample was screened for pregnancy (in females) and drug use amphetamines, MPH, barbiturates, benzodiazepines, cannabinoids, cocaine metabolites, methadone, opiates, and propoxyphene). Subjects had been informed that evidence of drug use other than therapeutic stimulant medication would result in sessions being cancelled and rescheduled, although no indication of nontherapeutic drug use was obtained in any of the samples collected on laboratory test days. The majority of subjects reported ongoing use of stimulants for therapeutic purposes; positive urine drug screens for therapeutic stimulants were considered an indication of immediate therapeutic use.
The 5-hour laboratory test days were conducted in a hospital room containing a chair, table, computer to present a Conners’ Continuous Performance Test (CPT), an automated blood pressure machine (Dinamap; GC Medical), television, videotapes, and hand-held games. Subjects received a low-fat breakfast (cereal, toast, jelly, juice, and skim milk) at 8:30 a.m., 60 minutes before drug administration, to allow for standardized gastrointestinal (GI) and nutritional conditions. An ActiTrac (IM Systems, Inc.) wrist activity monitor was applied immediately after the meal. Assessments, which required approximately 30 minutes to complete, were presented prior to drug administration (i.e., baseline) and 1 and 2 hours postdrug administration. VAS and cardiovascular measures were also collected 3 hours postdose. Between assessments, subjects were allowed to play hand-held games or watch videotapes.
Drug
MPH (0.00 and 0.25 mg/kg) capsules were prepared on the basis of body weight by the University of Kentucky Investigational Pharmacy and administered under randomized, double-blind conditions. Thirteen subjects received placebo and 11 received active drug on the first laboratory test day; subjects received the alternate dose on the second laboratory test day.
Assessment Measures
Self-report measures of drug effect were obtained in each assessment and included modified ARCI, POMS, and VAS items. A modified version of the ARCI Short Form (Martin et al. 1971) consisted of true–false questions from three subscales: The MBG scale, a measure of euphoria (see Table 1); the benzedrine group scale (BG), an amphetamine-sensitive scale that assesses intellectual efficiency and energy; and the amphetamine (A) scale, a stimulant-sensitive scale. Items from four scales of the POMS (Depression, Anger, Vigor, and Confusion) (McNair et al. 1971) were presented. Subjects rated individual items using a 5-point scale ranging from “not at all” to “extremely.” VAS items were answered by placing a mark on a 10-cm line with the left end point of the continuum labeled “not at all” and the right end point labeled “extremely.” VAS items included “Energetic,” “Sleepy,” “High,” “Anxious,” “Down,” “Hungry,” “Stressed,” and “Can Concentrate.” Two additional items (“How sure are you that you got the medication today?” and “I like the way the medication [I took for this study today] is making me feel.”) were presented during assessments occurring after capsule administration. To maintain on-task behavior, questions were recorded on an audiotape, and subjects were instructed to read and answer items as they were presented audibly. Subjects were also instructed that they would receive 50¢ for completing all questions in each assessment and encouraged to review their answers to assure completion. Subjects were observed through the entire protocol, and the observer gave subjects an extra 10¢ at the completion of the questionnaires if they had answered questions as they were presented on the audiotape. This payment supplemented their payment for participating in the study.
Physiological measures included heart rate, systolic and diastolic blood pressure, and physical movement (i.e., activity). Physical movement was measured in units of mG of gravitational acceleration.
Subjects completed a 15-minute version of the CPT, a vigilance task in which letters are flashed on a screen at a rate of 1 per second (Conners 1994). Subjects were instructed to respond following specific patterns of letter presentation. Six measures were obtained from this task: (1) Omissions, number of targets to which the subject did not respond; (2) commissions, number of times the subject responded to a nontarget; (3) hit response time (RT), the mean response time for all accurate target responses; (4) hit RT standard error (SE), the consistency of response times, expressed in terms of SE for responses to targets; (5) attentiveness (d’), a measure of how well the subject discriminated between targets and nontargets; and (6) risk taking (β), a subject’s response tendency to either not respond often or respond frequently.
