Abstract
Wounding usually causes considerable cell damage, and released ATP promotes migration of nearby epithelium. ATP binds to purinergic receptors on the cell surface and induces transactivation of the EGF receptor through signaling by the Src family kinases (SFKs). Here we tested whether ATP activates these kinases through Pyk2, a member of the focal adhesion kinase family. Pyk2 was rapidly and potently activated by treating corneal epithelial cells with ATP, and physical interaction of Pyk2 with the SFKs was enhanced. Disruption of Pyk2 signaling either by siRNA or by expression of a dominant-negative mutant led to inhibition of ATP-induced activation of the SFKs and the EGF receptor. Inhibiting Pyk2 activity also blocked ATP stimulation of healing of wounds in epithelial cell sheets. These data suggest that ATP stimulates sequential activation of Pyk2, SFKs, and the EGFR to induce cell migration.
Keywords: proline-rich tyrosine kinase 2, protein tyrosine kinase 2 beta, PTK2B, cell migration
1. Introduction
Epithelia respond robustly to damage by migrating and closing wounds in order to maintain integrity. Wounding induces many potential stimuli that can promote motility including release of growth factors and cytokines from nearby tissues and blood, exposure of extracellular matrix allowing formation of new interactions with cell surface receptors, mechanical forces, and even the very presence of unconstrained edges in the epithelial cell sheets [1–6]. Wounding invariably involves at least some cell breakage, and released ATP acts as an extracellular signaling molecule, which has been known for some time to induce motility in many cell types [7–11]. ATP exists in high concentrations (low mM) inside cells, and after cell breakage the released ATP binds to purinergic receptors on cells to elicit numerous cellular responses.
Activation of the epidermal growth factor receptor (EGFR) is a common requirement for induction of motility in many cell types after wounding [12–17]. Like many other cues that induce motility, ATP triggers activation of the EGFR through activation of the Src family kinases (SFKs), which leads to proteolytic release of transmembrane ligands such as heparin-binding EGF-like growth factor (HB-EGF) and amphiregulin in a mechanism known as the “triple-membrane-passing” mode of transactivation of the EGFR by G-protein coupled receptors [18–21].
Pyk2 is a member of the focal adhesion kinase (FAK) family [22, 23], which are well-established activators of the SFKs. We have previously reported that proline-rich tyrosine kinase 2 (Pyk2) rather than focal adhesion kinase is activated by wounding in conditions that excluded signaling by extracellular ATP [24]. In this paper we examined whether Pyk2 also mediates ATP-induced SFK activation and whether this is necessary for induction of motility by the nucleotide.
2. Materials and methods
2.1. Materials
Antibodies against a C-terminal epitope of the EGFR, the EGFR phosphorylated on tyrosine 1173, Pyk2 phosphorylated on tyrosine 402, FAK phosphorylated on tyrosine 397, and c-Src (mouse monoclonal used for immunoprecipitation) were from Santa Cruz Biotechnology; antibodies against SFK phosphorylated on tyrosine 419, SFK non-phosphorylated on tyrosine 419, c-Src (rabbit polyclonal used for immunoblotting), and the EGFR phosphorylated on tyrosine 845 were from Cell Signaling Technology; antibodies against Pyk2 were from BD Biosciences; antibodies against β-actin were from Sigma. Tyrphostin AG 1478 and Src Kinase Inhibitor-I were from EMD Biosciences. ATP was from Sigma. Cell culture reagents were from MediaTech, and all other supplies and reagents were from ISC BioExpress or ThermoFisher, unless otherwise noted.
2.2 Cell culture, ATP treatment, and wounding models
Human corneal limbal epithelial (HCLE) cells have been immortalized by abrogation of p16INK4A/Rb and p53 functions and overexpression of the catalytic subunit of the telomerase holoenzyme [25]. HCLE cells were cultured in keratinocyte serum-free medium (Invitrogen) supplemented with 0.3 mM CaCl2, 25 μg/ml bovine pituitary extract, 0.1 ng/ml human recombinant EGF, 50 IU/ml penicillin, and 50 μg/ml streptomycin. At least 16 hours prior to experiments, cells were washed and cultured in the same medium without pituitary extract and EGF. The standard ATP treatment was 5 μM ATP for 5 minutes. For wound healing assays, cells were cultured to confluence around a single agarose strip [14] and induced to differentiate into a stratified epithelium by culturing in DMEM:F-12 1:1 with 10% newborn calf serum [25]. Three days after transfection with small interfering RNA (siRNA), the agarose strips were removed and cultures were allowed to heal for 18 hours before fixation and staining with Gentian violet [14]. Micrographs of cultures were obtained before and after wounding and wound areas were quantified using the region tracing utility in MetaMorph® software (Universal Imaging).
