Abstract
Unraveling the highly interconnected nature of complex biological systems is fundamental to a wide range of modern research questions. At the heart of any coordinated biological network is cell-cell communication, and researching the means by which different cell types communicate is an essential prerequisite to fully understanding many aspects of biology. One major mechanism of cell signaling is the regulated release of chemical messengers from preformed vesicles in the cytoplasm. The process of transporting these vesicles to the exterior of the cell and the subsequent release of vesicular contents via membrane fusion is known as exocytosis. In recent decades, carbon-fiber microelectrodes have become increasingly useful for the measurement and study of exocytosis in a variety of biological contexts. This article details the critical background concepts of carbon-fiber microelectrode amperometry (CFMA) and carbon-fiber microelectrode fast scan cyclic voltammetry (FSCV) and reviews a variety of applications for monitoring exocytosis from single in vitro cells. Although the authors recognize the importance of several other complimentary methods including various electron microscopy and patch-clamp techniques, the scope of this article will focus only on CFMA and FSCV and their contributions to the field of single cell exocytosis measurements.
The use of carbon as an electrode material was first popularized by Ralph Adams in the 1950s.1 Since its inception, carbon has been a popular choice for electrochemical applications, as carbon electrodes demonstrate a wide working potential window, stable background currents, low noise, and high sensitivity. The development of carbon electrodes with active surfaces on the scale of several microns introduces several additional advantages. For voltammetry experiments, microelectrodes offer the ability to observe diffusion-limited currents at high scan rates and thus provide very fast time resolution.2 The small surface area of microelectrodes enables the use of high scan rates with relatively low background currents. Because of the small number of molecules that must be detected at the single cell level, however, most electrochemical measurements made with carbon microelectrodes produce currents in the nanoampere or picoampere range, depending on the method used.2 These current levels require electrodes that are both sensitive and exhibit low levels of noise, and carbon microelectrodes meet both of these criteria. Conveniently, using microelectrodes requires only a two-electrode system, as such low currents render the use of an auxiliary electrode unnecessary. Perhaps most importantly, microelectrodes provide much greater spatial resolution, and thus permit electrochemical measurements to be made from single cells.3 Although other forms of carbon can also be used to fabricate microelectrodes, carbon-fiber microelectrodes are often favored because they can be fabricated in large quantities and are relatively inexpensive.
R. Mark Wightman and coworkers first demonstrated the utility of carbon-fiber microelectrodes for the detection of exocytosis from single cells in 1990.4 Carbon-fiber microelectrodes of different geometries are used depending on the intended application; for single-cell electrochemical techniques, beveled and polished disc microelectrodes are most widely used. While Wightman's initial demonstration explored exocytosis from chromaffin cells, the cells responsible for the release of electroactive adrenaline, exocytosis is highly conserved across several cell types including neurons, chromaffin cells, platelets, and a variety of immune cells such as mast cells and macrophages. Accordingly, the technique has the capacity to reveal critical mechanistic details about exocytosis in these cell types, providing new insight into the fundamental behavior of normal and dysfunctional secretory cells.
Exocytosis
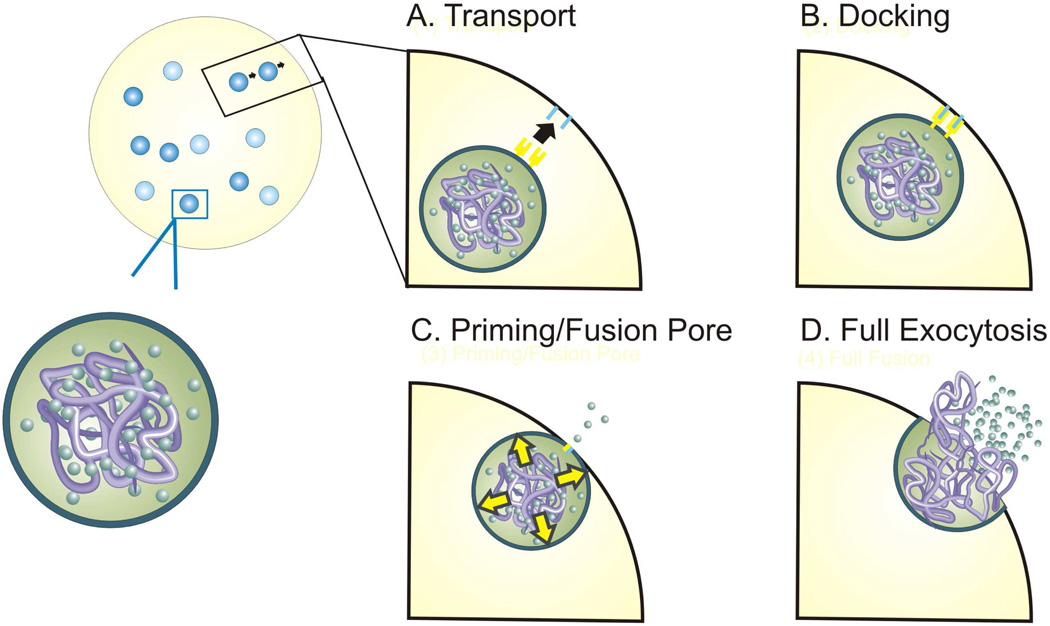
The basic function of exocytosis is comprised of several distinct sub-mechanisms that operate in an organized manner to facilitate the secretion of vesicular contents from the cell (Figure 1). In the presence of an appropriate stimulus, intracellular signaling first initiates the rearrangement of preformed vesicles within the cell. The result of this process is the transport and trafficking of vesicles from cytoplasmic sites to the cell membrane in preparation for fusion. Because electrochemical measurements monitor the release of chemical species, and thus the endpoint of exocytosis, conclusions regarding vesicle trafficking are difficult to make using this technique. Once a vesicle has been transported to a position immediately proximal to the plasma membrane, specialized proteins embedded in both the vesicle and cell membranes interact through a docking mechanism, securing the vesicle for fusion. Fusion is the last sub-mechanism of exocytosis, and is considered distinct from vesicle docking both because of its dependence on separate molecular machinery as well as its sensitivity to cytosolic calcium concentration. The initial stimulus and subsequent intracellular signaling results in an increase in cytosolic calcium ion concentration, causing the fusion of the two membranes, creating a small opening between the interior of the vesicle and the exterior of the cell called the fusion pore.5,6 Once the fusion pore is formed, small amounts of the vesicular contents are able to leak to the exterior of the cell. In general, this phase of exocytosis is followed rapidly by full exocytosis with the cell membrane, resulting in release of the vesicle’s contents. Sub-millisecond resolution microelectrochemical methods are uniquely suited to distinguish between the separate contributions of individual sub-mechanisms of cellular exocytosis.
