Abstract
Single antigen bead (SAB) testing permits reassessment of immunologic risk for kidney transplantation. Traditionally, high panel reactive antibody (PRA), retransplant and deceased donor (DD) grafts have been associated with increased risk. We hypothesized that this risk was likely mediated by (unrecognized) donor-specific antibody (DSA).
We grouped 587 kidney transplants using clinical history and SAB testing of day of transplant serum as 1) unsensitized; PRA=0 (n= 178), 2) 3rd party sensitized; no DSA (n=363), or 3) donor sensitized; with DSA (n=46), and studied rejection rates, death censored graft survival (DCGS), and risk factors for rejection.
Antibody-mediated rejection (AMR) rates were increased with DSA (p<0.0001), but not with PRA in the absence of DSA. Cell-mediated rejection (CMR) rates were increased with DSA (p<0.005); with a trend to increased rates when PRA>0 in the absence of DSA (p=0.08). Multivariate analyses showed risk factors for AMR were DSA, worse HLA matching, and female gender; for CMR: DSA, PRA>0 and worse HLA matching. AMR and CMR were associated with decreased DCGS.
The presence of DSA is an important predictor of rejection risk, in contrast to traditional risk factors. Further development of immunosuppressive protocols will be facilitated by stratification of rejection risk by donor sensitization.
Keywords: Sensitization, Retransplant, Risk, Antibody Mediated Rejection
Methods used to identify alloantibodies have become increasingly more sensitive and specific over time, evolving from complement dependent cytotoxicity (CDC) assays, to anti-human globulin enhanced CDC (AHG/CDC), enzyme-linked immunosorbent assay (ELISA), and solid phase assays performed on flow cytometric and Luminex platforms. Consequently the identification, characterization and to a limited degree, quantification of preformed HLA antibodies has improved, as single antigen bead (SAB) testing identifies a much lower level of antibody than required to cause a positive AHG crossmatch(1–6).
At the same time, the definition, diagnosis, and monitoring of rejection, particularly AMR, has also been refined(7–9). Collectively, these advances provide an opportunity to more accurately assess risk factors for kidney allograft rejection. Traditionally, high PRA, retransplant, and DD grafts have been associated with increased risk of rejection (10–17). We hypothesized that this risk was likely mediated by (previously unrecognized) donor-specific antibody (DSA). In this study we grouped kidney transplant recipients by immunologic history (i.e., sensitizing events and PRA) and presence or absence of DSA at the time of transplant, and examined the impact of previously identified risk factors for rejection and immunologic graft loss.
Patients and Methods
Nine-hundred five kidney transplants were performed at the University of Minnesota between January 1, 2004 and December 31, 2007. Transplants were done on the basis of a negative final AHG/CDC crossmatch (AHG-XM) in most circumstances. For retransplant candidates, repeat mismatches were not avoided a priori. All donor and recipient characteristics and post-transplant outcomes were prospectively recorded in our center’s database. Importantly, a repository of stored sera was maintained on all transplant candidates. For recipients with negative final AHG-XM, we studied the impact of immunologic history (i.e., prior sensitizing events) and pretransplant DSA on transplant outcome.
Among the 905 transplants, 318 were excluded from this analysis for the following reasons: 1) prior or simultaneous extrarenal transplants; 2) blood group antigen incompatible transplants, 3) transplants with positive final AHG-XM, 4) transplants done after desensitization, 5) incomplete sensitization history or XM data, or 6) patients who declined participation in outcomes research. The remaining 587 transplants were studied under an approved institutional review board protocol (#0711E22165). Recipients that underwent a subsequent extrarenal transplant (i.e. pancreas after kidney) were censored at the time of the extrarenal transplant.
HLA typing
All patients and donors were molecularly typed for HLA-A*, B*, C*, DRB1*/3*/4*/5*, DQB1*, and DQA1* antigens. Recipients and their donors were further typed for HLA-DPB1 antigens whenever testing revealed the presence of DP antibodies in recipient day of transplant (D0) sera. Molecular HLA typing was by reverse SSO (LABType® SSO, One Lambda, Inc) or, for DPB1, by SSP (OLERUP SSP™, QIAGEN).
PRA and DSA testing
Routine clinical HLA antibody profiling of renal candidate sera using solid phase, SAB technology for PRA and DSA (sequential testing with LABScreen® Mixed Antigen, PRA and Single Antigen assays (One Lambda, Inc.)) began in March 2006 at our center. For transplants in this study done prior to this date, D0 sera were retrospectively tested for PRA and DSA (by the same methods as those prospectively tested) using serum samples stored at −70°C. Peak (pk) PRA was defined as the historical or current serum sample with highest %PRA, as determined by AHG/CDC, ELISA, or LABScreen® PRA, depending on acquisition era. Peak serum samples had %PRA re-determined by LABScreen® PRA assay (if not already applied), but were not retrospectively tested for DSA.
D0 serum was tested for DSA. The presence of DSA was defined as a baseline-adjusted, mean fluorescence intensity (MFI) ≥500 for any SAB representing an A, B, C, DRB1/3/4/5, DQB1, DQA1, DPB1 antigen/allele present in the kidney donor. DQA1 specificities were assigned according to Deng et al. or by analysis of pattern of reactivity(18).
Crossmatching
The cytotoxic T and B cell AHG-XM assay was used to determine organ compatibility. AHG-XMs were performed prospectively with D0 sera and the historic peak sample as described previously(19). Positive AHG-XM with historical serum was not a contraindication to transplant, as long as the D0 serum XM was negative.
Immunosuppression and rejection
All patients received anti-lymphocyte globulin (Thymoglobulin®, Genzyme, Cambridge, MA) induction (4–6 mg/kg), calcineurin inhibitor and mycophenolic acid based immunosuppression and rapid discontinuation of prednisone(20). Calcineurin inhibitor 12 hour trough targets and steroid protocols were were uniformly applied to all patients (tacrolimus 8–10 mcg/L or Neoral® 175–200 mcg/L, as measured by high performance liquid chromatography).
Allograft biopsies were triggered by graft dysfunction, defined as serum creatinine >25% of baseline. All cellular rejection episodes were biopsy proven and described as per Banff criteria(7, 8). AMR was diagnosed when 2 of 3 findings (light microscopic changes of AMR, diffuse (>50%) C4d staining, or circulating DSA) were present; or when staining was diffusely C4d positive with otherwise unexplained graft dysfunction(9). Cell mediated rejection (CMR) was treated with steroid pulse and recycle, or with lymphocyte depleting antibody if steroid resistant (Thymoglobulin®) or Banff 2A or higher (muromonab-CD3). AMR was treated with steroids and 5 or more treatments of plasma exchange/IVIg, as needed.
Patient grouping
Transplants were categorized into groups based on sensitization history and detection of HLA antibodies by SAB in D0 serum. Group 1 (“unsensitized”) was comprised of 178 patients with no history of pregnancy, transfusion or transplant, PRA=0 in all available pretransplant sera, and negative AHG-XM on historical and D0 serum samples. Group 2 (“3rd party sensitized”) included 363 patients with a history of a sensitizing event, negative AHG-XM on historical and D0 serum, and no DSA by SAB testing in D0 serum. Of these 363 sensitized recipients, 214 (59%) had peak PRA=0 (Group 2A) and 149 (41%) had peak PRA>0 (Group 2B). Group 3 (“donor sensitized”) included 46 recipients with a negative AHG-XM but with DSA in D0 sera. Of these, 7 (15%) had a positive AHG-XM with historical serum.
