Abstract
The effects of ethanol exposure on fetal lungs remain under investigation. Previously, we demonstrated that lambs exposed to ethanol during gestation had impaired expression of pulmonary surfactant protein A, a crucial component of lung immunity. In this study, we investigated the effects of in utero exposure to ethanol on maturation and immunity of the fetal lung. Pregnant ewes were surgically implanted with an abomasal cannula and administered 1 g ethanol/kg (n = 8) or water (n = 8) during the last trimester of pregnancy. Lambs were delivered prematurely or naturally. Neonatal lungs were assessed for maturation markers (hypoxia-inducible factor-1α [HIF-1α], HIF-2α, HIF-3α, vascular endothelial growth factor-A [VEGF-A], VEGFR-1, VEGFR-2, glycogen, and lung protein levels) and immunity (cytokines and chemokines). Preterm animals exposed to ethanol had significantly reduced VEGF-A mRNA (p = .066) and protein levels, HIF-1α (p = .055), HIF-2α (p = .019), VEGFR-1 (p = .088), and VEGFR-2 (p = .067) mRNA levels but no changes in HIF-3α mRNA. No significant changes occurred in full-term animals exposed to ethanol. Glycogen levels were significantly higher in preterm animals exposed to ethanol (p = .006) but not in full-term animals. Ethanol exposure was associated with significantly lower lung protein levels in preterm (p = .03) but not full-term animals. Preterm animals exposed to ethanol had significantly reduced TNF-α (p = .05), IL-10 (p = .03), chemokine (C-C motif) ligand 5 (CCL5) (p = .017), and monocyte chemotactic protein-1 (MCP-1) (p = .0004) mRNA. In full-term animals exposed to ethanol, the immune alterations were either sustained (TNF-α, p = .009; IL-10, P = .03) or returned to near baseline levels (CCL5 and MCP-1). The ethanol-mediated alterations in fetal lung maturation and immunity may explain the increased incidence of respiratory infections in neonates exposed to ethanol in utero.
Keywords: Alcohol, Lung, Neonate, Sheep, Immune response, VEGF
Introduction
Alcohol (ethanol) consumption during pregnancy has been associated with adverse health effects in the developing fetus, including premature birth, low birth weight, multiple birth defects, and neurodevelopmental disorders, together named fetal alcohol spectrum disorder (Parazzini et al., 2003; Peadon et al., 2007). Along with these well-documented health consequences, there have been a few reports suggesting that fetal alcohol exposure has detrimental effects on the developing fetal lung, such as suppressed pulmonary innate immunity (Sozo et al., 2009) and an increased incidence of neonatal upper respiratory infections (Church and Gerkin, 1988). Studies that support these findings include impaired interstitial and alveolar macrophage differentiation and phagocytic function in neonatal mice (Gauthier et al., 2010), impaired terminal differentiation of interstitial and alveolar macrophages in neonatal guinea pigs (Brown et al., 2009).
Previously, we developed an ovine model of alcohol exposure in utero by administering moderate levels of alcohol to pregnant ewes during the last trimester of pregnancy (Lazic et al., 2007). In that study, the maternal blood alcohol levels achieved in pregnant sheep were similar to those of individuals who consume alcohol in moderation (Brien et al., 1987; Lazic et al., 2007). Exposure to alcohol had a negative impact on the fetal lung, as indicated by the reduction in the expression of pulmonary surfactant protein A (SP-A) mRNA in preterm born lambs, the decreased protein expression of SP-A in full-term lambs, and the reduced ciliary beat frequency in full-term lambs. Recently, another group has used a similar ovine model to demonstrate additional alterations in fetal lung because of alcohol exposure in utero (Sozo et al., 2009). In this study, Sozo et al. demonstrated that daily exposure to alcohol during the last trimester of pregnancy significantly reduced mRNA expression of SP-A, SP-C, and proinflammatory cytokines IL-1β and IL-8 in the lung of full-term lambs. Although alcohol has deleterious effects on the fetal lung, the exact mechanisms by which alcohol alters pulmonary SP-A and other immune parameters are yet to be determined.
One mechanism by which alcohol can impede lung development and immunity is through alterations in the expression of growth factors, which are vital for lung development (Fatayerji et al., 1996). Fetal lung development is a complex process, which is tightly regulated, in part, by hormones and growth factors (Harding and Hooper, 1996) such as vascular endothelial growth factor (VEGF) (Bhatt et al., 2001). VEGF-A is crucial for pre- and postnatal lung development as it stimulates angiogenesis and vascular permeability (Ferrara, 2001; Ferrara et al., 2003). VEGFR blockade results in inhibition of angiogenesis and alveolar formation in the developing rat lung (Jakkula et al., 2000), and VEGF gene deletion results in less complex alveolar patterning (Gerber et al., 1999). Furthermore, VEGF is essential in pulmonary epithelial cell maturation and pulmonary SP production, as inhibition of VEGF in fetal mice results in respiratory distress syndrome (RDS) because of decreased pulmonary SP production (Compernolle et al., 2002).