Data analysis
Data analysis was conducted using a repeated-measures analysis of covariance (ANCOVA) with drug (placebo, active) and time post dose (0, 1, 2 hours) as within-subject factors, and urine drug screen for therapeutic stimulants (positive or negative) and order of active drug versus placebo administration were entered as between-subject factors. In the case of the VAS, heart rate, and blood pressure, there was an additional 3-hour post-drug assessment. Interactions were examined using simple-effects models. Statistical analyses were conducted using SPSS GLM. An alpha level of 0.05 was used as the cutoff for statistical significance. The Huynh–Feldt adjustment was used to take into account nonsphericity of the co-variance. Effect sizes were determined for the within-subjects factors of drug and drug by time interactions using partial eta squared (η2), an estimate of the degree of association for within-subjects designs (Keppel 1991). MPH effects were determined based on significant F values (ANCOVA) for the main effects of drug and drug-by-time interactions.
RESULTS
Self-report measures
Addiction Research Center Inventory
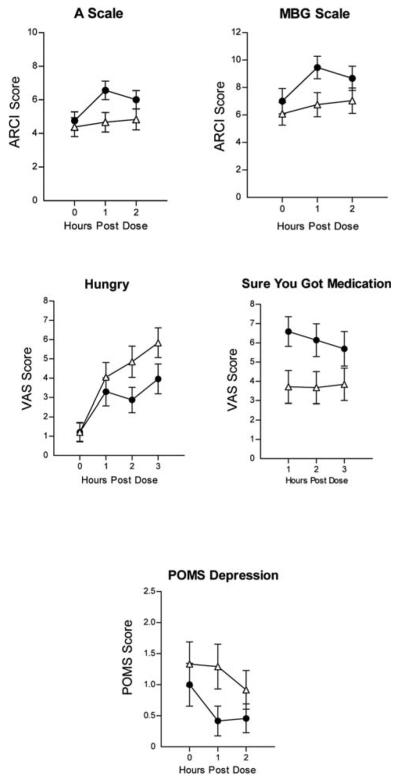
Figure 1 (top panels) presents the effects of MPH on self-report measures on the ARCI A and MBG scales scores. Significant main effects of drug [F(1, 20) = 5.98; p < 0.05; η2 = 0.36 and time [F(1, 40) = 3.44; p < 0.05] were observed on the A scale; significant main effects of drug [F(1, 21) = 8.93 p = 0.01; η2 = 0.38] and time [F(2, 42) = 6.24; p < 0.01] were observed on the MBG scale; and significant main effects of drug [F(1, 21) + 13.10; p < 0.01; η2 = 0.37; data not shown] were observed on the BG scale. Peak effects were observed 1 hour postdose on all three scales.
FIG. 1.
MPH effects on the A and MBG scales of the ARCI, “Hungry,” and “Sure You Got the Medication” on the VAS and POMS Depression Scale as a function of time following dose administration. Error bars = ± 1 SEM. (●) Active Drug; (△) placebo. MPH = Methylphenidate; MBG = morphine–benzedrine group; ARCI = Addiction Center Research Inventory; VAS = Visual Analogue Scales; POMS = Profile of Mood States; SEM = standard error of the mean.
Visual Analogue Scale
Figure 2 (middle panels) presents the effects of MPH on VAS ratings of “Hungry” and “How sure are you that you got the medication today?” MPH decreased VAS ratings of “Hungry” (significant dose × time effect [F(3, 63) = 2.56; p < 0.05; η2 = 0.15]) and increased ratings of “How sure are you that you got the medication today?” (dose effect [F(1, 19) = 6.05; p < 0.05; η2 = 0.29]). Note: This last measure was only obtained after medication administration. Peak increases in “How sure are you that you got the medication today?” occurred 1 hour postdose, whereas peak effects on hunger occurred 2–3 hours postdose. No significant effects of MPH were observed on the “Full of Energy,” “High,” “Anxious,” “Down,” “Able to Concentrate,” “The medication (I took for this study today) is making me feel different,” and “I like the way the medication (I took for this study today) is making me feel” VAS items.