Statistical Analysis
Donor and recipient characteristics for each group were compared by Chi-square and Kruskal-Wallis tests for discrete and continuous variables respectively. Actuarial rates of patient and death-censored graft survival, as well as CMR and AMR free survival rates were estimated by Kaplan-Meier methods and compared using log-rank tests. The inverse of death censored graft survival (DCGS) was used to approximate immunologic graft loss.
Graft function was estimated by the Cockcroft-Gault method and reported as creatinine clearance (CrCl) in ml/min. Linear regression analyses of inverse serum creatinine versus time (following the first post-transplant year) were applied to individual patient data. Resulting slope estimates were then compared between the groups using the Kruskal-Wallis test. Graft failure was defined as return to dialysis or kidney retransplantation. Cox proportional hazard modeling was applied to evaluate the relative risk of pretransplant characteristics toward the development of CMR and AMR; and of pretransplant risk factors, CMR and AMR toward death censored graft loss. A forward, stepwise procedure was used in constructing Cox regression models to adjust for contributions of recipient and donor characteristics. P values <0.05 were interpreted as statistically significant.
Results
Donor and recipient characteristics for the groups are shown in Table 1. Overall, Group 1 (unsensitized) recipients were more likely to be male, first transplant recipients, and receive a preemptive or living donor (LD) graft. Group 3 (donor sensitized) recipients were more likely to be female, retransplants, have higher PRA and more HLA mismatches. The sensitized groups spent more time on dialysis than the unsensitized group (Group 1=19.9, Group 2A 46.9, Group 2B 74.3, Group 3 113.2 months, p<0.0001). Median graft followup for all groups was between 43.8–56.0 months (range 0–78.2 months) (p=0.004). Median patient followup was 46.3–57.9 months (range 3.4–78.2 months) (p=0.01). Table 2 summarizes 1 year post-transplant outcomes.
Table 1.
Recipient and donor demographics.
| Group 1 Unsensitized N=178 |
Group 2A 3rd Party Sensitized pkPRA=0 N=214 |
Group 2B 3rd Party Sensitized pkPRA>0 N=149 |
Group 3 Donor Sensitized N=46 |
p value | |
|---|---|---|---|---|---|
| LD (%) | 78 | 67 | 60 | 70 | 0.003 |
| Female (%) | 21 | 51 | 50 | 61 | <0.0001 |
| Mean age | 42.8 +/− 18.1 | 43.2 +/− 21.6 | 47.4 +/− 17.3 | 42.5 +/− 17.0 | 0.08 |
| Recipient race (%C) | 83 | 88 | 84 | 76 | 0.56 |
| Diabetic (%) | 29.2 | 37.8 | 30.9 | 13.0 | 0.008 |
| Retransplant (%) | 0 | 12 | 22 | 44 | <0.0001 |
| Mean # Tx | 1.0 +/− 0 | 1.1 +/− 0.4 | 1.3 +/− 0.6 | 1.6 +/− 0.8 | <0.0001 |
| Mean # pregnancies | 0 | 2.0 +/− 1.98 | 3.2 +/− 3.49 | 3.2 +/− 2.67 | <0.0001 |
| Mean %PRA (peak) | 0 | 0 | 32.8 +/− 33.4 | 50.6 +/− 36.3 | <0.0001 |
| Mean %PRA (current) | 0 | 0 | 23.3 +/− 31.8 | 40.4 +/− 36.0 | <0.0001 |
| Donor gender (%female) | 57 | 51 | 50 | 54 | 0.55 |
| Donor Race (%C) | 89 | 94 | 93 | 89 | 0.63 |
| Mean donor age | 39.9 +/−12.5 | 39.9 +/− 12.8 | 40.1 +/− 13.8 | 42.7 +/− 12.5 | 0.46 |
| Mean HLA mismatch | 3.6 +/− 1.7 | 3.3 +/− 1.8 | 3.0 +/− 2.1 | 4.0 +/− 1.6 | 0.002 |
| 0 HLA mismatch (%) | 6.8 | 13.6 | 23.6 | 4.4 | <0.0001 |
| Cause of graft failure | 0.25 | ||||
| Cause of recip death | 0.85 | ||||
| Dialysis at Tx (%) | 50.6 | 70.4 | 64.9 | 71.7 | 0.0002 |
| Months since 1st ever dialysis | 19.9 | 46.9 | 74.3 | 113.2 | <0.0001 |
| Median followup (m, SD) | 51.0 +/−16.4 | 52.1 +/−17.1 | 43.8 +/−18.4 | 56.0 +/−17.7 | 0.004 |
pk=peak, PRA=panel reactive antibody, LD=living donor, C=Caucasian, Tx=transplant, current=day of transplant, m=months, SD=standard deviation.
Table 2.
Summary of one year post-transplant outcomes.
| n= | CMR Free | AMR Free | DCGS | PS | ||
|---|---|---|---|---|---|---|
| Grp 1 | Unsensitized, pkPRA=0 | 178 | 78.9% | 93.2% | 97.2% | 100.0% |
| Grp 2A | Sensitized, pkPRA=0 | 214 | 79.4% | 95.3% | 97.6% | 99.0% |
| Grp 2B | Sensitized, pkPRA>0 | 149 | 69.2% | 94.6% | 96.0% | 98.6% |
| Grp 3 | Donor Sensitized | 46 | 65.2% | 65.1% | 100.0% | 95.6% |
| Overall p= | 0.02 | <0.0001 | 0.50 | 0.08 | ||
CMR=acute cellular rejection, HR=hazard ratio, AMR=antibody mediated rejection, DCGS=death censored graft survival, PS=patient survival. Pk=peak, PRA=panel reactive antibody.