The VEGF gene transcription is upregulated by three transcription factors; hypoxia-inducible factor-1α, 2α, and 3α (HIF-1α, HIF-2α, HIF-3α, respectively) (Ferrara et al., 2003; Rajatapiti et al., 2008). VEGF binds two related tyrosine kinase receptors; VEGFR-1 (also known as fmstyrosine kinase-1 receptor [Flt-1] in rodents) and VEGFR-2 (also known as kinase insert-domain-containing receptor in humans or fetal liver kinase-1 receptor in rodents). VEGFR-2 has been considered as a main VEGF signaling receptor (Voelkel et al., 2006).
There are very few studies that focus on the direct effect of alcohol on VEGF. However, it has been reported that chronic alcohol exposure downregulates nitric oxide system and VEGF expression by blood vessel endothelial cells resulting in systemic hypertension in rats (Husain et al., 2007). In addition, acute alcohol intoxication has been associated with increased morbidity and mortality in traumatic injuries (Bird et al., 2009). Although the precise mechanism by which alcohol influences the posttraumatic outcomes is not known, there are some speculations that alcohol alters the VEGF signaling and slows down angiogenesis, and, therefore, wound healing (Radek et al., 2005). To the best of our knowledge, this is the first study analyzing the effect of alcohol on VEGF in the developing fetal lung.
Although a few studies have looked at the effects of alcohol on lung growth, maturation, and immunity (Lazic et al., 2007; Sozo et al., 2009), more studies are needed to address the consequences of maternal alcohol use on both pre- and postnatal lung function, maturation, and immune status.
As VEGF and the factors HIF-1α, HIF-2α, HIF-3α are known to have key roles in lung function and maturation, and as there is evidence of the deleterious effects of alcohol on lung function, development, and immunity, we tested four hypotheses: (1) maternal alcohol ingestion during the third trimester of pregnancy will significantly alter lung maturation through reduced expression of HIF-1α, HIF-2α, HIF-3α and their downstream target genes VEGF, VEGFR-1, and VEGFR-2; (2) maternal alcohol ingestion during the third trimester of pregnancy will significantly alter lung maturation by affecting lung glycogen and level of protein; (3) alcohol-related lung development alterations will be more severe in preterm animals than full-term animals; and (4) compared with control animals, those exposed to alcohol in utero will display dysregulated expression of genes involved in host defense and the inflammatory response.
Methods
Experimental procedure
Animal use and experimental procedures were approved by Iowa State University’s Animal Care and Use Committee according to the requirements of the NIH guide for the Care and Use of Laboratory Animals. Sixteen mixed (1/2 Rambouillet, 1/4 Dorset, and 1/4 Finnsheep), date-mated, pregnant ewes were assigned in four groups: alcohol-exposed preterm and full-term groups and control preterm and full-term groups. Alcohol was administered through surgically implanted abomasal cannula. This procedure was previously described in detail (Lazic et al., 2007). Briefly, ewes assigned to alcohol group received 40% ethanol at 1 g/kg, vol/vol (Sigma, St. Louis, MO). Control ewes received the equivalent amount of water. The ewes received alcohol or water 5 days a week for 5 weeks. Serum alcohol (ethanol) concentrations were determined at 6, 12, and 24 h after a single infusion by headspace gas chromatography analysis (University of Iowa Diagnostic Laboratories, Iowa City, IA). The lambs in the preterm group were delivered via Cesarean section at 138 days of gestation (term is ~147 days). The lambs in the full-term group were allowed to deliver naturally (~147 days); there were a total of 24 lambs, and we assigned six lambs to each group (n = 6). After lambing or uterotomy, all lambs were euthanized by intravenous administration of sodium pentobarbital. The lung samples from the lateral portions of the right cranial lobes were collected in cryovials, snap frozen in liquid nitrogen, and stored at −80°C until RNA extraction for quantitative polymerase chain reaction (qPCR) analysis or protein extraction for Western blot analysis. The remaining tissue from the same lung lobes was fixed in 10% buffered formalin, processed, and embedded in paraffin.