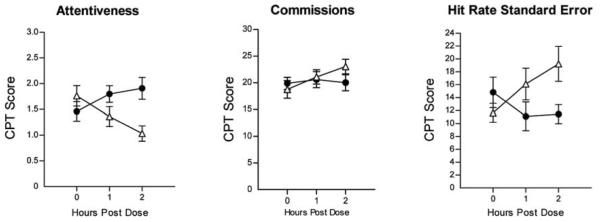
FIG. 2.
Effects of MPH on CPT performance as a function of time following dose administration. Error bars = ± 1 SEM. (●) Active drug; (△) placebo. MPH = Methylphenidate; CPT = Continuous Performance Test; SEM = standard error of the mean.
Profile of Mood States
Figure 1 (bottom panel) presents the effects of MPH on POMS “Depression,” with MPH reducing ratings. Significant main effects of drug were seen on the POMS Depression subscale [F(1, 21) = 5.32; p < 0.05; η2 = 0.21]. No significant effects of MPH were observed on the Anger, Vigor, or Confusion scales.
Continuous Performance Task
Figure 2 presents the effects of MPH on dimensions of CPT performance. Significant dose × time increases in Attentiveness (F[2, 40] = 11.88; p < 0.001; η2 = 0.37) and decreases in Hit Rate Standard Error (F[2, 38] = 8.91; p < 0.001; η2 = 0.31) and Errors of Commission (F[2, 40] = 3.59; p < 0.05; η2 = 0.15) were observed. Peak effects on performance on the CPT were observed 2 hours postdose. No significant effects of MPH were observed on the Errors of Omission or Hit Rate.
Activity
Figure 3 presents the effects of MPH on activity. MPH decreased physical activity (significant dose × time effects [F(2, 36) = 7.01; p < 0.01; η2 = 0.31] with peak effects occurring 2 hours postdose.
FIG. 3.
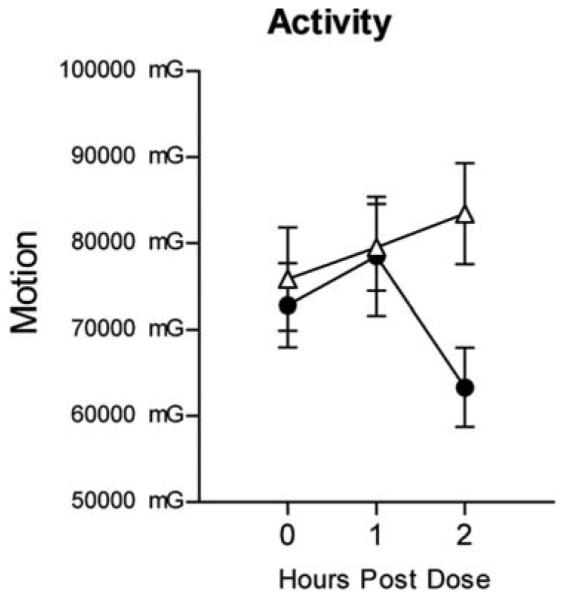
Effects of MPH on activity as a function of time following dose administration. Error bars = ± 1 SEM. (●) Active drug; (△) placebo. MPH = methylphenidate; SEM = standard error of the mean.
Cardiovascular measures
MPH increased cardiovascular activity, with significant dose × time effects occurring on heart rate [F(3, 57) = 3.00; p < 0.05; η2 = 0.12] and significant main drug effects on systolic [F(1, 19) = 6.05; p < 0.05; η2 = 0.34; data not shown] and diastolic [F(1, 19) 6.74; p < 0.05; η2 = 0.21; data not shown] blood pressure. Figure 4 presents the effects of MPH on heart rate. Peak increases of 9 beats per minute occurred 2 hours postdose.
FIG. 4.