Rejection
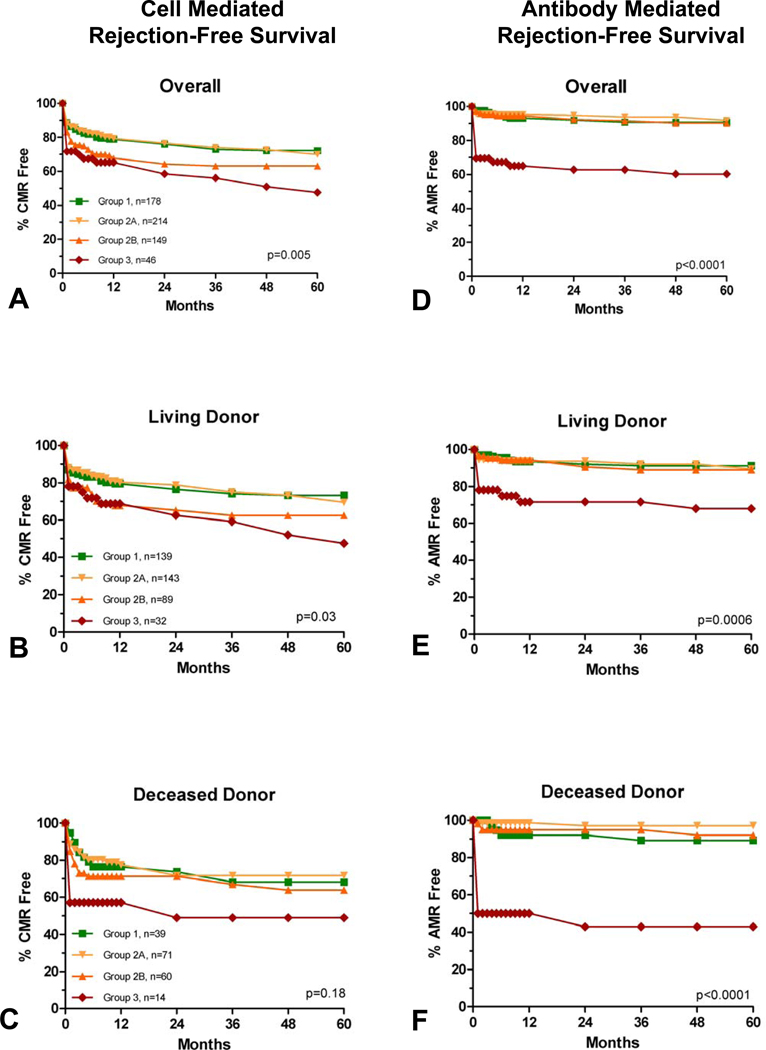
Rejection-free graft survival is shown, by group, in Figure 1, panels A–F. Episodes of AMR and CMR occurred primarily in the first 3 and 6 post-transplant months respectively, with little change in rate thereafter. Compared with Group 1, Group 2A (3rd party sensitized, pkPRA=0) had similar CMR and AMR rates. Group 2B (3rd party sensitized, pkPRA>0) had a trend toward more CMR (p=0.08), but AMR rates were not different than Group 1. Group 3 had significantly increased rates of CMR (HR=2.1, p=0.003) and AMR (HR=5.5, p<0.0001) compared to Group 1.
Figure 1.
CMR (panels A–C) and AMR (panels D–F) free survival. P values are overall. Compared with Group 1, pairwise analysis showed Group 3 to have significantly more CMR (p=0.003, HR 2.2) and AMR (p<0.0001, HR 5.5). There was a trend toward more CMR in Group 2B (p=0.08).
Median time to AMR was significantly longer for Group 1 (152 days interquartile range (IQR) 300), compared with Group 2A (22 days, IQR 880), Group 2B (84 days, IQR 596), and Group 3 (16.5 days, IQR 18) (p=0.02). Median time to CMR was not different between the groups (p=0.33) (Group 1=69 days, IQR 288, Group 2A=89 days, IQR 396, Group 2B=30 days, IQR 155, Group 3=27 days, IQR 531).
Patient Survival and Immunologic Graft Loss
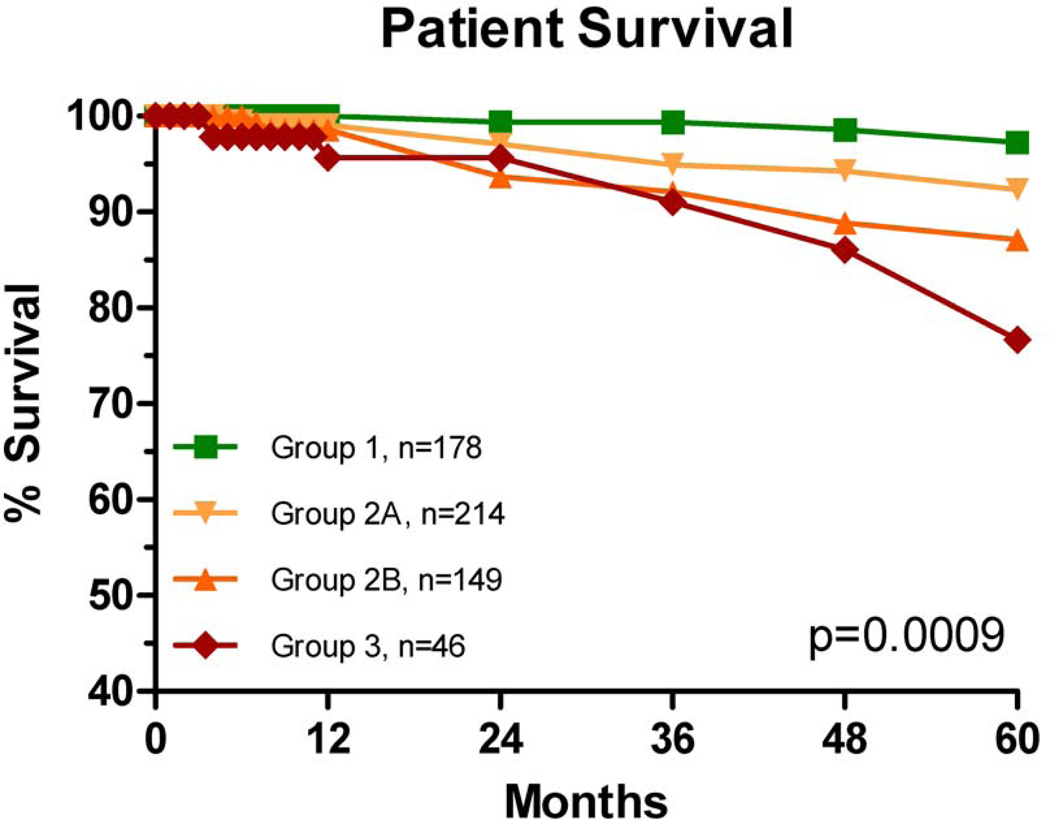
We observed no difference in patient and graft survival between groups at 1 year post transplant (Table 2), however, with longer followup, there were differences in actuarial patient and graft survival rates (Figures 2 and 3). Compared to Group 1, patient survival was significantly decreased (pairwise analysis) for Group 2A (p=0.02, HR=4.4), Group 2B (p=0.004, HR=6.3), and Group 3 (p=0.0006, HR=10.0). We did not identify any trends in cause of death.
Figure 2.
Actuarial patient survival rates. Compared with Group 1, pairwise analysis showed significant differences in patient survival rates for Group 2A (p=0.02, HR 4.4), Group 2B (p=0.004, HR=6.3) and Group 3 (p=0.0006, HR=10.0).