Gene expression analysis
The fluorogenic real-time qPCR was used to measure alcohol-mediated changes in gene expression of pulmonary maturation markers HIF-1α, HIF-2α, HIF-3α, VEGF-A, VEGFR-1, and VEGFR-2 and gene expression of major groups of immune genes in the lung, including the proinflammatory cytokine TNF-α, anti-inflammatory cytokine IL-10, chemokine (C-C motif) ligand 5 (CCL5), and monocyte chemotactic protein-1 (MCP-1). This procedure has been previously described in detail (Lazic et al., 2007). Briefly, total RNA was isolated from lung tissues sampled from lateral portions of the right cranial lobe, followed by DNase treatment (TURBO DNA-free kit; Ambion, Austin, TX). A test plate for all targets was run to determine optimal sample and standard dilutions for each target. The DNase-treated RNA isolates were diluted 1:10 with nuclease-free water, then diluted to their ideal ranges (according to what was learned from the test plate analysis) and placed in duplicate into 96-well qPCR plates (Applied Biosystems, Forest City, CA). Each sample contained 7.8 μL of DNase-treated, appropriately diluted RNA isolate, 15 μL of a commercially available Applied Biosystems One-Step Master Mix, 775 nM primers, 150 nM TaqMan (5′-6-carboxyfluorescein, 3′-carboxytetramethylrhodamine quenched) probe, and nuclease-free water. The negative no-template control wells contained nuclease-free water (Ambion) instead of RNA isolate. About 27 μL of each reaction was used per well in the 96-well reaction plates. The plates were run in the GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA). The results were analyzed by using the GeneAmp 5700 software in conjunction with our own custom Excel files.
Western blot analysis
The Western blot analysis was used to measure pan-VEGF protein levels. The terms “pan-VEGF” and “VEGF-A” are interchangeably used because antibodies that can bind and detect multiple VEGF-A isoforms are named as “pan-VEGF” antibodies (Bevan et al., 2008). The sections of the lateral portion of the right cranial lung lobes (0.1 g) were homogenized in 2 mL T-PER® Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL) and Complete TM Protease Inhibitor Cocktail (Santa Cruz Biotechnology, Santa Cruz, CA). The tissue homogenates were centrifuged at 13,000× rpm for 5 min at 4°C, protein extracts were boiled for 5 min with sodium dodecyl sulfate sample buffer, loaded onto polyacrylamide gel (8–16%, Precise™ Protein gels, Thermo scientific, Rockford, IL), and then transferred to polyvinylidene fluoride transfer membrane (Millipore, Bedford, MA). The membranes were then blocked with 5% nonfat dry milk and incubated overnight at 4°C with the mouse monoclonal antirecombinant VEGF 189 protein antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The next day, the membranes were incubated for 90 min at room temperature with secondary Alexa Fluor 488 goat anti-mouse normal IgG antibodies (Invitrogen, Carlsbad, CA). To demonstrate the equal loading of the protein samples, the proteins were stripped and membranes were probed with monoclonal mouse anti-β-actin antibodies (Sigma, St. Louis, MO) and secondary antibody goat anti-mouse Alexa Fluor 488. The fluorescence was detected by using Typhoon imaging system (GE Healthcare, Baie-d’Urfe, Quebec, Canada) using excitation wavelength 488 and 520 nm BP 40 emission filter.
Staining and microscopic scoring of cytoplasmic glycogen
To determine the glycogen content in type II pneumocytes, the slides were subjected to the periodic acid–Schiff stain procedure as follows: on collection, the lung tissue was fixed in 10% buffered formalin for 24 h, and 3-μm-thick tissue sections were cut and placed on glass slides. The slides were then deparaffinized, rehydrated, and placed in 1% periodic acid for 10 min (Sigma, St. Louis, MO) followed by a thorough wash. After the wash, slides were placed in Sigma Schiff’s reagent (Rowley Biochemical Institute, Denver, MA) for 15 min followed by another wash after which they were counterstained with MCB reagent (Manufacturing Chemists, Inc., Gibbstown, NJ), rinsed in distilled water, dehydrated, and coverslipped using Permount (Fisher, Hanover, IL). Ten alveoli were scored from each of the three tissue sections sampled from the left cranial, right cranial, and right caudal lung lobes of each lamb. Alveoli were similar in size, which ranged between 100 and 200 mm in diameter. Scoring was based on predetermined scale: 0, no detectable stain; 1, earliest detectable stain; 2, cytoplasmic glycogen granules present in less than 30% of alveolar epithelial cells; and 3, cytoplasmic glycogen granules present in more than 60% of alveolar epithelial cells.
Total lung protein concentration analysis
The total protein concentration in the lungs of experimental lambs was determined by Coomassie Plus (Bradford) Assay Kit (Pierce Biotechnology, Rockford, IL). The bovine serum albumin (BSA) standards provided by the kit were serially diluted in water. The BSA standards, a water blank, and the diluted lung protein samples (1:100) were mixed with equal amounts (150 μg) of Coomassie Plus reagent and loaded into microplate wells. The microplate was placed for 30 s on microplate shaker and incubated for 10 min at room temperature. The absorbance was measured at 595 nm on a plate reader. The standard curve was determined by plotting the average blank-corrected 595-nm measurements for each BSA standard. The total lung protein concentration was estimated by using the standard curve and correcting for the initial dilution gradient.
