Abstract
The matrix metalloproteinase (MMP) family is believed to play a role in the ovulatory process because MMP inhibitors block oocyte release. However, little is known about the mechanisms by which the MMPs affect ovulation. The present study investigated the degradomic actions of the gelatinases, MMP2 and MMP9, by identifying gelatinolytic targets in periovulatory granulosa cells. Granulosa cells were collected from immature rats 48 h after equine chorionic gonadotropin treatment and were cultured with human chorionic gonadotropin (hCG) in the absence or presence of a specific MMP2/9 inhibitor ((2R)-2-[(4-biphenylylsulfonyl)amino]-3-phenylpropionic acid) for an additional 24 h. The conditioned media was analyzed for gelatinolytic activity, progesterone, and peptide profiles. Gelatinolytic activity and progesterone were induced in response to hCG; however, there was no difference in progesterone between cells treated with or without the inhibitor. Peptide fragments of proteins altered in the presence of the gelatinase inhibitor were identified by two-dimensional gel electrophoresis and mass spectrometry. Protein disulfide isomerase A3 (PDIA3), which plays a role in protein folding, was identified as a peptide that decreased in the presence of inhibitor while the serine protease hepsin, was found to increase with inhibitor treatment. Subsequent experiments established that PDIA3 and hepsin were targets of MMP2/9 action by cleavage with MMP2 and Western blot analysis, respectively. Additionally, hepsin was identified as a gelatinolytic target in ovarian cancer cells. In the present study, proteomics has identified proteins that may be involved in novel ways in the complex cascades that are mediated by gelatinolytic MMPs during the periovulatory period.
Keywords: corpus luteum, hepsin, matrix metalloproteinase, ovulation, protein disulfide isomerase A3
Gelatinases from rat granulosa cells degrade hepsin and protein disulfide isomerase A3.
INTRODUCTION
The mammalian ovary undergoes dynamic structural changes throughout the reproductive cycle with remarkable transformations occurring during the periovulatory period. These structural changes encompass extensive remodeling of the extracellular matrix (ECM) to facilitate breakdown of the follicular wall, release of the oocyte, and transformation of the postovulatory follicle to form the corpus luteum. These rapid changes in the periovulatory follicular ECM are postulated to occur through the action of the matrix metalloproteinases (MMPs), a family of structurally related enzymes capable of cleaving components of the ECM [1–6]. Support for such a postulate comes from numerous observations, including 1) fragmentation and a loss of the collagenous matrix of the follicular wall after the luteinizing hormone (LH) surge, especially at the apex [7, 8], 2) induction of members of the MMP family after an LH/hCG (human chorionic gonadotropin) stimulus [9–13], and 3) the ability of chemical MMP inhibitors to block follicular rupture in vitro [9, 14, 15] and in vivo [16]. These observations suggest that follicular rupture requires focal degradation of the apical ECM that is controlled, in part, by ovarian MMPs.
The actions of the ovarian MMPs have been hypothesized to be directed toward components of the follicular ECM such as the collagens, laminins, and fibronectins [3–6]. This concept is based, in part, on the observation that interstitial type I collagen found in the follicular wall is fairly resistant to cleavage by proteinases other than collagenase. Additionally, the capacity of the gelatinases, such as MMP2 and MMP9, to degrade ECM basement membrane components such as laminin and fibronectin is well documented [17, 18]. Investigators have demonstrated that the collagenous matrix in the follicular wall, as well as the follicular and apical basement membranes, become fragmented and lose their structural integrity as ovulation approaches [7, 19]. These morphological observations in conjunction with the known actions of the MMPs on the ECM have led to the hypothesis that MMPs are involved in the LH-induced breakdown of the ECM components present in the wall of the preovulatory follicle to facilitate oocyte release and luteal formation [3–6]. For example, the marked induction of Mmp2 mRNA at 24 h after hCG in the forming corpus luteum has been suggested to support a role for the gelatinases in early luteinization of the postovulatory follicle [20].
MMPs have been observed to act on non-ECM substrates. The ability of MMPs to act on other MMPs, growth factors, binding proteins (e.g., insulin-like growth factor-binding proteins), receptors, integrins, and cytokines has expanded the repertoire of MMP action outside the classical action on the ECM [1, 17, 21]. For example, MMPs are able to release growth factors by cleaving binding proteins and the extracellular domains of growth factors, thus acting as so-called sheddases to control growth factor bioavailability or action. The breadth of MMP actions, therefore, has exploded to include modulation of cell growth, cell proliferation, cell migration, chemotaxis, and apoptosis through their ability to cleave non-ECM substrates [1, 17, 21]. Surprisingly, with all of these targets of MMP action, we are uncertain as to the actual ovarian substrates targeted during follicular rupture and thus the overall comprehensive role that the MMP system plays in the process of ovulation and luteal formation. The current study has begun to address this question of identifying the ovarian targets of MMP action by inhibiting the action of the gelatinases and exploring which proteins may be targets of MMP2 or MMP9. We report on two proteins that are regulated by MMP2/9: protein disulfide isomerase A3 (PDIA3) and hepsin. We further explore the action of MMP2/9 on hepsin in human ovarian cancer.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all the chemicals and reagents were purchased from Sigma-Aldrich or Thermo Fisher Scientific.
Animals: Granulosa Cell Model
All the animal procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee. The changes in expression patterns of MMP2 and MMP9 activity were determined in rat granulosa cells during the periovulatory period. Granulosa cells were isolated from ovaries collected from equine chorionic gonadotropin (eCG)-primed immature rats as described previously with the modifications noted below [22]. Briefly, immature female Sprague Dawley rats (Harlan Laboratories, Inc.) were provided with water and rat chow ad libitum and maintained on a 12L:12D cycle. At 22–23 days of age, animals were injected with 10 IU (international units) of eCG to stimulate and synchronize follicular growth. Ovaries from five rats were removed 48 h later, and granulosa cells were isolated by follicular puncture. The cells from these 10 ovaries were pooled, pelleted by centrifugation, and resuspended in defined medium consisting of Opti-MEM I (Invitrogen) supplemented with 28.6 mM sodium bicarbonate, 0.05 mg/ml of gentamicin, and 1× ITS (insulin, transferin, and selenium) (Invitrogen). The cells were distributed into 6-well plates at a density of approximately 1 × 106 viable cells in 2 ml of the defined medium per well (100 000 cells/cm2) and cultured with or without 1–2 IU hCG/ ml. This represents a single experiment. Conditioned media was collected at 0, 12, and 24 h after hCG treatment (n = 3 separate, independent experiments for each time point) and analyzed by gel zymography.
To determine the ovarian substrates of MMP2 and MMP9 action, rat granulosa cells were cultured in the absence or presence of a MMP2/9 inhibitor. Granulosa cells were isolated from ovaries of eCG-primed immature rats as described above. The cells were distributed at a density of approximately 1 × 106 viable cells per 2 ml of defined medium per well and treated with 2 IU hCG/(1 × 106 cells) in the absence or presence of 100 μM of (2R)-2-[(4-biphenylylsulfonyl)amino]-3-phenylpropionic acid (Calbiochem-EMD BioSciences), a specific MMP2/9 inhibitor [23], for 24 h (five wells pooled per time point, n = 3 separate, independent experiments for each time point). At the end of the culture period, conditioned media were removed and an aliquot analyzed for progesterone (P4). The remaining portion of the conditioned media was frozen and later subjected to two-dimensional (2D) gel electrophoresis.