2.3 Western Blotting and immunoprecipitation
Following stimulation, cells were washed in ice-cold phosphate-buffered saline (171 mM NaCl, 10.1 mM Na2HPO4, 3.35 mM KCl, and 1.84 mM KH2PO4, pH 7.2) and lysed in either SDS (1% in H2O) or immunoprecipitation buffer (50 mM Tris-Cl, 260 mM NaCl, 0.02% NaN3, 5 mM EDTA, 1% Triton X-100, 0.5 mM Na3VO4, 50 mM NaF, 1 tablet/10 ml of protease inhibitor cocktail (Roche Diagnostics GmbH), and 5 μM pepstatin A, pH 7.5). Protein contents of extracts, determined with the bicinchoninic acid assay (ThermoFisher), were normalized prior to SDS-PAGE. For immunoprecipitation, approximately 250 μg of cellular protein was incubated with 30 μl of protein-A Sepharose slurry and 1 μg of antibody on an end-over-end rotator at 4°C overnight. Pellets were washed twice with immunoprecipitation buffer and three times with immunoprecipitation buffer with Triton X-100 reduced to 0.1% before addition of SDS-PAGE sample buffer. Multiple exposures of Western Blots were collected, and densitometry of appropriate images was performed using QuantityOne software (BioRad).
2.4 siRNA transfection and adenoviral infection
siRNA duplexes targeting Pyk2 (10 nM) were transfected using siPORT™ NeoFX™ transfection reagent (Applied Biosystems) according to manufacturer’s protocol. Cells were then re-seeded after two days at experimental densities and used four days after transfection. Multiple Pyk2 siRNA oligonucleotides were used: CACAUGAAGUCCGAUGAGAdTdT (Sigma) and a Pyk2 SMARTpool that contains at least four duplexes of undisclosed sequence (Millipore). As a control siRNA, MISSION® siRNA Universal Negative Control #1 (Sigma) was used. Pyk2 and PRNK cloned into adenovirus vectors were from Dr. Joseph C. Loftus (Mayo Clinic, Scottsdale, AZ). Adenovirus encoding tet-OFF (used as adenovirus control) was from Dr. Ora A. Weisz (University of Pittsburgh, Pittsburgh, PA). For signaling studies, cells were infected at a multiplicity of 10 for 1 hr and were used the following day.
2.5 Statistical analysis
All experiments were performed a minimum of three times with similar results. Representative immunoblots are shown below the bar graphs. Densitometry were from at least four replicates, and quantitative data were plotted (means +/− S.D.) and analyzed by t-test or one-way ANOVA followed by Bonferroni’s multiple comparisons test using Prism (GraphPad Software).
3. Results
1.1 Stimulation with ATP activates Pyk2
We and others have previously reported that ATP stimulates activation of the EGFR, and that it occurs through a triple-membrane-passing mode of signaling that is mediated by the SFKs [7, 11, 17]. Members of the focal adhesion kinase (FAK) family are activated by autophosphorylation creating binding sites for the SFKs, which subsequently are activated by another autophosphorylation event. We tested the activation state of FAK with an antibody that we have previously verified recognizes FAK autophosphorylated on tyr-397 in immortalized human corneal limbal epithelial (HCLE) cells [25]. As is illustrated in Fig. 1A no activation was observed after stimulation with ATP. In contrast, strong activation of Pyk2 was readily detected by an antibody that recognizes its major autophosphorylation site on tyr-402. A time course analysis showed detectable activation of Pyk2 up to two hours after stimulation (Fig. 1B), whereas activation of FAK was not observed at any time point.
Fig 1.
