Abstract
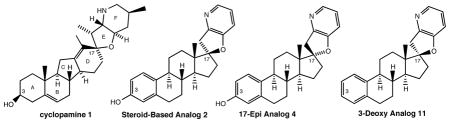
Previous work from our laboratory has established that the readily available steroid-based analog 2 of cyclopamine 1 is, like 1, a highly potent inhibitor of Hedgehog signaling. The first structure-activity relationship studies on 2, i.e., the synthesis and biological evaluation of both the C-17 epi analog 4 and the C-3 deoxy analog 11, both of which are more potent than cyclopamine 1 are described. The implications of these results for the emerging pharmacophore of these Sonic Hedgehog signaling inhibitors is discussed.
The alkaloid teratogen cyclopamine 1 has emerged as an important lead structure in the development of cancer chemotherapeutic agents that act via inhibition of the Sonic Hedgehog (SHH) signaling pathway,1 specifically at the level of the transmembrane protein Smoothened (SMO).2 The teratogenicity associated with cyclopamine has not hampered interest in the development of this important SHH signaling inhibitor.3 Cyclopamine 1 has been shown to be effective against a number of human cancers, including basal cell carcinomas4 and brain tumors, i.e., medulloblastomas and gliomas.5 Activation of the SHH signaling pathway has also been linked to melanoma,6 lung adenocarcinoma,7 as well as prostate,8 small cell lung,9 and pancreatic cancers.10
However, the considerable cost of the natural product which is isolated from the California corn lily, Veratrum californicum,11 and the metabolic instability observed in vivo (t1/2 ca. 30 sec in the presence of stomach acid)12 have precluded its development as a clinical candidate. Instead, the development of cyclopamine mimics has been a subject of intense study.13 We have recently reported that the replacement of the C-nor-D-homo ring system of 1 with the ABCD steroidal system in 2 leads to cyclopamine analogs with activity comparable to that of 1 in two different cellular assays.14 We describe herein our preliminary results directed toward the identification of the pharmacophore that is responsible for the potent activity of the metabolically stable cyclopamine analog 2 and related structures.
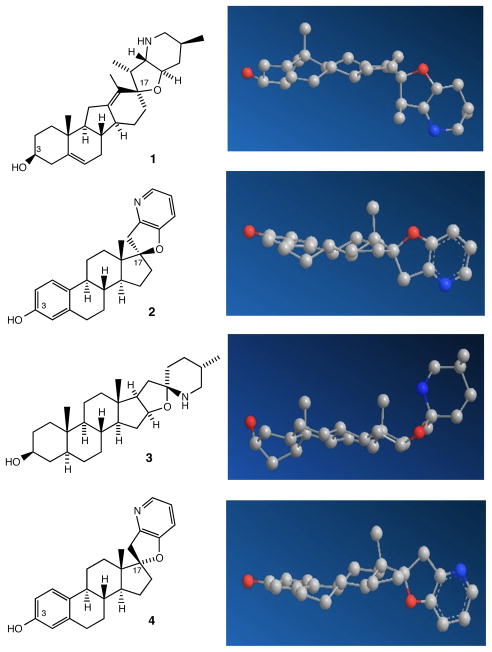
The difference in biological activity between cyclopamine 1 and the close structural analog tomatidine 3 (Figure 1; no SHH inhibition with 3) has been attributed to the difference in the orientation of the nitrogen atom (blue) relative to the steroid plane in 1 and 3. The C-nor-D-homo framework of 1 can thus be viewed as a scaffold that orients the E/F heterobicyclic moiety orthogonal to the steroidal ring system, with the F-ring nitrogen atom on the α-face of the steroid plane relative to the C-3β hydroxyl group, as highlighted in the three-dimensional model of 1.15 In contrast, the tetrahydrofuran ring of 3 (oxygen in red) lies in the steroid plane and the nitrogen atom of 3 is on the β-face of the steroid plane, as illustrated in the three-dimensional model of 3.
Figure 1.