Tissues and Cells
Rat ovaries were removed from adult Sprague Dawley rats and immediately frozen for Western blot analysis. Human ovaries were collected from five women, 28–49 years of age, undergoing hysterosalpingo-oophorectomy and ovariectomy for various reasons, including uterine leiomyoma, abnormal uterine bleeding, pelvic pain, and endometriosis. All the samples were from nonmalignant tissues, and the ovaries exhibited no gross pathology (e.g., ovarian endometrioma). Ovaries were bisected, and a portion of each ovary was immediately frozen for Western blot analysis. All the procedures for these experiments utilizing human tissue were approved by the University of Kentucky Institutional Review Board.
All the ovarian cancer cell lines (Ovcar-3, Caov-3, and Skov-3) and cell culture media were obtained from the American Type Culture Collection ATCC. Ovcar-3 cells were cultured in RPMI 1640 media, supplemented with 20% fetal bovine serum (FBS), penicillin (1 U/ml)/streptomycin (100 μg/ml), and 0.25 μg/ml amphotericin B (Gibco-Invitrogen). Caov-3 and Skov-3 cells were cultured in Dulbecco modified Eagle medium and McCoy 5A medium, respectively, both supplemented with 10% FBS and antibiotics as described above. Cells were plated in 6-well plates and maintained until the cells reached ∼60%–90% confluence. Cells were then starved of serum for 24 h and treated for an additional 24 h with 0.1% dimethyl sulfoxide (DMSO) (i.e., control) or 100 μM of MMP2/9 inhibitor. At the end of the incubation period, five wells were pooled, and the cells and conditioned media were collected and processed for Western blot analysis. This experiment was repeated with four sets of cells cultured in separate, independent experiments.
Protein Separation: 2D Gel Electrophoresis
Approximately 10 ml of granulosa cell conditioned culture media was pooled from five wells per treatment (n = 1), acetone precipitated, and the pellet dissolved in 150 μl of 7 M urea, 2 M thiourea, 50 mM dithiothreitol (DTT), 4% CHAPS, 1% NP-40, 0.2% ampholytes pH 3–10, and 0.0002% bromophenol blue. A 125-μl aliquot of the protein solution was applied to a 7-cm Bio-Rad pH 3–10 IPG (immobilized pH gradient) strip by overnight passive rehydration. After focusing in a Bio-Rad Protean IEF cell, the proteins in the IPG strip were reduced by DTT and alkylated by iodoacetamide. The second-dimension SDS-PAGE separation was performed, the gels were stained by Sypro Ruby (Invitrogen), and an image was obtained using a Typhoon scanner (GE Healthcare Life Sciences). Two-dimensional gel electrophoretic separation was performed from three separate, independent cell culture experiments (n = 3 where n = conditioned media from one experiment). To determine changes in spots representing protein or peptide fragments between cells treated with or without the MMP2/9 inhibitor, gel profiles were analyzed using the PDQuest software program (Bio-Rad, Version 7.3.1).
Peptide Separation: Tryptic Digestion and Mass Spectrometry
Selected protein spots were excised using a Bio-Rad ProteomeWorks Plus Spot Cutter System and washed with 50 mM NH4HCO3 in 50% CH3CN. After dehydration, the gel pieces were reduced and then alkylated for 30 min. The gel pieces were washed with 50 mM NH4HCO3, dehydrated, rehydrated in 5 μl Promega modified trypsin, and digested at 37°C. Peptides were extracted first with 0.1% HCOOH and then with 0.1% HCOOH in 50% CH3CN. The peptide solution volume was reduced to 12 μl for liquid chromatography-mass spectrometry (LC-MS) analysis.
Nano-flow reverse-phase LC-MS/MS was performed using a capillary HPLC system (LC Packings) coupled with a QSTAR XL quadruple time-of-flight (TOF) mass spectrometer (ABI/MDS Sciex) through a nanoelectrospray ionization source (Protana). Analyst QS software was used for the system control and data collection. The desired volume of protein solution was desalted on a C18 trap column and subsequently separated on a 75 μm × 15 cm C18 reverse-phase column (Vydac) at a flow rate of 220 nl/min. After LC separation, the sample was introduced into the MS through a 10-μm silica tip (New Objective) adapted with a nanoelectrospray source. Data were acquired in information-dependent acquisition mode. Each cycle typically consisted of a 1-sec TOF MS survey from 400 to 1600 (m/z) and two 2-sec MS/MS scans with a mass range of 65–1600 (m/z).
Peptide Identification and Western Blot Analysis
The LC-MS/MS data were submitted to a local MASCOT server (Matrix Science Inc.) for MS/MS ions search. The pI (isoelectric point) and molecular weight (MW) data were also submitted for further identification using the TagIdent program from ExPASy (www.expasy.org). Protein matches at the 95%-confidence level were selected for further identification by Western blot analysis and examination of susceptibility to gelatinolytic degradation.
For Western blot analysis, rat granulosa cells, whole rat ovary, human ovarian biopsies, and human ovarian cancer cells were lysed in RIPA buffer (Santa Cruz Biotechnology, Inc.) that was supplemented with a protease inhibitor cocktail (phenylmethylsulfonyl fluoride, aprotinin, and sodium orthovanadate). Twenty micrograms of protein was separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Western blot analysis was performed by first blocking nonspecific binding with 5% dry milk in Tris-saline buffer (10 mM Tris HCl, 150 mM NaCl; pH 8.0) containing 0.5% Tween-20 for 1 h. Blots were then incubated with 0.1 μg/ml of the primary antibody for hepsin (catalog no. 100022; Cayman Chemical) overnight at 4°C. After a series of washes, the blots were incubated with a secondary antibody (goat anti-rabbit HRP; catalog no. sc-2030; Santa Cruz) at 1:2000 dilution linked to horseradish peroxide, washed extensively, and analyzed using an enhanced ECL chemiluminescence detection system (GE Healthcare Life Sciences). To ensure the specificity of the Western blot analysis, the antibody was incubated with blocking peptide at 4°C overnight before performing Western blot analysis as described above. To ensure equal sample concentrations, membranes were stripped and incubated with β-actin (1:2000) for 1 h at room temperature and processed for Western blot analysis. All the data were normalized relative to the actin control.