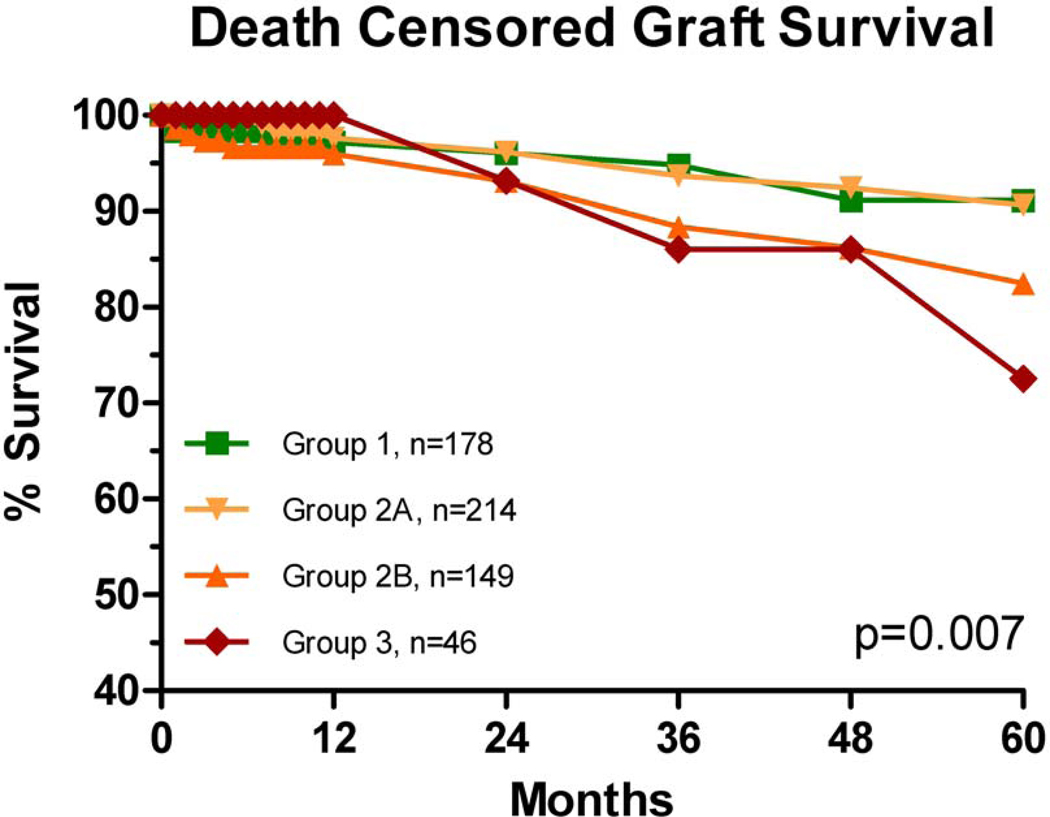
Figure 3.
Actuarial death censored graft survival rates were different overall. Pairwise analysis showed only Group 3 to be different from Group 1 (p=0.008, HR 2.9).
There was no difference in DCGS between Groups 1 and 2A (p=0.98); but there was a trend to worse survival for Group 2B (p=0.06). Group 3 recipients had significantly worse DCGS (p=0.008, HR=2.9).
Graft Function and Impact of AMR
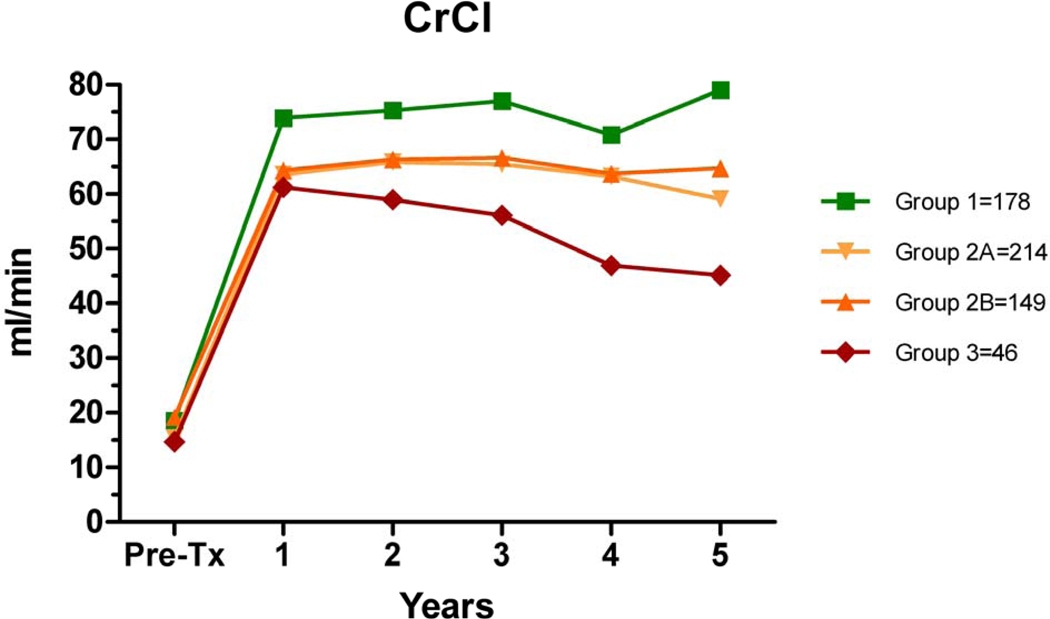
CrCl was significantly different between the groups (Figure 4). In addition, after the first year, the slope of 1/Cr vs time was more negative for Group 3 vs. Group 1 (−0.056 vs −0.017, p<0.0001). Further insight is gained when evaluating the impact of AMR on graft function over time; in the absence of AMR, the CrCl was stable, even in Group 3 (data not shown).
Figure 4.
Creatinine clearance (Cockroft-Gault) over time. Differences between groups were significant at each post-transplant anniversary (year 1 p=0.01, year 2 =0.002, year 3 =0.0005, year 4 p=0.0048, and year 5 p=0.003).
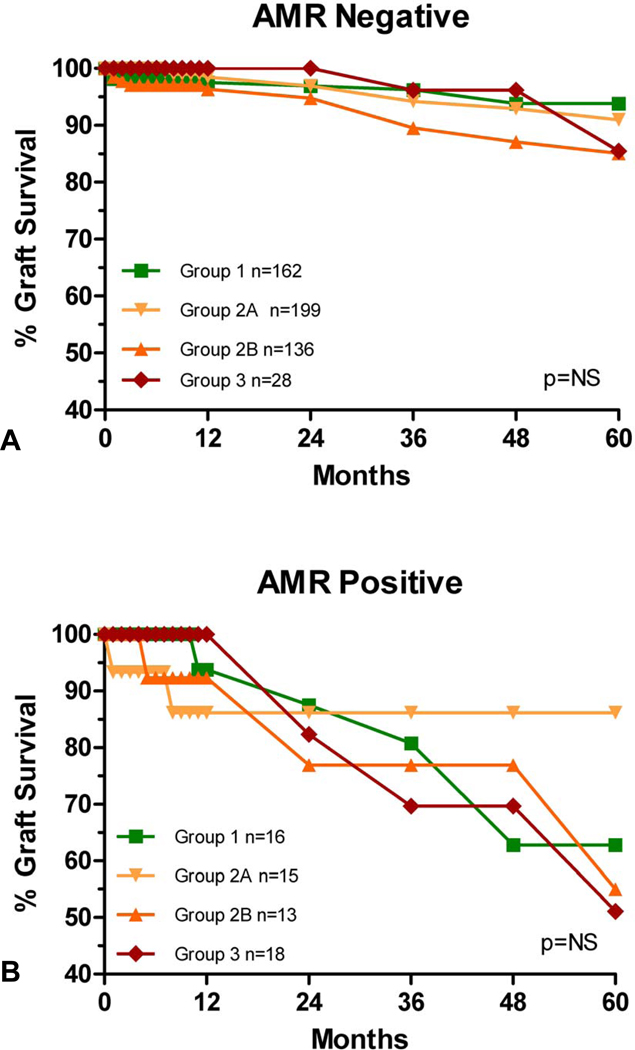
In the absence of AMR, there was no difference between groups in DCGS (Fig 5A) whereas the development of AMR was associated with decreased DCGS (Figure 5B). Similarly, the development of pure CMR was associated with decreased DCGS in all sensitized groups (data not shown).
Figure 5.