Structures and Energy Minimized Models of Cyclopamine 1, Steroid-Based Analog 2, Tomatidine 3, and C-17-Epi Analog 4
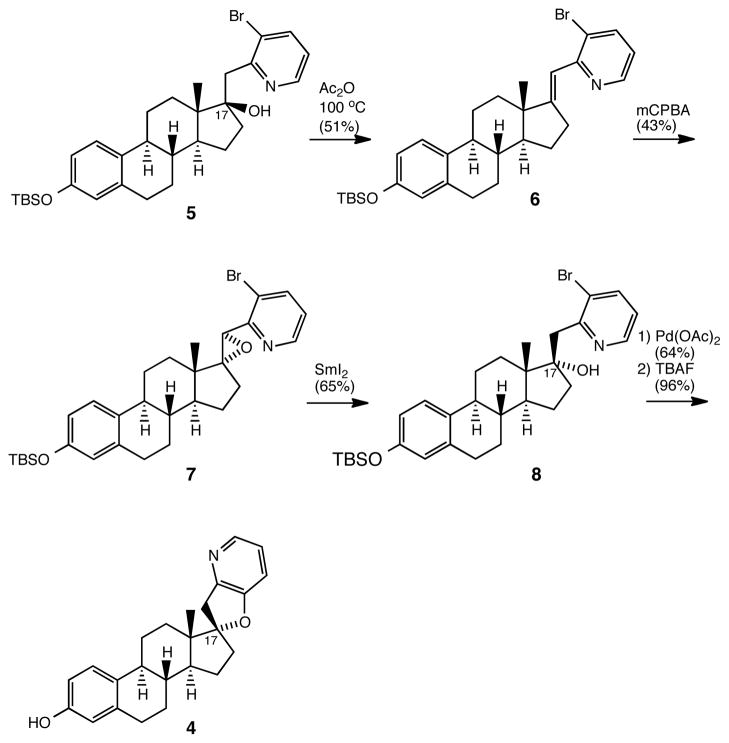
The energy-minimized structures in Figure 1 suggest an important role for the C-17 stereochemistry common to both 1 and 2, which, unlike 3, share the orientation of the C-17 oxygen substituent on the β-face of the steroid plane. In contrast, the C-17 oxygen atom of 4, the C-17 epimer of 2, is oriented on the α-face of the steroid plane, which leads to the orientation of the F-ring nitrogen atom of 4 on the β-face of the steroid plane, the same orientation that is found in tomatidine 3, a naturally occurring compound which displays no activity as a Hedgehog signaling inhibitor. To test the hypothesis that the three-point recognition of the C-3 oxygen, C-17 oxygen and C-21 nitrogen heteroatoms as oriented in 1 and 2 is required for recognition at SMO, we have synthesized 4, the C-17 epimer of 2, as outlined in Scheme 1.
Scheme 1.
Synthesis of C-17 Epi Analog 4
Dehydration of 514 selectively afforded Z-alkene 6, the stereochemistry of which was established by 1H NMR spectroscopy.16 Epoxidation of 6 led to the exclusive formation of epoxide 7, via reaction of 6 from the sterically less hindered α-face. While reaction of 7 with hydride donors did not lead to the desired epoxide ring opening to give 8, the C-17 epimer of 5, we were delighted to find that exposure of 7 to samarium diiodide afforded the desired product 8 (65% yield), based on Kato’s work on the deoxygenation of pyridine methanols.17 Exposure of 8 to the Buchwald-Hartwig cyclization conditions employed to generate 214 led, after deprotection of the silyl ether, to the formation of 4, the C-17 epimer of 2.
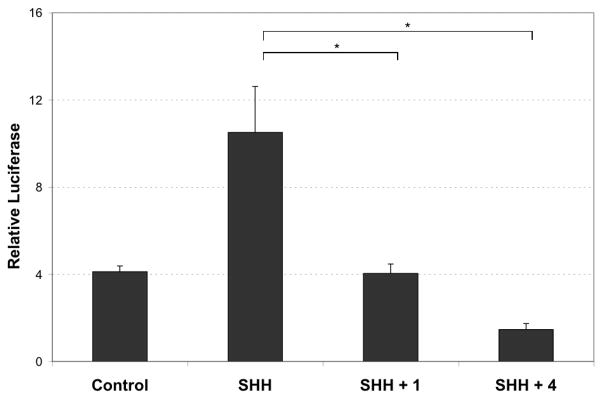
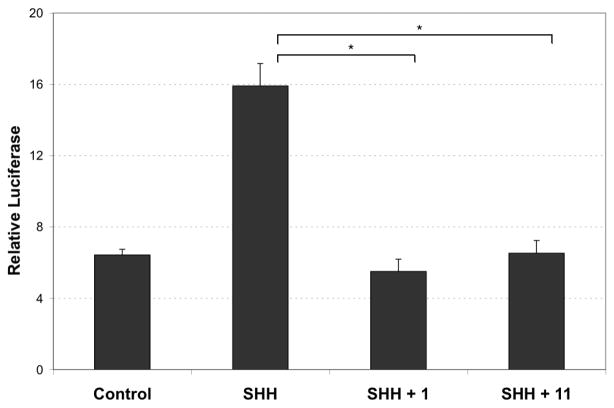
To determine whether 4 inhibits SHH signaling, we first examined the effect of 4 on the signaling pathway using the SHH-Light2 cells: this cell line is a 3T3 clone that stably expresses a GLI-dependent reporter.18 Treatment of these cells with recombinant SHH activates GLI-dependent firefly luciferase expression and this SHH-induced activation is inhibited when cells are also treated with cyclopamine 1 (Figure 2).18 Treatment of SHH-light2 cells with SHH (200 ng/ml, R&D) in combination with 4 (5 μM) led to a ca. 3-fold increase in signaling inhibition relative to that observed with cyclopamine, 1. These data show that 4 is a more potent inhibitor of SHH signaling than cyclopamine 1.