Protein Disulfide Isomerase A3 Digestion
To demonstrate that PDIA3 was a substrate for MMP2, 0.1 μg purified PDIA3 (P3818; Sigma-Aldrich) was incubated alone or with increasing concentrations (0.01–0.5 μg) of purified active MMP2 (aMMP2; Calbiochem-EMD Biosciences) at 37°C for 24 h; all the incubations were in a 10-μl volume. At the end of the incubation, the PDIA3 (0.1 μg), aMMP2 (0.5 μg), or the PDIA3 + aMMP2 mixture was separated by 10% SDS-PAGE, silver stained, and photographed. A similar experiment was performed incubating PDIA3 and 0.9 μg of nonactive MMP2 (nMMP2; Abcam) for 24 h. Gel zymography was performed with both aMMP2 and nMMP2 using 10% SDS-PAGE gels with 10 mg gelatin and incubated for 24 h before staining with Coomassie. In order to visualize the cleavage products of PDIA3 by MMP2; a protein gradient gel (4%–20%; Pierce) was run with samples of aMMP2 (0.5 μg), purified PDIA3 (0.1 μg), and PDIA3 incubated with aMMP2 (0.5 μg) for 4, 8, 12, and 24 h at 37°C.
Gelatin Zymography
Ten milliliters of conditioned media from granulosa cell cultures was concentrated using Centricon filter devices (Millipore Corp.) with a 10 000 MW cutoff, and 15 μl of the concentrated conditioned media were diluted with nonreducing sample buffer (final concentration, 1% SDS and 5% glycerol) and subjected to SDS-PAGE for 4 h. After electrophoresis, the gel was washed in 2% Triton X-100 for 2 h to elute the SDS, rinsed briefly in Tris incubation buffer (50 mM Tris-HCl, 1 mM CaCl2, 0.05% Brij 35, pH 7.4), and then incubated for at least 24 h at 37°C in Tris incubation buffer. Subsequently, the gels were stained with Coomassie brilliant blue R250 dye. Gelatin-degrading enzymes were identified by their ability to digest the gel.
Progesterone Assay
Concentrations of P4 in the granulosa cell conditioned media after 24 h of culture in the presence or absence of the MMP2/9 inhibitor were assayed using an Immulite kit on an Immulite 1000 (Siemens Healthcare Diagnostics). The assay sensitivity was 0.2 ng P4/ml, and the intraassay and interassay coefficients of variation were 3.5% and 6.3%, respectively.
Statistical Analysis
Differences in mRNA, protein, or activity between groups were analyzed by one-way ANOVA and post hoc comparisons were performed when appropriate using least significant differences, with P < 0.05 being considered significant. Analysis of the gelatinolytic activity in the gel zymograms was performed using the Nikon NIS-Elements AR program (Version 3.13; Nikon Instruments Inc.). The regions of digestion were thresholded according to the binary intensity, and the percent area of the thresholded band region was calculated. Because of the variability in gelatinolytic activity and the background intensity of the gel zymograms between experiments, the 24-h +hCG sample was assigned a value of 1 in each experiment, and all the other treatments were expressed relative to the 24-h +hCG sample.
RESULTS
Changes in Gelatinase Activity after hCG Treatment of Granulosa Cells
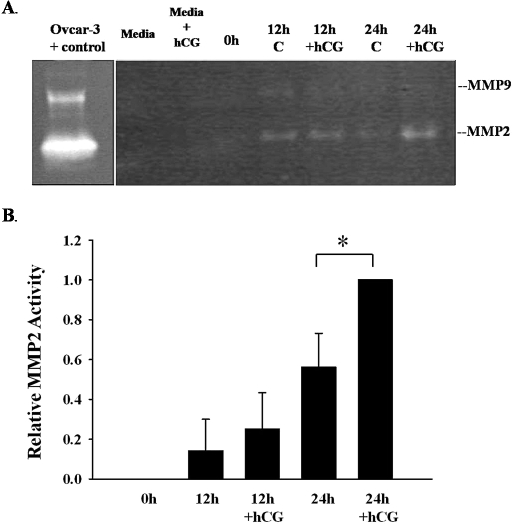
Induction of Mmp2 and Mmp9 mRNA expression in granulosa cells after hCG has been previously demonstrated in vivo [4–6, 20]. To validate our rat granulosa cell culture model, MMP2 and MMP9 activity in the conditioned media was assessed by gel zymography. Granulosa cells were isolated 48 h after eCG (0 h) and cultured for 12 or 24 h in the absence or presence of hCG. Gelatinolytic activity in the conditioned media was observed at both 12 and 24 h of culture (Fig. 1). The predominate gelatinase from rat granulosa cells was MMP2. The active form of MMP2 was highly abundant in conditioned media at 24 h after hCG treatment (Fig. 1B). MMP9 activity was detected at 12 h but was almost undetectable by 24 h. MMP9 activity varied between experiments but was always less than the activity of MMP2 (n = 5). There was no MMP activity detected in the media, media +hCG, or in the supernatants removed from the granulosa cells at the start of the experiment (0 h).
FIG. 1.
A) Gelatinolytic activity of granulosa cell supernatants following hCG treatment. Conditioned media in the absence (C) or presence of hCG (+hCG) were collected at 12 and 24 h and subjected to gel zymography. The predominate gelatinase from rat granulosa cells was MMP2. The active form of MMP2 was readily abundant at 24 h after hCG treatment. MMP9 activity was detected at 12 h and almost undetectable by 24 h. There was no MMP activity detected in the media, media +hCG, or in the supernatants removed from the granulosa cells at the start of the experiment (0 h). B) Graphical representation of the MMP2 activity for each band on the gelatin zymography (n = 5 separate, independent experiments). MMP2 activity was significantly increased (*) at 24 h after hCG in the conditioned media (P < 0.05).
The MMP2/9 Inhibitor Does Not Affect P4 Levels
Measurement of P4 production in the granulosa cell conditioned media after 24 h of culture in the presence or absence of the MMP2/9 inhibitor (Fig. 2) revealed that, as expected, hCG induced P4 production (∼70-fold increase: 0 h = 0.7 ± 0.11 ng/ml, 24 h hCG control = 48.0 ± 1.59 ng/ml). Treatment with the MMP2/9 inhibitor had no effect on this hCG-stimulated P4 production (24-h hCG control = 48.0 ± 1.59 ng/ml vs. 24-h hCG inhibitor = 44.1 ng/ml ± 2.20 ng/ml).
FIG. 2.
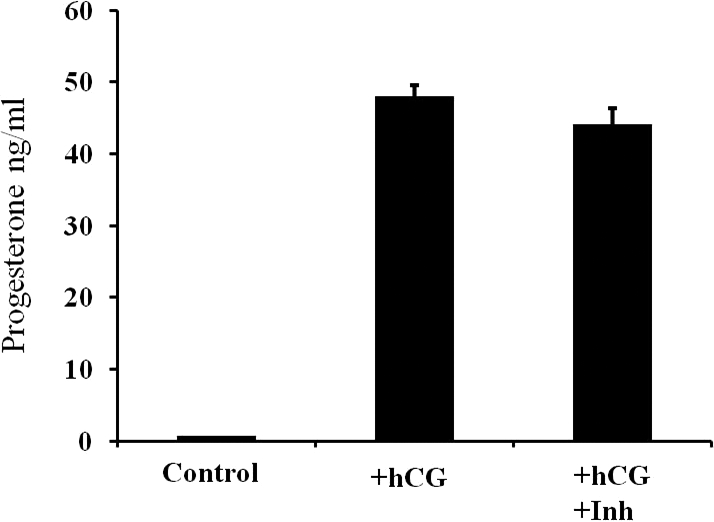
Measurement of progesterone (P4) production in the granulosa cell conditioned media after 24 h of culture in the presence or absence of the MMP2/9 inhibitor. Human chorionic gonadotropin-induced P4 production (mean ± SEM) resulting in ∼70-fold increase; 0 h (control) = 0.7 ± 0.11 ng/ml, 24 h hCG (+hCG) = 48.0 ± 1.59 ng/ml. Treatment with the MMP2/9 inhibitor (hCG+Inh) had no effect on hCG-stimulated P4 production.