Figure 1.
Steps of exocytosis.
Experimental Section
As stated, electrochemical methods using carbon-fiber microelectrodes (CFMs) are the gold-standard for studying single cell exocytosis. CFMA and FSCV are the most widely used, as these methods allow the direct measurement of secreted molecules without damaging cells or interfering with endocytosis, which is a common issue in capacitance-based measurement methods such as patch-clamp techniques.7
In FSCV, individual voltammograms are obtained by repeated application of triangular waveform scans through a range of potentials at regular intervals.2,8–15 Any electroactive species that can be oxidized/reduced in the potential window are monitored “simultaneously”. Each scan starts with the electrode placed on a single cell and held at a resting potential to relax the diffusive layer at the electrode tip. Potential sweep in a positive direction results in an oxidative peak in the trace. The potential then comes back in the negative direction, and if the species has a reversible redox process, it results in a reduction peak in the voltammogram. The amplitude of the current signal depends on the number of analyte molecules diffusing to the electrode surface.2 As the scan rate is hundreds of volts per second, a large background current occurs during the measurement.11,15 This background current is relatively stable for CFMs and can be subtracted from later scans.11 In single cell exocytosis studies, time-resolved chemical changes in the local environment of the electrode tip are obtained for each scan as the cell of interest is stimulated. Voltammograms obtained before stimulation of the cell are subtracted from voltammograms containing information related to the local chemical change after stimulation.2 This method provides chemical identification of oxidized/reduced species and also can be used to monitor multiple electroactive species simultaneously based on their oxidation potential.12,14 Accordingly, FSCV is most commonly used to identify molecules secreted from a cell. The temporal resolution of single cell FSCV measurements can reach the 100 millisecond level15,16, limiting insight into the real-time kinetics of exocytosis; as a result, CFMA is commonly employed as a complimentary technique for in-depth exocytosis kinetics studies.
In CFMA experiments, a constant potential sufficient to oxidize/reduce the chemical messenger of interest is supplied at the tip of CFM.3,7,17,18 Current at the electrode tip is monitored as a function of time. Oxidative current from the analyte at a positive potential is monitored while detection of reductive current at a negative potential can be troublesome due to the interference from oxygen. Upon stimulation of a single cell, exocytotic behavior of the cell is revealed as current spikes that represent vesicles of chemical messenger molecules stored in a quantal fashion, meaning that the concentration in each vesicle is approximately the same.13,19,20 Each spike may represent one vesicle but it is also possible, though statistically improbably, to simultaneously detect multiple vesicles. As electron transfer is faster than diffusion of chemical messengers to the electrode surface, redox reactions occurring at the electrode are limited by diffusion of chemical messengers.3,19 This allows the CFMA method to achieve sub-millisecond temporal resolution and enables real-time monitoring of exocytotic events. In an amperometric trace, each spike has a fast rising and slower decaying phase due to the convolution effects of diffusion and dissociation of chemical messenger molecules.17 Analysis of various spike characteristics reveals several aspects of exocytosis such as vesicle release kinetics and membrane properties.3,17
With the purpose of providing practical guidelines for electrochemical measurements on single cells, a concise and concrete review on how to perform a FSCV/CFMA experiment is provided herein.
Instrumentation (Experimental Setup)
The experimental setups for amperometry and voltammetry techniques share many common features. CFMs are typically fabricated in-house, and the fabrication methods may vary slightly by laboratory. Generally, a single carbon fiber with 5 ~ 10 µm diameter is aspirated into a glass capillary, which is then pulled using a capillary puller that applies thermal and magnetic fields. Using a microscope, the extruded carbon-fiber is cut back as desired depending on application. The carbon-fiber is next sealed in the capillary using epoxy resin for insulation and cured at high temperatures (100–150°C). Prior to electrochemistry experiments, cured electrodes are polished at 45° (angle can vary depending on applications) using a micropipette polisher and stored tip-down in isopropyl alcohol to remove any surface contaminants.