Figure 2. Luciferase Based Assay for SHH Activity.
SHH-Light2 cells were treated as described.18 GLI-binding site luciferase activities were measured using the luciferase reporter assay system with the luciferase kit (Promega). Treatment of SHH-Light2 cells with recombinant SHH (200 ng, R&D) resulted in the strong induction of reporter activity, which was largely blocked by co-treatment with either cyclopamine 1 or with 4 at 5 μM (in DMSO), both P < 0.01 [SHH vs. SHH + 1; SHH vs. SHH + 4]
This result contradicts our hypothesis that the three-point recognition of the C-3 oxygen and each of the other E and F ring heteroatom functionalities in 2 is required for recognition at SMO, the cellular target of cyclopamine, since structures with either orientation at C-17, i.e., both 2 and 4, are potent inhibitors of SHH signaling. The relative orientations of the tetrahydrofuran oxygen and pyridine nitrogen relative to the steroid plane do not appear to be important features for recognition of these cyclopamine analogs at SMO, suggesting that the C-3 oxygen functionality may not be required for recognition at SMO.
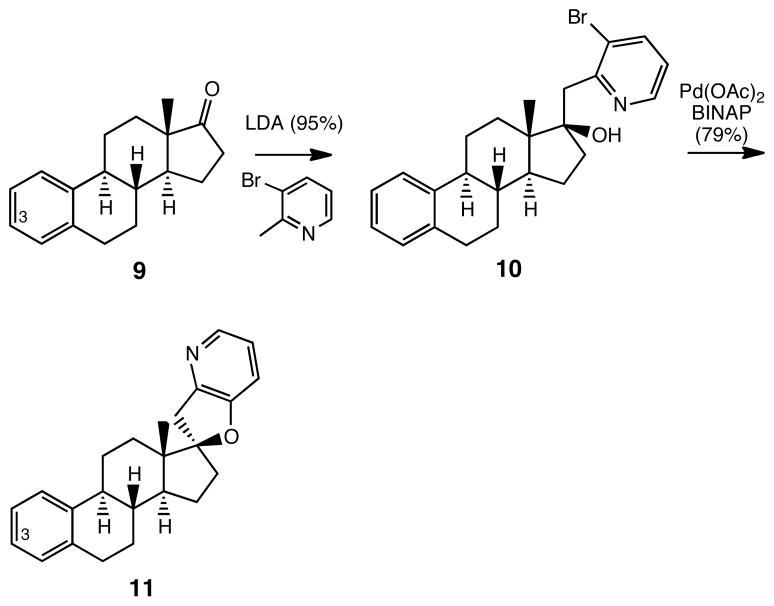
To establish the role, if any, of the C-3 oxygen functionality that is present in 2 and 4 on the biological activity of these estrone-derived analogs of cyclopamine 1, we have prepared the C-3 deoxy compound 11, the synthesis of which is shown in Scheme 2. Addition of the conjugate base of 3-bromopicoline to the known 3-deoxyestrone19 gave 10, which on reaction under Buchwald-Hartwig conditions afforded 11, the C-3 deoxy analog of 2 (Figure 1).
Scheme 2.
