Contrast agents for Magnetic Resonance Imaging (MRI) provide anatomical and functional detail and increasingly can convey information at the molecular level.[1] The field of molecular MRI has advanced to the point that clinical studies with molecularly targeted agents are now appearing.[2] Despite the tremendous strengths of molecular MRI (molecular specificity superimposed on a high spatial resolution anatomical image, deep tissue penetration, three dimensional imaging, and lack of ionizing radiation), the field remains limited by the relatively low sensitivity for contrast agent detection.[1b, 3] Sensitivity of contrast agents is typically described by the extent to which they can induce relaxation of tissue water, and this is termed relaxivity (r1). Molecular relaxivity can be increased either by increasing the number of paramagnetic ions in the molecule, or optimizing the molecular factors that influence relaxation, or some combination of both.
For targets present at high concentrations, such as fibrin or extracellular matrix components, it is possible to develop effective peptide targeted agents with one or more gadolinium chelates for positive signal enhancement.[4] Unlike nanoparticles, these relatively small molecules can rapidly reach targets in extravascular spaces and can be readily excreted through the kidneys to reduce/avoid long-term gadolinium retention and toxicity.
Clinical MRI is performed at relatively low fields (0.2 – 3T, with majority of scanners at 1.5T) compared to NMR spectroscopy. One of most effective ways to increase relaxivity at these field strengths is to slow the rotational dynamics of the contrast agent.[1b, 3] For targeted agents, binding to the protein target slows rotation and can increase relaxivity several fold over the unbound agent. While protein binding generally increases relaxivity, the gains are often limited because of internal motion. This is especially true for peptide-based agents where there may be many single bonds between the rigidly bound peptide pharmacophore and the gadolinium chelate, resulting in increased flexibility at the gadolinium ion. For agents that employ multiple chelates, it is a challenge to conjugate these chelates in a way that minimizes internal motion and yet does not deleteriously impact targeting.
One relaxation enhancing strategy is to introduce two binding moieties to further rigidify the molecule upon protein binding.[5] This was successfully applied in a serum albumin-targeted gadolinium tetramer where it was demonstrated that a tetramer with two binding groups resulted in ca. 50% higher relaxivity than the analogous tetramer with one binding group when relaxivity was measured in albumin solution.[6] We recently reported two GdDTPA tetramers targeted to fibrin containing either one or two fibrin-specific peptides.[7] While the agent with two peptides had higher affinity to fibrin, the relaxivity of both compounds bound to fibrin was approximately the same. This suggested that both peptide moieties were not simultaneously bound to fibrin.
Recently, we showed that the relaxivity of a fibrin-bound peptide conjugated to four GdDTPA moieties (Gd2-Pep-Gd2) was limited by internal motion.[8] Although the relaxivity of Gd2-Pep-Gd2 increased when it was bound to fibrin, this increase was much lower than theoretically possible due to the flexibility inherent in the molecule. To increase the sensitivity of this agent one could increase the number of gadolinium chelates per molecule. However this would also increase the relaxivity of the unbound agent (background signal). Further, it is challenging to add more chelates in a way that does not decrease fibrin affinity nor increase internal motion and lower per gadolinium relaxivity. Another approach would be to use a second peptide and vary the linker length between the peptides to identify a molecule where both peptides are bound and the molecule is further rigidified. Both approaches are uncertain and significantly increase the complexity of the molecule.