Identification of Peptides Altered after MMP2/9 Inhibitor Treatment of Granulosa Cells
Granulosa cell conditioned media from cells cultured for 24 h in the presence of hCG with or without the MMP2/9 inhibitor was analyzed for peptide patterns by 2D gel electrophoresis. Analysis of the spots representing peptide fragments on the 2D gels was performed on three separate samples of granulosa cell conditioned media using the PDQuest software. When gelatinase activity was inhibited, there were a total of 274 different spots on the gel. Of those 274, 55% were up-regulated in the presence of the MMP2/9 inhibitor, 29% were down-regulated, and 16% were unchanged (by more than 10% over the control). Those peptides that were present in all three samples were visually assessed for relative abundance (absolute density greater than 5000), clarity of the peptide fragment, and ease of fragment isolation for subsequent analysis. There were 15 spots that met these criteria. From this analysis, we selected five peptides that were subjected to tryptic digest and mass spectrometry analysis; they were tentatively identified using peptide mass fingerprint with MASCOT. One of the spots selected was unchanged between the control and inhibitor-treated gels and served as a landmark to align the gels for accurate peptide excision. The MW of the landmark peptide was 36.4 kDa, its pI was 5.70, and it had 23% sequence coverage; this peptide was identified as the B-chain of L-lactate dehydrogenase. The remaining four peptide fragments differed in pI, MW, and relative abundance. One spot that increased in the presence of inhibitor had a MW of 27.7 kDa, a pI of 6.7, and 12% sequence coverage, and was identified as hepsin, a type II membrane-associated serine proteinase. The only spot selected that decreased had a MW of 56.5 kDa, a pI of 5.88, and 9% sequence coverage, and was identified as PDIA3, which functions in the formation of disulfide bonds and protein folding. We were unable to recover sufficient quantities of the tryptic digests for accurate analysis of the remaining two spots that were increased following inhibitor treatment.
Validation of Peptides Altered after MMP2/9 Inhibitor Treatment
The proteomic approach identified three proteins present in granulosa cells, two of which appear to be modulated by gelatinolytic action. To confirm that hepsin and Pdia3 mRNA are present in the ovary and are potential targets of MMP2/9 action, the expression of mRNA was analyzed using Affymetrix microarray data from our rat ovarian gene expression database (rOGED) [24]. The mRNAs for hepsin (NM 017112.1) and Pdia3 (NM 017319.1) were present in extracts of intact ovaries as well as in isolated granulosa cells collected at the time of hCG administration (i.e., 48 h after eCG or 0-h hCG). Hpn mRNA increased 20% after hCG (6 h) before returning to control levels at 12 h after hCG whereas Pdia3 was constitutively expressed at 6 and 12 h after hCG (data not shown).
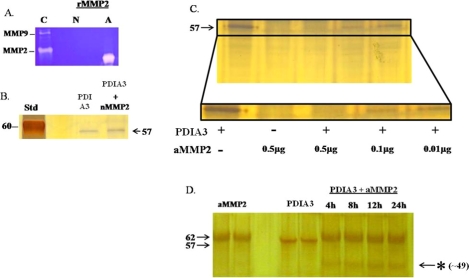
Our proteomic analysis identified PDIA3 as a protein that was decreased by inhibition of MMP2/9 action, which suggests that PDIA3 may be a target of the gelatinases. PDIA3 belongs to a class of disulfide isomerases that fold cell surface and secretory proteins [25]. To validate PDIA3 as a target of the gelatinases, PDIA3 was incubated with active recombinant MMP2. The activity of the recombinant MMP2 was first authenticated by gel zymography (Fig. 3A). When PDIA3 was incubated with a nMMP2 for 24 h, the inactive MMP2 was unable to cleave PDIA3 (Fig. 3B). Subsequently, PDIA3 was incubated with increasing concentrations of recombinant aMMP2 for 24 h. Analysis of the PDIA3:MMP2 incubation reaction by gel electrophoresis demonstrated that with increasing concentrations of aMMP2, the cleavage of PDIA3 was enhanced (Fig. 3C) and additional peptides were present, suggesting PDIA3 cleavage products (Fig. 3D, * indicates cleavage products at ∼49 kDa). These data demonstrate that MMP2/9 is able to release the intact protein as well as degrade PDIA3 (see schematic representation in Fig. 7).
FIG. 3.
Validation of protein disulfide isomerase A3 as a target of MMP2 action. A) Gel zymography demonstrating gelatinolytic activity of an active recombinant MMP2 (A) and a nonactive MMP2 (N). Conditioned media from Ovcar-3 cells was used as a positive control (C) for MMP2 and MMP9 activity. B) Protein gel of purified PDIA3 (0.1 μg) and PDIA3 incubated with nMMP2 (0.9 μg) for 24 h. The nMMP2 was unable to cleave PDIA3. The molecular weight of monomeric PDIA3 is 57 kDa. C) Protein gel of purified PDIA3 (0.1 μg) incubated with increasing concentrations of aMMP2 (0.01, 0.1, 0.5 μg) for 24 h. The single band of PDIA3 was diminished after incubation with aMMP2. Similar results were obtained in five different experiments. D) Protein gradient gel (4%–20%) of active MMP2 (aMMP2, 0.5 μg), purified PDIA3 (0.1 μg), and PDIA3 incubated with aMMP2 (0.5 μg) for varying amounts of time. The asterisk (*) indicates a breakdown product of PDIA3 at approximately 49 kDa.
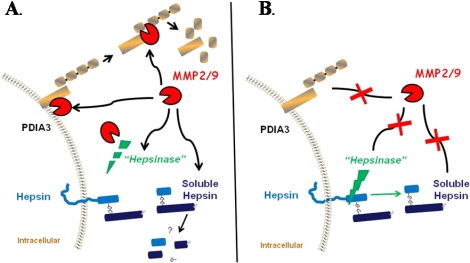
FIG. 7.
Schematic representation of gelatinolytic activity in the ovary. MMP2/9 is able to degrade PDIA3 on the cell surface or, once released, it is able to be further degraded in the conditioned media (A). When gelatinolytic activity is inhibited (B), PDIA3 is not degraded, resulting in an increase in PDIA3 on the cell surface and a decrease in PDIA3 in the conditioned media. For hepsin, inhibition of gelatinolytic activity resulted in an increase in the soluble fragment of hepsin in the conditioned media (B). This could occur by one of several mechanisms. The gelatinases could act directly on the soluble hepsin fragment or could act to degrade an intermediary proteinase (i.e., a hepsinase) that regulates the cleavage of hepsin (A). Inhibiting MMP2/9 would block degradation of the 29-kDa fragment or would protect the hepsinase, allowing it to cleave hepsin and release the soluble form of the protein into the conditioned media.