Synthesis of C-3 Deoxy Analog 11
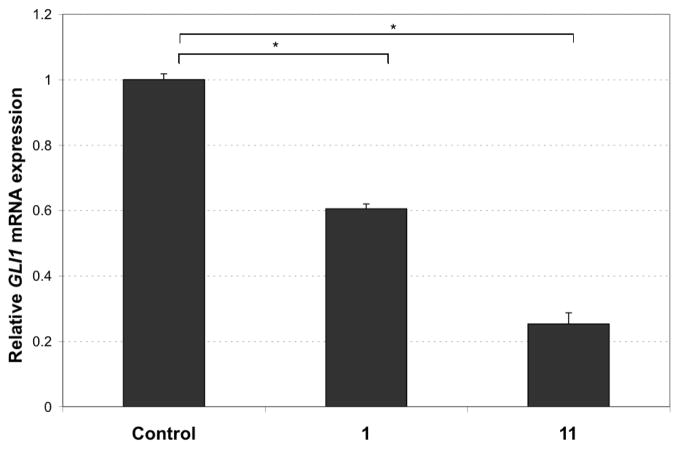
Biological evaluation of 11 using the same luciferase assay described for 4 (Figure 2) reveals that it is a potent inhibitor of SHH signaling. In this assay, the C-3 deoxy analog 11 (Figure 3) leads to a strong inhibition of SHH signaling activity (80% inhibition at 5 μM; compared to 70% inhibition in the same assay with 2).14 We have also demonstrated that 11 acts on the SHH pathway, as there is a significant decrease of the expression of GLI1, a transducer and a target of this pathway, following treatment of human medulloblastoma cells with 11 (Figure 4).
Figure 3. Luciferase Based Assay for SHH Activity.
SHH-Light2 cells were treated as described.18 GLI-binding site luciferase activities were measured using the luciferase reporter assay system with the luciferase kit (Promega). Treatment of SHH-Light2 cells with recombinant SHH (200 ng, R&D) resulted in the strong induction of reporter activity, which was largely blocked by co-treatment with either cyclopamine 1 or with 11 at 5 μM (in DMSO), both P < 0.001 [SHH vs. SHH + 1; SHH vs. SHH + 11].
Figure 4.
11 decreases GLI1 mRNA levels in human MB DAOY cells. DAOY cells were treated with either cyclopamine 1 or with 11 (10 μM each in DMSO) for 4 hours and levels of GLI1 mRNA were assessed by quantitative RT-PCR and normalized over the expression of GAPDH. Control level is set arbitrarily at 1 for comparison, both P < 0.001 [control vs. 1; control vs. 11]
We have also demonstrated that 11 has a potent inhibitory effect on brain tumor cell growth in vitro (Figure 5), further validating this approach to the development of steroid-based cyclopamine mimics as brain cancer chemotherapeutic candidates.
Figure 5.
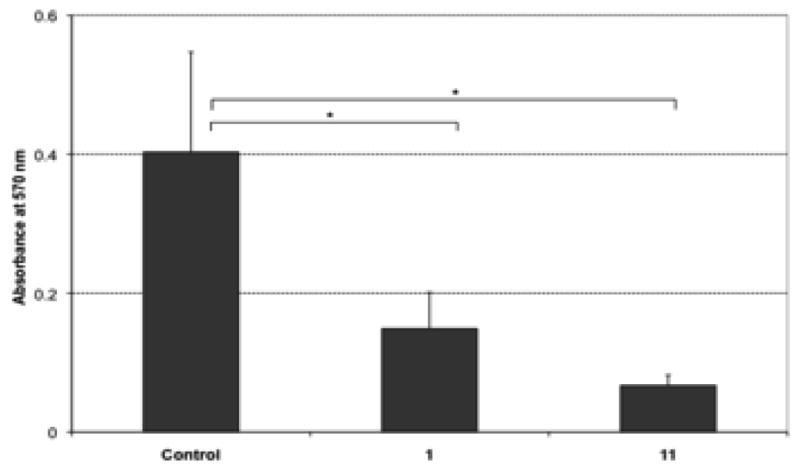
Analog 11 reduces DAOY medulloblastoma cell viability DAOY human medulloblastoma cells were treated with either carrier DMSO (Control), cyclopamine 1 (10 μM in DMSO) or 11 (10 μM in DMSO) for 3 days. The histogram measures cell viability assessed by the MTT assay (absorbance at 570 nm) (asterisk indicates p < 0.05). Similar results were obtained with U87GBM cells (not shown).
