Abstract
The requirement of the developing mammalian embryo for retinoic acid is well established. Retinoic acid, the active form of vitamin A, can be generated from retinol and retinyl ester obtained from food of animal origin, and from carotenoids, mainly β-carotene, from vegetables and fruits. The mammalian embryo relies on retinol, retinyl ester and β-carotene circulating in the maternal bloodstream for its supply of vitamin A. The maternal-fetal transfer of retinoids and carotenoids, as well as the metabolism of these compounds in the developing tissues are still poorly understood. The existing knowledge in this field has been summarized in this review in reference to our basic understanding of the transport and metabolism of retinoids and carotenoids in adult tissues. The need for future research on the metabolism of these essential lipophilic nutrients during development is highlighted.
Keywords: β-carotene, retinoid, β-carotene cleavage enzymes, developing tissues, retinol-binding protein (RBP), maternal-fetal metabolism
1. VITAMIN A METABOLISM
1.1 Absorption
Vitamin A is an essential nutrient that controls many crucial biological functions such as vision, reproduction, development, growth and immunity [1]. All retinoids (vitamin A and its derivatives) in animals are derived from the diet either as preformed vitamin A [retinyl esters (RE), retinol (ROH) and very small amounts of retinoic acid (RA)] from animal products or as carotenoids, mainly β-carotene, from vegetables and fruits [2]. Within the intestinal mucosa all ROH, regardless of its dietary origin, is re-esterified with long-chain fatty acids primarily by the action of the enzyme lecithin:retinol acyltransferase (LRAT), which is widely expressed in tissues [3-5]. Together with other dietary lipids, the newly synthesized RE are packaged into chylomicrons and secreted into the lymphatic system [6]. Once in the general circulation, lipoprotein lipase (LPL), which is bound to the luminal surface of the vascular endothelium, catalyzes the lipolysis of triglycerides to generate free fatty acids and chylomicron remnants [7]. After chylomicron remnants acquire apolipoprotein E, either in the plasma or in the space of Disse, approximately 75% of chylomicron remnant-RE is cleared by the liver, the major site of vitamin A storage and metabolism [8]. The remaining 25% is cleared by extrahepatic tissues [9].
1.2 Transport, tissue uptake and metabolism
Once taken up by the hepatocytes, RE are hydrolyzed again to ROH to be transferred to stellate cells and then re-esterified by LRAT for storage. Alternatively, ROH can bind to its sole specific serum transport protein, retinol-binding protein (RBP), to be secreted into the bloodstream [10, 11]. RBP is a 21 kDa protein with a single binding site for one molecule of all-trans-ROH. It is mainly, but not exclusively, synthesized within the hepatocytes [10, 11]. RBP circulates in the blood as a 1:1 molar complex with another serum protein, transthyretin (TTR) [12]. The major function of RBP is to mobilize hepatic retinoid stores and deliver ROH to peripheral tissues [10, 13], such as embryos [14, 15]. In the fasting circulation, ROH-RBP accounts for approximately 95-99% of all serum retinoids. Upon vitamin A intake the concentration of retinoids in chylomicrons and chylomicron remnants can greatly exceed that of plasma ROH. Blood levels of ROH-RBP in both humans and animals are maintained very constant, except in extreme cases of insufficient intake of vitamin A, protein, calories and zinc; or in response to hormonal factors, stress; and in certain disease states [11, 16, 17]. The mechanisms that regulate the secretion of the complex ROH-RBP from the liver have yet to be fully elucidated.
Most tissues acquire vitamin A primarily from ROH-RBP. The peripheral uptake of vitamin A from the complex ROH-RBP seems to be mediated by Stra6, a large membrane protein that has been recently identified as the receptor for the circulating complex [18], at least in those tissues that do express Stra6. The precise molecular mechanism of this uptake still needs to be clarified. Emerging evidence supports the hypothesis that Stra6 may have other functions in addition to being the RBP receptor [19]. Berry and colleagues [20] recently reported that Stra6 acts as a cytokine receptor to transduce signaling by holo-RBP that can ultimately regulate insulin response. In addition, in vitro experiments [21] and observations made in the developing embryo [22] suggest that Stra6 may also mediate the efflux of retinol from the cell and thus act as a bi-directional transporter of retinol, with intracellular retinol concentration determining the polarity of transport. Interestingly, in some tissues with high retinoid content, such as skin and liver, Stra6 is expressed at very low levels [18]. Thus, other mechanisms of retinol uptake from the complex likely occur in these tissues. Spontaneous transfer of free retinol across the phospholipid bilayer is one of the possible mechanisms supported by experimental evidence [23-25]. It should also be noted that even in the fasting state there are low concentrations of RE associated with circulating lipoproteins (in VLDL and LDL) and small amounts of circulating RA bound to albumin [6].
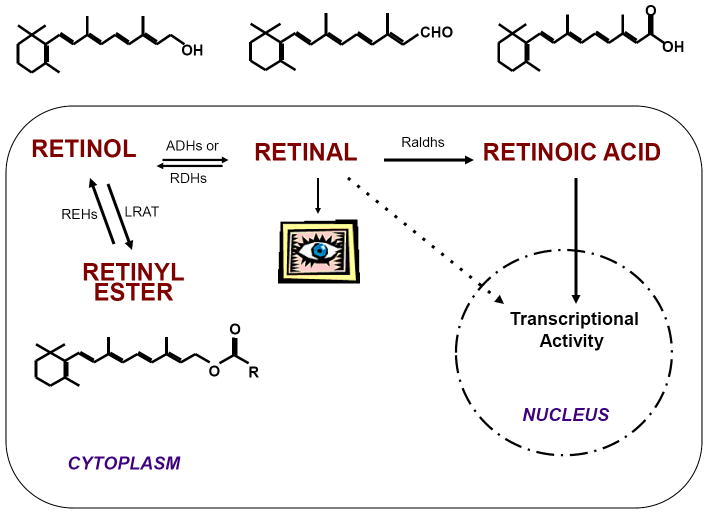
Vitamin A exerts its activity through oxidized metabolites of ROH: retinaldehyde (RAL), involved in the visual cycle [26], and RA, which regulates the expression of many target genes through receptor-mediated events (Figure 1) [27-32]. RA is the biologically active form of vitamin A and it functions as a ligand for specific nuclear receptors, retinoic acid receptor (RAR) or retinoid X receptor (RXR), that regulate the transcription of numerous target genes, as homo- or hetero-dimers [27-32]. More than 500 genes are known to be regulated by RA [33]. A great number of these genes have been shown to control embryonic development [34]. When RA signaling needs to be turned off, RA is degraded by members of the cytochrome P450 family of enzymes, such as Cyp26A1, to produce more polar compounds, like 4-hydroxy or 4-oxo RA, which are believed to be non-transcriptionally active [35]. Within cells, ROH is reversibly oxidized to RAL by enzymes members of the alcohol dehydrogenases (ADHs) of medium-chain dehydrogenase/reductase (MDRs) and retinol dehydrogenases (RDHs) of short-chain dehydrogenase/reductase (SDRs) superfamily [36]. RAL is further oxidized to RA by the action of retinal dehydrogenases (Raldhs) [37]. Being lipid molecules, retinoids must be bound to proteins within cells. Several intracellular binding proteins for ROH, RAL and RA have been identified and extensively characterized. These include cellular retinol-binding proteins (CRBPI, II and III), cellular retinaldehyde-binding protein (CRalBP) and cellular retinoic acid-binding proteins (CRABPI and CRABPII). Each of these retinoid-binding proteins has a distinct expression pattern and plays a specific role in vitamin A transport and metabolism [38].
Figure 1. Retinol is as a metabolic precursor of active compounds.
Inside the cell, retinol (ROH), the vitamin A alcohol, is first oxidized and converted to retinal (RAL), which is known to be active in the visual cycle, even though recent data suggest that endogenous aldehydes can also control gene expression [152, 153]. Further oxidation of retinaldehyde generates retinoic acid (RA), which acts as a ligand for specific nuclear receptors, such as RAR and RXR, to regulate the transcription of a wide variety of target genes. Alternatively, ROH can also be converted to RE, which, inside the cells, represents the storage form of vitamin A. All these vitamin A derivatives are also called retinoids. The enzyme or class of enzymes catalyzing each reaction are abbreviated as follows: ADHs, alcohol dehydrogenases; RDHs, retinol dehydrogenases; Raldhs, retinal dehydrogenases; LRAT, lecithin:retinol acyltransferase; REHs, retinyl ester hydrolases.