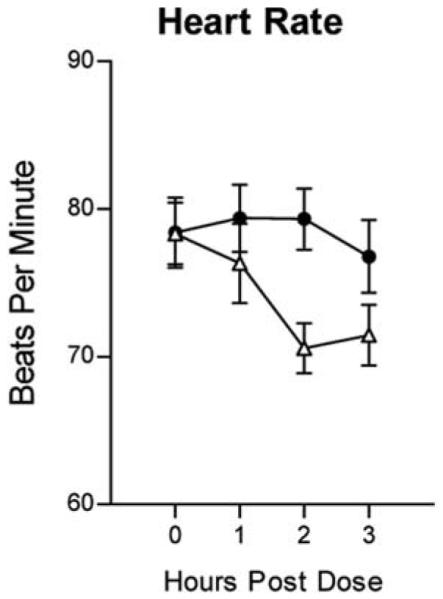
Effects of MPH on heart rate as a function of time following dose administration. Error bars = ± 1 SEM. (●) Active drug; (△) placebo. MPH = Methylphenidate; SEM = standard error of the mean.
Urine drug screen for stimulants and order of active or placebo drug administration
In an effort to control for recency of stimulant treatment, the urine drug screen for stimulants was entered as a factor. This variable did not impact any measure of drug effect. In addition, the order of active or placebo drug administration did not impact on any measure of drug effect.
CONCLUSIONS
The major finding of this study is that reliable MPH-induced (0.25 mg/kg) changes in subjective drug effects were obtained in adolescents with ADHD. Significant effects were obtained on the ARCI A, MBG, and BG scales, as well as on VAS ratings of “Hungry” and “How sure are you that you got the medication today?” and the POMS Depression scale. The time course for MPH-induced changes in self-report measures paralleled drug effects on cardiovascular, task, and activity measures in the current study. In addition, peak MPH effects on these measures typically occurred at 2 and 3 hours postdose, which is similar to the time course of MPH effects on self-report measures often reported by adults (Heil et al. 2002) and on other behavioral effects in children (Pelham et al. 1999). In addition, the time course and magnitude of drug effects on heart rate were consistent with those engendered in adults (Martin et al. 1971; Foltin and Fischman 1991). The range of effect sizes for self-report effects were comparable to effects seen with the CPT, activity, and cardiovascular measures. Cohen (1988) considers an η2 of approximately 0.14 to constitute a large effect size; nearly all effect sizes found in this study were at this level or even larger. These observations support the conclusion that a pharmacologically active dose of MPH was tested and that drug-induced changes were obtained on subjective, physiological, and performance measures in the adolescent ADHD subjects.
We are aware of few previous studies in which subjective stimulant drug effects have been reported by adolescents. The results of this study suggest that the absence of drug-induced changes in subjective drug effects in previous studies with adolescents (MacKay et al. 1973; Rapaport et al. 1980) may reflect the use of insensitive self-report measures, rather than the absence of subjective effects. In the present study, standard procedures used to measure subjective drug effects in adults were modified for use with an ADHD adolescent population. Modifications included using words and phrases that were understood by adolescents; training adolescents to recognize and report changes in subjective states prior to the study; presenting self-report items on audiotape; and providing intermittent reinforcement (i.e., money) for answering self-report items at the pace of the audiotape. Future studies will be required to determine which, if any, of these modifications contributed to enhancing the sensitivity of the self-report measures.