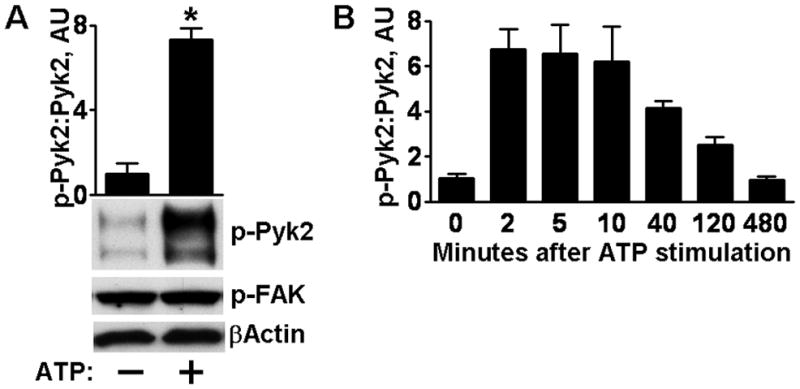
Activation of Pyk2 by exogenous ATP. (A) HCLE cells were untreated or subjected to the standard treatment with ATP and cell extracts were analyzed by immunoblotting for Pyk2 phosphorylated on tyr-402 (p-Pyk2) or for FAK phosphorylated on tyr-397 (p-FAK). Due to various splicing and post-translational events, the antibody for phosphorylated Pyk2 detects multiple bands, all of which are used in the analysis. The ratio of p-Pyk2:Pyk2 (not shown) was determined by densitometry and * denotes significant differences from untreated cells (p<0.001) using the Student’s t-test. Columns are means and error bars are S.D. in this and the following figures. (B) Time course of Pyk2 activation. AU, arbitrary units.
Pyk2 can be activated downstream of the EGFR in some systems [26], and we therefore examined the effects of addition of the EGFR kinase inhibitor tyrphostin AG 1478. Although the inhibitor abolished autophosphorylation of the EGFR, no effects were seen on Pyk2 phosphorylation (Fig. 2A). Pyk2 contains tyrosines that can be phosphorylated by the SFKs, and some studies indicate that SFK-mediated phosphorylation regulates Pyk2 activity [27–29]. However, ATP-induced Pyk2 autophosphorylation was unaffected by treatment with the SFK inhibitors Src Kinase Inhibitor-I (SKI) or PP2 (Figs. 2B and C) [30]. The SFK inhibitors blocked EGFR phosphorylation of tyr-845, which is catalyzed by the SFKs [31, 32], demonstrating the efficacy of the inhibitors. Together, these results indicate that Pyk2 is activated by exogenous ATP independently of the EGFR and the SFKs.
Fig 2.
Characterization of ATP-induced Pyk2 activation. (A) Cells received no treatment or were stimulated with ATP. Where indicated, cells were treated with 100 nM tyrphostin AG 1478 (AG) for 30 min before addition of ATP. Western Blots were probed for p-Pyk2 and then stripped and re-probed for total levels of Pyk2 and EGFR phosphorylated on tyr-1173 (p-EGFR). The ratio of p-Pyk2:Pyk2 was determined by densitometry and * denotes significant differences from unstimulated control groups (p<0.001). (B, C) Cells were treated as in (A), except 1 μM PP2 or 1 μM of Src Kinase Inhibitor-I (SKI) was added for 30 min before addition of ATP, as indicated. The blots were analyzed with antibodies to p-Pyk2 and Pyk2 as in A, and with an antibody that recognizes the EGFR phosphorylated on tyr-845 (p-EGFR-Y845). (D) Extracts from control and ATP-treated cells were blotted with an antibody that recognizes all isoforms of the SFKs phosphorylated on tyr-419. The blots were stripped and blotted with an antibody that recognizes all isoforms not phosphorylated on tyr-419. * indicates significant stimulation of SFK by ATP (p<0.0001). (E) Extracts from cells treated as indicated were precipitated with c-Src antibodies, which removed approximately 90% of the c-Src present in the extracts (not shown), and the precipitates were subjected to Western Blotting for SFK phosphorylated on tyr-419 (p-SFK) and subsequently for total c-Src. * indicates significant increases of phosphorylated c-Src compared to the corresponding controls (p<0.001). (F) The extracts from (E) were blotted with antibodies to Pyk2 phosphorylated on tyr-402 (p-Pyk2) and total levels of c-Src. * indicates significant increases of phosphorylated c-Src compared to the corresponding controls (p<0.01).