Immunologic graft loss by group when AMR negative (A) or positive (B). There were no differences between groups when the AMR outcome was the same. Graft survival within study groups was significantly worse when AMR occurred (Group 1p<0.0001, Group 2B p=0.04, Group 3, p=0.003).
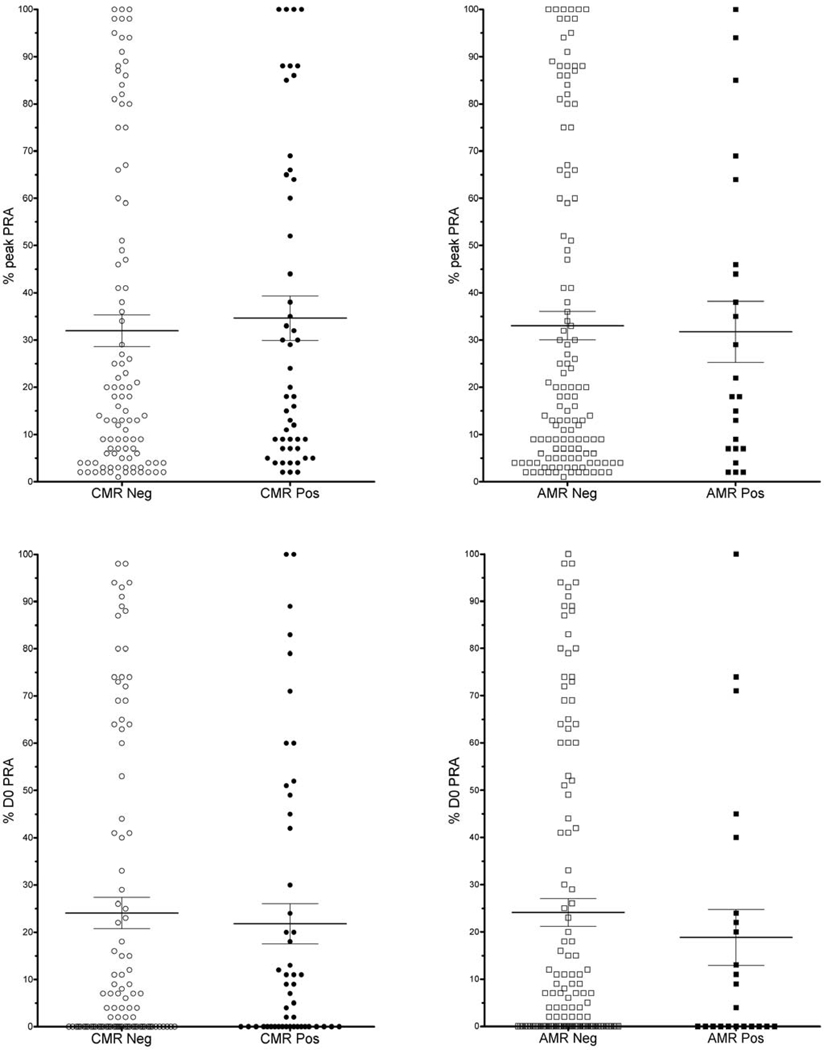
Effect of peak and D0 PRA in recipients without DSA
In Group 2B (3rd party sensitized with peak PRA>0), mean peak PRA was not different for those who had CMR (35% +/−4.7) vs. those CMR-free (32% +/−3.4) (p=0.65) (Figure 6). Similarly, there was no difference in mean peak PRA in those who had AMR (32% +/−6.5) vs. those AMR-free (32% +/−3.0) (p=0.86). Likewise, mean D0 PRA did not differ between rejecters and non-rejecters: CMR positive = 22% +/−4.3 vs. CMR-free = 24% +/−3.3, (p=0.68); AMR positive = 19% +/−5.9 vs. AMR-free= 24% +/−2.9, (p=0.46).
Figure 6.
CMR and AMR outcomes for 3rd party sensitized recipients with pkPRA>0 (Group 2B). There were no significant differences in mean PRA (peak or D0 ) between nonrejecters and rejecters.
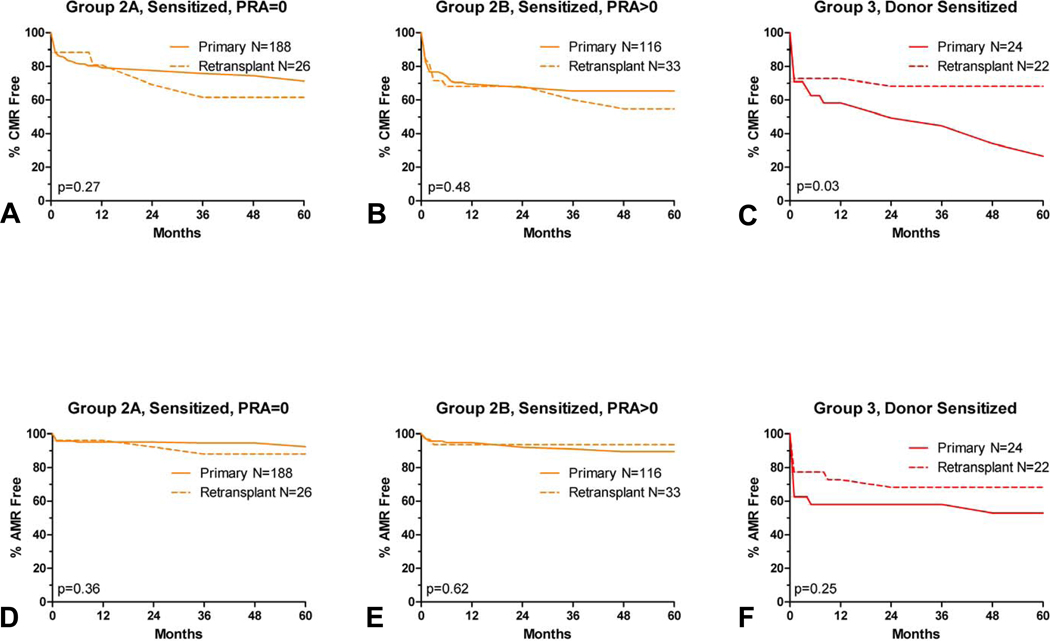
Effect of Retransplant Status and Donor Type
For both Groups 2A and 2B, there was no significant difference between primary and retransplants in either AMR or CMR (Figure 7). Group 3 (donor sensitized) retransplants actually had a higher rate of CMR-free graft survival compared to donor sensitized primary transplants (p=0.03); while AMR-free survival was also higher for retransplants in this group, but did not meet statistical significance (p=0.25).
Figure 7.
Primary vs retransplant rejection rates: CMR (panel A–C) and AMR (panel D–F). Rejection free graft survival is similar for third party sensitized recipients, and significantly worse for donor sensitized primary transplants.