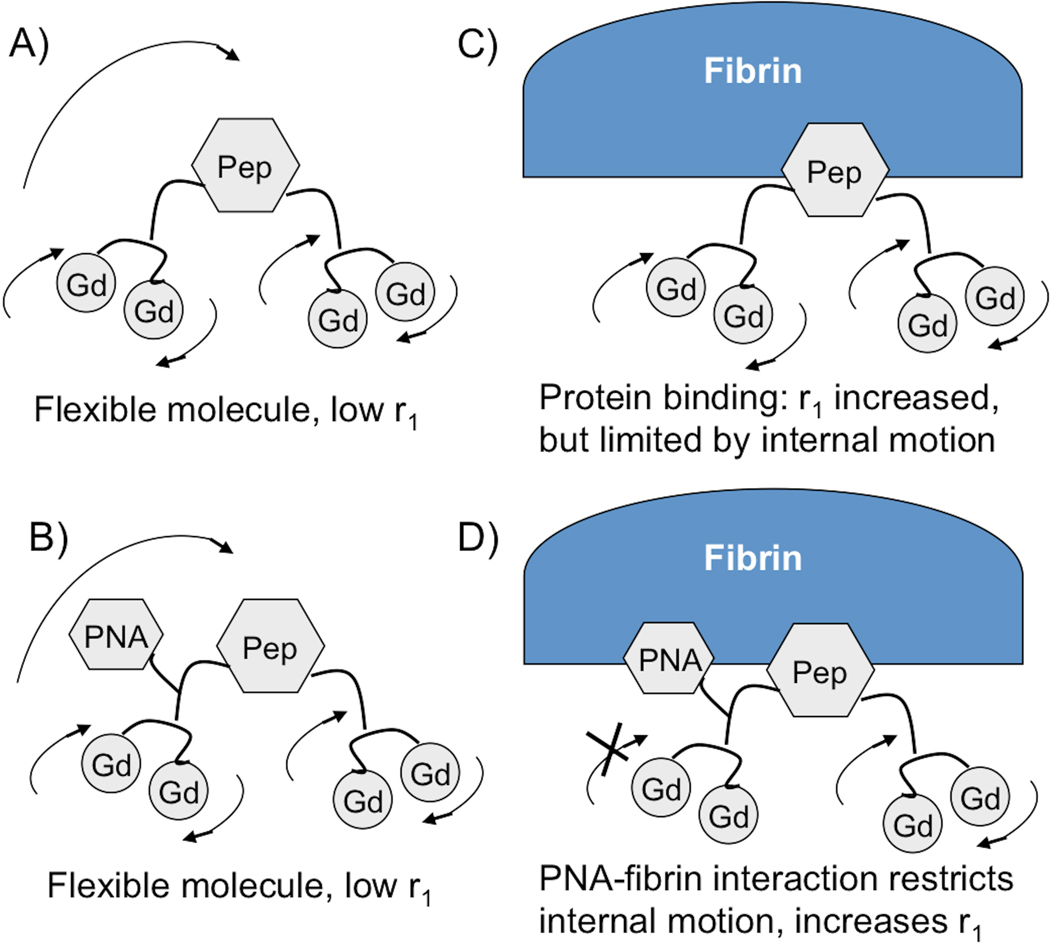
In this report we employ a much simpler approach that was inspired by the success in the fragment based drug discovery field.[9] We reasoned that it is possible to identify a second small pharmacophore at the peptide N-terminus by screening a small library of peptides with N-terminal variation. The binding of this second pharmacophore would serve to rigidify the N-terminal part of the molecule and boost relaxivity while at the same time increasing overall affinity for the target. This is shown conceptually in Figure 1. A peptide nucleic acid (PNA) group is a DNA-mimicking molecule and these have been widely used in molecular biology procedures, diagnostic assays and antisense therapies. PNA moieties can render the molecule more resistant to endo- and exonuclease –mediated degradation, as well as to protease digestion, and can also introduce hydrogen bonding and π-π interactions with protein targets to improve affinity. We synthesized 4 new peptides (T-Pep, G-Pep, C-Pep, and A-Pep, Figure 2) and compared their fibrin affinity to the parent peptide (NH2-Pep). Peptides were screened for binding to the soluble fibrin fragment DD(E) and the results are given in Table 1. For all four PNA derivatives a modest increase in affinity was observed. The thymine derivative 5 showed the greatest increase in affinity (160%) relative to the parent peptide.
Figure 1.
Mechanism of increased relaxivity by heteroditopic protein binding. A and B) In absence of protein (fibrin), molecule is flexible, undergoes fast tumbling, relaxivity is low. C) Fibrin binding reduces rotational motion and relaxivity is increased, but local motion limits relaxivity. D) Addition of second binding group PNA limits internal motion upon fibrin binding and boosts relaxivity.