Our proteomic screen identified hepsin as a protein that was increased after inhibition of MMP2/9 action. Hepsin is reported to be a 51-kDa type II membrane-associated serine proteinase [26]. However, by proteomic analysis, we identified hepsin as an MMP target from a peptide fragment obtained by 2D gel electrophoresis with a MW of 27.7 kDa. To resolve this apparent discrepancy in molecular size, we performed Western blot analysis to identify hepsin in the ovary. In whole ovary (Fig. 4A) and granulosa cell conditioned media (Fig. 4B, control), hepsin was present as a peptide of approximately 45 and 29 kDa. The 45-kDa band represents the intact protein, which matches the reported size 45.3 kDa, while the 29-kDa fragment represents the cleaved extracellular domain [27, 28]. We observed a nonspecific band at 36 kDa that remained even in the presence of an excess of hepsin peptide (data shown only for whole ovary, Fig. 4A).
FIG. 4.
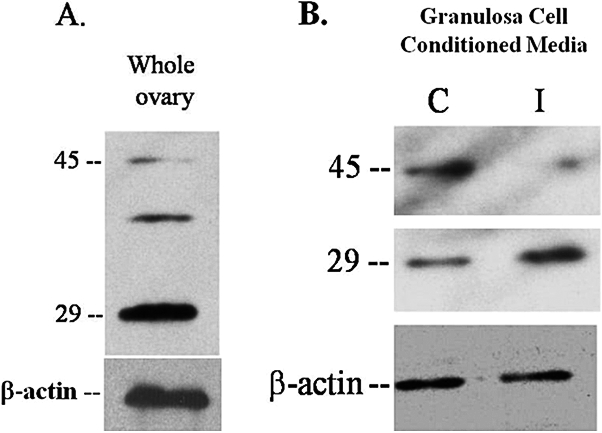
Western blot analysis examining the presence of hepsin in the rat ovary. Extracts of whole ovaries or granulosa cell conditioned media were subjected to Western blot analysis for hepsin. Hepsin was present as a peptide of molecular weight approximately 45 kDa and a fragment of approximately 29 kDa, representing the cleaved extracellular domain in both whole ovaries (A) and granulosa cell conditioned media (B, control). B) Inhibition of gelatinolytic activity increased the 29-kDa hepsin fragment in granulosa cell conditioned media. Granulosa cells were cultured for 24 h in the presence of 1–2 IU hCG and 100 μM MMP2/9 inhibitor (I, inhibitor) or DMSO vehicle (C, control); n = 3 separate, independent experiments.
To confirm our findings from the proteomic screening that hepsin was increased in granulosa cell conditioned media after inhibition of MMP2/9 action, granulosa cells were cultured for 24 h with or without the MMP2/9 inhibitor and Western blot analysis was performed on the conditioned media. In the presence of the MMP2/9 inhibitor, there was an increase in the 29-kDa hepsin fragment (Fig. 4B), which is similar to our findings by proteomic screening, while the 45-kDa hepsin peptide decreased in the conditioned media.
Hepsin Is Altered after MMP2/9 Inhibitor Treatment in Human Ovarian Cancer Cells
We next extended our present findings in the rat and explored hepsin expression in the human and whether hepsin was a gelatinolytic target in the human ovary. The rationale for this set of experiments was that hepsin has been reported to be absent in the normal ovary [29] but yet hepsin has been suggested to play a role in ovarian cancer [29–31]. Thus, we were interested to discover whether our findings in the rodent were applicable to the human. First, hepsin protein expression was examined in extracts of intact human ovaries (Fig. 5A) as well as in Caov-3, Ovcar-3, and Skov-3 cancer cells (Fig. 5B). Both the intact and cleaved forms of hepsin were present in the human ovary, however, the relative abundance varied between the five different human specimens and the three different cell lines (Fig. 5, A and B). As the ovarian cancer cells contained an abundance of the 29-kDa hepsin fragment, we next investigated whether gelatinolytic activity was responsible for the presence of this fragment. After culture of the ovarian cancer cells with the MMP2/9 inhibitor for 24 h, there was no change in the cleaved form of hepsin in the conditioned media from the Caov-3 and Skov-3 cancer cells; however, the 29-kDa hepsin fragment increased in Ovcar-3 cell conditioned media (Fig. 6).
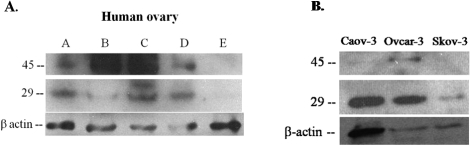
FIG. 5.
Hepsin is present in normal human ovary and in ovarian cancer cells. A) Human ovaries (A–E) display varying levels of hepsin protein expression in both the 45- and 29-kDa bands (n = 5). B) Western blot analysis of three ovarian cancer cell lines (Caov-3, Ovcar-3, and Skov-3) demonstrating varying levels of hepsin protein expression in the cells in both the 45- and 29-kDa bands; n = 4 separate, independent experiments for each time point.
FIG. 6.
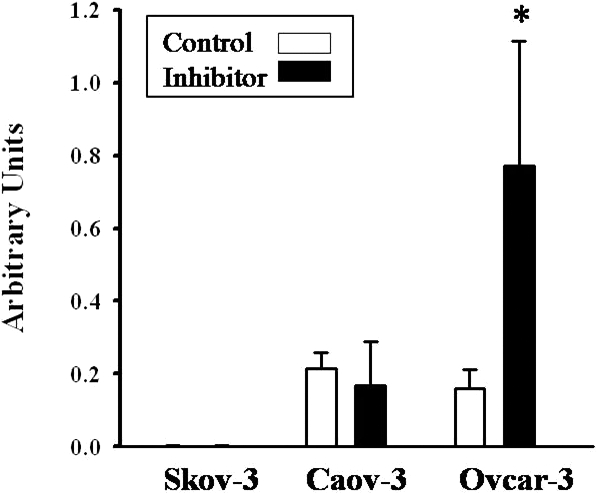
Inhibition of gelatinolytic activity increases hepsin in Ovcar-3 cell conditioned media. Graphical representation of Western blot analysis results of the 29-kDa fragment of hepsin in Skov-3, Caov-3, and Ovcar-3 cells treated with either DMSO (control) or 100 μM of MMP2/9 inhibitor (inhibitor) for 24 h. The 29-kDa fragment was significantly increased in the conditioned media of Ovcar-3 cells treated with MMP2/9 inhibitor compared to Ovcar-3 cells treated with vehicle (*); n = 4 separate, independent experiments, and all the values were normalized to actin in each sample.