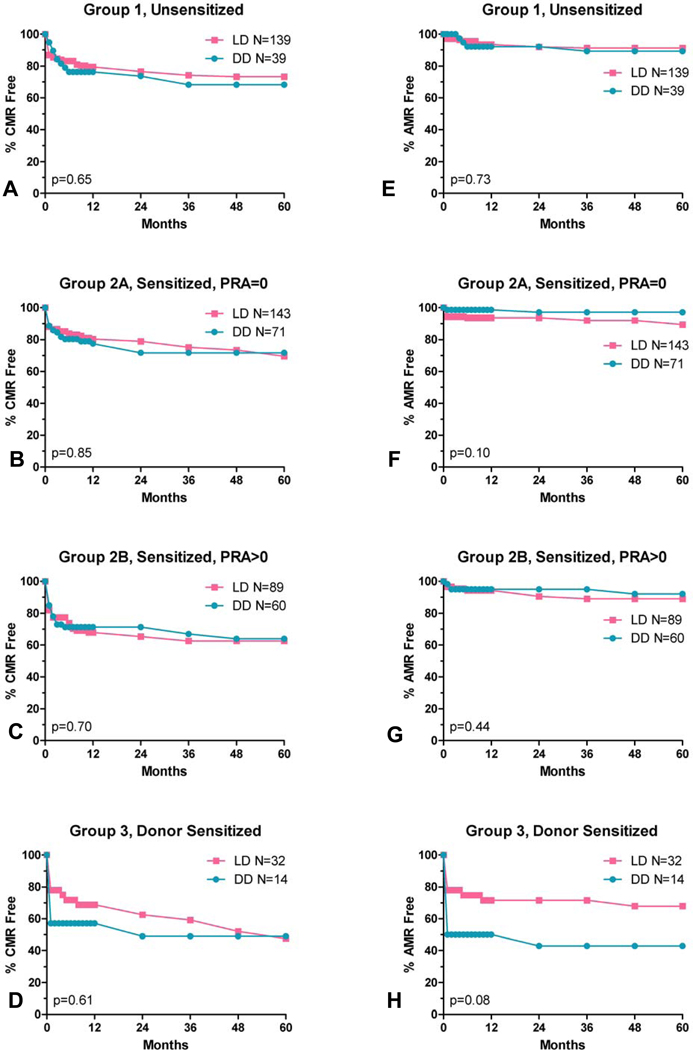
We found no significant difference in rejection rates within study groups for recipients of living vs deceased donor transplants (Figure 8).
Figure 8.
CMR (panel A–D) and AMR (panel E–H) rates by donor source. LD=living donor, DD=deceased donor.
Multivariate Analyses
In order to identify pretransplant risk factors associated with the development of rejection (CMR and AMR), we applied Cox regression modeling and adjusted for differences in key characteristics between study groups. Retransplant status was considered in the stepwise selection of the multivariate analyses of the entire cohort, but was not significant so was not included in the final model. The most important risk factors for CMR were the presence of donor-specific sensitization (p=0.016), 3rd party sensitization with PRA>0 (p=0.03), and worse HLA matching (p=0.001) (Table 3). Risk factors for AMR were donor-specific sensitization, worse HLA matching, and female gender. Recipient age >50 was protective.
Table 3.
Cox Proportional Hazard modeling for risk of rejection, all groups (N=587).
| CMR | AMR | |||
|---|---|---|---|---|
| p | HR | p | HR | |
| Donor Sensitized vs Unsensitized | 0.016 | 1.87 | <0.0001 | 4.90 |
| Sensitized, pk PRA>0 vs Unsensitized | 0.030 | 1.59 | 0.720 | 1.15 |
| Sensitized, pk PRA=0 vs Unsensitized | 0.757 | 0.94 | 0.316 | 0.69 |
| Donor Source (living vs deceased) | 0.678 | 0.93 | 0.636 | 0.87 |
| African American Yes vs No | 0.265 | 1.36 | 0.807 | 0.88 |
| Female vs Male | 0.834 | 1.03 | 0.052 | 1.71 |
| Recipient age <18 vs 18–50 | 0.134 | 1.37 | 0.068 | 1.77 |
| Recipient age >50 vs 18–50 | 0.004 | 0.61 | 0.007 | 0.40 |
| HLA ABDR Mismatch 3–6 vs 0–2 | 0.001 | 1.86 | 0.001 | 5.00 |
| Dialysis <180 days vs None | 0.984 | 1.00 | 0.798 | 1.10 |
| Dialysis >180 days vs None | 0.167 | 1.29 | 0.846 | 1.06 |
pk=peak, PRA=panel reactive antibody.
In a subanalysis of the 3rd party sensitized patients (Group 2) (n=363), risk factors for CMR were PRA>0, age <18 and worse HLA matching, while risk factors for AMR included only age <18 and worse HLA matching (Table 4).
Table 4.
Cox Proportional Hazard modeling for risk of rejection for (3rd party) sensitized patients (n=363).
| CMR | AMR | |||
|---|---|---|---|---|
| p | HR | p | HR | |
| Donor Source (living vs deceased) | 0.609 | 0.89 | 0.397 | 1.54 |
| Retransplant vs Primary Transplant | 0.120 | 1.50 | 0.403 | 1.59 |
| Group 2B (pkPRA>0) vs Group 2A (pkPRA=0) | 0.010 | 1.69 | 0.178 | 1.73 |
| African American Yes vs No | 0.883 | 1.06 | 0.679 | 0.65 |
| Female vs Male | 0.820 | 0.96 | 0.820 | 0.91 |
| Recipient age <18 vs 18–50 | 0.011 | 2.00 | 0.028 | 3.07 |
| Recipient age >50 vs 18–50 | 0.114 | 0.69 | 0.576 | 0.76 |
| HLA ABDR Mismatch 3–6 vs 0–2 | 0.004 | 1.96 | 0.018 | 4.31 |
| Dialysis <180 days vs None | 0.952 | 0.98 | 0.597 | 1.35 |
| Dialysis >180 days vs None | 0.661 | 1.11 | 0.659 | 1.24 |
pk=peak, PRA=panel reactive antibody.
Importantly, by multivariate analysis, risk factors for DCGS were history of dialysis >180 days (p=0.022), pure CMR (p=0.0005), and mixed rejection (CMR+AMR) (p<0.0001) (Table 5). With rejection included in the analysis, donor-specific sensitization was not significant.
Table 5.
Cox Proportional Hazard modeling for risk of death censored graft loss, all groups (N=587).
| p | HR | |
|---|---|---|
| Donor Sensitized vs Unsensitized | 0.237 | 1.70 |
| Sensitized, pk PRA>0 vs Unsensitized | 0.083 | 1.93 |
| Sensitized, pk PRA=0 vs Unsensitized | 0.770 | 1.12 |
| Donor Source (living vs deceased) | 0.994 | 1.00 |
| African American Yes vs No | 0.680 | 1.20 |
| Female vs Male | 0.162 | 0.67 |
| Recipient age <18 vs 18–50 | 0.097 | 0.50 |
| Recipient age >50 vs 18–50 | 0.060 | 0.56 |
| HLA ABDR MM 3–6 vs 0–2 | 0.850 | 0.94 |
| Dialysis <180 days vs None | 0.174 | 1.85 |
| Dialysis >180 days vs None | 0.022 | 2.26 |
| CMR only | 0.0005 | 3.00 |
| CMR + AMR | <0.0001 | 6.70 |
pk=peak, PRA=panel reactive antibody.
Discussion
Detection of preformed HLA antibody by SAB testing has revolutionized the pretransplant evaluation of donor and recipient compatibility. Immunologic risk has been previously assessed in retrospective analyses noting associations between high PRA, retransplants, and DD grafts and increased rates of rejection and/or graft loss. These studies were done before the advent of SAB testing for DSA. DSA present in low or even moderate amounts may not cause a positive AHG-XM and it is likely that many XM-negative recipients had DSA. Therefore it is quite probable that historically accepted risk factors such as retransplantation or increased PRA were surrogates for (unrecognized) donor specific sensitization(1, 17, 21).