2. VITAMIN A AND DEVELOPMENT
2.1 Functions
Retinoids are crucial for the health of the mother, are required for maintenance of the placenta, and are absolutely essential for the developing embryo. Indeed, RA, which is mainly synthesized within embryonic tissues, controls the expression of numerous key developmental target genes [39], thus, influencing the pattern formation of various organs, including hindbrain [40-43], spinal cord [44, 45], eye [46], heart [47, 48], kidney [49], lung [50] and limb buds [51, 52]. Perturbations of the amount of RA available to the embryo lead to abnormal development. Animal model studies have established that severe maternal vitamin A deficiency results in early embryonic death (reviewed in [39]). Less severe vitamin A deficiency, however, induces fetal developmental malformations. These have been described in several animal systems, and are known collectively as vitamin A deficiency (VAD) syndrome (rat: [53-57], pig: [58], quail: [47], and mouse: [59]). The features of the VAD syndrome include: cleft face, palate and lip; small or absent eye; abnormality of the urogenital system; abnormality of the heart and great vessels; and malformation of the forelimbs. Mice RAR compound null mutants and RAR/RXR double mutants display phenotypes that resemble those seen during gestational vitamin A deficiency [60-62]. Severe embryonic malformations have also been described in mutant mice lacking Raldh2, an enzyme that controls the irreversible oxidation of RAL to RA [63], or in mice lacking Cyp26A1, an enzyme that metabolizes RA into more polar hydroxylated and oxidized derivatives [35, 64, 65]. Retinoid excess during development also results in major embryonic defects which often overlap with those observed in retinoid deficiency [59, 66-69].
2.2 Sources of vitamin A for the embryo
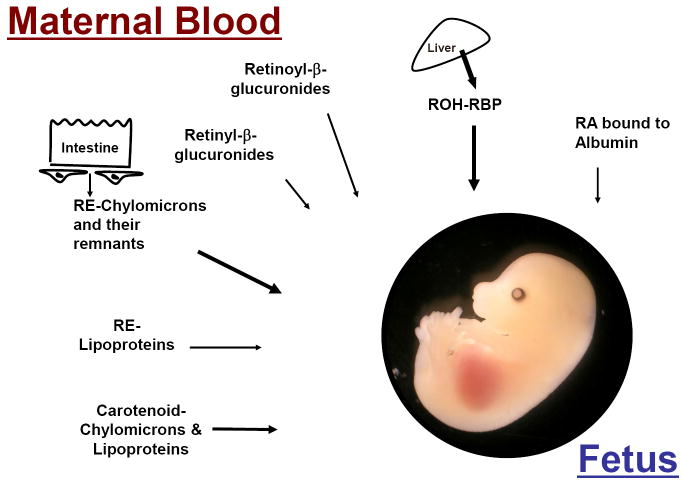
Since there is no de novo fetal synthesis of vitamin A, to meet its requirement for retinoids, the developing mammalian embryo relies on circulating maternal vitamin A that reaches the embryo through the maternal-fetal barrier, i.e. placenta and yolk sac [70]. In the maternal bloodstream, two major retinoid forms can be identified: ROH bound to RBP is the major form in the fasting state, when most of ROH is secreted into the circulation from the liver stores; RE packaged in chylomicrons and their remnants may account for the majority of circulating retinoids upon dietary vitamin A intake [6]. Although at lower concentrations, other forms of vitamin A circulate in the bloodstream: 1. RE incorporated in lipoprotein particles [9, 71]; 2. RA (both all-trans and 13-cis) in the fasting plasma of human, rodents and cow [72]; 3. Fully water soluble glucuronides of both ROH and RA [73, 74] and 4. Provitamin A carotenoids [75-77] (Figure 2). Overall, the levels of these circulating retinoids reflect the maternal vitamin A status, which is determined by both their concentration within the stores and the recent dietary retinoid intake. Hence, alterations in maternal status and/or dietary vitamin A intake ultimately affect circulating retinoid levels and thus the amount of vitamin A available to cross the placenta towards the fetus.
Figure 2. Maternal circulating forms of retinoid and carotenoid available to the developing embryo.
The mammalian embryo is entirely dependent on maternal circulating retinoids for its vitamin A supply. In the maternal bloodstream, we can identify two major retinoid forms: 1. ROH bound to RBP is the major form in the fasting state, when most of retinol is secreted into the circulation from the liver stores. 2. RE packaged in chylomicrons and their remnants may account for the majority of circulating retinoids upon dietary vitamin A intake. Although at lower concentrations, other forms of vitamin A circulate in the bloodstream, such as intact β-carotene in chylomicrons and lipoprotein particles. ROH, retinol; RE, retinyl ester; RA, retinoic acid; RBP, retinol-binding protein.
2.3 Changes in endogenous retinoids during pregnancy and embryogenesis
Fetal acquisition of vitamin A remains relatively stable over a wide range of maternal dietary vitamin A intake, presumably as a result of maternal serum homeostatic mechanisms involving RBP [78]. Early studies from Satre and colleagues [79] examined changes in endogenous vitamin A levels in the maternal circulation and developing embryo over the course of normal pregnancy in mouse. They observed a large transient decrease in maternal plasma ROH levels and an apparent increase in mobilization from hepatic stores to the conceptus coinciding with the period of organogenesis (E9-14). Embryonic ROH levels increased with little or no change in RE and RA concentrations. Patterns of retinoid accumulation in embryonic liver indicate that the onset of vitamin A storage occurs by mid-organogenesis. In contrast, placental retinoid levels remained unchanged throughout organogenesis. Analysis of the conceptus as a developmental unit revealed that during early organogenesis the majority of retinoid is contained in the placenta (8-fold more than in the embryo). However, by mid-organogenesis the retinoid content of the embryo exceeds that of the placenta. The data reported from mouse are in general agreement with those reported for humans and other species [80, 81].
2.4 Transplacental transfer of retinol
The presence of measurable fetal hepatic vitamin A stores at birth indicates the efficiency of placental ROH transport during pregnancy [78, 79]. It is clear that ROH crosses the placenta [82], but, at the cellular level, the mechanism by which it is transferred to the fetus is poorly understood. Three possible mechanisms have been proposed: 1. direct transfer of ROH-RBP involving cellular uptake and release of the protein-ligand complex; 2. transfer of free ROH; and 3. cellular uptake of ROH by its specific receptor Stra6. It is noteworthy that the visceral endoderm of the yolk sac is a major site of RBP [83, 84] and Stra6 [18] synthesis and of ROH uptake [85]. Thus, it has been proposed that maternally-derived ROH passes from maternal blood circulating in the trophoblastic blood sinuses into the yolk sac cavity. There it comes into direct contact with the endodermal surface of the visceral yolk sac, where uptake occurs [85]. Once within the endodermal cells, ROH must be transferred to the yolk sac vasculature for transport to the embryo. This process would depend on RBP synthesized in the endodermal cells [86]. This RBP, located in the endoplasmic reticulum, binds and exports ROH [85]. The expression of RBP at later stages of gestation remains localized to the visceral endoderm of the yolk sac membranes. However, RBP expression is also detected (as early as E13.5) at the junction of the uterine wall and placenta (deciduas basalis) [84]. These data indicate that a second site for ROH uptake may correspond to the area that eventually forms the definitive placenta. However, active uptake by the yolk sac membranes may continue after formation of the definitive placenta. It is of note that maternal circulating RBP has been shown not to cross the maternal-fetal barrier [14].
2.5 Transplacental transfer of other retinoids and their placental metabolism
Early studies have investigated the ability of retinoids to pass through the placental barrier in human, mouse, rat and monkey [79, 87-89]. Although retinyl palmitate has been shown not to be transferred [90], other retinoids (ROH; 13-cis, 9-cis, all-trans RA and their glycuronoconjugates) are transferred with specific kinetics [91]. These studies suggest that the transfer of each retinoid from mother to embryo is promoted by a specific pathway. Furthermore, the placenta can metabolize some retinoids (ROH acetate can be oxidized by a placenta lipoxygenase; [92]) and thus may produce retinoids from maternally derived precursors [93]. The villous mesenchymal fibroblasts of the placenta are also able to esterify ROH to RE [94]. The placenta is suggested to serve as a site of vitamin A stores until the embryonic liver becomes functional. The placenta has also been proposed to buffer retinoid delivery, by releasing ROH to the fetus when maternal intake is deficient and by storing it to protect the embryo from a potential toxic excess of maternal retinoids. Vitamin A reserves can also be used in placental tissue [94].
2.6 The retinol-RBP pathway versus the postprandial retinyl ester pathway
Mice lacking RBP (RBP-/-) are a very useful model to study the transfer of retinoid from mother to fetus [10]. RBP-/- mice are unable to efficiently mobilize their hepatic retinoids stores, and must continuously acquire vitamin A from the diet to support normal physiological processes, including embryonic development [10, 14, 15]. Indeed, when maintained on a vitamin A-sufficient diet (containing no less than 22 IU vitamin A/g of diet [95]), RBP-/- mice yield viable embryos that display only mild and transient cardiac embryonic developmental anomalies [13, 96]. The unremarkable phenotype of the RBP-/- embryos reflects the existence of an alternative pathway(s) of vitamin A delivery to the fetus. Indeed, in mice lacking ROH-RBP, high levels of RE incorporated in maternal circulating chylomicrons and/or VLDL particles provide the embryos with sufficient amounts of vitamin A to enable relatively normal embryonic development [14, 96]. However, the inability of the RBP-/- mice to mobilize efficiently the hepatic retinoid reserve makes them a good model to study embryonic vitamin A deficiency due to the limiting amount of retinoids available to the developing embryo when the dams are maintained on a vitamin A-deficient diet. Under normal circumstances (normal maternal vitamin A status and presence of RBP), the ROH-RBP pathway is the primary contributor to fetal development, while the RE pathway is largely responsible for the accumulation of fetal retinoid stores. This is especially true when maternal dietary vitamin A is limiting, as retinol would be released from the maternal liver bound to RBP to be delivered to fetus [15]. In contrast, fetal offspring from RBP-/- dams maintained on a vitamin A-deficient diet display a wide range of embryonic VAD phenotypes, dependent upon the period of maternal dietary vitamin A deprivation. Moreover, early embryonic lethality is observed when RBP-/- dams are maintained on a regimen of severe vitamin A deprivation [15]. In all cases, embryonic RBP rescues the embryos from early lethality [92]. Overall, RBP-/- dams deprived of dietary vitamin A mimic the status of a vitamin A-deficient pregnant woman, thus, representing a unique model system to analyze the metabolic links between maternal nutrition and developmental abnormalities.