DISCUSSION
The current studies are the first to characterize the ovarian actions of the gelatinases induced by hCG during the periovulatory period. It is well documented that the endogenous LH surge or exogenous hCG stimulates the induction of members of the MMP family that are thought to degrade the apical extracellular matrix to facilitate oocyte release and subsequent luteal formation [3, 5, 6, 9–13]. The specific MMPs induced by the LH surge or an hCG stimulus appear to be species-specific as well as dependent upon the individual experimental model system employed. The gelatinases, MMP2 and MMP9, are induced by an LH/hCG stimulus and exhibit a different spatiotemporal pattern of expression among species as well as the experimental model systems employed [13, 32–40]. In the rat, Mmp2 in intact ovaries is stimulated by LH while Mmp9 mRNA expression is unchanged throughout the periovulatory period [13, 32, 33]. Our laboratory and others have demonstrated that the increase in Mmp2 mRNA results in an increase in gelatinolytic activity in extracts of intact rat ovaries during the periovulatory period and that this activity is predominately due to MMP2 [13, 32, 33]. In the present study, we have extended these observations in intact ovaries to demonstrate that there is an increase in MMP2 activity in cultured granulosa cells. This observation is in concordance with in situ hybridization studies that revealed that Mmp2 mRNA expression in granulosa cells from healthy rat follicles prior to an hCG stimulus was extremely low but as ovulation approached, the mRNA expression of Mmp2 increased in the granulosa-luteal compartment and remained elevated in the forming corpus luteum [20]. However, differences exist with cultured granulosa cells as the appearance of MMP2 activity in conditioned media is not as robust as activity observed from intact ovaries collected in vivo.
The gelatinases have a high affinity for components of the ECM, especially those of the basement membranes. The ability of MMP2 and MMP9 to degrade ECM components present in the follicular wall along with their induction by an LH/hCG stimulus has led to the concept that the gelatinases are involved in the LH-induced breakdown of connective tissue in the preovulatory follicle. The current approach has begun to elucidate some of the non-ECM targets of gelatinolytic action during the periovulatory period. One of the targets of MMP2/9 action identified in this study was PDIA3, which belongs to a family of 17 different PDIs that are capable of formation (oxidation), reduction, and rearrangement (isomerization) of the disulfide-bonding patterns of proteins, often as part of the process of folding of nascent proteins [25, 41] The PDIA3 identified in the present study acts as an oxidase, reductase, isomerase, and chaperone in protein folding, is fairly ubiquitous in distribution, and is induced by stress [25].
Our observation that inhibition of MMP2/9 action results in a decrease in PDIA3 in the conditioned media suggests that PDIA3 is a direct target of MMP2/9 (Fig. 7). Initially our findings raised the question of the mechanism by which MMP2/9 targets PDIA3 cleavage because PDIs are classically described as being anchored to the endoplasmic reticulum (ER) via KDEL-receptor proteins [41]. However, there is a growing body of literature suggesting additional functions for the PDIs on the surface of cells, where they participate in receptor activation and remodeling as well as substrate processing [42]. A number of cell types, including murine sperm [43], rat pancreatic cells [44], bovine aortic endothelial cells [45], rat hepatocytes [46], human B cells [47], and human platelets [41] have been shown to secrete PDIs, which associate with the cell surface. The membrane-associated PDIs have been proposed to function in sperm:oocyte fusion [43], platelet aggregation and activation [41], and activation of integrins [48]. Another potential function of PDIs is as hormone reservoirs. PDI has an affinity for 17β-estradiol, and the high concentrations of PDI in the ER has led to the proposal that PDI may act to sequester estradiol intracellularly [49]. Turano and colleagues [42] hypothesized that the relatively low abundance of PDI in the cell membrane makes it unlikely that PDI localizes estradiol to the cell surface. Although the actual role of PDIA3 in ovarian function is unknown, PDIs have been observed in the sea urchin egg [50], mouse oocytes [51] and periovulatory human follicular fluid (HFF) [52]. Of relevance to the present study, PDI was found in the oocyte and in follicular fluid via proteomic screening. In HFF collected from normal ovulatory women undergoing assisted reproductive techniques due to male infertility factor, PDI was 1 of 27 proteins out of a total of 695 peptide fragments that was present in HFF but was absent in plasma [52]. Furthermore, the PDI in HFF represented almost the entire intact protein (52 kDa), similar to our findings in the conditioned media from rat granulosa cells. It is tempting to speculate that because PDI was increased in HFF collected after hCG, a time when MMP2 and MMP9 activities increase [13, 20, 32, 33], that the PDIA3 found in HFF resulted from the actions of MMP2/9 as observed in the present study.
Another target of MMP2/9 action identified in the present study was hepsin, which is a member of the type II transmembrane serine proteinase family [53]. This family is estimated to contain more than 20 members and share similarities with proteinases of the plasminogen activation, blood coagulation, and complement systems [54]. The C-terminal domain of these serine proteinases is localized at the cell surface and interacts with extracellular matrix components and transmembrane molecules. The N-terminal cytoplasmic domain associates with intracellular molecules and participates in signaling [55]. Although the biological function and potential substrates for the majority of the hepsins have yet to be identified, hepsin has been proposed to degrade components of the ECM, including basement membranes [54, 55].
One of the intriguing observations from the current study is that the 45-kDa hepsin peptide decreased while the 29-kDa fragment of hepsin increased in the conditioned media after inhibition of MMP2/9 activity. If the gelatinases were acting directly to cleave hepsin from the cell surface, then inhibition of MMP2/9 would increase or stabilize the 45-kDa peptide and decrease the release of the hepsin fragment into the conditioned media. The current finding of a decrease in the 45-kDa peptide and an increase in the soluble fragment of hepsin in the conditioned media could occur by one of several mechanisms (Fig. 7). The gelatinases could act directly to degrade the soluble hepsin fragment present in the conditioned media. Alternatively, the gelatinases could act to degrade an intermediary proteinase (i.e., a hepsinase) that regulates the cleavage and release of hepsin from the cell surface [27]. Inhibiting MMP2/9 would block degradation of the soluble hepsin fragment or would protect the hepsinase, which would then cleave hepsin from the cell surface releasing the 29-kDa fragment into the conditioned media (Fig. 7).
It is well recognized that both the MMPs and hepsin are highly overexpressed in numerous cancers, including ovarian cancer [29–31, 56–58], which led us to explore the expression and potential regulation of hepsin by the gelatinases in the human. The gelatinases have been proposed to facilitate migration and invasion as well as cleavage of growth factors and cell surface proteins to regulate tumor growth or differentiation [56–58]. For hepsin, Tanimoto and coworkers [29] reported that hepsin mRNA is overexpressed in 60% of low-grade ovarian tumors and in 80% of ovarian carcinomas. The functional significance of high levels of hepsin in ovarian cancer is unknown, although hepsin may be acting to cleave growth factors or degrade basement membranes to facilitate cell invasion. Support for this idea comes from reports that hepsin acts to disorganize basement membranes [55], cleave precursors of blood coagulation factors, and cleave the precursor of the single-chain hepatocyte growth factor [59]. Evidence for a potential function of hepsin in ovarian tumor biology has also been examined using antibodies that neutralize hepsin's proteolytic activity. Utilizing this approach, inhibition of hepsin's proteolytic activity reduced the invasive capacity of Caov-3 ovarian tumor cells by approximately 50%, suggesting a role of hepsin in tumor cell invasion [30].