Our practice of setting a very low threshold (MFI>500) for a definition of the presence of DSA permitted a stringent criterion of those free from DSA at the time of transplant, thus facilitating investigation of the contribution of historically accepted risk factors for poor immunologic outcome.
In this study, we reassessed three previously described risk factors for rejection and graft loss after kidney transplantation (namely sensitization, retransplantation, and DD grafts) in the context of solid phase antibody detection. By univariate analysis, none of these risk factors were significantly associated with AMR. However, we did confirm previous studies that noted recipients with PRA seemed to have an increased risk of CMR (although this did not reach statistical significance, perhaps due to underpowering). These findings expand on the existing literature in that we found PRA remained a significant risk factor for CMR even after excluding recipients with DSA in the day of transplant sera.
Retransplant status and DD grafts were not associated with a higher risk of rejection or graft loss in both univariate and multivariate analyses. In addition, PRA (in the absence of DSA) was not a significant risk factor for AMR. Our data suggests that these factors alone should not be criteria for the application of more intense post-transplant immunosuppression protocols. These findings call into question a) the general practice of using certain historically reported risk factors to identify patients at risk for rejection (especially AMR), and b) challenges the rationale for including non-donor sensitized patients in study protocols for AMR prevention due to low event rates. An important limitation to our observations is that all patients were treated with polyclonal antibody induction, calcineurin inhibitor, and antimetabolite. It may be that these traditional risk factors would remain relevant if other immunosuppressive protocols were utilized.
Importantly, we showed that donor specific sensitization was a risk factor for rejection (Figure 1) and rejection was a risk factor for death-censored graft loss (Figure 5). Differences in graft and patient survival were not seen at one year post-transplant (Table 2), however were evident at longer followup (Figures 2 and 3). However, in the absence of AMR, donor sensitization did not lead to increased graft loss (Figure 5A) and multivariate analysis revealed rejection to be the most significant risk factor for graft loss (Table 5). Additionally, CrCl was preserved in donor sensitized recipients who did not experience AMR (data not shown). Together, these observations suggest that rejection, especially AMR, is the most important risk factor for decreased DCGS and CrCl after kidney transplantation; thus avoidance or prevention of AMR may result in improved long-term outcomes.
Our hypothesis was that DSA would be associated with increased AMR. It is interesting that the donor sensitized group (Group 3) had more CMR than any other group; this may be due to AMR usually presenting as a mixed rejection (both CMR and AMR). A missed AMR diagnosis (C4d negative AMR in the era before SAB testing) for those in Group 2B may at least partly contribute toward the observation of increased CMR rates for Group 2B compared to groups 2A or 1(22, 23). Alternatively, a cell mediated memory response may play a role, as suggested by Poggio et al(24, 25).
Median time to AMR was longer for Group 1 compared to other groups, suggesting that the AMR in unsensitized recipients is likely due to de novo antibody production. This later presentation may be due to reductions in immunosuppression or the potential emergence of medication non-adherence. The mean time to AMR in the sensitized groups was much shorter, suggesting that the early presentation of AMR is primarily driven either by an anamnestic response (Groups 2A and 2B) or DSA present at transplant (Group 3). Further examination of historical (peak PRA) serum samples for DSA in Group 2B recipients may elucidate the risk of historical DSA alone toward the development of AMR.
Strengths of our study include complete sensitization history, a stringent cut point for the determination of DSA (by SAB testing), 6 locus HLA typing for donors and recipients, a standardized induction and maintenance immunosuppression protocol, and uniform post-transplant monitoring. This is in contrast to previous reports from registry data or single centers with heterogenous pretransplant histocompatibility testing and induction immunosuppression protocols. Our approach facilitated analysis of pretransplant characteristics for contribution toward immunologic risk. However, as noted above, the uniform use of a rapid discontinuation of prednisone protocol and anti-lymphocyte globulin induction in our largely Caucasian population precludes extrapolation to prednisone maintenance or non-induction protocols; as lymphodepletion alters the natural milieu and specifically increases early T regulatory cell proliferation, potentially altering T memory or donor specific humoral responses(26, 27). Expanding our approach to a diverse, multi-center cohort with non-depleting induction is needed to confirm our findings.
Potential limitations of this study include our definition of AMR and lack of protocol biopsy data. Our definition of AMR may be considered somewhat liberal compared to very recent Banff guidelines(9), since we initially categorized biopsies as having AMR when there was diffusely positive C4d staining as the sole finding in the setting of otherwise unexplained graft dysfunction. This practice predominated before SAB testing was employed at our center and may have overestimated AMR. However, Haas et al. has shown that the majority of early posttransplant C4d+ biopsies (92%) correlate with the presence of DSA in HLA-incompatible grafts(28). It is possible (and even probable) that the actual AMR rate is higher than reported due subclinical (and chronic) AMR, the presentation of which may be insidious and fail to trigger a biopsy. Nonetheless, we found AMR and DCGS rates for Group 3 to be similar to other centers reports of incompatible kidney transplant outcomes(29–32).
Univariate analysis showed unexpected similarities in long-term rejection free graft and patient survival for some some study groups. These may be due to additional posttransplant events not controlled for in this study. Separating the effect of time on dialysis toward the risk of rejection presents a challenge, as longer time on dialysis is associated with multiple other variables of interest. We tried to address this by comparing patients with short (<180 days) and longer (>180 days) time on hemodialysis with those who were preemptively transplanted. It appeared that longer time on dialysis was not associated with AMR, but did impact risk of graft loss.
SAB testing is now a ubiquitous tool in modern histocompatibility labs. Data from pre and post-transplant SAB testing has shed light on risk that was previously not appreciated. Using SAB in combination with the AHG-XM has increased the transplant rate of sensitized candidates with increasingly compatible grafts, which is a laudable advance in transplantation(33, 34). This has facilitated the dual goal of increasing access to transplant as well as minimizing early and midterm graft loss from rejection; thus optimizing each recipient’s long term outcome.
Our study, although retrospective and single center, underscores that the best short and long-term immunologic outcomes occur when donor sensitization is avoided, and that historically accepted risk factors such as PRA, retransplant and DD grafts do not necessarily confer significant immunologic risk.
Questions remain as to the clinical significance of risk contributed by low-level DSA and positive flow XM in predicting long-term allograft survival. Further refinements in risk assessment of donor sensitized (HLA incompatible) transplants are needed in order for patients and clinicians to weigh the morbidities of rejection and shortened graft survival against the increased mortality rate of remaining on dialysis.
Acknowledgement
Funding Sources: NIH DK 13083.
Special recognition to Hang McLaughlin, M.Ed for assistance with manuscript preparation.