2.7 Retinoid homeostasis in the developing tissues
The mechanisms through which the developing tissues maintain retinoid homeostasis despite the changes in the maternal vitamin A status have just recently been investigated. LRAT is a key enzyme in the generation of vitamin A stores [3, 4], and has been proposed to play a crucial role in maintaining retinoid homeostasis by diverting ROH away from its oxidative activation to RA in adult mammalian tissues [97]. This action of LRAT has been proposed to be important especially under conditions of excessive retinoid intake [97]. Using mice lacking both LRAT and RBP (LRAT-/-RBP-/-), our laboratory [22] showed that LRAT plays a crucial role in maintaining a tight regulation of retinoid levels during embryonic development and that this regulation is achieved by striking a balance between RE synthesis and RA degradation via Cyp26A1 activity. When dams were fed the vitamin A-excess diet, embryonic Cyp26A1 mRNA expression was upregulated, not only in the absence of LRAT but also in wild-type mice [22], suggesting that there is a limit to the retinoid storage capability of the embryo, at least at 14.5 days post coitum (dpc). In this model system, we found little evidence to support the notion that RA synthesis is regulated to counter the changes in maternal dietary vitamin A intake, as shown by the relatively constant gene expression levels of Raldh2 in the absence of LRAT or in wild type animals maintained on different regimens of dietary vitamin A intake [22]. We also demonstrated that, similar to the embryonic tissues, an upregulation of RA catabolism is a compensatory mechanism that maintains retinoid homeostasis in the extraembryonic tissues (placenta) in the absence of LRAT for dams fed either the vitamin A-sufficient or -excess diet [22]. In addition, the lack of a Raldh2 expression response to variations in maternal vitamin A intake in wild-type placenta confirmed that, also in extraembryonic tissues, regulation of RA homeostasis does not normally involve modulating the synthesis of retinoic acid but rather its degradation [22]. Only when severe maternal-fetal retinoid deficiency occurs, as observed for LRAT-/-RBP-/- dams maintained on the vitamin A-deficient diet, placental expression levels of Raldh2 were downregulated [22].
Interestingly, we also found that the expression levels of Stra6, the specific receptor for the complex ROH-RBP, were upregulated in response to maternal dietary vitamin A excess in wild-type embryos. Similarly, Stra6 mRNA levels were also elevated in LRAT-/-RBP-/- embryos compared to wild-type. This apparent discrepancy could be explained by the hypothesis, put forward by Isken and colleagues [21], that Stra6 acts as a bidirectional transporter of retinol. In this case, in times of excess retinoic acid, Stra6 expression would increase to drive retinol efflux and reduce the generation of potentially toxic levels of retinoic acid within the cell. Therefore, our data suggest an important role of Stra6 in maintaining retinoid homeostasis in the developing tissues. Furthermore, Stra6 was upregulated in the placenta of LRAT-/-RBP-/- mice maintained on the vitamin A-sufficient or -excess diets. However, in contrast to the embryo, vitamin A supplementation of wild-type dams did not upregulate the placental expression levels of Stra6, indicating that the placenta might have a larger retinoid storage capacity compared to the embryo and that the coordinated action of LRAT and Cyp26A1 may be sufficient to maintain retinoid homeostasis in this organ.
Of note, in this report, it was also shown that the impairment of LRAT activity predisposes embryonic tissues to develop vitamin A deficiency [22].
3. PROVITAMIN A CAROTENOID (β-CAROTENE) METABOLISM
3.1 Absorption and transport
For the majority of the world population, provitamin A carotenoids are the main reliable source of vitamin A [26]. Several provitamin A compounds, including β-carotene, α-carotene, β-cryptoxanthin, and as many as 50 other carotenoids, are found in the human diet mainly in orange, red, yellow, and leafy fruits and vegetables [77, 98]. β-carotene (bC), the main provitamin A carotenoid, is absorbed by the intestine within mixed micelles. The intestinal absorption, solubility, transport and cleavage of bC depend crucially on the presence of bile salts, as well as the size of micelles [99]. As a lipophilic molecule, bC is most readily absorbed in oil [98], and its absorption is influenced by many of the same factors as dietary lipids. bC is more readily absorbed from fruits than from vegetables, and its bioavailability in oil is generally greater than in plants [98]. Also, polymorphisms in the genes encoding apolipoprotein B, hepatic lipase, lipoprotein lipase, and SR-BI have been shown to alter the intestinal uptake of bC and its distribution in body tissues [100]. Cholesterol and fatty acid transport proteins on both the apical side of enterocytes (SR-BI, NPC1L1, and CD36) and the basolateral side (ABCA1), are thought to facilitate the uptake of bC [101-103].
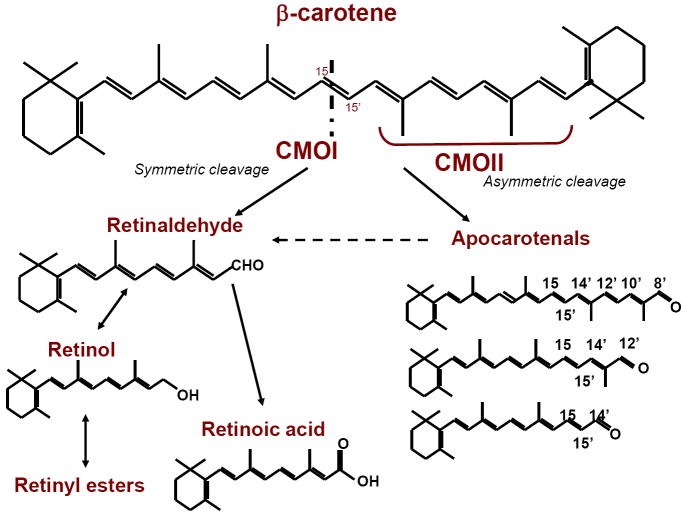
Within the enterocytes, the first step of the conversion of bC to vitamin A is its symmetric cleavage by the enzyme β-carotene 15-15’-monoxygenase (CMOI), yielding two molecules of RAL [77] (Figure 3). A second carotenoid cleavage enzyme, β-carotene 9’,10’-monooxygenase (CMOII), has been cloned and has been shown to cleave bC asymmetrically generating apocarotenals, which in turn can yield one molecule of RAL by chain shortening [104, 105] (Figure 3). Although both enzymes are expressed in the intestine [106], the contribution of the asymmetric cleavage enzyme is thought to be minor compared to the role of CMOI in the generation of vitamin A from its carotenoid precursor [77, 107]. Within the enterocytes, retinol dehydrogenases convert RAL into ROH, which is then converted into RE mainly by the action of LRAT [108]. These esters are packaged into chylomicrons and secreted into the lymph ducts before ultimately reaching the systemic circulation [108]. The fate of the chylomicrons containing retinoid has been described in detail above (sections 1.1 and 1.2).
Figure 3. Cleavage of β-carotene and its conversion into retinoids.
When β-carotene is cleaved symmetrically by the action of the 15,15’-oxygenase enzyme (CMOI) two molecules of retinaldehyde are produced. Retinaldehyde can then be oxidized to form retinoic acid or can be reduced to retinol, which is further converted into retinyl ester, the storage form of vitamin A in tissues. In vivo, 95% of retinoids arising from β-carotene are produced by this pathway. However, β-carotene can also be cleaved asymmetrically by the action of 9’,10’-oxygenase enzyme (CMOII) producing apocarotenals, which can be converted to retinaldehyde by chain shortening. Through this pathway, one molecule of β-carotene yields one molecule of retinaldehyde. Interestingly, Ziouzienkova and colleagues [153] showed that apocarotenals may also function as ligands for PPAR and RXR receptors, inhibiting vitamin A signaling.
In humans, a fraction of the dietary bC (25-45%) is not cleaved in the intestine, but rather enters the circulation intact [77, 109]. The bioavailability of bC depends on a variety of conditions, including species of carotenoid (favoring all-trans isomers), dosage, food matrix in which the carotenoid is incorporated, nutrient status of the host (including dietary intake of fat, fiber, vitamin A and other carotenoids), and host factors such as gender (favoring females), BMI and gut integrity [98]. Mounting evidence suggests that genetic polymorphisms in the enzymes involved in carotenoid cleavage strongly contribute to the inter-individual differences in carotenoid bioconversion [98, 110]. As a result, among several double tracer studies, 27-45% of participants were found to be “poor converters” [98]. This phenotype is likely due to one of four prevalent single-nucleotide polymorphisms in CMOI [77]; for instance, R267S variant T is found in 42% of the Caucasian population, and is known to decrease bC cleavage in the intestine [98].