The ARCI MBG scale has been widely used to assess the abuse potential of drugs in adults (Martin et al. 1971). The finding that MPH increased MBG scale ratings in ADHD adolescents may have two implications. First, to the extent that the stimulant effects measured by the modified MBG are associated with the reinforcing effects of drugs in adolescents, as they are in adults, it is possible to interpret these data as suggesting that MPH may have abuse potential in the adolescent ADHD population. Although anecdotally ADHD adolescents rarely seek increases in their therapeutic medication, and, in general, treatment of ADHD with stimulants is associated with decreased risk for substance abuse (Biederman et al. 1999), therapeutic stimulants are used for nontherapeutic purposes and are diverted in the ADHD adolescent and young adult collegeaged populations (Upadhyaya et al. 2005). Additional studies are needed to clarify further whether the modified version of the ARCI MBG scale captures subjective effects that are associated with the reinforcing effects of drug. These could include: Combining self-report measures reported here with self-administration procedures; broadening the study to include varied doses of MPH and other stimulants; and focusing on ADHD adolescents with current stimulant abuse histories. The second implication may be that in adolescents with ADHD, the MBG euphoria scale may capture therapeutic effects of stimulants, such as “feelings of well-being and confidence” (Martin 1973). This interpretation would be consistent with the MPH-induced decrease on the POMS Depression scale and with the observations of Fredericks and Kollins (2005) that choosing MPH over placebo was more closely associated with MPH-induced reductions in ADHD symptoms than the subjective effects of the drug. Not all self-report scales were altered by MPH. It is important to note that stimulants do not engender changes in self-report measures associated with drug effects uniformly across or within scales in adults. For example, in a study of stimulant effects in subjects who had been trained to discriminate the effects of D-amphetamine, Rush et al. (1998) reported that D-amphetamine and MPH increased the ARCI BG subscale but had no effect on other ARCI subscale scores (e.g., A, MBG) and none of the POMS subscales. The fact that there were changes in the MBG scale but not the VAS “High” scale or the “I like the way the medication (I took for this study today) is making me feel” VAS item is not inconsistent with other pharmacological studies of MPH effects in adults.
Limitations of the current study are apparent. Investigation of stimulant effects in adolescents is hampered by ethical and practical concerns associated with the administration of stimulants to stimulant–medication naive adolescents. Because of these concerns, the current study was performed on an ADHD population that had previously received stimulant medication for clinical purposes. In addition, regular drug users were excluded from the study participation because of concerns that active drug use would compromise assessment of MPH effects. These selection criteria may have limited the generalizability of the study results. Another limitation to this study is the use of only a single dose of MPH. The extent of prior therapeutic stimulant use also varied among adolescents. Current medication status was controlled statistically in the present study. However, because the sample size was limited and the duration of exposure was variable among participants, the effects of medication history were not examined in a thorough manner. In addition, there was variability in the time between sessions and time since last therapeutic dose. Finally, although training procedures, age-appropriate terms and contingencies for answering questions at a steady rate were used to enhance the sensitivity of the self-report measures, the effects of these procedures were not examined.
Stimulants are a highly effective treatment for ADHD (Rushton et al. 2004). Although the performance effects of stimulants have been well documented in this population, the subjective effects of stimulants have received limited attention. Subjective measures may be useful in both the research and clinical settings. First, with rapid development of new medications for ADHD, the strategies developed here could be used along with self-administration procedures in ADHD adolescents for initial assessment of abuse potential, as has been done in adults (Frederick and Kollins 2004). This may be particularly important for adolescents with ADHD because they are moving into an age when risk for drug experimentation increases. Second, stimulants may engender subjective effects that may be related to the therapeutic effects of the drug and may engender feelings of “well-being and confidence” (Martin, 1973) in a group that may be vulnerable to feelings of self-doubt as natural sequelae of their compromised functioning. Whether these self-reported drug effects are associated with therapeutic and/or reinforcing effects of stimulant drugs requires further investigation.
Understanding the subjective effects of stimulants is of critical importance when developing and ultimately choosing optimal pharmacological interventions in adolescents with ADHD when the cost of under treatment is high and yet there are concerns these ADHD adolescents are moving into an age when risk for drug diversion and misuse increases (McCabe et al., 2004).
Acknowledgments
This study was supported by NIDA grants 1 KO8 DA00333; P50 DA05312; 1 R03 DA015413-01A2 and National Institutes of Health grant number M01 RR02602.
Footnotes
DISCLOSURES
Catherine Martin has served as an educational consultant for Cephalon. No other author has any disclosures to report.