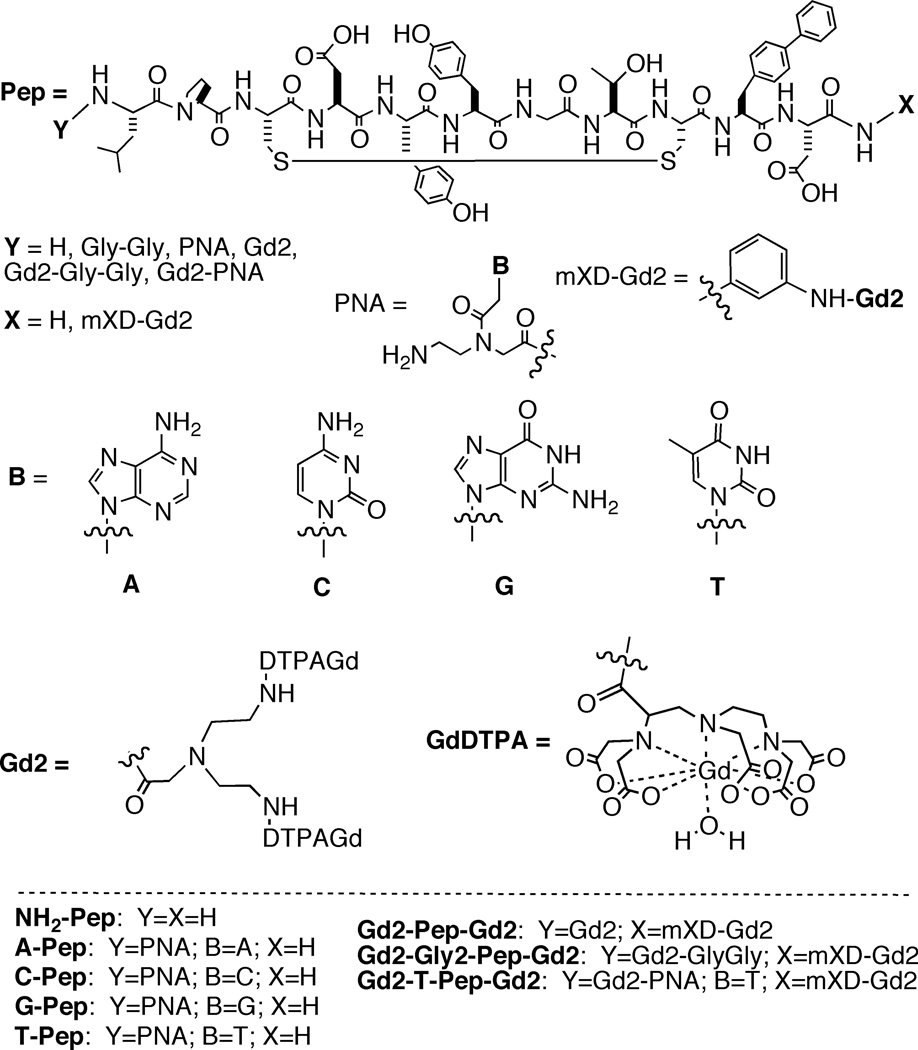
Figure 2.
Compounds synthesized and discussed in this study. Gd2-Pep-Gd2 was described previously.[8]
Table 1.
Inhibition constants (Ki), standard deviation in parentheses, showing binding of peptides to soluble fibrin fragment DD(E).
| Compound | NH2-Pep | A-Pep | C-Pep | G-Pep | T-Pep |
|---|---|---|---|---|---|
| Ki (µM) | 1.8 (0.2) | 1.3 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.7 (0.1) |
Based on the peptide screen, we incorporated the thymine PNA into the contrast agent Gd2-T-Pep-Gd2, Figure 2, using the same synthetic approach that was used to prepare Gd2-Pep-Gd2.[8] As a control Gd2-Gly2-Pep-Gd2 was also synthesized. This latter compound has the same number of bonds between the peptide and the bis(GdDTPA) moiety as the PNA derivative, i.e. the same degree of rotational flexibility in the absence of protein binding. These compounds were compared to data for Gd2-Pep-Gd2 reported previously.[8] Conjugation of 4 GdDTPA moieties to the peptide results in somewhat lower fibrin affinity compared to the peptide itself, as may be expected (Table 2). However addition of the PNA group results in greater affinity as compared to the two other contrast agents suggesting a positive binding interaction between the PNA and the protein.