In addition to genetic factors, the vitamin A status of the individual strongly influences bC conversion, by a recently elucidated feedback mechanism. Initial studies in transgenic mice demonstrated that intestinal CMOI and SR-BI expression were elevated in mice lacking functional ISX, an intestine-specific homeobox transcription factor [111]. In addition, ISX and CMOI expression were shown to depend on vitamin A status, with vitamin A deficiency reducing ISX mRNA levels and increasing CMOI levels [111]. This work suggested that vitamin A production from bC increases when dietary vitamin A is limiting, by an ISX-dependent mechanism. More recent data have further clarified this mechanism [112]: RA, the biologically active form of vitamin A, binds to retinoic acid receptors (RARs) in the promoter of ISX to increase its expression. Then, ISX down-regulates the expression of SR-BI and CMOI. The dependence of this regulatory pathway on RA production was confirmed by genetic and dietary manipulations. In vitamin A-deficient CMOI-/- mice, in which RA production was presumably negligible, ISX expression was diminished, whereas SR-BI expression and tissue bC levels increased. However, when CMOI-/- mice were supplemented with dietary retinoids, ISX expression increased, while SR-BI expression and bC accumulation were reduced. Overall, these data demonstrate that when sufficient dietary vitamin A or provitamin A is converted to RA, the uptake and cleavage of bC (by SR-BI and CMOI, respectively) are reduced, preventing accumulation of toxic levels of vitamin A [112].
The most recent data suggest that the bioefficacy of bC conversion to vitamin A in humans is 12:1 [98]. However, it is important to note that the efficiency of intestinal bC cleavage varies greatly across species. Ferrets, gerbils, pre-ruminant calves, and some non-human primates, are more similar to humans in their ability to absorb and metabolize bC, compared with other model animals such as mice and rats, which cleave almost all bC in the intestine rather than absorbing some of it in an intact form [113]. Thus, careful discretion is needed in choosing the appropriate model system for studying bC metabolism. Several transgenic mouse models have facilitated bC research, and are discussed later in this review.
3.2 Tissue uptake and metabolism
bC circulating in lipoprotein particles in the fed state may be taken up by the liver or target tissues, such as lung, adipose tissue, muscle, corpus luteum, and adrenal gland [114-116]. bC can also be re-secreted from the liver within VLDL and thus found in association with LDL or even HDL [99]. Therefore, tissue uptake of bC may occur by a variety of mechanisms, depending on the lipoprotein particle with which it is associated. As in the intestine, it has been demonstrated that SR-BI mediates the uptake of bC in RPE cell lines [103] and in the trunks, heads, and eyes of D. melanogaster [117]. The specific roles of other proteins mediating the uptake of bC from lipoproteins in other tissues have not been investigated, but probably include LPL, SR-BI, and LDLr, all of which facilitate lipid absorption in extrahepatic tissues [118-120].
Following cellular uptake, bC may be stored in its intact form [121], or cleaved by CMOI and/or CMOII. Both enzymes are expressed in liver and a variety of extrahepatic tissues [106, 122], suggesting that dietary bC may be delivered to these tissues and be cleaved to provide local vitamin A to adult tissues [76, 116, 123].
Carotenoid cleavage is regulated not only at the level of the intestine, but also throughout the body. CMOI is a PPAR target gene; indeed, a PPARα/γ agonist increases hepatic CMOI expression [124]. Accordingly, recent in vitro data demonstrated that CMOI expression is induced during adipogenesis, and that bC supplementation induced CMOI expression in inguinal white adipose tissue in a mouse model of vitamin A deficiency [125]. In rats, lycopene feeding down-regulated CMOI expression in adrenal gland and kidney, which the investigators attributed to lycopene metabolites [126]. A recent study revealed that intestinal, hepatic, and testicular CMOI expression all increased in diabetic rats compared to controls [127]. The regulation of CMOII expression has not been thoroughly investigated; however, a recent study reported an increase in CMOII mRNA following zeaxanthin feeding [107]. Interestingly, hepatic CMO2 expression was shown to increase in CMOI-/- mice [128]. Also, both hepatic CMOI and CMOII expression increased following chronic alcohol consumption in rats [129]. Overall, the tissue uptake and metabolism of bC depend on a variety of genetic, dietary, and health conditions, which warrant further study.
3.3 Lessons from CMOI knockout mice
Significant insight into the function of CMOI has been gained from loss-of-function studies in mice. In 2007, Hessel and colleagues [115] generated CMOI-/- mice, and found that they accumulated large amounts of intact bC in serum, liver, adipose and intestine when the diet contained 1 mg/g bC but no preformed vitamin A. Correspondingly, vitamin A levels were reduced in several tissues known to express CMOI (lung, testis, uterus), confirming that this is the primary enzyme that generates retinoid from bC in adult mammalian tissues. Regardless of the diet, CMOI-/- mice accumulated lipids in serum and liver, and this phenomenon intensified on a high-fat diet. PPARγ target gene expression was elevated in CMOI-/- visceral adipose, suggesting that bC conversion to RAL and RA influences lipid metabolism through nuclear receptors bound to PPARγ [115]. More recently, van Helden et al. [130] discovered that markers of lung inflammation were elevated in female CMOI-/- mice, which was ameliorated when supplemental bC was provided. These results suggested that bC cleavage by CMOII could produce RA, which would modulate expression of inflammatory genes. Interestingly, ROH and RE formation were increased to similar degrees in the lungs of both wild-type and CMOI-/- females supplemented with bC, questioning the role of CMOI as the primary bC cleavage enzyme in all tissues [130]. Overall, CMOI has been clearly indicated as the main bC cleavage enzyme in many adult tissues, with key roles in generating local vitamin A and modulating lipid metabolism. Although CMOI is important for preventing lung inflammation, it appears that other mechanisms exist to ensure vitamin A homeostasis in lung and perhaps other tissues when preformed vitamin A is limiting in the diet.
3.4 Lessons from CMOII knockout mice
Although some in vitro studies have investigated the function of CMOII, until very recently the physiological role of CMOII in mammals was elusive. In 2010, Amengual and colleagues [107] showed that CMOII cleaves a broad range of carotenoids, including not only bC, but also zeaxanthin, and lutein. The authors also generated CMOII-/- mice, which appeared viable and fertile. When fed diets containing 50 mg/kg of zeaxanthin or lutein, CMOII-/- mice accumulated these carotenoids and their metabolites in serum, heart, adipose, and liver. The lutein-fed CMOII-/- animals displayed hepatic mitochondrial abnormalities, including yellow coloring, up-regulation of genes indicating mitochondrial stress, and diminished cellular respiration. Likewise, oxidative stress pathways were stimulated in the livers and hearts of the zeaxanthin-fed CMOII-/- mice. Overall, this study unveiled a novel physiological role of CMOII in cleaving carotenoids that would otherwise damage mitochondria [107]. In spite of this progress, the function of CMOII in mammalian vitamin A metabolism has yet to be shown in vivo.
4. PROVITAMIN A CAROTENOID β-CAROTENE AND DEVELOPMENT
Being the most abundant vitamin A precursor, bC is considered important during embryonic development. However, much of the current knowledge in this field pertains to the role of maternal circulating preformed retinoids. The function of provitamin A carotenoids has remained elusive.
4.1 Human studies
bC from the maternal circulation is able to reach the fetus, potentially via placental uptake of bC-containing chylomicrons, VLDL and LDL [70]. Several human studies have found significant correlations between bC levels in the maternal serum and umbilical cord, and reported that maternal serum bC levels were consistently higher than cord levels [93, 131-133]. Dimenstein and colleagues postulated that the dramatic difference in maternal and fetal circulating bC levels might reflect either enhanced metabolism of bC by the embryo, or else reduced placental uptake and transport of bC compared to other nutrients [93]. Interestingly, these authors observed that placental bC levels were nearly twice those of the maternal liver [93]. In a study of preterm rupture of fetal membranes, lower levels of bC were detected in amniotic fluid from women who had preterm ruptures [134]. In addition, fetal bC metabolism appears to depend on the maternal vitamin A status: Dimenstein and colleagues reported a correlation between maternal serum bC and both cord serum and placental retinol levels, but only in women with subadequate serum ROH levels. On the other hand, maternal serum and placental bC levels were correlated only in women with adequate serum ROH [93].
Although trials of vitamin A supplementation during pregnancy are far more prevalent, several reports on bC supplementation have been published. Weekly oral supplementation with bC was shown to reduce maternal mortality during pregnancy by 49% in a large trial in Nepal [135], although it did not significantly reduce maternal night blindness [136], and had no effect on fetal or early infant mortality [137]. Another study investigated the role of maternal antioxidant status on birth weight, and found a significant correlation between birth weight and the combined intake of bC and vitamin E [138]. In Tanzania, combined bC and vitamin A supplementation had no effect on fetal deaths, birth weight or size, and maternal T-cell count among HIV-positive pregnant women [139]. A small study of bC supplementation during the first month postpartum found no change in milk bC in the treatment group, which the authors postulated was due to saturation of the milk with bC [140]. Simultaneous daily supplementation with zinc and bC during pregnancy was shown to improve plasma ROH levels in newborns and breast milk in Indonesia [141]. Considering the variety of outcomes of these bC supplementation trials depending on the population, more studies must be done to understand the role of maternal vitamin A status on the efficacy of bC supplements during pregnancy, to improve the design of supplementation programs.