The remarkable potency of the C-3 deoxy analog 11 suggests that the two-point interaction (of the C-3β hydroxyl and the EF heterobicyclic ring) suggested by the three-dimensional structures of cyclopamine 1 (Figure 1) is not a critical recognition feature for biological activity, thereby pointing the way to further simplification of the structure of the cyclopamine analogs. Further studies on the synthesis and biological evaluation of such structures are currently underway in our laboratory and our results will be reported in due course.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the NIH (CA-134983) to J.D.W. and N.D.
Footnotes
Supporting Information Available Full experimental details, characterization data and NMR spectra of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Incardona J, Gaffield W, Kapur R, Roelink H. Development. 1998;125:3553–62. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]; b) Cooper M, Porter J, Young K, Beachy P. Science. 1998;280:1603–7. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Taipale J, Cooper M, Beachy P. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Firestone A, Chen J. Hedgehog Singaling Activation in Human Cancer and Its Clinical Implications. Springer; New York: 2011. Small Molecule Inhibitors of the Hedgehog Pathway; pp. 163–168. [Google Scholar]; b) Gould A, Missailidis S. Mini Reviews in Medicinal Chemistry. 2011;11:200–213. doi: 10.2174/138955711795049871. [DOI] [PubMed] [Google Scholar]
- 4.Dahmane N, Lee J, Robbins P, Heller P, Ruiz A, Altaba I. Nature. 1997;389:876–81. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 5.a) Berman D, Karhadkar S, Hallahan A, Pritchard J, Eberhart C, Watkins D, Chen J, Taipale J, Olson J, Beachy P. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]; b) Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz i Altaba A. Development. 2001;128:5201–12. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 6.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz A. Proc Natl Acad Sci USA. 2007;104:5895–900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Z, Goetz J, Singh S, Ogden S, Petty W, Black C, Memoli V, Dmitrovsky E, Robbins D. Oncogene. 2007;26:1046–55. doi: 10.1038/sj.onc.1209860. [DOI] [PubMed] [Google Scholar]
- 8.a) Karhadkar S, Bova G, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs J, Berman D, Beachy P. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]; b) Sanchez P, Hernandez A, Stecca B, Kahler A, DeGueme A, Barrett A, Beyna M, Datta M, Datta S, Ruiz A. Proc Natl Acad Sci U S A. 2004;101:12561–6. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins D, Berman D, Burkholder S, Wang B, Beachy P, Baylin S. Nature. 2003;422:313–7. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 10.Berman D, Karhadkar S, Maitra A, Montes D, Gerstenblith M, Briggs K, Parker A, Shimada Y, Eshleman J, Watkins D, Beachy P. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 11.Oatis J, Brunsfeld P, Rushing J, Moeller P, Bearden D, Gallien T, Cooper G. Chem Cent J. 2008;2:1–6. doi: 10.1186/1752-153X-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay M, Nevalainen M, Nair S, Porter J, Castro A, Behnke M, Yu L, Hagel M, White K, Faia K, Grenier L, Campbell M, Cushing J, Woodward C, Hoyt J, Foley M, Read M, Sydor J, Tong J, Palombella V, McGovern K, Adams J. J Med Chem. 2008;51:6646–6649. doi: 10.1021/jm8008508. [DOI] [PubMed] [Google Scholar]
- 13.Dolgin E. Nature Medicine. 2011;17:523. doi: 10.1038/nm0511-523. [DOI] [PubMed] [Google Scholar]
- 14.Winkler J, Isaacs A, Holderbaum L, Tatard V, Dahmane N. Org Lett. 2009;11:2824–7. doi: 10.1021/ol900974u. [DOI] [PubMed] [Google Scholar]
- 15.Keeler R. Lipids. 1978;13:708–15. doi: 10.1007/BF02533750. [DOI] [PubMed] [Google Scholar]
- 16.a) Benn W, Dodson R. J Org Chem. 1964;29:1142. [Google Scholar]; b) Mackella F, Slomp G. Steroids. 1968;11:787. doi: 10.1016/s0039-128x(68)80094-7. [DOI] [PubMed] [Google Scholar]; c) Tanabe M, Peters R. J Org Chem. 1971;36:2403. doi: 10.1021/jo00816a004. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Mase T. Tetrahedron Lett. 1999;40:8823–8826. [Google Scholar]
- 18.Taipale J, Chen J, Cooper M, Wang B, Mann R, Milenkovic L, Scott M, Beachy P. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou KC, Barnette W, Ma P. J Org Chem. 1980;45:1463–1470. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.