Table 2.
Inhibition constants for fibrin fragment DD(E) binding, Ki (µM), and per gadolinium relaxivities (r1 (mM−1s−1) determined at 1.5T, 37 °C in Tris buffered saline (TBS), human plasma, or bound to human fibrin for the contrast agents.a % inc refers to the percentage increase in r1 going from plasma to fibrin.
| Compound | Ki | r1 TBS | r1 plasma | r1 fibrin | % inc |
|---|---|---|---|---|---|
| Gd2-Pep-Gd2 | 4.7 | 13.0 | 13.1 | 18.0 | 37% |
| Gd2-Gly2-Pep-Gd2 | 4.0 | 12.4 | 13.0 | 17.8 | 37% |
| Gd2-T-Pep-Gd2 | 3.5 | 13.3 | 13.1 | 28.0 | 114% |
Uncertainties estimated at ±10%.
The relaxivity of the new contrast agents was measured in pH 7.4 Tris buffered saline (TBS), human plasma, or a 30 µM fibrin gel in TBS. Per gadolinium relaxivities at 1.5T, 37°C are listed in Table 2. The relaxivities of the 3 compounds in buffer or plasma were very similar and quite high compared to GdDTPA itself (3.3 or 4.1 mM−1s−1 respectively).[10] This increase can be traced to the much larger size of the peptide-gadolinium multimers which results in a longer rotational correlation time.[8] As expected, the relaxivities increase when the peptide-chelate conjugates are bound to fibrin, due to a further increase in the correlation time. However Gd2-T-Pep-Gd2 showed greater than 50% higher fibrin bound relaxivity than the other two compounds. Since Gd2-T-Pep-Gd2 and Gd2-Gly2-Pep-Gd2 have an equivalent number of single bonds between the peptide pharmacophore and the bis(GdDTPA) moiety, an equivalent relaxivity would be expected if the PNA group did not interact with the protein. The much higher relaxivity for Gd2-T-Pep-Gd2 bound to fibrin is most likely due to a positive binding interaction between the PNA and the protein which serves to reduce rotational flexibility at the N-terminus and increase relaxivity.
The relaxivities in Table 2 are per Gd ion, but on a molecular basis, the relaxivity is four times higher. Thus the relaxivity of Gd2-T-Pep-Gd2 bound to fibrin is 112 mM−1s−1 at 1.5T, which should enable sensitive thrombus detection. We note that the fibrin-bound relaxivity of Gd2-T-Pep-Gd2 is over 50% higher than that of EP-2104R, another peptide-based agent that has shown thrombus imaging efficacy in human trials.[2, 4d]
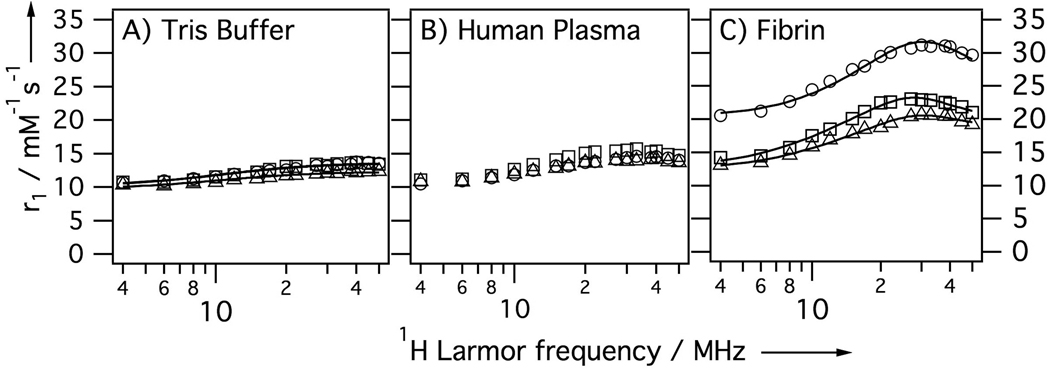
To better understand the mechanism of increased relaxivity, nuclear magnetic relaxation dispersion (NMRD) measurements were performed. Here relaxivity is measured as a function of applied field and this technique is very sensitive to the rotational dynamics at the Gd(III) ion. Figure 3A shows NMRD profiles for the three compounds in Tris buffered saline (TBS) at 35 °C. In the absence of protein, the NMRD profiles of the three compounds are very similar as may be expected for their similar structures. These profiles are flat and indicative of fast rotation. Figure 3B shows relaxivities in human plasma. The relaxivities are slightly increased in plasma suggesting some weak plasma protein binding. Again, all three compounds show very similar NMRD profiles in plasma, suggesting that all compounds show the same degree of weak binding. Figure 3C shows the NMRD profiles of the compounds bound to fibrin. Fibrin binding causes an increase in relaxivity for all 3 compounds. For Gd2-Gly2-Pep-Gd2 and Gd2-Pep-Gd2 the increase in relaxivity upon fibrin binding ranged from 30 – 70% depending on field. For the PNA derivative the relaxivity increase was much more remarkable: 90 – 140% higher than in the absence of fibrin. Comparing the relaxivity of each compound in fibrin we note that the introduction of the PNA group increases relaxivity by 30 – 60% when compared to the other two agents.