4.2 Animal Studies
For ethical reasons, humans are not the most appropriate model to address questions related to the metabolism of bC in the developing tissues. Nevertheless, only a few studies have been conducted on this topic by using animal models, to date. In vitamin A deficient gilts, weekly injection of bC and vitamin A prior to and during pregnancy increased maternal plasma vitamin A and bC, reduced embryonic mortality, and increased litter size, whereas dietary supplementation of these nutrients had no effect [142]. In cows, uterine bC levels were shown to decline throughout the estrous cycle, while ROH levels increased. Interestingly, bC levels were unequally distributed between the horns of the bovine uterus, with ipsilateral levels exceeding contralateral levels [143]. In Holstein cows, bC supplementation had no effect on reproductive performance at a dose of 400 mg/day in one study [144], although another study found that milk production and pregnancy rate increased with the same dose of bC for <90 days [145]. bC may be more effective in increasing pregnancy rates in younger cows, according to one report [146].
4.3 Expression of CMOI and CMOII during development
Both CMOI and CMOII enzymes have been detected in the developing tissues, in numerous vertebrate species. In the vertebrate model organism, zebrafish, Lampert and colleagues identified CMOI transcripts during early segmentation in the cranial/neural crest and eye prior to embryonic day 2. In contrast, CMOII was only detected in the embryonic heart after day 2 [147]. CMOI expression was detected in chick embryos as early as 1.5 days of gestation. bC was detected in the egg yolk of chicks at 5 days of gestation, when CMOI transcripts were still detectable[148]. The human uterine endometrium and amniotic membranes express both CMOI and CMOII [70, 106, 122], although neither enzyme was detected in the human term placenta [70].
In mice, CMOI expression was found in different developing tissues, depending on gestational stage and analytical technique. Specifically, Paik and colleagues performed in situ hybridization during gestational days 7.5- 8.5, and detected CMOI only in the maternal tissues surrounding the fetus (i.e. the uterus) [149]. In contrast, Redmond and colleagues successfully detected embryonic CMOI during gestational days 7-15 by Northern blotting [150]. Very recently, our laboratory performed an extensive analysis of CMOI and CMOII expression levels in mouse embryos, placentae and yolk sacs from 6.5- to 14.5 dpc [95]. CMOI was shown to have a very distinct pattern of mRNA expression levels during gestation that was mirrored by that of the protein. Its expression was detected in the embryonic tissues from 8.5 dpc. In contrast, CMOII mRNA levels were steadier and less abundant compared to CMOI [95].
Overall, these data argue in favor of the hypothesis that maternally circulating bC can be delivered to the developing tissues and there cleaved to generate RA in situ. This local pathway of vitamin A synthesis could be important, for example, during times of insufficient maternal dietary vitamin A intake.
4.4 Transfer of β-carotene from mother to fetus and its metabolism in the developing tissues
Our laboratory has recently provided novel insights into the ability of the developing embryo to metabolize and use bC by investigating the function of CMOI specifically in mouse embryonic tissues [95]. Firstly, loss of CMOI function studies in an established model of mouse embryonic vitamin A deficiency (VAD), such as the RBP-/- animals, revealed that lack of CMOI in the developing tissues further exacerbates the severity of VAD and thus the embryonic malformations of the RBP-/- mice. Indeed, in addition to the RBP-/- like phenotype (i.e. eye malformations and peripheral edema), the embryos of the double-knockout mice (CMOI-/-RBP-/-) showed a certain percentage of cleft face and palate or exencephaly. The severe developmental defects of the double-knockout mice (CMOI-/-RBP-/-) on a vitamin A-deficient diet during pregnancy were accompanied by reduced levels of ROH, RE and RA and were due to the lack of CMOI in the developing tissues, rather than to a more severe maternal vitamin A-deficient status. This study also showed that CMOI deficiency manifests itself in an autosomal dominant fashion, but with different degrees of penetrance depending upon the gene copy number. The double knockout model used in this study was instrumental to unequivocally demonstrate in vivo the ability of intact bC circulating in the maternal bloodstream to cross the placenta toward the fetus, as well as the ability of embryonic CMOI to generate locally, i.e. in the developing tissues, vitamin A from bC. Indeed, CMOI+/-RBP-/- embryos from double knockout dams deprived of vitamin A throughout gestation and supplemented with bC from 6.5 to 9.5 dpc, showed a reduced frequency of developmental defects compared to un-supplemented animals. Accordingly, the maternal supplementation also increased embryonic levels of ROH, RE and RA [93].
These studies also provided evidence for a potential novel role of CMOI, independent from its major known function of bC cleavage [95]. Indeed, unless supplemented, bC was not present in the diet of our experimental animals. Nevertheless, CMOI deficiency not only increased the severity of VAD-associated embryonic malformations, but also reduced LRAT mRNA expression and activity thus decreasing RE levels in the developing tissues. Therefore, it seems that CMOI may impact retinoid metabolism, specifically influencing RE formation, at least in the developing tissues. At the moment, the molecular mechanisms responsible for this effect have not been elucidated and are under investigation.
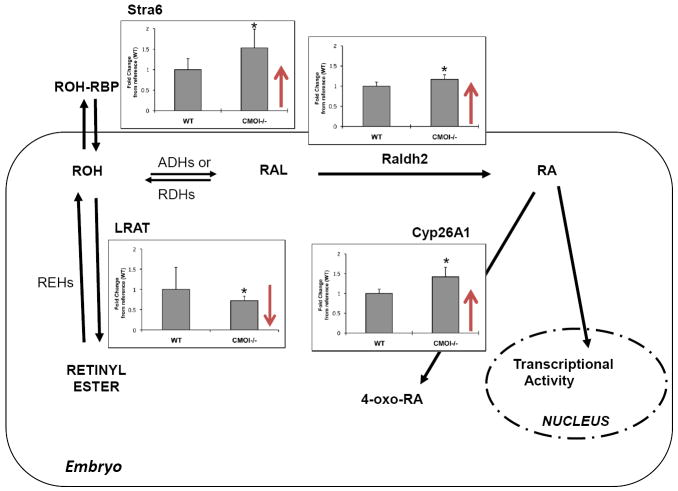
Interestingly, despite the reduced levels of retinoids, embryos lacking CMOI from dams on a vitamin A-sufficient diet did not show any gross morphological defects (and were also viable; [95]). In agreement, embryonic RA levels were similar between CMOI-/- and wild-type animals [95]. To investigate the mechanisms preventing the appearance of signs of retinoid toxicity in embryos lacking CMOI (and hence with reduced levels of RE) on a vitamin A-sufficient diet, we measured by real-time RT-PCR analysis the expression levels of other key enzymes and receptors that we have previously shown to be important in maintaining retinoid homeostasis in the developing tissues, namely Raldh2, Cyp26A1 and Stra6 [22]. As shown in Figure 4, when LRAT expression is severely impaired, mRNA levels of all the above-mentioned genes are upregulated. These data confirm that RE synthesis via LRAT, RA degradation via Cyp26A1 activity, and possibly elimination of excess of ROH via the RBP-ROH specific receptor Stra6 contribute to maintaining RA homeostasis in the developing tissues. In contrast to our previous published data [22], in this model, the pathway of synthesis of RA mediated by Raldh2 also seems to contribute to the homeostatic mechanisms that maintain a tight regulation of retinoid levels during embryonic development. Since all these genes are transcriptionally regulated by RA [22], it has been proposed that increased mRNA levels of LRAT and Cyp26A1 correspond to elevated levels of RA in tissues [4, 97, 151]. In contrast, our data (Figure 3 and [95]) suggest that changes in the expression levels of the above-mentioned genes reflect a flux of retinoids aimed at maintaining homeostatic levels of tissue RA. The effectiveness of these regulatory mechanisms, and thus the tissue levels of RA, may depend upon numerous factors, including, for example, the whole-body vitamin A status and the dietary intake of vitamin A.
Figure 4. Mechanisms of retinoic acid homeostasis in embryo lacking CMOI.
Real time RT-PCR and statistical analyses for LRAT, Cyp26A1, Raldh2 and Stra6 were performed as described [22, 95] by using CMOI-/- and wild-type E14.5 (n=7 embryos/genotype) from dams maintained on a vitamin A-sufficient diet (vitamin A=25-28 IU/g of diet). * indicates p< 0.05. In CMOI-/- embryos, showing reduced levels of LRAT mRNA, Raldh2, Cyp26A1 and Stra6 mRNA levels are significantly elevated. We interpret these data as an indication that, in spite of the reduced levels of retinyl ester, increased synthesis and catabolism of retinoic acid as well as increased efflux of intracellular retinol, prevent retinoid toxicity and maintain normal levels of RA in CMOI-/- embryos.
Overall, this work has provided novel insights into the role of CMOI and bC metabolism during embryogenesis and has raised many intriguing questions. For instance, what is the mechanism(s) through which CMOI regulates embryonic development and ROH esterification independent of bC cleavage? What mechanisms mediate and regulate bC uptake in placenta and its transfer to the developing tissues? Is CMOI expression regulated in the developing tissues as it is in adult tissues? and, if so, how? These are all key topics that require further studies and that will certainly expand our knowledge about the role of a metabolic pathway that is crucial during embryonic development.