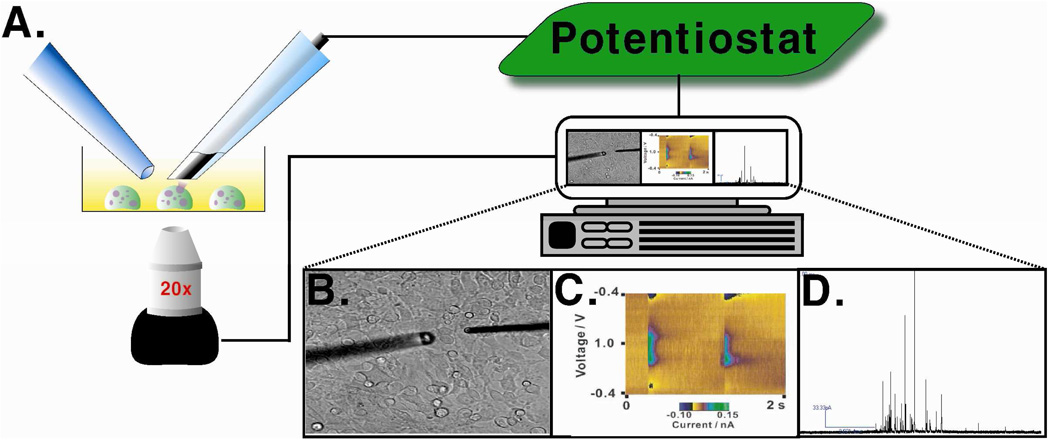
A typical instrument for CFMA and FSCV techniques includes a working electrode (the CFM) and a reference electrode (often a purchased Ag/AgCl electrode). Both the CFM and the reference electrode are connected to the potentiostat that controls the voltage between electrodes. Because currents due to the contents of single vesicles are quite small (nA to pA level), a low-noise measuring device or patch-clamp amplifier is often employed between the CFM and the potentiostat. Usually, the cells of interest are cultured in a Petri dish and are placed in a culture dish warmer to maintain physiological temperature. An inverted microscope is used to visualize cells and position the CFM. For cell stimulation, a pulled capillary containing stimulant is connected to a pressure-driven flow controller and positioned near the cell of interest. Positioning of the carbon–fiber microelectrode (adjacent to the single cell) and stimulating pipette is accomplished using micromanipulators. (Figure 2)
Figure 2.
Instrumental setup for a CFM electrochemistry experiment. (A) CFM (right) is placed on a single cell with the stimulating pipette (left) near the cell. (B) Bright field image of the experimental setup showing the CFM and stimulating pipette positioned on a single mast cell. (C) Representative color plot obtained by FSCV. (D) Representative amperometric trace obtained using CFMA on a mast cell. Figure (C) modified with permission from American Chemical Society from Reference 25.
Sensitivity, Selectivity, and Resolution
With regard to sensitivity and selectivity, the chemical messengers that are most commonly analyzed using CFM have an adsorption affinity for the carbon surface. As more adsorption of analyte onto the electrode surface occurs, measured current increases at the cost of temporal resolution.8,10,21 Adjusting the potential range, especially the resting potential in FSCV measurements, can control adsorption.8,10,22 Surface coatings of the carbon electrode have been used to increase selectivity and sensitivity of the measurement for specific chemical messengers but these methods add another layer for analyte to diffuse through, sacrificing temporal resolution. The dimensions of the CFM can also significantly affect the measurement. During CFMA experiments, the use of small diameter CFMs significantly reduces the number of superimposed spikes in a trace but it also reduces the total number of events detected.23 In summary, there are the expected trade-offs between sensitivity, selectivity, and resolution but adjusting the parameters mentioned above will facilitate optimal outcomes.
FSCV Data Analysis
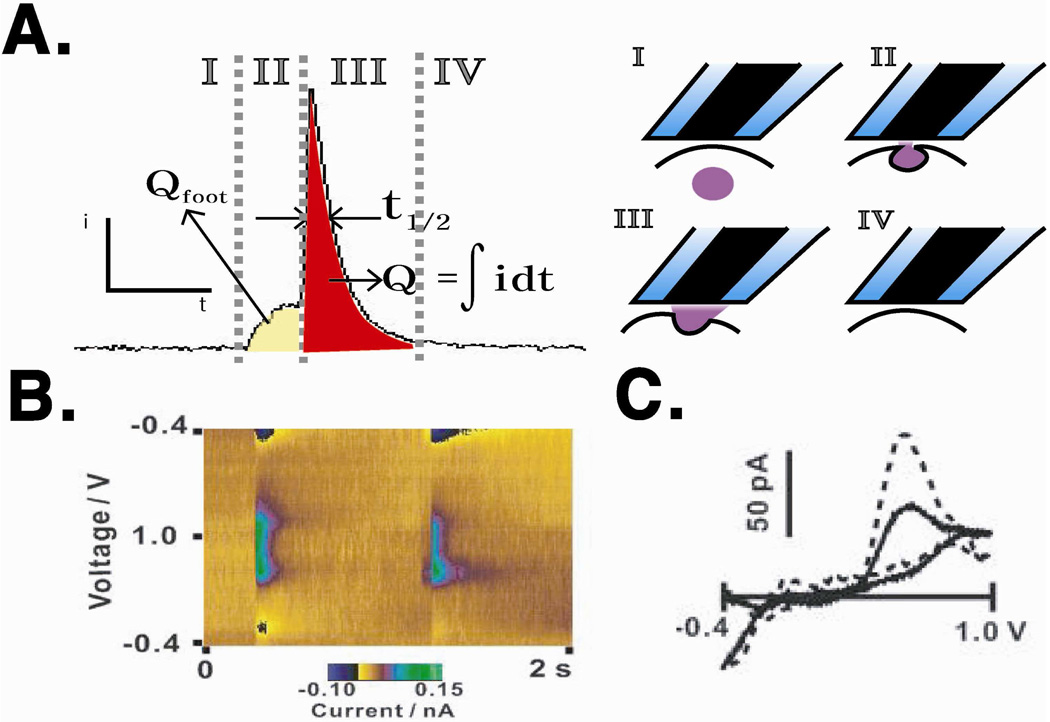
In FSCV, properties of detected molecules, such as electron transfer rate, stability, and carbon adsorption affinity, contribute to the CV shape, peak position, and the ratio of forward and reverse peak intensities; therefore, confirmation of the identity of chemical messengers from single cells by FSCV is normally done by comparing obtained voltammograms to those of control solutions.17,19,24 FSCV data is often represented in the format of a color plot where voltammograms (current versus potential) obtained as a function of time are stacked together with time on the x-axis, potential on the y-axis, and current coded in false color (Figure 2C).11,25 In this color plot format, identification of a specific chemical messenger is done by extracting individual CVs and comparing them to standards. Identity of a chemical messenger is then confirmed using a calculated correlation coefficient, and once identified, quantification is done by measuring the peak current and comparing the value with the values in a calibration curve previously prepared.22 As shown in Figure 2C, this color-plot representation facilitates visualization of the redox reactions occurring over the entire measurement in one 2-D plot and has been widely used to interpret FSCV data, though not those measured from complex mixtures. Addressing the difficulty of complex mixture detection, the Wightman group has recently employed chemometric methods such as principal component analysis and principal component regression to identify feature variation within voltammograms and successfully resolved and quantified nine components in a mixture.15,26
Spike Determination and Analysis in CFMA