Figure 3.
NMRD showing per Gd relaxivity at 35 °C of Gd2-Pep-Gd2 (squares), Gd2-Gly2-Pep-Gd2 (triangles) and Gd2-T-Pep-Gd2 (circles) in A) pH 7.4 Tris buffer, B) human plasma, or C) bound to human fibrin. Solid lines are fits to the data as described in the text.
The NMRD profiles were modeled using Solomon-Bloembergen-Morgan theory (see Supporting Information for more details). We used the simplest model that could reproduce the NMRD data and results in averaged correlation times for the 4 GdDTPA moieties. In the absence of protein, the data could be well fit by an isotropic model with a rotational correlation time of about 400 ps. Fibrin binding results in restricted motion and here a simple isotropic model was not sufficient to reproduce the data; instead the Lipari–Szabo formalism was used.[11] Here two correlation times describe rotational diffusion: a slow, global correlation time (τg) for the protein-bound compound and a shorter, local correlation time (τl) for internal motion. These are weighted by an order parameter, 1 ≥ F2 ≥ 0, where F2 = 1 represents isotropic global motion and F2 = 0 represents local motion decoupled from the slow global motion. Both F2 and τl increased in the order Gd2-T-Pep-Gd2 > Gd2-Pep-Gd2 > Gd2-Gly2-Pep-Gd2 indicating that the increased relaxivity of Gd2-T-Pep-Gd2 was due to restricted internal motion likely caused by binding of the PNA residue to the protein.
In summary, we have shown that the small structural perturbation of incorporating a PNA group into a fibrin-targeted contrast agent has a profound impact on relaxivity. The PNA moiety increases molecular weight by 3% but increases relaxivity by 50% compared to Gd2-Gly2-Pep-Gd2. The effect of the PNA group on relaxivity is the equivalent of synthesizing an agent with 6 GdDTPA moieties to achieve equivalent relaxivity. The PNA group has a modest positive impact on fibrin binding and serves to rigidify the N-terminal portion of the molecule upon fibrin binding. Importantly, the PNA group does not increase non-specific protein binding. As a result, relaxivity of Gd2-T-Pep-Gd2 bound to fibrin is more than 50% increased compared to Gd2-Pep-Gd2 while the relaxivity of the two compounds in plasma is comparable. This should result in much greater clot:blood contrast for Gd2-T-Pep-Gd2.
Overall, this strategy of rigidifying peptide-based MR contrast agents upon binding should be broadly applicable. Focused libraries combining N- or C-terminal fragments can be rapidly prepared and screened to identify elongated peptides with enhanced affinity. Derivatization of the N- or C-terminus with metal chelates would then show restricted internal motion upon binding and enhanced relaxivity.
Experimental Section
Details of compound syntheses, protein binding and relaxivity assays are given in the Supporting Information.
Supplementary Material
Table 3.