Highlights.
Maternal serum retinol, retinyl ester and β-carotene supply vitamin A to the embryo
Retinoid homeostasis is maintained in the developing tissues
Intact β-carotene can be used as a local source of retinoic acid in the developing tissues
Acknowledgments
The work form the author’s laboratory presented in this review was supported by grants R01HD057493 and R01HD057493-02S1 from the U.S. national Institute of Health (NIH) and by NRI award #2006-35200-16580 from USDA-CSREES, Bioactive Food Component for Optimal Health (31.0).
ABBREVIATIONS
- ROH
retinol
- RE
retinyl ester
- RA
retinoic acid
- RAL
retinaldehyde
- bC
β-carotene
- dpc
days post coitum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 2.Sporn MB, Roberts AB, Goodman DS. The Retinoids: Biology, Chemistry, and Medicine. 2. Raven Press; New York: 1994. [Google Scholar]
- 3.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 5.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel S, Gamble MV, Blaner WS. Biosynthesis, Absorption, Metabolism and Transport of Retinoids. In: Nau H, Blaner WS, editors. Handbook of experimental pharmacology Retinoids. The biochemical and molecular basis of vitamin A and retinoid action. Springer Verlag Publishing; Heidelberg, Germany: 1999. pp. 31–95. [Google Scholar]
- 7.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase - and CD36-mediated pathways. J Lipid Res. 2009;50:S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 9.Goodman DS, Huang HS, Shiratori T. Tissue distribution of newly absorbed vitamin A in the rat. J Lipid Res. 1965;6:390–396. [PubMed] [Google Scholar]
- 10.Quadro L, Hamberger L, Colantuoni V, Gottesman M, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003;24:421–430. doi: 10.1016/s0098-2997(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 11.Soprano DR, Blaner WS. Plasma retinol-binding protein. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids, Biology, Chemistry and Medicine. Raven Press; New York, NY: 1994. pp. 257–282. [Google Scholar]
- 12.Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268:1039–1041. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- 13.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J. 1999;17:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am J Physiol Endocrinol Metab. 2004;286:E844–851. doi: 10.1152/ajpendo.00556.2003. [DOI] [PubMed] [Google Scholar]
- 15.Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- 16.Biesalski HK, Frank J, Beck SC, Heinrich F, Illek B, Reifen R, Gollnick H, Seeliger MW, Wissinger B, E Z. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol-binding protein. Am J Clin Nutr. 1999;69:931–936. doi: 10.1093/ajcn/69.5.931. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contibutes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 19.Blaner WS. STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell metab. 2007;5:164–166. doi: 10.1016/j.cmet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci U S A. 2011;108:4340–4345. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin: Retinol acyltransferase (LRAT) is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem. 2008;283:5611–5621. doi: 10.1074/jbc.M708885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noy N, Xu ZJ. Thermodynamic parameters of the binding of retinol to binding proteins and to membranes. Biochemistry. 1990;29:3888–3892. doi: 10.1021/bi00468a014. [DOI] [PubMed] [Google Scholar]
- 24.Noy N, Xu ZJ. Kinetic parameters of the interactions of retinol with lipid bilayers. Biochemistry. 1990;29:3883–3888. doi: 10.1021/bi00468a013. [DOI] [PubMed] [Google Scholar]
- 25.Noy N, Xu ZJ. Interactions of retinol with binding proteins: implications for the mechanism of uptake by cells. Biochemistry. 1990;29:3878–3883. doi: 10.1021/bi00468a012. [DOI] [PubMed] [Google Scholar]
- 26.von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010;35:400–410. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld MG, Glass CK. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 29.Leblanc BP, Stunnenberg HG. 9-cis retinoic acid signaling: changing partners causes some excitement. Genes Dev. 1995;9:1811–1816. doi: 10.1101/gad.9.15.1811. [DOI] [PubMed] [Google Scholar]
- 30.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 31.Pfahl M, Chytil F. Regulation of metabolism by retinoic acid and its nuclear receptors. Annu Rev Nutr. 1996;16:257–283. doi: 10.1146/annurev.nu.16.070196.001353. [DOI] [PubMed] [Google Scholar]
- 32.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 33.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 34.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pares X, Farres J, Kedishvili N, Duester G. Medium- and short-chain dehydrogenase/reductase gene and protein families: Medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol Life Sci. 2008;65:3936–3949. doi: 10.1007/s00018-008-8591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348:481–495. [PMC free article] [PubMed] [Google Scholar]
- 39.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 40.Maden M. Retinoids in neural development. In: Nau H, Blaner WS, editors. Handbook of experimental pharmacology Retinoids The biochemical and molecular basis of vitamin A and retinoid action. Spring Verlag; Heidelberg: 1999. pp. 399–442. [Google Scholar]
- 41.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- 42.White JC, Highland M, Kaiser M, Clagett-Dame M. Vitamin A deficiency results in the dose-dependent acquisition of anterior character and shortening of the caudal hindbrain of the rat embryo. Dev Biol. 2000;220:263–284. doi: 10.1006/dbio.2000.9635. [DOI] [PubMed] [Google Scholar]
- 43.Gould A, Itasaki N, Krumlauf R. Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron. 1998;21:39–51. doi: 10.1016/s0896-6273(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 44.Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- 45.Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- 46.Wagner E, McCaffery P, Drager U. Retinoic acid in the formation of the dorsoventral retina and its central projection. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- 47.Dersch H, Zile MH. Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev Biol. 1993;160:424–433. doi: 10.1006/dbio.1993.1318. [DOI] [PubMed] [Google Scholar]
- 48.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- 49.Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet. 2001;27:74–78. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- 50.Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- 51.Power SC, Lancman J, Smith SM. Retinoic acid is essential for shh/hoxd signaling during rat limb outgrowth but not for limb initiation. Dev Dyn. 1999;216:469–480. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<469::AID-DVDY15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Stratford T, Logan C, Zile MH, Maden M. Abnormal anteroposterior and dorsoventral patterning of the limb bud in the absence of retinoids. Mech Dev. 1999;81:115–125. doi: 10.1016/s0925-4773(98)00231-7. [DOI] [PubMed] [Google Scholar]
- 53.Thompson JN, Howell JM, Pitt GAJ. Vitamin A and reproduction in rats. Proc R Soc Lond B Biol Sci. 1964;159:51–35. doi: 10.1098/rspb.1964.0017. [DOI] [PubMed] [Google Scholar]
- 54.Warkany J, Schraffenberger E. Congenital malformations induced in rats by maternal vitamin A deficiency. Defects of the eye. Arch Ophthalmol. 1946;35:150–169. doi: 10.1001/archopht.1946.00890200155008. [DOI] [PubMed] [Google Scholar]
- 55.Wilson JG, Warkany J. Epithelial keratinisation as evidence of fetal vitamin A deficiency. Proc Soc Exp Biol Med. 1947;64:419–422. doi: 10.3181/00379727-64-15814. [DOI] [PubMed] [Google Scholar]
- 56.Wilson JG, Warkany J. Malformation in the genitourinary tract induced by maternal vitamin A deficiency in the rat. Am J Anat. 1948;83:357–408. doi: 10.1002/aja.1000830303. [DOI] [PubMed] [Google Scholar]
- 57.Wilson JG, Warkany J. Aortic arch and cardiac anomalies in the offspring of vitamin A deficient rats. Am J Anat. 1949;85:113–155. doi: 10.1002/aja.1000850106. [DOI] [PubMed] [Google Scholar]
- 58.Hale F. Relation of maternal vitamin A deficiency to microphtalmia in pigs. Texas State J Med. 1937;33:228–232. [Google Scholar]
- 59.Morriss-Kay GM, Ward SJ. Retinoids and mammalian development. Int Rev Cytol. 1999;188:73–131. doi: 10.1016/s0074-7696(08)61566-1. [DOI] [PubMed] [Google Scholar]
- 60.Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 61.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P. Function of the retinoic acid receptors (RARs) during development. (II) Multiple abnormalities at various stage of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- 62.Kastner P, Mark M, Ghyselinck NB, Krezel W, Dupe V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:316–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 63.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post- implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 64.Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the antero-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–225. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottesman ME, Quadro L, Blaner WS. Studies of vitamin A metabolism in mouse model systems. Bioessays. 2001;23:409–419. doi: 10.1002/bies.1059. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi YI, Smith JE, Winick M, Goodman DS. Vitamin A deficiency and fetal growth and development in rat. Am J Physiol. 1975;233:E263–E271. doi: 10.1093/jn/105.10.1299. [DOI] [PubMed] [Google Scholar]