After obtaining amperometric traces, the data analysis first requires identifying spikes and the corresponding baseline current. Usually, the root-mean-square current noise is measured before cell stimulation, and the spike detection threshold is set using a multiple of the measured noise.27 In the case of overlapping spikes or spikes with abnormal shape, spikes are visually selected. Upon baseline determination and spike selection, each trace is analyzed for basic spike parameters to characterize single cell exocytosis. The area underneath individual spikes (Q), full-width at half maximum (t1/2), and frequency of spikes in a trace (f) are commonly used as basic spike parameters to describe each cell’s exocytotic behavior (Figure 3). Applying Faraday’s law, the number of moles of detected redox-active species (N) can be quantified.13 T1/2 yields kinetic information about the time required to release a vesicle’s chemical messenger content.17 f is a measure of the vesicle release pattern of a single cell upon stimulation, and it provides information about how vesicles are recruited to the plasma membrane during exocytosis. These parameters are reliably used to quantify the exocytotic processes of single cells under various circumstances.17,27,28 As full exocytosis of a vesicle generates a fast rising slope and slower decaying slope in a spike,17 several additional kinetic parameters can be extracted from each spike, including trise,, time spent from 10% to 90% of maximum intensity in the rising phase, and tdecay, time spent from 90% to 10% of the maximum intensity in the decaying phase.3 Trise is especially interesting as it provides important information about the kinetics of the release event, such as the rate of dilation of the fusion pore to the full exocytosis state immediately following vesicle-cell membrane fusion and dynamics of molecules escaping the matrix that they are stored in42,43. The flux of redox-active chemical messengers before full exocytosis results in a characteristic pre-spike feature known as a pre-spike foot.17,27 Pre-spike foot features, which were first investigated by Chow and co-workers44 are diverse in magnitude, duration, and shape, yielding information about the dynamic nature of the fusion site. Analysis of pre-spike foot features often includes consideration of the number of spikes preceded by a foot event, charge within the foot portion of the feature (Qfoot), and duration of the foot before full exocytosis occurs (tfoot) relative to the corresponding value for the full exocytosis spike since, for a given condition, the portion of the messengers released through the fusion pore should be the similar.16,25 While much insight is accessible through detailed foot analysis, the analysis methods are not nearly as well established as those for full exocytosis amperometric spikes. Standard statistical procedures are used to compare the mean amperometric trace characteristics for different populations of single cells.3,22
Figure 3.
(A) Correlation of spike parameters and phases of exocytosis. (B) Example false-color plot of serotonin release. (C) Voltammogram obtained from serotonin release of a single platelet (solid line) and serotonin standard solution (dotted line). Figures (B) and (C) modified with permission from American Chemical Society from Reference 25.
Chemical Messengers and Cell Types
As many cell types release electroactive chemical messengers stored within vesicles through exocytosis, FSCV and CFMA have been used to monitor these important biological processes. Table 1 provides an overview of the cell types and secreted chemical messenger species that have been examined with CFMA and FSCV on the single cell level, and more detail regarding electroactive chemical messengers and the cells from which they are secreted is provided here. This article covers only experiments on single cells cultured in vitro, but there are also many examples of microelectrode work in situ or in vivo.45
| Class of Cell | Cell Type | Chemical Messenger (Detected by CFM) |
Molecules per Granule (zeptomoles)† |
Reference |
|---|---|---|---|---|
| Neuroendocrine/Endocrine Cells | Adrenal Chromaffin | Epinephrine, norepinephrine | 5,000–10,000 | 4,29 |
| Pancreatic β-Cell | Insulin | 600 | 30 | |
| Melanotrophs | α-melanocyte stimulating hormone | 320 | 31 | |
| Neurons | Retinal Amacrine | Dopamine | 8–170 | 32 |
| Somatic Leech | Serotonin | 7.8 (small vesicles) 13.3 (large vesicles) | 33 | |
| Large Dopamine | Dopamine | 1360 | 34 | |
| Superior Cervical Ganglion | Dopamine | 58 | 35 | |
| Cultured Midbrain | Dopamine | 5 | 36 | |
| Hematopoetic | Mast Cells | Serotonin, Histamine* | 1.24 × 106 ** | 37 |
| Platelets | Serotonin | 1000 | 38 | |
| Dendritic Cells | Serotonin | 49.8 – 215.9 | 39 | |
| Immortal Cell Lines | PC-12 | Dopamine | 190 | 40 |
| MN9D | Dopamine | 82 | 41 |
Histamine is co-stored and co-secreted with 5-HT, but has yet to be quantified per vesicle, due to the concomitant oxidation of serotonin at potentials sufficient to oxidize histamine.