Statistical analysis
The summary statistics (mean and standard error of the mean) was calculated for each experimental group. Student’s t-test was performed using GraphPad Sigma software (San Diego, CA). One-way ANOVA was performed using SAS version 9.1 (SAS Institute, Cary, NC). Mean values of relative mRNA expression levels of genes from preterm and full-term animals exposed to ethanol were compared with control animals of the same age group at *P ≤.1, **P ≤.01, and ***P ≤.001 and expressed as means ± standard error of the mean (S.E.M.). Considering we used a large animal model in this study and consequently dealt with small sample size, the difference in which P value was ≤.1 was accepted as significant.
Results
Serum alcohol concentration
These results have been previously published (Lazic et al., 2007).
Pulmonary HIF, VEGF-A, and VEGFR gene expression
Compared with control animals, the preterm animals exposed to ethanol had significantly decreased expression of HIF-1α (P = .055), HIF-2α (P = .0187) (Fig. 1), VEGF-A (P = .066) (Fig. 2), VEGFR-1 (P = .088), and VEGFR-2 mRNA (P = .067) (Fig. 3); no significant changes were seen with HIF-3α. In full-term animals exposed to ethanol, no significant changes in the expression of HIF-1α, HIF-2α, VEGF-A, VEGFR-1, and VEGFR-2 mRNA were seen when compared with animals of the same age group.
Fig. 1.
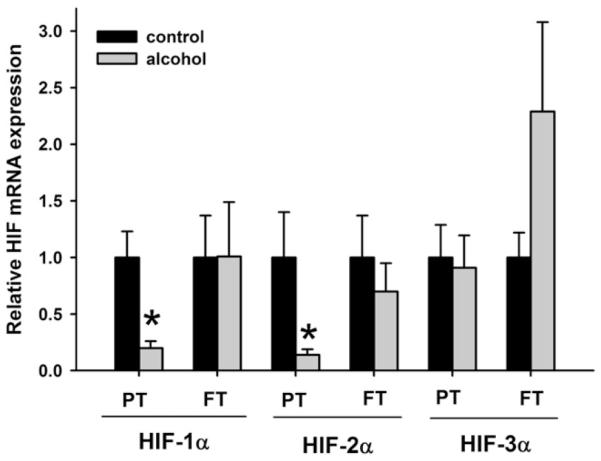
Relative mRNA expression of hypoxia-inducible factor-1α, 2α, and 3α (HIF-1α, HIF-2α, and HIF-3α, respectively) in the neonatal ovine lung. Exposure to ethanol in utero reduces mRNA expression of HIF-1α and HIF-2α in the lungs of preterm (PT) lambs when compared with the control lambs of the same age group. Such changes are not observed in the lungs of full-term (FT) lambs exposed to ethanol in utero. No significant changes are seen with HIF-3α mRNA expression levels in both PT and FT lambs (*P < .1, significance was established at 90% confidence interval).
Fig. 2.
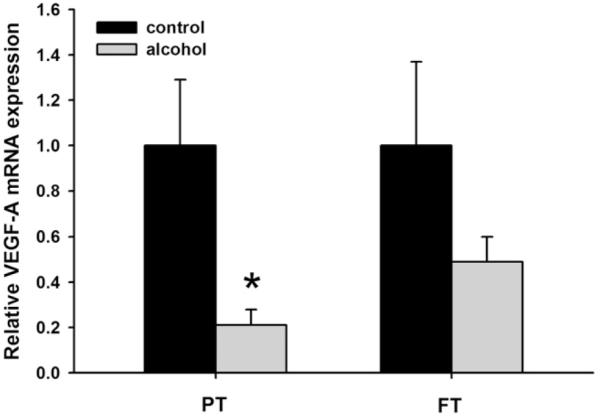
Relative mRNA expression of vascular endothelial growth factor-A (VEGF-A) in the neonatal ovine lung. Exposure to ethanol in utero reduces mRNA expression of VEGF-A in the lungs of preterm (PT) lambs when compared with the control lambs of the same age group. Such changes are not observed in the lungs of full-term (FT) lambs exposed to ethanol in utero (*P < .1, significance was established at 90% confidence interval).
Fig. 3.
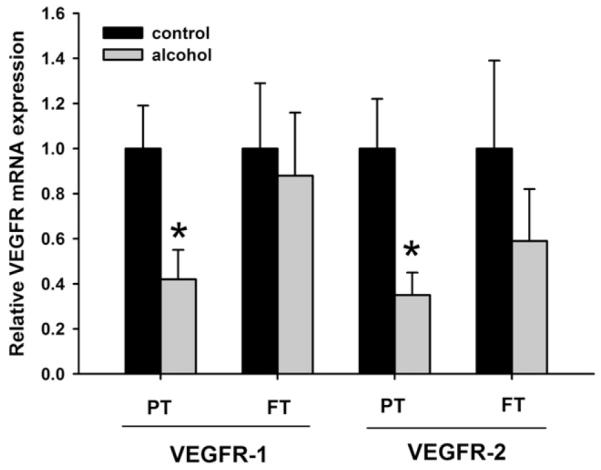
Relative mRNA expression of vascular endothelial growth factor receptors 1 and 2 (VEGFR-1 and VEGFR-2, respectively) in the neonatal ovine lung. Exposure to ethanol in utero reduces mRNA expression of VEGFR-1 and VEGFR-2 in the lungs of preterm (PT) lambs when compared with the control lambs of the same age group. Such changes are not observed in the lungs of full-term (FT) lambs exposed to ethanol in utero (*P < .1, significance was established at 90% confidence interval).