Abbreviations
- SAB
single antigen bead
- PRA
panel reactive antibody
- DD
deceased donor
- DSA
donor specific antibody
- DCGS
death censored graft survival
- AMR
antibody mediated rejection
- CMR
cell mediated rejection
- HLA
human leukocyte antigen
- CDC
complement dependent cytotoxicity
- AHG
anti-human globulin
- ELISA
enzyme-linked immunosorbent assay
- XM
crossmatch
- D0
day of transplant
- pk
peak
- MFI
mean fluorescence intensity
- CrCl
creatinine clearance
- LD
living donor
- IQR
interquartile range
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Bryan CF, Baier KA, Nelson PW, Luger AM, Martinez J, Pierce GE, et al. Long-term graft survival is improved in cadaveric renal retransplantation by flow cytometric crossmatching. Transplantation. 1998 Dec 27;66(12):1827–1832. doi: 10.1097/00007890-199812270-00043. [DOI] [PubMed] [Google Scholar]
- 2.Mulder A, Kardol MJ, Kamp J, Uit Het Broek C, Schreuder GM, Doxiadis II, et al. Determination of the frequency of HLA antibody secreting B-lymphocytes in alloantigen sensitized individuals. Clin Exp Immunol. 2001 Apr;124(1):9–15. doi: 10.1046/j.1365-2249.2001.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerman RH, Orosz CG, Lorber MI. Clinical relevance of anti-HLA antibodies pre and post transplant. Am J Med Sci. 1997 May;313(5):275–278. doi: 10.1097/00000441-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gebel HM, Bray RA. Sensitization and sensitivity: defining the unsensitized patient. Transplantation. 2000 Apr 15;69(7):1370–1374. doi: 10.1097/00007890-200004150-00027. [DOI] [PubMed] [Google Scholar]
- 5.Zeevi A, Girnita A, Duquesnoy R. HLA antibody analysis: sensitivity, specificity, and clinical significance in solid organ transplantation. Immunol Res. 2006;36(1–3):255–264. doi: 10.1385/IR:36:1:255. [DOI] [PubMed] [Google Scholar]
- 6.Leffell MS, Zachary AA. Antiallograft antibodies: relevance, detection, and monitoring. Curr Opin Organ Transplant. 2010 Feb;15(1):2–7. doi: 10.1097/MOT.0b013e3283342798. [DOI] [PubMed] [Google Scholar]
- 7.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999 Feb;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 8.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003 Jun;3(6):708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 9.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008 Apr;8(4):753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 10.Salvatierra O, Jr, Perkins HA, Amend W, Jr, Feduska NJ, Duca RM, Potter DE, et al. The influence of presensitization on graft survival rate. Surgery. 1977 Feb;81(2):146–151. [PubMed] [Google Scholar]
- 11.Koka P, Cecka JM. Sensitization and crossmatching in renal transplantation. Clin Transpl. 1989:379–390. [PubMed] [Google Scholar]
- 12.Stratta RJ, Oh CS, Sollinger HW, Pirsch JD, Kalayoglu M, Belzer FO. Kidney retransplantation in the cyclosporine era. Transplantation. 1988 Jan;45(1):40–45. [PubMed] [Google Scholar]
- 13.Almond PS, Matas AJ, Gillingham K, Troppmann C, Payne W, Dunn D, et al. Risk factors for second renal allografts immunosuppressed with cyclosporine. Transplantation. 1991 Aug;52(2):253–258. doi: 10.1097/00007890-199108000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Flechner SM, Lorber MI, Kerman RH, Van Buren CT, Wideman C, Kahan BD. Does cyclosporine influence the results of cadaver retransplantation? Hum Immunol. 1985 Nov;14(3):305–313. doi: 10.1016/0198-8859(85)90238-1. [DOI] [PubMed] [Google Scholar]
- 15.House AA, Chang PC, Luke PP, Leckie SH, Howson WT, Ball EJ, et al. Re-exposure to mismatched HLA class I is a significant risk factor for graft loss: multivariable analysis of 259 kidney retransplants. Transplantation. 2007 Sep 27;84(6):722–728. doi: 10.1097/01.tp.0000281398.41670.1f. [DOI] [PubMed] [Google Scholar]
- 16.Gruber SA, Brown KL, El-Amm JM, Singh A, Mehta K, Morawski K, et al. Equivalent outcomes with primary and retransplantation in African-American deceaseddonor renal allograft recipients. Surgery. 2009 Oct;146(4):646–652. doi: 10.1016/j.surg.2009.05.020. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- 17.Sanfilippo F, Vaughn WK, LeFor WM, Spees EK. Multivariate analysis of risk factors in cadaver donor kidney transplantation. Transplantation. 1986 Jul;42(1):28–34. doi: 10.1097/00007890-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Deng CT, El-Awar N, Ozawa M, Cai J, Lachmann N, Terasaki PI. Human leukocyte antigen class II DQ alpha and beta epitopes identified from sera of kidney allograft recipients. Transplantation. 2008 Aug 15;86(3):452–459. doi: 10.1097/TP.0b013e3181804cd2. [DOI] [PubMed] [Google Scholar]
- 19.Noreen HJ, McKinley DM, Gillingham KJ, Matas AJ, Segall M. Positive remote crossmatch: impact on short-term and long-term outcome in cadaver renal transplantation. Transplantation. 2003 Feb 27;75(4):501–505. doi: 10.1097/01.TP.0000048225.98745.64. [DOI] [PubMed] [Google Scholar]
- 20.Khwaja K, Asolati M, Harmon J, Melancon JK, Dunn T, Gillingham K, et al. Outcome at 3 years with a prednisone-free maintenance regimen: a single-center experience with 349 kidney transplant recipients. Am J Transplant. 2004 Jun;4(6):980–987. doi: 10.1111/j.1600-6143.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 21.Cho YW, Cecka JM. Crossmatch tests--an analysis of UNOS data from 1991–2000. Clin Transpl. 2001:237–246. [PubMed] [Google Scholar]
- 22.Halloran PF, Schlaut J, Solez K, Srinivasa NS. The significance of the anti-class I response. II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation. 1992 Mar;53(3):550–555. [PubMed] [Google Scholar]
- 23.Cosio FG, Lager DJ, Lorenz EC, Amer H, Gloor JM, Stegall MD. Significance and implications of capillaritis during acute rejection of kidney allografts. Transplantation. 2010 May 15;89(9):1088–1094. doi: 10.1097/TP.0b013e3181d368f1. [DOI] [PubMed] [Google Scholar]
- 24.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, et al. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation. 2007 Apr 15;83(7):847–852. doi: 10.1097/01.tp.0000258730.75137.39. [DOI] [PubMed] [Google Scholar]
- 25.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005 Mar;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 26.Lynch RJ, Platt JL. Accommodation in renal transplantation: unanswered questions. Curr Opin Organ Transplant. 2010 Aug;15(4):481–485. doi: 10.1097/MOT.0b013e32833b9c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010 Sep;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006 Aug;6(8):1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 29.Pollinger HS, Stegall MD, Gloor JM, Moore SB, Degoey SR, Ploeger NA, et al. Kidney transplantation in patients with antibodies against donor HLA class II. Am J Transplant. 2007 Apr;7(4):857–863. doi: 10.1111/j.1600-6143.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 30.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008 Feb;8(2):324–331. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 31.Amico P, Honger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009 Jun 15;87(11):1681–1688. doi: 10.1097/TP.0b013e3181a5e034. [DOI] [PubMed] [Google Scholar]
- 32.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009 Nov;9(11):2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 33.Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003 Dec;3(12):1488–1500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 34.Bingaman AW, Murphey CL, Palma-Vargas J, Wright F. A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation. 2008 Dec 27;86(12):1864–1868. doi: 10.1097/TP.0b013e318191404c. [DOI] [PubMed] [Google Scholar]