To determine whether the SFKs are activated by stimulation with ATP in HCLE cells as has been seen in other systems [33–35], extracts were blotted with an antibody that recognizes all isofoms of the SFKs phosphorylated on tyr-419, the major activating autophosphorylation site. This confirmed the expectation that ATP activates SFKs in these cells (Fig. 2D). To determine whether stimulation by extracellular ATP enhances formation of SFK-Pyk2 complexes, we analyzed anti-c-Src immunoprecipitates. We first probed with the phospho-SFK antibody, which showed that the c-Src isoform is activated by addition of extracellular ATP (Fig. 2E). Importantly, increased amounts of Pyk2 were present in the immunoprecipitates of extracts from ATP-treated cells, demonstrating an increased association of c-Src and Pyk2 (Fig. 2F). The interaction was not blocked by the presence of tyrphostin AG 1478, in agreement with the expectation that association occurs upstream of the EGFR.
3.2. Pyk2 mediates EGFR activation by ATP
To examine whether Pyk2 mediates EGFR activation, we tested the effects of reducing expression of Pyk2. Cells were transfected with 10 nM of a commercial pool of siRNAs of undisclosed sequences targeted to Pyk2. Transfection significantly inhibited activation of the EGFR by added ATP (Supplementary Content Fig. 1). To confirm and to better control for off-target effects, we also used a siRNA with a defined sequence. This similarly resulted in 80% reduction in Pyk2 expression. Whereas ATP induced 250% activation in cells transfected with control siRNA, only 20% activation was seen in cells transfected with Pyk2 siRNA (Fig. 3A). In the same experiments we verified that transfection of the siRNA did not interfere with activation of the EGFR by EGF, indicating that the reduction of activation is not due to unspecific effects on the receptor (Fig. 3B).
Fig. 3.
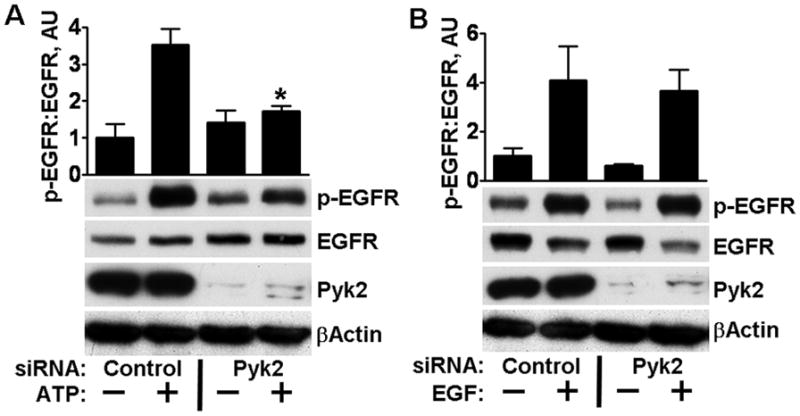
Knockdown of Pyk2 with siRNA inhibits EGFR activation after ATP stimulation but not after stimulation with EGF. (A) HCLE cells transfected with 10 nM of control or Pyk2 siRNA were treated with ATP as indicated. Immunoblots were probed for EGFR phosphorylated on tyr-1173, stripped and reprobed with antibodies that recognize total EGFR, Pyk2, or β–actin. * denotes significant decrease from ATP-treated controls (p<0.001). (B) Cells were treated as in (A), stimulated with 10 ng/ml EGF for 5 minutes where indicated, and extracts immunoblotted as in A.
As an independent approach to block Pyk2 signaling, we used a dominant negative construct, Pyk2-related non-kinase (PRNK), which consists of the 228 C-terminal amino acids of Pyk2 and lacks a kinase domain, and which acts as a dominant-negative that blocks Pyk2 signaling [36]. Expression of this construct blocked Pyk2 activation by ATP (Fig. 4A) as expected. Importantly, activation of the EGFR by addition of ATP was also strongly inhibited by the presence of PRNK (Fig. 4B). Controls showed that activation of the EGFR by EGF was not altered by PRNK (Fig. 4C).
Fig. 4.