Pulmonary cytokines and chemokines gene expression
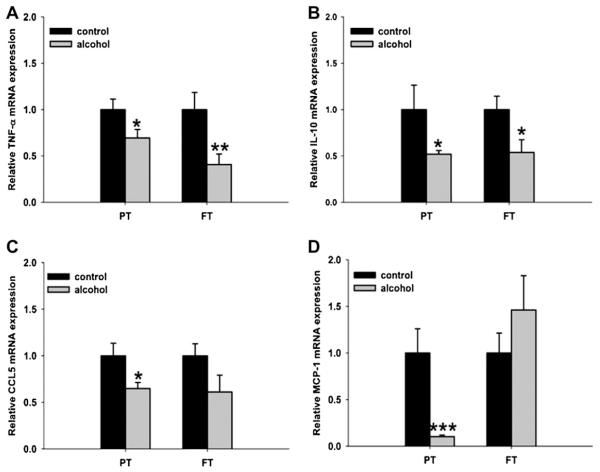
Preterm animals exposed to ethanol had significantly reduced levels of TNF-α mRNA (P = .05), IL-10 mRNA (P = .03), CCL5 mRNA (P = .017), and MCP-1 mRNA (P = .0004) when compared with control animals of the same age group (Fig. 4). In full-term animals exposed to ethanol, a significant decrease in the expression of TNF-α mRNA (P = .009) and IL-10 mRNA (P = .03) was observed. However, no significant changes in the expression of CCL5 mRNA (P = .12) and MCP-1 mRNA (P = .33) occurred when compared with control full-term animals.
Fig. 4.
Relative mRNA expression of chemokines and cytokines in the neonatal ovine lung. Exposure to ethanol in utero reduces mRNA expression of (A) TNF-α, (B) interleukin (IL)-10, (C) chemokine (C-C motif) ligand 5 (CCL5), (D) and monocyte chemotactic protein-1 (MCP-1) in the lungs of preterm (PT) lambs when compared with the control lambs of the same age group. In the lungs of full-term (FT) lambs, ethanol exposure in utero reduces mRNA expression of (A) TNF-α and (B) IL-10 (*P < .1, significance was established at 90% confidence interval).
Pulmonary VEGF-A protein levels
The Western blot analysis demonstrated significantly decreased levels of VEGF-A protein in the lungs of preterm animals exposed to ethanol when compared with control animals of the same age group (Fig. 5).
Fig. 5.

Vascular endothelial growth factor-A (VEGF-A) protein levels in the lung of preterm lambs. Exposure to ethanol in utero reduces VEGF-A protein level in the lungs of preterm lambs when compared with the control lambs of the same age group. β-act β-actin.
Pulmonary glycogen content
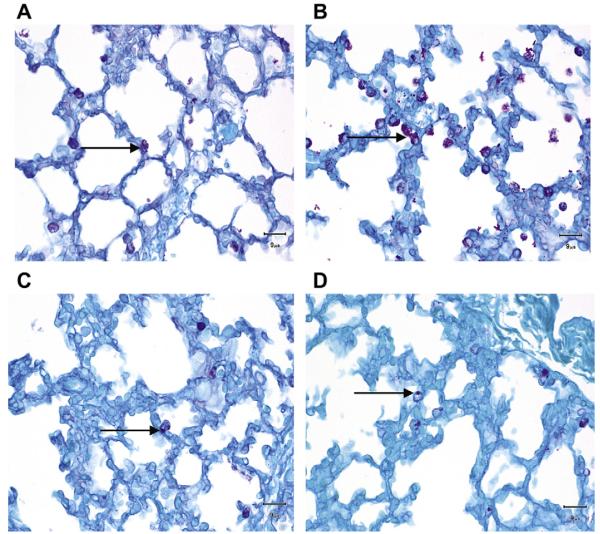
We found that preterm animals exposed to alcohol expressed more glycogen retention in the lungs (score = 1.408 ± 0.12, mean ± S.E.M., Fig. 6) when compared with the preterm control animals (score = 0.96 ± 0.04, P = .006). Although the glycogen content in the lungs of full-term alcohol-exposed animals was slightly increased (score = 0.73 ± 0.04) when compared with the full-term control animals (score = 0.64 ± 0.05), this difference was not statistically significant (P = .21) (Fig. 6).