Zmol serotonin in table refers to mast cells cultured alone. When co-cultured with fibroblasts, mast cell secretion of serotonin per vesicle increases to 1 × 107 zmol.37
The oxidations of epinephrine, norepinephrine, dopamine and serotonin are two electron processes. The oxidation reaction of histamine has yet to be conclusively deciphered. For insulin and α-melanocyte stimulating hormone, although several oxidizable moieties are present, the oxidation reactions for the two proteins in Table 1 are two electron processes.
Neuroendocrine/Endocrine Cells
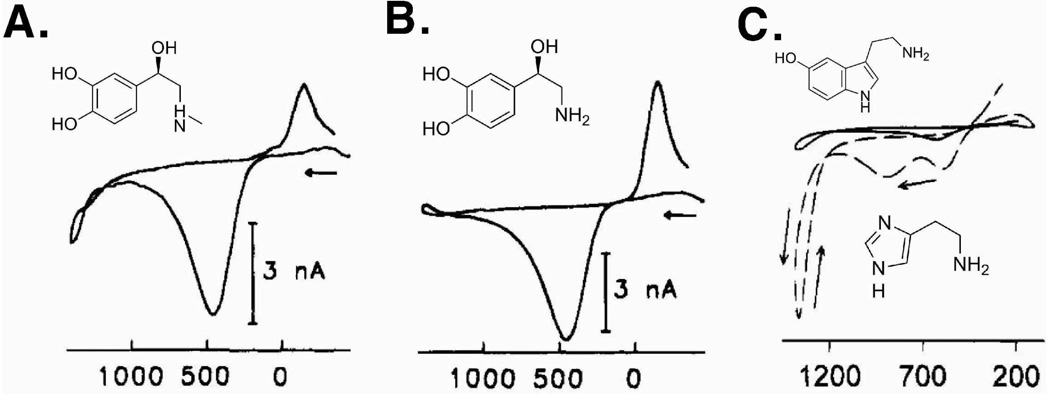
Adrenal chromaffin cells are neuroendocrine cells located in the central layer of the adrenal gland, and are of interest because of their role in the fight-or-flight response.4,29,46 Additionally, the similarities between chromaffin cells and neurons make chromaffin cells a good model for studying exocytosis.4 The first effort in monitoring single cell exocytosis was performed using stimulated single bovine chromaffin cells, confirming that epinephrine and norepinephrine are co-stored and released during chromaffin cell exocytosis, with an oxidation to an o-quinone at approximately +460 mV and reduction at approximately −150 mV vs Ag/AgCl.4,5 However, due to the structural similarities of norepinephrine and epinephrine (Figures 4A and B), discrimination of these two compounds was only achieved at a very high potential (+1400 mV vs Ag/AgCl) where the oxidation of the secondary amine of epinephrine was observed.14 This approach revealed that some chromaffin cells store either epinephrine or norepinephrine while others store both. Norepinephrine and epinephrine have been similarly detected from murine and rat adrenal medullary cells.47
Figure 4.
Molecular structures of (A) epinephrine, (B) norepinephrine, and (C) serotonin (upper, solid line in the voltammogram) and histamine (lower, dotted line in the voltammogram) with corresponding voltammograms. Figures (A) and (B) modified with permission from American Chemical Society from Reference 40, and Figure (C) modified with permission from American Chemical Society from Reference 52.
Melanotrophs are neuroendocrine cells located in the pituitary gland that secrete alpha-melanocyte stimulating hormone (alpha-MSH). Kennedy and colleagues detected peptide exocytosis from rat melanotrophs, and used both CFMA and FSCV to probe the exocytosis of these cells. Their results indicated that the oxidation of alpha-MSH was the primary source of the amperometric spikes.31
Neurons
As communication between neurons is achieved through exocytosis, the chemical signaling among neurons with a variety of origins and functions are of broad interest. Both dopaminergic and serotonergic neurons have been isolated and examined with CFMA. Zhou and Misler isolated cervical ganglia from rats, cultured individual dopaminergic and serotenergic neurons, and used CFMA to record amperometric spikes, the amplitudes of which were approximately 1% of the amperometric spike amplitudes of chromaffin cells.35 Sulzer and co-workers detected dopamine exocytosis from cultured midbrain neurons of rat pups.36,48 Dopamine has also been detected from retinal amacrine cells, which are dopaminergic neurons located in the retina, by Wightman and colleagues.32 Serotonergic leech neurons, called Retzius cells, have also been isolated and examined with CFM techniques.33 Retzius cells are large (80 µm diameter) and can be co-cultured with a receptor cell to form a serotonergic connection.49 Bruns and Jahn used CFMA to examine the exocytosis of both large dense core vesicles and small clear vesicles from isolated Retzius cells.33
Hematopoetic Cells
Mast cells are a tissue-bound granulated cell found in organs as diverse as skin, brain, lungs, and connective tissue. These cells have a complex function in the immune system, providing a line of defense against parasitic organisms but also playing a primary role releasing mediators of allergic response. Millar and co-workers used FSCV to detect exocytosis from single mast cells and to identify the substance exocytosed as serotonin.50 de Toledo, et al. used amperometry as well as FSCV to probe mast cell exocytosis and determined the amount of serotonin secreted was 1.24 × 106 zmol/granule.51 Wightman and co-workers later employed FSCV and CFMA to determine that histamine and serotonin are co-stored in mast cell granules.52 The CVs of histamine and serotonin each have unique features that were used to discriminate between the two compounds.52 Histamine release has yet to be detected amperometically due to the concomitant oxidation of serotonin at potentials sufficient to oxidize histamine, but concentration of secreted histamine has been quantified voltammetrically using its oxidation peak at +1250mV vs Ag/AgCl.52
Platelets are an anuclear circulating cell type with critically important roles in blood clot formation and hemostasis. Haynes and co-workers used FSCV to detect the exocytosis of two electroactive species from individual rabbit platelets and identify them as serotonin and histamine.38 By maintaining the potential of the electrode below the oxidation potential of histamine, they were able to use CFMA to explore the biophysical properties of platelet exocytosis with the well-understood serotonin redox reaction both in rabbit and human platelets.25,53
Immortal Cell Lines