- 67.Mason KE. Foetal death, prolonged gestation and difficult parturition in the rat as result of vitamin A. Am J Anat. 1935;57:303–311. [Google Scholar]
- 68.Cohlan SQ. Excessive intake of vitamin A as a cause of congenital anomalies in rat. Science. 1953;117:535–536. doi: 10.1126/science.117.3046.535. [DOI] [PubMed] [Google Scholar]
- 69.Wallingford JC, Underwood BA. Vitamin A deficiency in pregnancy, lactation and the nursing child. In: Bauernfeind CJ, editor. Vitamin A deficiency and its control. Academic Press; New York: 1986. pp. 47–64. [Google Scholar]
- 70.Marceau G, Gallot D, Lemery D, Sapin V. Metabolism of retinol during mammalian placental and embryonic development. Vitam Horm. 2007;75:97–115. doi: 10.1016/S0083-6729(06)75004-X. [DOI] [PubMed] [Google Scholar]
- 71.Harrison EH, Hussain MM. Mechanisms involved in the intestinal digestion and absorption of dietary vitamin A. J Nutr. 2001;131:1405–1408. doi: 10.1093/jn/131.5.1405. [DOI] [PubMed] [Google Scholar]
- 72.Blaner WS, Olson JA. Retinol and retinoic acid metabolism. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids, Biology, Chemistry and Medicine. Raven Press; New York, NY: 1994. pp. 229–256. [Google Scholar]
- 73.Barua AB, Batres RO, Olson JA. Synthesis and metabolism of all-trans-[11-3H]retinyl beta-glucuronide in rats in vivo. Biochem J. 1988;252:415–420. doi: 10.1042/bj2520415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barua AB, Olson JA. Chemical synthesis of all-trans-[11-3H]retinoyl beta-glucuronide and its metabolism in rats in vivo. Biochem J. 1989;263:403–409. doi: 10.1042/bj2630403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olson JA. Provitamin A functions of carotenoids: the conversion of β-carotene into vitamin A. J Nutr. 1989;119:105–108. doi: 10.1093/jn/119.1.105. [DOI] [PubMed] [Google Scholar]
- 76.Napoli JL, Race KR. Biogenesis of retinoic acid from β-carotene. J Biol Chem. 1988;263:17372–17377. [PubMed] [Google Scholar]
- 77.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr. 2010;30:35–56. doi: 10.1146/annurev-nutr-080508-141027. [DOI] [PubMed] [Google Scholar]
- 78.Ross AC, Gardner EM. The function of vitamin A in cellular growth and differentiation, and its role during pregnancy and lactation. Adv Exp Med Biol. 1994;352:187–200. doi: 10.1007/978-1-4899-2575-6_15. [DOI] [PubMed] [Google Scholar]
- 79.Satre MA, Ugen KE, Kochhar DM. Developmental changes in endogenous retinoids during pregnancy and embryogenesis in mouse. Biol Reprod. 1992;46:802–810. doi: 10.1095/biolreprod46.5.802. [DOI] [PubMed] [Google Scholar]
- 80.Shah RS, Rajalkshmi R, Bhat RV, Hazra MN, Patel BC, Swamy NB, Patel PV. Liver stores of vitamin A in human fetuses in relation to gestational age, fetal size and maternal nutritional status. Br J Nutr. 1987;58:181–189. doi: 10.1079/bjn19870085. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi YI, Smith JE, Goodman DS. Vitamin A and retinol-binding protein metabolism durign fetal development in the rat. Am J Physiol. 1977;233:E263–E272. doi: 10.1152/ajpendo.1977.233.4.E263. [DOI] [PubMed] [Google Scholar]
- 82.Lorente CA, Miller SA. Fetal and maternal vitamin A levels in tissues of hypervitaminotic A rats and rabbits. J Nutr. 1977;107:1816–1821. doi: 10.1093/jn/107.10.1816. [DOI] [PubMed] [Google Scholar]
- 83.Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein and transthyretin mRNA levels in visceral yolk sac and liver during fetal development in the rat. Proc Natl Acad Sci U S A. 1986;83:7330–7334. doi: 10.1073/pnas.83.19.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sapin V, Ward SJ, Bronner S, Dolle P, Chambon P. Differential expression of transcripts encoding retinoid binding proteins and retinoic acid receptors during placentation of the mouse. Dev Dyn. 1997;208:199–210. doi: 10.1002/(SICI)1097-0177(199702)208:2<199::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 85.Ward SJ, Chambon P, Ong DE, Bavik C. A retinol-binding protein receptor-mediated mechanism for uptake of vitamin A to postimplantation rat embryos. Biol Reprod. 1997;57:751–755. doi: 10.1095/biolreprod57.4.751. [DOI] [PubMed] [Google Scholar]
- 86.Bavik C, Ward SJ, Chambon P. Developmental abnormalities in cultured mouse embryos deprived of retinoic by inhibition of yolk-sac retinol binding protein synthesis. Proc Natl Acad Sci U S A. 1996;593:3110–3114. doi: 10.1073/pnas.93.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Creek Kraft J, Nau H, Lammer E, Olney A. Human embryo retinol concentrations after maternal intake of isotretinoin. N Engl J Med. 1989;321:262. [PubMed] [Google Scholar]
- 88.Ward SJ, Morriss-Kay G. Distribution of all-trans, 13-cis, 9-cis-retinoic acid to whole rat embryos and maternal serum following oral administration of a teratogenic dose of all-trans retinoic acid. Pharmacol Toxicol. 1995;76:196–201. doi: 10.1111/j.1600-0773.1995.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 89.Tzimas G, Collins MD, Burgin H, Hummler H, Nau H. Embryotoxic doses of vitamin A to rabbits result in low plasma but high embryonic concentrations of all-trans-retinoic acid: risk of vitamin A exposure in humans. J Nutr. 1996;126:2159–2171. doi: 10.1093/jn/126.9.2159. [DOI] [PubMed] [Google Scholar]
- 90.Geelen JAG. The localization of vitamin A in the pregnant rat by means of fluorescence microscopy. Teratology. 1972;6:19–26. doi: 10.1002/tera.1420060104. [DOI] [PubMed] [Google Scholar]
- 91.Nau H, Elzamar MM, Rhul, Ruhl R, Thiel R, Sass JO. All-trans-retinoyl-glucuronide is a potent teratogen in the mouse because of extensive metabolism to all-trans-retinoic acid. Teratology. 1996;54:150–156. doi: 10.1002/(SICI)1096-9926(199609)54:3<150::AID-TERA5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 92.Datta K, Kulkarni AP. Co-oxidation of all-trans-retinol acetate by human term placental lipoxygenase and soybean lipoxygenase. Reprod Toxicol. 1996;10:105–112. doi: 10.1016/0890-6238(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 93.Dimenstein R, Trugo NM, Donangelo CM, Trugo LC, Anastacio AS. Effect of subadequate maternal vitamin A status on placental transfer of retinol and β-carotene to the human fetus. Biol Neonate. 1996;69:230–234. doi: 10.1159/000244315. [DOI] [PubMed] [Google Scholar]
- 94.Sapin V, Chaib S, Blanchon L, Alexandre-Gouabau MC, Lemery D, Charbonne F, Gallot D, Jacquetin B, Dastugue B, Azais-Braesco V. Esterification of vitamin A by the human placenta involves villous mesenchymal fibroblasts. Pediatr Res. 2000;48:565–572. doi: 10.1203/00006450-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 95.Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. {beta}-Carotene and its cleavage enzyme {beta}-carotene-15,15’-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. 2011 doi: 10.1096/fj.10-175448. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wendler CC, Schmoldt A, Flentke GR, Case LC, Quadro L, Blaner WS, Lough J, Smith SM. Increased fibronectin deposition in embryonic hearts of retinol-binding protein-null mice. Circ Res. 2003;92:920–928. doi: 10.1161/01.RES.0000069030.30886.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic acid oxidation. J Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 98.Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. Beta-carotene is an important vitamin A source for humans. J Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Erdman JW, Jr, Bierer TL, Gugger ET. Absorption and transport of carotenoids. Ann N Y Acad Sci. 1993;691:76–85. doi: 10.1111/j.1749-6632.1993.tb26159.x. [DOI] [PubMed] [Google Scholar]
- 100.Lietz G, Lange J, Rimbach G. Molecular and dietary regulation of beta,beta-carotene 15,15’-monooxygenase 1 (BCMO1) Arch Biochem Biophys. 2010;502:8–16. doi: 10.1016/j.abb.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 101.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–4525. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- 102.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- 103.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–1724. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 105.Wang XD, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI. Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids. J Biol Chem. 1996;271:26490–26498. [PubMed] [Google Scholar]
- 106.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9’,10’-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 107.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2010 doi: 10.1096/fj.10-173906. Epub ahaed of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vogel S, Piantedosi R, O’Byrne SM, Kako Y, Quadro L, Gottesman ME, Goldberg IJ, Blaner WS. Retinol-binding protein-deficient mice: biochemical basis for impaired vision. Biochemistry. 2002;41:15360–15368. doi: 10.1021/bi0268551. [DOI] [PubMed] [Google Scholar]
- 109.Hickenbottom SJ, Follett JR, Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability in conversion of beta-carotene to vitamin A in men as measured by using a double-tracer study design. Am J Clin Nutr. 2002;75:900–907. doi: 10.1093/ajcn/75.5.900. [DOI] [PubMed] [Google Scholar]