Fig. 6.
Periodic acid–Schiff (PAS) stain for glycogen granules in the type II pneumocytes (ATII). Glycogen granules in the ATII are more abundant in the preterm lambs exposed to ethanol in utero (B) when compared with the control lambs of the same age group (A). Glycogen content in the ATII is similar in the full-term lambs exposed to ethanol in utero (D) and the control lambs of the same age group (C).
Pulmonary protein level
We found that in utero exposure to ethanol led to a statistically significant reduction of protein content in pre-term animals when compared to the age-matched control group (P = .03) (Fig. 7). This difference was not statistically significant in full-term animals.
Fig. 7.
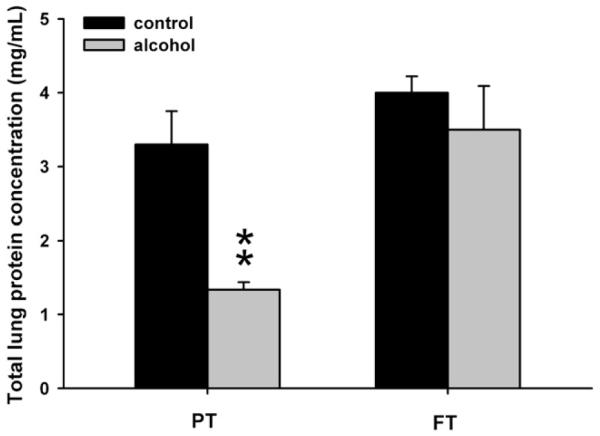
Coomassie Plus (Bradford) Assay for total lung protein determination. Exposure to ethanol in utero reduces total lung protein concentration in preterm (PT) lambs when compared with the control lambs of the same age group. Such changes are not observed in the lungs of full-term (FT) lambs exposed to ethanol in utero (*P < .1, significance was established at 90% confidence interval).
Discussion
The present study demonstrated alcohol (ethanol)-mediated alterations in lung maturation, which include decrease in the expression of VEGF-A, gene expression of its receptors (VEGFR-1 and VEGFR-2), and gene expression of its regulatory components (HIF-1α and HIF-2α). This may explain our previously reported decrease in SP-A mRNA expression in preterm lambs exposed to alcohol in utero (Lazic et al., 2007). VEGF mediates conversion of glycogen in type II pneumocytes (ATII) into SPs (Compernolle et al., 2002), and our finding of increased glycogen content in the lung of preterm lambs exposed to alcohol is consistent with the suppressing effect of alcohol on VEGF and consequently SP-A expression. The most recent study done by Sozo et al. demonstrated similar effect of alcohol on the fetal lung. In that study, preterm lambs that were chronically exposed to alcohol had SP-A and SP-B mRNA levels decreased to one-third of control levels (Sozo et al., 2009). Alcohol administration during the entire length of pregnancy would more closely resemble the drinking habit in pregnant women. However, our goal was to investigate the influence of alcohol primarily on the lung maturation that occurs during the last trimester of pregnancy and avoid teratogenic effect on the central nervous system (Joshi and Kotecha, 2007; Peadon et al., 2007). In addition, alcohol did not alter the gene expression of VEGF-A, its receptors, and regulatory transcription factors in full-term lambs when compared with the age-matched controls. We suspect that additional gestational time allowed lung epithelial cells, including the ATII cells to further mature and reach its maturation level necessary for VEGF-A secretion.
The exact mechanism by which alcohol impairs lung maturation in neonates is not clear. Although there are no published data describing in utero effect of alcohol on pulmonary VEGF, recent studies have pointed to the impairing properties of alcohol on the HIF–VEGF–VEGFR pathway. The well-documented role of VEGF during fetal lung development involves blood vessel branching and growth (Zachary, 1998). However, there are studies suggesting that in addition to blood vessel growth, VEGF coordinates the development of pulmonary epithelial cells; particularly ATII that are the major source of VEGF in the lung (Acarregui et al., 1999). Incubation of fetal human lung explants with VEGF resulted in increased ATII cell differentiation, SP-A and SP-C mRNA levels, and SP-A protein level in ATII cells (Brown et al., 2001). Furthermore, inhibition of VEGF resulted in impaired lung maturation and fetal RDS in mice (Compernolle et al., 2002). The same study suggested that VEGF stimulates conversion of glycogen stores in ATII cells into SPs. These results are in accordance with our finding that the lungs of preterm lambs contained more abundant glycogen stores within type II alveolar cells, indicating suppressed conversion of glycogen to SPs because of reduced maturation/differentiation. In our studies, we suspect that fetal alcohol exposure may have a direct downregulating effect on the expression (transcription or protein stabilization) of HIF-1α and HIF-2α, which consequently leads to the downregulation of VEGF and VEGFR expression. Another possible mechanism involves increased oxidative stress by alcohol in the fetal lung, which accelerates apoptosis, especially in the ATII cells (Brown et al., 2001). Additional studies are required to further elucidate the underlying mechanistic basis for these findings.