In addition to primary culture cells, single cell detection of exocytotic events has been achieved from immortal cell lines as well, which are advantageous in that cells are homogenous, proliferate in culture indefinitely, and require no animal sacrifice. The most frequency examined cell line is the rat pheochromocytoma (PC12) cell line, which is derived from an adrenal medullary tumor and widely used as a model nerve cell for examining neurochemical processes. Unlike chromaffin cells, PC12 cells have dopamine levels 2–300 times higher than that of norepinephrine levels.54,55 Ewing and colleagues used electrochemical methods to probe the exocytosis of PC12 cells for the first time and established the identity of the exocytosed mediator as dopamine using FSCV.54,55
Fundamentals of Exocytosis Revealed
Beyond the utility of quantitative detection of chemical messengers released from the vesicles of single cells for fundamental characterization, CFMs have been used to probe more general exocytotic phenomena such as chemical messenger storage and release mechanisms, fusion pore formation, and intragranular matrix swelling. Correlated single cell electron microscopy and CFMA studies have revealed that the concentration of chemical messengers within vesicles, across cell types, is 0.5–1.0 M, which is much higher than previously thought possible.56 Most cell vesicles have two phases: a dense core and liquid halo. Highly sulfonated biopolymers, such as negatively charged chromogranins in chromaffin cells and heparin and chondroitin sulfate in mast cells, constitute the dense core matrix. Ionic interactions between the matrix and chemical messengers and hydrogen bonding between the two at the acidic granular pH are suggested as the possible driving forces that provide the interior of the vesicle to be isosmotic with the cell cytoplasm by allowing only minute amount of chemical messenger solubilized in halo region. It has been shown that the rate of the release is strongly influenced by the dissociation of the chemical messengers from the matrix in chromaffin and mast cells.5,6,57
During exocytosis, formation of a fusion pore does not always lead to full exocytosis; in some cases, the fusion pore recloses in a process known as “kiss-and-run” exocytosis. This “kiss-and-run” process may be a method for cells to send a less than quantal signal and it also represents a more efficient vesicle recycling process. Recently, Ewing and coworkers showed that even during the full exocytosis process vesicles do not extrude all their content, in fact, only 40% of the vesicle is released during PC12 cell exocytosis.58 CFMA experiments show that midbrain neurons release dopamine multiple times from the same vesicle through this mechanism. Moreover, “kiss-and-run” exocytosis has also been observed in chromaffin, mast, and PC12 cells. While it is likely that swelling and dissociation of the intragranular matrix play a role in determining the fate of the fusion pore5,6 the species or processes that regulate whether a fusion pore reseals or dilates is currently unresolved.
During fusion pore formation, the vesicular and plasma membranes fuse, mixing membrane components and distributing proteins that affect the physical and chemical properties of the membrane. Although the lipid and protein composition of the fusion pore is still under debate, CFMA results show that both membrane proteins and lipids influence exocytotic process. The previously mentioned SNARE proteins and their subgroups are often implicated in fusion pore formation models.Chapman and coworkers studied the exocytotic release from PC12 cells with genetically altered synaptotagmin using CFMA and concluded that a synaptotagmin-SNARE complex during exocytosis triggers formation of the fusion pore and sub-groups of synaptotagmin (I and IV) regulate the fate of that fusion pore.59 Amperomety studies accompanied by capacitance measurements on chromaffin cells60,61 demonstrated that both synaptotagmin I and VII may act as a Ca2+ sensor which regulates the fusion machinery and especially the later one increases the release through kiss-and-run exocytosis.M oreover, syntaxin (another SNARE protein) has been shown as a necessary component of fusion pore formation and exocytosis.59 Synaptobrevin and SNAP-25 are the other two SNARE proteins whose function in fusion pore formation and stability is studied.62,63
As some groups explore the role of protein components, others focus on the role of the lipids themselves. The effect of membrane phospholipid components and distribution on exocytosis has been studied using CFMA in both PC12 and chromaffin cells by adding exogeneous lipid species to the membrane.64,65 Results showed that phosphotidylserine, phosphatidylethanolamine, sphingomyelin and phosphatidylcholine influence the exocytotic process either by changing the frequency, quantity, and/or kinetics of release. In addition to membrane phospholipids, cholesterol is another major species found in the membrane, and has both structural and functional importance in exocytosis. It has been shown that SNARE proteins are localized in cholesterol-rich lipid rafts, and removal of cholesterol disrupts these rafts, reducing exocytosis in PC12 cells, chromaffin cells, and platelets.25,66 2001 Since cholesterol stabilizes the negative curvature of the fusion pore, removal of cholesterol decreases fusion pore stability, and decreases the percentage of spikes with a fusion pore (foot) events.