Average NMRD parameters for the three contrast agents bound to fibrin at 35 °C. The global correlation time, τg, was >20 ns for all compounds. The water residency time τm was assumed to be 140 ns as determined previously for Gd2-Pep-Gd2.[8]
| Compound | τv [ps] |
Δ2 [×10−18 s2] |
F2 | τl [ps] |
|---|---|---|---|---|
| Gd2-Pep-Gd2 | 18.2±1.5 | 9.6±0.3 | 0.06±0.01 | 818±18 |
| Gd2-Gly2-Pep-Gd2 | 16.0±1.6 | 10.6±0.4 | 0.04±0.01 | 720±20 |
| Gd2-T-Pep-Gd2 | 10.4±0.6 | 8.3±0.4 | 0.10±0.02 | 1507±31 |
Acknowledgment
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering, R01EB009062.
Contributor Information
Zhaoda Zhang, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, 149 13th St, Suite 2301, Charlestown, MA 02129 (USA).
Andrew F. Kolodziej, Epix Pharmaceuticals, 4 Maguire Road, Lexington, MA 02124 (USA)
Matthew T. Greenfield, Epix Pharmaceuticals, 4 Maguire Road, Lexington, MA 02124 (USA)
Peter Caravan, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, 149 13th St, Suite 2301, Charlestown, MA 02129 (USA).
References
- 1.a) Uppal R, Caravan P. Future Med Chem. 2010;2:451. doi: 10.4155/FMC.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Terreno E, Castelli DD, Viale A, Aime S. Chem Rev. 2010;110:3019. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 2.Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, Parsons EC., Jr Invest Radiol. 2009;44:697. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 3.Caravan P. Chem Soc Rev. 2006;35:512. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 4.a) Burtea C, Laurent S, Port M, Lancelot E, Ballet S, Rousseaux O, Toubeau G, Vander Elst L, Corot C, Muller RN. J Med Chem. 2009;52:4725. doi: 10.1021/jm9002654. [DOI] [PubMed] [Google Scholar]; b) Amirbekian V, Aguinaldo JG, Amirbekian S, Hyafil F, Vucic E, Sirol M, Weinreb DB, Le Greneur S, Lancelot E, Corot C, Fisher EA, Galis ZS, Fayad ZA. Radiology. 2009;251:429. doi: 10.1148/radiol.2511080539. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ye F, Jeong EK, Jia Z, Yang T, Parker D, Lu ZR. Bioconjug Chem. 2008;19:2300. doi: 10.1021/bc800211r. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Overoye-Chan K, Koerner S, Looby RJ, Kolodziej AF, Zech SG, Deng Q, Chasse JM, McMurry TJ, Caravan P. J Am Chem Soc. 2008;130:6025. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]; e) Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, Zhang Z. Angew Chem Int Ed Engl. 2007;46:8171. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 5.Kielar F, Tei L, Terreno E, Botta M. J Am Chem Soc. 2010;132:7836. doi: 10.1021/ja101518v. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Greenfield MT, Spiller M, McMurry TJ, Lauffer RB, Caravan P. Angew Chem Int Ed Engl. 2005;44:6766. doi: 10.1002/anie.200502245. [DOI] [PubMed] [Google Scholar]
- 7.Nair S, Kolodziej AF, Bhole G, Greenfield MT, McMurry TJ, Caravan P. Angew Chem Int Ed Engl. 2008;47:4918. doi: 10.1002/anie.200800563. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Kolodziej AF, Qi J, Nair SA, Wang X, Case AW, Greenfield MT, Graham PB, McMurry TJ, Caravan P. New J Chem. 2010;2010:611. doi: 10.1039/b9nj00787c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Congreve M, Chessari G, Tisi D, Woodhead AJ. J Med Chem. 2008;51:3661. doi: 10.1021/jm8000373. [DOI] [PubMed] [Google Scholar]; b) Hajduk PJ, Greer J. Nat Rev Drug Discov. 2007;6:211. doi: 10.1038/nrd2220. [DOI] [PubMed] [Google Scholar]
- 10.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Invest Radiol. 2005;40:715. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 11.Lipari G, Szabo A. J Am Chem Soc. 1982;104:4546. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.