To support the finding of decreased lung maturation process in the preterm lambs exposed to alcohol, we have determined the total lung protein content. Exposure to alcohol during in utero development is a known risk factor for fetal growth retardation (Peadon et al., 2007). We found that total lung protein content was reduced in preterm lambs exposed to alcohol in utero, which is consistent with other studies performed in fetal rats (Inselman et al., 1985). Although the exact mechanism of fetal organ growth retardation by alcohol is still not completely elucidated, several mechanisms might be involved, including impaired transport of nutrients or oxygen because of placental vasocon-striction (Fisher et al., 1983), suppression of insulin-like growth factor-1 (IGF-1) (Breese et al., 1993), or direct toxicity of alcohol and its metabolites on organs, such as lung (Bernstein, 1982). IGF-1 appears to be of particular importance in the lung development considering that IGF-1 receptor knockout mice have 45% decrease in lung weight at birth and soon after die because of respiratory failure (Warburton et al., 2000). We speculate that suppression of HIF–VEGF–VEGFR pathway may also contribute to pulmonary growth retardation, but the exact mechanism remains to be determined.
Our last objective of this study was to determine whether in utero exposure to alcohol may have an immunomodulatory effect in the fetal lung through the alteration of inflammatory cytokines. There is a report of increased incidence of neonatal upper respiratory infections related to alcohol consumption during pregnancy (Church and Gerkin, 1988). Sozo et al. (2009) had demonstrated that ethanol-exposed prematurely delivered neonatal lambs had decreased pulmonary expression of IL-1β and IL-8. However, we extended our studies by analyzing the effects of ethanol on lung immunity in lambs that have reached full-term gestation and by comparing the gene expression of specific cytokines and chemokines with that of preterm animals. In preterm animals exposed to ethanol, some genes displayed significant alterations in expression that either persisted to full-term gestation (as seen with TNF-α and IL-10) or were restored to levels similar to those of control animals at full-term gestation (as seen with CCL5 and MCP-1). Our findings that ethanol also decreased the expression of the C-C chemokines CCL5 and MCP-1 are supported by other studies that showed in vitro suppressive effects of ethanol on chemokine production (Szabo et al., 1999). However, the data on inflammatory cytokines presented in this manuscript are on mRNA level. Additional studies are needed to determine whether the altered gene expression consequentially altered the protein expression and test the biological relevance of this change, such as respiratory infectious agent challenge and/or pulmonary leukocyte count.
Conclusions
In conclusion, we have demonstrated that in utero exposure to alcohol alters the expression of VEGF in the lung of the prematurely born lambs, one of the crucial growth factors involved in alveolar epithelial cell maturation. This finding was supported by increased glycogen content in the alveolar type II cells of the immature lungs and decreased total protein content. These findings may explain a novel mechanism by which alcohol impairs fetal lung maturation and may explain the previously reported decrease in SP-A expression by prematurely born lambs. Our study also suggests that maternal alcohol consumption may have significant immunological effects on fetal lambs. To the best of our knowledge, this is the first study to report such findings. Considering the importance of VEGF in fetal lung maturation, these results may open a new venue for therapeutics targeting VEGF pathways in pediatric patients suffering from deleterious effects of in utero alcohol exposure.
Acknowledgments
The authors thank other individuals involved with the project including Drs. David Merkley, Linda Nelson, Rachel Derscheid, Pam Knake, Francis Zacharakis-Jutz, Beth Hafkey, Jenny Groeltz-Thrush, Diane Gerjets, and the Laboratory Animal Resources at Iowa State University. This work was funded, in part, by the JG Salsbury Endowment and NIH NIAID grant R01AI062787.