- 110.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15’-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 111.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15’-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–4911. doi: 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- 112.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee CM, Boileau AC, Boileau TW, Williams AW, Swanson KS, Heintz KA, Erdman JW., Jr Review of animal models in carotenoid research. J Nutr. 1999;129:2271–2277. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 114.Fierce Y, de Morais Vieira M, Piantedosi R, Wyss A, Blaner WS, Paik J. In vitro and in vivo characterization of retinoid synthesis from beta-carotene. Arch Biochem Biophys. 2008;472:126–138. doi: 10.1016/j.abb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 116.von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta. 2005;1740:122–131. doi: 10.1016/j.bbadis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 117.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron etinyl esters. J Lipid Res. 1999;40:565–574. [PubMed] [Google Scholar]
- 119.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Inves. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lakshman MR, Asher KA, Attlesey MG, Satchithanandam S, Mychkovsky I, Coutlakis PJ. Absorption, storage, and distribution of beta-carotene in normal and beta-carotene-fed rats: roles of parenchymal and stellate cells. J Lipid Res. 1989;30:1545–1550. [PubMed] [Google Scholar]
- 122.Lindqvist A, Andersson S. Cell type-specific expression of beta-carotene 15,15’-mono-oxygenase in human tissues. J Histochem Cytochem. 2004;52:491–499. doi: 10.1177/002215540405200407. [DOI] [PubMed] [Google Scholar]
- 123.Wang XD, Krinsky NI, Tang GW, Russell RM. Retinoic acid can be produced from excentric cleavage of beta-carotene in human intestinal mucosa. Arch Biochem Biophys. 1992;293:298–304. doi: 10.1016/0003-9861(92)90399-h. [DOI] [PubMed] [Google Scholar]
- 124.Boulanger A, McLemore P, Copeland NG, Gilbert DJ, Jenkins NA, Yu SS, Gentleman S, Redmond TM. Identification of beta-carotene 15,15’-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J. 2003;17:1304–1306. doi: 10.1096/fj.02-0690fje. [DOI] [PubMed] [Google Scholar]
- 125.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, Palczewski K, von Lintig J. Beta,beta-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a beta,beta-carotene oxygenase 1-dependent manner. J Biol Chem. 2010;285:27891–27899. doi: 10.1074/jbc.M110.132571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaripheh S, Nara TY, Nakamura MT, Erdman JW., Jr Dietary lycopene downregulates carotenoid 15,15’-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136:932–938. doi: 10.1093/jn/136.4.932. [DOI] [PubMed] [Google Scholar]
- 127.Takitani K, Zhu CL, Inoue A, Tamai H. Molecular cloning of the rat beta-carotene 15,15’-monooxygenase gene and its regulation by retinoic acid. Eur J Nutr. 2006;45:320–326. doi: 10.1007/s00394-006-0601-3. [DOI] [PubMed] [Google Scholar]
- 128.Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, Blaner WS. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys. 2010;504:3–10. doi: 10.1016/j.abb.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luvizotto RA, Nascimento AF, Veeramachaneni S, Liu C, Wang XD. Chronic alcohol intake upregulates hepatic expression of carotenoid cleavage enzymes and PPAR in rats. J Nutr. 2010;140:1808–1814. doi: 10.3945/jn.110.123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Helden YG, Heil SG, van Schooten FJ, Kramer E, Hessel S, Amengual J, Ribot J, Teerds K, Wyss A, Lietz G, Bonet ML, von Lintig J, Godschalk RW, Keijer J. Knockout of the Bcmo1 gene results in an inflammatory response in female lung, which is suppressed by dietary beta-carotene. Cell Mol Life Sci. 2010;67:2039–2056. doi: 10.1007/s00018-010-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scaife AR, McNeill G, Campbell DM, Martindale S, Devereux G, Seaton A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br J Nutr. 2006;95:771–778. doi: 10.1079/bjn20051718. [DOI] [PubMed] [Google Scholar]
- 132.Yeum KJ, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr. 1998;17:442–447. doi: 10.1080/07315724.1998.10718791. [DOI] [PubMed] [Google Scholar]
- 133.Karakilcik AZ, Aksakal M, Baydas G, Sozen R, Ayata A, Simsek M. Plasma beta-carotene concentrations in pregnancies, newborn infants and their mothers. J Pak Med Assoc. 1996;46:77–80. [PubMed] [Google Scholar]
- 134.Barrett BM, Sowell A, Gunter E, Wang M. Potential role of ascorbic acid and beta-carotene in the prevention of preterm rupture of fetal membranes. Int J Vitam Nutr Res. 1994;64:192–197. [PubMed] [Google Scholar]
- 135.West KP, Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Connor PB, Dali SM, Christian P, Pokhrel RP, Sommer A. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. B M J. 1999;318:570–575. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Christian P, West KP, Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, Shrestha SR. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. J Nutr. 1998;128:1458–1463. doi: 10.1093/jn/128.9.1458. [DOI] [PubMed] [Google Scholar]
- 137.Katz J, West KP, Jr, Khatry SK, Pradhan EK, LeClerq SC, Christian P, Wu LS, Adhikari RK, Shrestha SR, Sommer A. Maternal low-dose vitamin A or beta-carotene supplementation has no effect on fetal loss and early infant mortality: a randomized cluster trial in Nepal. Am J Clin Nutr. 2000;71:1570–1576. doi: 10.1093/ajcn/71.6.1570. [DOI] [PubMed] [Google Scholar]
- 138.Osorio JC, Cruz E, Milanes M, Ramirez Y, Sierra M, Cruz M, Sanfiel L. Influence of maternal redox status on birth weight. Reprod Toxicol. 2011;31:35–40. doi: 10.1016/j.reprotox.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 139.Fawzi WW, Mbise RL, Fataki MR, Herrera MG, Kawau F, Hertzmark E, Spiegelman D, Ndossi G. Vitamin A supplementation and severity of pneumonia in children admitted to the hospital in Dar es Salaam, Tanzania. Am J Clin Nutr. 1998;68:187–192. doi: 10.1093/ajcn/68.1.187. [DOI] [PubMed] [Google Scholar]
- 140.Gossage CP, Deyhim M, Yamini S, Douglass LW, Moser-Veillon PB. Carotenoid composition of human milk during the first month postpartum and the response to beta-carotene supplementation. Am J Clin Nutr. 2002;76:193–197. doi: 10.1093/ajcn/76.1.193. [DOI] [PubMed] [Google Scholar]
- 141.Dijkhuizen MA, Wieringa FT, West CE, Muhilal Zinc plus beta-carotene supplementation of pregnant women is superior to beta-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am J Clin Nutr. 2004;80:1299–1307. doi: 10.1093/ajcn/80.5.1299. [DOI] [PubMed] [Google Scholar]
- 142.Brief S, Chew BP. Effects of vitamin A and beta-carotene on reproductive performance in gilts. J Anim Sci. 1985;60:998–1004. doi: 10.2527/jas1985.604998x. [DOI] [PubMed] [Google Scholar]
- 143.Costello LM, O’Boyle P, Godkin JD, Diskin MG, Hynes AC, Morris DG. Retinol-binding protein (RBP), retinol and beta-carotene in the bovine uterus and plasma during the oestrous cycle and the relationship between systemic progesterone and RBP on day 7. Reprod Fertil Dev. 2010;22:1198–1205. doi: 10.1071/RD10034. [DOI] [PubMed] [Google Scholar]
- 144.Akordor FY, Stone JB, Walton JS, Leslie KE, Buchanan-Smith JG. Reproductive performance of lactating Holstein cows fed supplemental beta-carotene. J Dairy Sci. 1986;69:2173–2178. doi: 10.3168/jds.S0022-0302(86)80650-6. [DOI] [PubMed] [Google Scholar]
- 145.Arechiga CF, Staples CR, McDowell LR, Hansen PJ. Effects of timed insemination and supplemental beta-carotene on reproduction and milk yield of dairy cows under heat stress. J Dairy Sci. 1998;81:390–402. doi: 10.3168/jds.S0022-0302(98)75589-4. [DOI] [PubMed] [Google Scholar]
- 146.Ascarelli I, Edelman Z, Rosenberg M, F Y. Effect of dietary carotene on fertility of high-yielding dairy cows. Anim Sci. 1985;40:195–207. [Google Scholar]
- 147.Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, von Lintig J. Provitamin A conversion to retinal via the beta,beta-carotene-15,15’-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development. 2003;130:2173–2186. doi: 10.1242/dev.00437. [DOI] [PubMed] [Google Scholar]
- 148.Mora O, Kuri-Melo L, Gonzalez-Gallardo A, Melendez E, Morales A, Shimada A, Varela-Echavarria A. A potential role for beta-carotene in avian embryonic development. Int J Vitam Nutr Res. 2004;74:116–122. doi: 10.1024/0300-9831.74.2.116. [DOI] [PubMed] [Google Scholar]
- 149.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem. 2001;276:32160–32168. doi: 10.1074/jbc.M010086200. [DOI] [PubMed] [Google Scholar]
- 150.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15’-dioxygenase. J Biol Chem. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 151.Liu L, Tang XH, Gudas LJ. Homeostasis of retinol in lecithin: retinol acyltransferase gene knockout mice fed a high retinol diet. Biochem Pharmacol. 2008;75:2316–2324. doi: 10.1016/j.bcp.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, Duester G, Plutzky J. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ziouzenkova O, Orashanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of RXR and PPAR responses. Mol Endocrinol. 2007;21:77–88. doi: 10.1210/me.2006-0225. [DOI] [PubMed] [Google Scholar]