Summary and Future Directions
In summary, CFM techniques have illuminated the underpinnings of exocytosis and provided fundamental understanding of this important cellular process. As with any analytical technique, however, CFMA and FSCV have limitations. Exciting studies are currently underway to improve these methods for broader application. Current limitations that should be addressed include further improvement of spatial resolution and access to non-electroactive species. Although CFM techniques have been useful for detecting exocytosis from single cells, single electrode measurements yield no information regarding if and how exocytotic sites are spatially organized within the cellular membrane. To explore this question, individually addressable microelectode arrays have been used by Ewing and coworkers. CFMs with diameters of 2.5 µm were employed in arrays of 5–7 CFMs simultaneously to probe the spatial organization of exocytosis from PC12 cells. Using this technique, they detected "hot-spots" for exocytosis, and found that these locations are dynamic during the exocytotic process. Options for increasing the range of detectable species include direct derivatization with electrochemical tags or detection of electroactive side-products from an induced chemical reaction. Both of these options, however, will lead to decreased temporal resolution but facilitate quantitative measurement. Clearly, the CFMA and FSCV hold great potential for exploration of fundamental biological processes in normal, manipulated, and dysfunctional cells. As CFM technology continues to evolve, it will likely be possible to monitor exocytosis from a wider range of cells and chemical messenger types and reveal details about important cellular processes, including consideration of multi-cellular networks.
Acknowledgements
We would like to acknowledge Sara A. Love for providing Figure 1. This work is supported by NIH grant 1 DP2 OD004258-01.
Biographies
Donghyuk’s current research interests involve incorporation of analytical techniques, including microelectrochemical detection, into microfluidic platforms for the assessment of immune cell responses.
Secil’s research interests involve understanding fundamentals of platelet exocytosis using carbon-fiber microelectrochemistry and development of biosensors for detection of non-electroactive species secreted from cells.
Benjamin’s research employs carbon-fiber microelectrode amperometry to study the role of mast cells in a variety of different inflammatory conditions.
Audrey’s research interests include understanding the secretion behavior of mast cells that give rise to asthma and allergic response.
Christy L. Haynes is an Associate Professor of Chemistry at the University of Minnesota and advisor to all four co-authoring graduate students. Her main research interest lies in pushing the limits of analytical chemistry to explore complex biological and environmental questions.
References
- 1.Adams RN. Anal. Chem. 1958;30:1576. [Google Scholar]
- 2.Bard AJ, Faulkner LR. Electrochemical Methods. 2nd ed. John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 3.Wightman R, Jankowski J, Kennedy R, Kawagoe K, Schroeder T, Leszczyszyn D, Near J, Diliberto E, Viveros O. Proc. of the Natl. Acad. of Sci. of the U. S. A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leszczyszyn D, Jankowski J, Viveros O, Diliberto E, Near J, Wightman R. J. Biol. Chem. 1990;265:14736–14737. [PubMed] [Google Scholar]
- 5.Troyer KP, Wightman RM. J.Biol. Chem. 2002;277:29101–29107. doi: 10.1074/jbc.M202856200. [DOI] [PubMed] [Google Scholar]
- 6.Borges R, Diaz-Vera J, Dominguez N, Arnau M, Machado J. J. Neurochem. 2010;114:335–343. doi: 10.1111/j.1471-4159.2010.06786.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakmann B, Neher E. Single-Channel Recording. 2nd ed. ed. New York: Plenum Press; 1995. pp. 245–276. [Google Scholar]
- 8.Robinson D, Venton B, Heien M, Wightman R. Clin. Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 9.Omiatek D, Santillo M, Heien M, Ewing A. Anal. Chem. 2009;81:2294–2302. doi: 10.1021/ac802466g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venton B, Wightman R. Anal. Chem. 2003;75:414A–421A. [Google Scholar]
- 11.Michael D, Travis ER, Wightman RM. Anal. Chem. 1998;70:586A–592A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
- 12.Jackson B, Dietz S, Wightman R. Anal. Chem. 1995;67:1115–1120. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 13.Heien ML, Ewing AG. In Micro and Nano Tech. Bioanal. 2009;544:153–162. [Google Scholar]
- 14.Pihel K, Schroeder T, Wightman R. Anal. Chem. 1994;66:4532–4537. [Google Scholar]
- 15.Heien M, Johnson M, Wightman R. Anal. Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Heien M, Santillo M, Mellander L, Ewing A. Anal. Chem. 2011;83:571–577. doi: 10.1021/ac102502g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosharov EV, Sulzer D. Nat. Methods. 2005;2:651–658. doi: 10.1038/nmeth782. [DOI] [PubMed] [Google Scholar]
- 18.Chen T, Luo G, Ewing A. Anal. Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- 19.Jankowski J, Schroeder T, Ciolkowski E, Wightman R. J. Biol. Chem. 1993;268:14694–14700. [PubMed] [Google Scholar]
- 20.Schroeder T, Jankowski J, Kawagoe K, Wightman R, Lefrou C, Amatore C. Anal. Chem. 1992;64:3077–3083. doi: 10.1021/ac00048a003. [DOI] [PubMed] [Google Scholar]
- 21.Travis E, Wang Y, Michael D, Caron M, Wightman R. Proc. of the Natl. Acad. of Sci. of the U. S. A. 2000;97:162–167. doi: 10.1073/pnas.97.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heien M, Phillips P, Stuber G, Seipel A, Wightman R. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 23.Amatore C, Arbault S, Bouret Y, Guille M, Lemaitre F, Verchier Y. Anal. Chem. 2009;81:3087–3093. doi: 10.1021/ac900059s. [DOI] [PubMed] [Google Scholar]