REFERENCES
- Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: Clinical response to three dose levels of methylphenidate. Pediatrics. 1991;87:519–531. [PubMed] [Google Scholar]
- Biederman J, Wilens TE, Mick E, Faraone SV, Weber W, Curtis S, Thornell A, Pfister K, Jetton JG, Soriano J. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens TE, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- Bradley C. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94:577–585. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale (New Jersey): 1988. [Google Scholar]
- Conners CK. The Conners’ Continuous Performance Test. Multi-Health Systems, Inc; Toronto: 1994. [Google Scholar]
- Conners CK, Barkley RA. Rating scales and checklists for child psychopharmacology. Psychopharmacol Bull. 1985;21:809–811. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: A methodological survey. Drug Alcohol Depend. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Fredericks EM, Kollins SH. Assessing methylphenidate preference in ADHD patients using a choice procedure. Psychopharmacology. 2004;175:391–398. doi: 10.1007/s00213-004-1838-2. [DOI] [PubMed] [Google Scholar]
- Fredericks EM, Kollins SH. A pilot study of methylphenidate preference assessment in children diagnosed with Attention-Deficit/Hyperactivity Disorder. J Child Adolesc Psychopharmacol. 2005;15:729–741. doi: 10.1089/cap.2005.15.729. [DOI] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Johnson RE, Jasinski DR. Clinical procedures for the assessment of abuse potential. In: Bozarth MA, editor. Methods of Assessing Reinforcing Properties of Drugs. Springer-Verlag; Berlin: 1987. pp. 573–590. [Google Scholar]
- Jasinski DR, Johnson RE, Henningfield JE. Abuse liability assessment in human subjects. Trends Pharmacol Sci. 1984;5:196–200. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the future National survey results on drug use, 1975–2002. NIDA. 2003;1:386–391. [Google Scholar]
- Keppel G. Design and Analysis. Prentice Hall; Englewood Cliffs (New Jersey): 1991. [Google Scholar]
- Kollins SH, Shapiro SK, Newland MC, Abramowitz A. Discriminative and participant-rated effects of methylphenidate in children diagnosed with attention deficit hyperactivity disorder (ADHD) Exp Clin Psychopharmacol. 1998;6:375–389. doi: 10.1037/1064-1297.6.4.375. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and Non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Mackay MC, Beck L, Taylor R. Methylphenidate for adolescents with minimal brain dysfunction. NY State J Med. 1973;73:550–554. [PubMed] [Google Scholar]
- Martin WR. In: Drug abuse—the need for a rational pharmacologic approach. Brill L, Harms E, editors. Behavioral Publications; New York: 1973. [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ. The use, misuse and diversion of prescription stimulants among middle and high school students. J Psychoactive Drugs. 2004;39:1095–1116. doi: 10.1081/ja-120038031. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38:43–56. doi: 10.1080/02791072.2006.10399827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Profile of Mood States (manual) Educational and Industrial Testing Services; San Diego (California): 1971. [Google Scholar]
- Pelham WE, Arnoff HR, Midlam JK, Shapiro CJ, Gnagy EM, Chronis AM, Onyango AN, Gorehand G, Nguyen A, Waxmonsky J. A comparison of Ritalin and Adderall: Efficacy and time-course in children with Attention-Deficit/Hyperactivity Disorder. Pediatrics. 1999;104:1300–1311. doi: 10.1542/peds.103.4.e43. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine: Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Rushton JL, Fant KE, Clark SJ. Use of practice guidelines in the primary care of children with attention-deficit/hyperactivity disorder. Pediatrics. 2004;114:e23–28. doi: 10.1542/peds.114.1.e23. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Gupta S, Williams L, Agler D, Lerner M, Wigal S. Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: Effects on activity level in the classroom and on the playground. J Am Acad Child Adolesc Psychiatry. 2002;41:1306–1314. doi: 10.1097/00004583-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Upadhyaya HP, Rose K, Wang W, O’Rourke K, Sullivan B, Deas D, Brady KT. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15:799–809. doi: 10.1089/cap.2005.15.799. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Murphy DL. Attenuation of euphoriant and activating effects of D- and L-amphetamine by lithium carbonate treatment. Psychopharmacology. 1975;44:215–224. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- Walker MK, Sprague RL, Sleator EK, Ullmann RK. Effects of methylphenidate hydrochloride on the subjective reporting of mood in children with attention deficit disorder. Issues Ment Health Nurs. 1988;9:373–385. doi: 10.3109/01612848809140939. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. J Nerv Ment Dis. 1997;185:475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Wilens T, Faraone S, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]