References
- Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expres- sion in human fetal lung in vitro. Am. J. Respir. Cell Mol. Biol. 1999;20:14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- Bernstein J. The role of the lung in the metabolism of ethanol. Res. Commun. Chem. Pathol. Pharmacol. 1982;38:43–56. [PubMed] [Google Scholar]
- Bevan HS, van den Akker NM, Qiu Y, Polman JA, Foster RR, Yem J, et al. The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol. 2008;110:57–67. doi: 10.1159/000177614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- Bird MD, Choudhry MA, Molina PE, Kovacs EJ. Alcohol and trauma: a summary of the Satellite Symposium at the 30th Annual Meeting of the Shock Society. Alcohol. 2009;43:247–252. doi: 10.1016/j.alcohol.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, D’Costa A, Ingram RL, Lenham J, Sonntag WE. Long-term suppression of insulin-like growth factor-1 in rats after in utero ethanol exposure: relationship to somatic growth. J. Pharmacol. Exp. Ther. 1993;264:448–456. [PubMed] [Google Scholar]
- Brien JF, Clarke DW, Smith GN, Richardson B, Patrick J. Disposition of acute, multiple-dose ethanol in the near-term pregnant ewe. Am. J. Obstet. Gynecol. 1987;157:204–211. doi: 10.1016/s0002-9378(87)80381-2. [DOI] [PubMed] [Google Scholar]
- Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell prolifer- ation in human fetal lung in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1001–L1010. doi: 10.1152/ajplung.2001.281.4.L1001. [DOI] [PubMed] [Google Scholar]
- Brown SD, Gauthier TW, Brown LA. Impaired terminal differentiation of pulmonary macrophages in a Guinea pig model of chronic ethanol ingestion. Alcohol. Clin. Exp. Res. 2009;33:1782–1793. doi: 10.1111/j.1530-0277.2009.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- Fatayerji N, Engelmann GL, Myers T, Handa RJ. In ute- ro exposure to ethanol alters mRNA for insulin-like growth factors and insulin-like growth factor-binding proteins in placenta and lung of fetal rats. Alcohol. Clin. Exp. Res. 1996;20:94–100. doi: 10.1111/j.1530-0277.1996.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001;280:C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Atkinson M, Jacobson S, Sehgal P, Burnap J, Holmes E, et al. Selective fetal malnutrition: the effect of in vivo ethanol exposure upon in vitro placental uptake of amino acids in the non-human primate. Pediatr. Res. 1983;17:704–707. doi: 10.1203/00006450-198309000-00002. [DOI] [PubMed] [Google Scholar]
- Gauthier TW, Ping XD, Gabelaia L, Brown LA. Delayed neonatal lung macrophage differentiation in a mouse model of in utero ethanol exposure. Am. J. Physiol. Lung Cell Mol. Physiol. 2010;299:L8–L16. doi: 10.1152/ajplung.90609.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J. Appl. Physiol. 1996;81:209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- Husain K, Vazquez-Ortiz M, Lalla J. Down regulation of aortic nitric oxide and antioxidant systems in chronic alcohol-induced hypertension in rats. Hum. Exp. Toxicol. 2007;26:427–434. doi: 10.1177/0960327106072993. [DOI] [PubMed] [Google Scholar]
- Inselman LS, Fisher SE, Spencer H, Atkinson M. Effect of intrauterine ethanol exposure on fetal lung growth. Pediatr. Res. 1985;19:12–14. doi: 10.1203/00006450-198501000-00004. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveo- larization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Joshi S, Kotecha S. Lung growth and development. Early Hum. Dev. 2007;83:789–794. doi: 10.1016/j.earlhumdev.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Lazic T, Wyatt TA, Matic M, Meyerholz DK, Grubor B, Gallup JM, et al. Maternal alcohol ingestion reduces surfactant protein A expression by preterm fetal lung epithelia. Alcohol. 2007;41:347–355. doi: 10.1016/j.alcohol.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini F, Chatenoud L, Surace M, Tozzi L, Salerio B, Bettoni G, et al. Moderate alcohol drinking and risk of preterm birth. Eur. J. Clin. Nutr. 2003;57:1345–1349. doi: 10.1038/sj.ejcn.1601690. [DOI] [PubMed] [Google Scholar]
- Peadon E, O’Leary C, Bower C, Elliott E. Impacts of alcohol use in pregnancy—the role of the GP. Aust. Fam. Physician. 2007;36:935–939. [PubMed] [Google Scholar]
- Radek KA, Matthies AM, Burns AL, Heinrich SA, Kovacs EJ, Dipietro LA. Acute ethanol exposure impairs angiogen- esis and the proliferative phase of wound healing. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1084–H1090. doi: 10.1152/ajpheart.00080.2005. [DOI] [PubMed] [Google Scholar]
- Rajatapiti P, van der Horst IW, de Rooij JD, Tran MG, Maxwell PH, Tibboel D, et al. Expression of hypoxia- inducible factors in normal human lung development. Pediatr. Dev. Pathol. 2008;11:193–199. doi: 10.2350/07-04-0257.1. [DOI] [PubMed] [Google Scholar]
- Sozo F, O’Day L, Maritz G, Kenna K, Stacy V, Brew N, et al. Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:L510–L518. doi: 10.1152/ajplung.90532.2008. [DOI] [PubMed] [Google Scholar]
- Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates interleukin-8 (IL-8) and monocyte che- moattractant peptide-1 (MCP-1) induction in response to ex vivo stimulation. J. Clin. Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endo- thelial growth factor in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech. Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Zachary I. Vascular endothelial growth factor. Int. J. Biochem. Cell Biol. 1998;30:1169–1174. doi: 10.1016/s1357-2725(98)00082-x. [DOI] [PubMed] [Google Scholar]