The majority of the previous explorations of hepsin expression in the ovary have examined hepsin in ovarian tumors with a limited number of investigations comparing normal ovarian tissue with ovarian carcinoma. Tanimoto and colleagues [29] reported that the hepsin transcript was abundant in carcinoma but was almost never expressed in normal adult tissue, including normal ovary. The lack of hepsin expression in the human ovary is in marked contrast to our findings in the rat. We have observed by microarray analysis using rOGED [24] that hepsin mRNA is fairly abundant in intact rat ovaries, granulosa cells, and the tissue remaining after granulosa cell collection. Administration of hCG results in approximately a 1.2- to 1.4-fold increase in hepsin mRNA in these various ovarian compartments at 6 h after hCG (Dr. Chemyong Ko, rOGED database, unpublished results used with author's permission). The current findings suggest that the hepsin mRNA is transcribed into protein and hepsin is abundant in rat granulosa cells. The differences in hepsin expression patterns between the human and rat may be species related or may be related to the physiologic stage of the ovary. To explore this possibility, we examined normal human ovarian tissue and observed hepsin present in all of the ovarian extracts although the levels of hepsin varied between patients. The functional significance of hepsin in normal ovarian function is unknown; however, extrapolations from hepsin in ovarian cancer would suggest that hepsin may play a role in growth factor cleavage or ECM remodeling. Furthermore, the present findings suggest that hepsin in ovarian cancer cells is sensitive to gelatinolytic cleavage.
In conclusion, the present study has inhibited MMP2/9 activity and identified PDIA3 and hepsin as targets of gelatinolytic activity. Hepsin was further characterized as a MMP2/9 target in ovarian cancer cells, suggesting that the MMPs may modulate hepsin action during normal physiologic processes such as ovulation and/or luteinization as well as in altered physiologic states such as cancer.
ACKNOWLEDGMENTS
The authors are indebted to Dr. Carol Beach and Charlotte Randall in the Department of Biochemistry and Molecular Biology at the University of Kentucky for their assistance and guidance with the mass spectrometry identification of gelatinolytic targets. The authors thank Lauren McCord for her help with the gel zymography. The authors would also like to thank Dr. Jo, Dr. Liu, and Ms. Park for their efforts in reviewing the manuscript.
Footnotes
Supported by grants NIH HD057446 and NCRR P20 RR15592 to T.E.C.
REFERENCES
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006; 69: 562 573 [DOI] [PubMed] [Google Scholar]
- Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol 2008; 40: 1362 1378 [DOI] [PubMed] [Google Scholar]
- Smith MF, Ricke WA, Bakke LJ, Dow MPD, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol 2002; 191: 45 56 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med 2006; 24: 228 241 [DOI] [PubMed] [Google Scholar]
- Ny T, Wahlberg P, Brandstrom IJ. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol Cell Endocrinol 2002; 187: 29 38 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428 465 [DOI] [PubMed] [Google Scholar]
- Bjersing L, Cajander S. Ovulation and the mechanism of follicle rupture. V. Ultrastructure of tunica albuginea and theca externa of rabbit graafian follicles prior to induced ovulation. Cell Tissue Res 1974; 153: 15 30 [DOI] [PubMed] [Google Scholar]
- Espey LL, Lipner H. Ovulation. : Knobil E, Neill J. (eds.), The Physiology of Reproduction, 2nd ed. New York: Raven Press. 1994: 725 780 [Google Scholar]
- Reich R, Tsafriri A, Mechanic GL. The involvement of collagenolysis in ovulation in the rat. Endocrinology 1985; 116: 522 527 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Dean DD, Woessner JF, Jr, LeMaire WJ. The extraction of a tissue collagenase associated with ovulation in the rat. Biol Reprod 1985; 33: 981 991 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Mann JS, Huang MH, Keeble SC. Gelatinase and proteoglycanase activity during the periovulatory period in the rat. Biol Reprod 1992; 46: 256 264 [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Peterson TA, Van Kirk EA, Vincent DL, Inskeep EK. Interactive roles of progesterone, prostaglandins, and collagenase in the ovulatory mechanism of the ewe. Biol Reprod 1986; 35: 1187 1194 [DOI] [PubMed] [Google Scholar]
- Reich R, Daphna-Iken D, Chun SY, Popliker M, Slager R, Adelmann-Grill BC, Tsafriri A. Preovulatory changes in ovarian expression of collagenases and tissue metalloproteinase inhibitor messenger ribonucleic acid: role of eicosanoids. Endocrinology 1991; 129: 1869 1875 [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Woessner JF, Jr, Koos RD, Sear CHJ, LeMaire WJ. Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology 1988; 122: 1715 1721 [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Ohta M, Morioka H, Murao S. Blockage of ovulation in the explanted hamster ovary by a collagenase inhibitor. J Reprod Fertil 1983; 68: 17 19 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Van Kirk EA, Murdoch WJ. Role of matrix metalloproteinase 2 in the ovulatory folliculo-luteal transition of ewes. Reproduction 2002; 124: 347 352 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17: 463 516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner JF, Jr, Nagase H. Matrix Metalloproteinases and TIMPs. New York: Oxford University Press. 2000. [Google Scholar]
- Bjersing L, Cajander S. Ovulation and the mechanism of follicle rupture. VI. Ultrastructure of theca interna and the inner vascular network surrounding rabbit graafian follicles prior to induced ovulation. Cell Tissue Res 1974; 153: 31 44 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation and early luteal formation in the rat. Biol Reprod 2001; 65: 855 865 [DOI] [PubMed] [Google Scholar]
- Overall CM, Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev 2006; 25: 69 75 [DOI] [PubMed] [Google Scholar]
- Mann JS, Kindy MS, Edwards DR, Curry TE., Jr Hormonal regulation of matrix metalloproteinase inhibitors in rat granulosa cells and ovaries. Endocrinology 1991; 128: 1825 1832 [DOI] [PubMed] [Google Scholar]
- Tamura Y, Watanabe F, Nakatani T, Yasui K, Fuji M, Komurasaki T, Tsuzuki H, Maekawa R, Yoshioka T, Kawada K, Sugita K, Ohtani M. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J Med Chem 1998; 41: 640 649 [DOI] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry TE, Jr, Ko C. Development and application of a rat ovarian gene expression database (rOGED). Endocrinology 2004; 145: 5384 5396 [DOI] [PubMed] [Google Scholar]
- Maattanen P, Kozlov G, Gehring K, Thomas DY. ERp57 and PDI: multifunctional protein disulfide isomerases with similar domain architectures but differing substrate-partner associations. Biochem Cell Biol 2006; 84: 881 889 [DOI] [PubMed] [Google Scholar]