- 24.Cahill P, Walker Q, Finnegan J, Mickelson G, Travis E, Wightman R. Anal. Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- 25.Ge S, White J, Haynes C. ACS Chem. Biol. 2010;5:819–828. doi: 10.1021/cb100130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keithley R, Heien M, Wightman R. Trac-Trends Anal. Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travis E, Wightman R. Annu. Rev. Biophys. Biomol. Struct. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Segura F, Brioso M, Gomez J, Machado J, Borges R. J. Neurosci. Methods. 2000;151:4820–4829. doi: 10.1016/s0165-0270(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 29.Wightman R, Jankowski J, Kennedy R, Kawagoe K, Schroeder T, Leszczyszyn D, Near J, Diliberto EJ, Viveros O. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy RT, Huang L, Atkinson MA, Dush P. Anal.l Chem. 1993;65:1882–1883. doi: 10.1021/ac00062a012. [DOI] [PubMed] [Google Scholar]
- 31.Paras C, Kennedy R. Anal. Chem. 1995;67:3633–3637. doi: 10.1021/ac00116a003. [DOI] [PubMed] [Google Scholar]
- 32.Hochstetler S, Puopolo M, Gustincich S, Raviola E, Wightman R. Anal. Chem. 2000;72:489–496. doi: 10.1021/ac991119x. [DOI] [PubMed] [Google Scholar]
- 33.Bruns D, Jahn R. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Gavin P, Luo G, Ewing A. J. Neurosci. 1995;15:7747–7755. doi: 10.1523/JNEUROSCI.15-11-07747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, Misler S. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6938–6942. doi: 10.1073/pnas.92.15.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pothos E, Davila V, Sulzer D. J. Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marquis BJ, Haynes CL. Biophys. Chem. 2008;137:63–69. doi: 10.1016/j.bpc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Ge S, Wittenberg N, Haynes C. Biochemistry. 2008;47:7020–7024. doi: 10.1021/bi800792m. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell P, Wang X, Leon-Ponte M, Griffiths C, Pingle S, Ahern G. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerink R, Ewing A. Acta Physiol (Oxf) 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong Y, Heien ML, Maxson MM, Ewing AG. J. Neurochem. 2008;107:1589–1595. doi: 10.1111/j.1471-4159.2008.05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amatore C, Arbault S, Bonifas I, Guille M. Biophysical Chemistry. 2009;143:124–131. doi: 10.1016/j.bpc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Sombers L, Hanchar H, Colliver T, Wittenberg N, Cans A, Arbault S, Amatore C, Ewing A. J.Neurosci. 2004;24:303–309. doi: 10.1523/JNEUROSCI.1119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow R, Vonruden L, Neher E. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 45.Wightman RM. Science. 2006;311:1570. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]
- 46.Burgoyne RD. Biochim. Biophys. Acta (BBA) - Reviews on Biomembranes. 1991;1071:174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- 47.Borges R, Jaen R, Freire F, Gomez J, Villafruela C, Yanes E. Cell and Tissue Res. 2001;304:159–164. doi: 10.1007/s004410000320. [DOI] [PubMed] [Google Scholar]
- 48.Rayport S, Sulzer D, Shi W, Sawasdikosol S, Monaco J, Batson D, Rajendran G. J Neurosci. 1992;12:4264–4280. doi: 10.1523/JNEUROSCI.12-11-04264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruns D. Methods. 2004;33:312–321. doi: 10.1016/j.ymeth.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Tatham PER, Duchen MR, Millar J. Pflügers Archiv. 1991;419:409–414. doi: 10.1007/BF00371124. [DOI] [PubMed] [Google Scholar]
- 51.De Toledo G, Fernandezchacon R, Fernandez J. Nature. 1993;363:554–558. [Google Scholar]
- 52.Pihel K, Hsieh S, Jorgenson J, Wightman R. Anal. Chem. 1995;67:4514–4521. doi: 10.1021/ac00120a014. [DOI] [PubMed] [Google Scholar]
- 53.Ge S, Woo E, White JG, Haynes CL. Anal. Chem. 2011;83:2598–2604. doi: 10.1021/ac102929y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen T, Luo G, Ewing A. Anal. Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- 55.Kozminski K, Gutman D, Davila V, Sulzer D, Ewing A. Anal. Chem. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- 56.Amatore C, Arbault S, Bonifas I, Bouret Y, Erard M, Ewing AG, Sombers LA. Biophys. J. 2005;88:4411–4420. doi: 10.1529/biophysj.104.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monck J, Oberhauser A, De Toledo G, Fernandez J. Biophys. J. 1991;59:39–47. doi: 10.1016/S0006-3495(91)82196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omiatek DM, Dong Y, Heien ML, Ewing AG. ACS Chem. Neurosci. 2010;1:234–245. doi: 10.1021/cn900040e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson M, Chapman E. Nature Struct. Mol. Biolo. 2008;15:684–689. doi: 10.1038/nsmb.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voets T, Moser T, Lund P, Chow R, Geppert M, Sudhof T, Neher E. Proceedings of the National Academy of Sciences of the United States of America. 2001:11680. doi: 10.1073/pnas.201398798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Segovia M, Ales E, Montes M, Bonifas I, Jemal I, Lindau M, Maximov A, Sudhof T, de Toledo G. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19032–19037. doi: 10.1073/pnas.1014070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ngatchou A, Kisler K, Fang Q, Walter A, Zhao Y, Bruns D, Sorensen J, Lindau M. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18463–18468. doi: 10.1073/pnas.1006727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang Q, Berberian K, Gong L, Hafez I, Sorensen J, Lindau M. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15388–15392. doi: 10.1073/pnas.0805377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amatore C, Arbault S, Bouret Y, Guille M, Lemaître F, Verchier Y. Chembiochem. 2006;7:1998–2003. doi: 10.1002/cbic.200600194. [DOI] [PubMed] [Google Scholar]
- 65.Uchiyama Y, Maxson M, Sawada T, Nakano A, Ewing A. Brain Res. 2007;1151:46–54. doi: 10.1016/j.brainres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Xue R, Ong W, Chen P. Biophys J. 2009;97:1371–1380. doi: 10.1016/j.bpj.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]