- Wu Q, Yu D, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest 1998; 101: 321 326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T-KH, Liu RW, Haaksma CJ, Tomasek JJ, Howard EW. Identification and cloning of the membrane-associated serine protease, Hepsin, from mouse preimplantation embryos. J Biol Chem 1997; 272: 31315 31320 [DOI] [PubMed] [Google Scholar]
- Tsuji A, Torres-Rosado A, Arai T, Le Beau MM, Lemons RS, Chou SH, Kurachi K. Hepsin, a cell membrane-associated protease. Characterization, tissue distribution, and gene localization. J Biol Chem 1991; 266: 16948 16953 [PubMed] [Google Scholar]
- Tanimoto H, Yan Y, Clarke J, Korourian S, Shigemasa K, Parmley TH, Parham GP, O'Brien TJ. Hepsin, a cell surface serine protease identified in hepatoma cells, is overexpressed in ovarian cancer. Cancer Res 1997; 57: 2884 2887 [PubMed] [Google Scholar]
- Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y, Finster S, Vogel D, Mintzer B, Dinter H, Light D, Parry R, et al. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res 2006; 66: 3611 3619 [DOI] [PubMed] [Google Scholar]
- Bignotti E, Tassi RA, Calza S, Ravaggi A, Bandiera E, Rossi E, Donzelli C, Pasinetti B, Pecorelli S, Santin AD. Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am J Obstet Gynecol 2007; 196: 245.e1 e11 [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Komar CM, Burns PD, Nothnick WB. Periovulatory changes in ovarian metalloproteinases and tissue inhibitors of metalloproteinases (TIMPs) following indomethacin treatment. : Adashi EY. (ed.), Ovulation: Evolving Scientific and Clinical Concepts. New York: Springer Verlag. 2000: 265 276 [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A 2000; 97: 4689 4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driancourt MA, Quesnel H, Meduri G, Prunier A, Hermier D. Luteinization and proteolysis in ovarian follicles of Meishan and Large White gilts during the preovulatory period. J Reprod Fertil 1999; 114: 287 297 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Van Kirk EA, Murdoch WJ. Tumour necrosis factor alpha up-regulates matrix metalloproteinase-2 activity in periovulatory ovine follicles: metamorphic and endocrine implications. Reprod Fertil Dev 2000; 12: 75 80 [DOI] [PubMed] [Google Scholar]
- Hägglund AC, Ny A, Leonardsson G, Ny T. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology 1999; 140: 4351 4358 [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in Macaque periovulatory granulosa cells: time course and steroid regulation. Biol Reprod 1999; 61: 14 21 [DOI] [PubMed] [Google Scholar]
- Shalev E, Goldman S, Ben-Shlomo I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: comparison between women with PCOS and normal ovulatory women. Mol Hum Reprod 2001; 7: 325 331 [DOI] [PubMed] [Google Scholar]
- Bakke LJ, Dow MPD, Cassar CA, Peters MW, Pursley JR, Smith GW. Effect of the preovulatory gonadotropin surge on matrix metalloproteinase (MMP)-14, MMP-2, and tissue inhibitor of metalloproteinase-2 expression within bovine periovulatory follicular and luteal tissue. Biol Reprod 2002; 66: 1627 1634 [DOI] [PubMed] [Google Scholar]
- Lahav-Baratz S, Kraiem Z, Shiloh S, Koifman M. Decreased expression of tissue inhibitor of matrix metalloproteinases in follicular fluid from women with polycystic ovaries compared with normally ovulating patients undergoing in vitro fertilization. Fertil Steril 2003; 79: 567 571 [DOI] [PubMed] [Google Scholar]
- Jordan PA, Stevens JM, Hubbard GP, Barrett NE, Sage T, Authi KS, Gibbins JM. A role for the thiol isomerase protein ERP5 in platelet function. Blood 2005; 105: 1500 1507 [DOI] [PubMed] [Google Scholar]
- Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol 2002; 193: 154 163 [DOI] [PubMed] [Google Scholar]
- Ellerman DA, Myles DG, Primakoff P. A role for sperm surface protein disulfide isomerase activity in gamete fusion: evidence for the participation of ERp57. Dev Cell 2006; 10: 831 837 [DOI] [PubMed] [Google Scholar]
- Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. Localization of protein disulfide isomerase on plasma membranes of rat exocrine pancreatic cells. J Histochem Cytochem 1988; 36: 1069 1074 [DOI] [PubMed] [Google Scholar]
- Ejima K, Nanri H, Araki M, Uchida K, Kashimura M, Ikeda M. 17beta-Estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. Eur J Endocrinol 1999; 140: 608 613 [DOI] [PubMed] [Google Scholar]
- Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. Distribution of protein disulfide isomerase in rat hepatocytes. J Histochem Cytochem 1988; 36: 1533 1542 [DOI] [PubMed] [Google Scholar]
- Kroning H, Kahne T, Ittenson A, Franke A, Ansorge S. Thiol-proteindisulfide-oxidoreductase (proteindisulfide isomerase): a new plasma membrane constituent of mature human B lymphocytes. Scand J Immunol 1994; 39: 346 350 [DOI] [PubMed] [Google Scholar]
- Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal 2006; 8: 312 324 [DOI] [PubMed] [Google Scholar]
- Primm TP, Gilbert HF. Hormone binding by protein disulfide isomerase, a high capacity hormone reservoir of the endoplasmic reticulum. J Biol Chem 2001; 276: 281 286 [DOI] [PubMed] [Google Scholar]
- Lucero HA, Lebeche D, Kaminer B. ERcalcistorin/protein disulfide isomerase (PDI). Sequence determination and expression of a cDNA clone encoding a calcium storage protein with PDI activity from endoplasmic reticulum of the sea urchin egg. J Biol Chem 1994; 269: 23112 23119 [PubMed] [Google Scholar]
- Calvert ME, Digilio LC, Herr JC, Coonrod SA. Oolemmal proteomics—identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod Biol Endocrinol 2003; 1: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci S, Ciavardelli D, Di GF, Eleuterio E, Sulpizio M, Tiboni GM, Giampietro F, Palumbo P, Di IC. Proteome analysis of human follicular fluid. Biochim Biophys Acta 2006; 1764: 1775 1785 [DOI] [PubMed] [Google Scholar]
- Leytus SP, Loeb KR, Hagen FS, Kurachi K, Davie EW. A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry 1988; 27: 1067 1074 [DOI] [PubMed] [Google Scholar]
- Qiu D, Owen K, Gray K, Bass R, Roles Ellis V. and regulation of membrane-associated serine proteases. Biochem Soc Trans 2007; 35: 583 587 [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell 2004; 6: 185 195 [DOI] [PubMed] [Google Scholar]
- Stack MS, Ellerbroek SM, Fishman DA. The role of proteolytic enzymes in the pathology of epithelial ovarian carcinoma. Int J Oncol 1998; 12: 569 576 [DOI] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest 2008; 118: 1367 1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun JL, Cortez A, Commo F, Uzan S, Rouzier R, Darai E. Serous and mucinous ovarian tumors express different profiles of MMP-2, -7, -9, MT1-MMP, and TIMP-1 and -2. Int J Oncol 2008; 33: 1239 1246 [PubMed] [Google Scholar]
- Herter S, Piper DE, Aaron W, Gabriele T, Cutler G, Cao P, Bhatt AS, Choe Y, Craik CS, Walker N, Meininger D, Hoey T, Austin RJ. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem J 2005; 390: 125 136 [DOI] [PMC free article] [PubMed] [Google Scholar]