Expression of a Pyk2 dominant-negative mutant inhibits SFK and EGFR activation after wounding but not after stimulation with EGF. (A) Cells were infected with control adenovirus or adenovirus coding for PRNK and treated with ATP as indicated. Blots were probed with antibodies against p-Pyk2, stripped, and probed with antibodies against total Pyk2. * indicates significant increase over control (p<0.001). (B) Cells were treated as in (A). Western Blots were probed for EGFR phosphorylated on tyr-1173, stripped and re-probed with antibodies that recognize total EGFR, Pyk2, or β–actin. * denotes significant decrease from ATP-treated controls (p<0.001). (C) Cells were treated as in (A), stimulated with 10 ng/ml EGF for 5 minutes where indicated, and extracts immunoblotted as in B.
We have previously found that forcing Pyk2 signaling by overexpression strongly stimulates SFK and EGFR activities in agreement with an upstream role of Pyk2 [24]. Together with the blocking experiments reported here, this strongly supports that Pyk2 is a signaling intermediate that mediates ATP-induced transactivation of the EGFR..
3.3 Activation of Pyk2 is required for stimulation of cell motility by ATP
Since Pyk2 mediates EGFR activation by exogenous ATP, we tested the expectation that Pyk2 also mediates ATP-induced enhancement of cell motility. The presence of added ATP in the culture increased the speed of closure significantly in our wound healing assay (Fig. 5A). Acceleration of healing by exogenous ATP was previously reported to depend on EGFR signaling in an SV40 immortalized cell line [11], and this was confirmed with HCLE cells (data not shown). Transfection with the single Pyk2 siRNA, which was shown above to block EGFR activation, resulted in complete inhibition of ATP-stimulated healing. As previously reported, transfection with this oligonucleotide does not interfere with enhancement of wound closure by EGF [24], so the decreased healing is not due to universal effects on the cell motility machinery. Furthermore, transfection with the oligonucleotide did not result in reduced cell densities, and the result is therefore not due to the presence of fewer cells in the siRNA transfected cultures (Supplementary Content Fig. 2A).
Fig. 5.
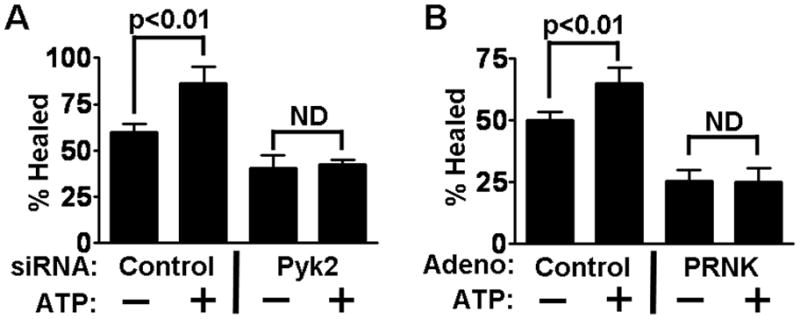
Pyk2 activation is required for stimulation of cell motility by ATP. (A) HCLE cells were transfected with control or Pyk2 siRNA prior to the wound healing assays, and were incubated with ATP as indicated. P values for relevant significant differences are indicated. ND, no significant difference. (B) A similar experiment was performed with cells infected with adenovirus coding for PRNK.
Infection with adenovirus coding for PRNK similarly blocked ATP stimulation of healing (Fig. 5B). In the presence of EGF, inhibition of motility could largely be rescued by EGF [24], and there was no effect on cell densities (Supplementary Content Fig. 2B). Unfortunately, infection with virus coding for wt-Pyk2 was toxic over the course of the healing assay, and no meaningful analysis could be made.
Since inhibition of Pyk2 signaling both by Pyk2 siRNA and the dominant negative PRNK resulted in inhibition of ATP-stimulated healing, we conclude that the effects of ATP on cell motility depend on Pyk2 to stimulate SFK/EGFR signaling.
4. Discussion
ATP is usually released in copious amounts from broken cells at the time of wounding, and it acts as an important extracellular messenger that enhances motility of many types of epithelial cells [7–11]. Activation of the EGFR is considered absolutely required for induction of motility in corneal epithelial cells and many other cell types [12–16], and ATP induces activation of the EGFR through SFK-initiated proteolytic mobilization of ligands of the receptor in a triple-membrane-passing type of mechanism. A great number of mechanisms exist for activation of the SFKs [37–39]. Here we have determined that Pyk2 but not FAK is activated by ATP stimulation, that Pyk2 and SFKs associate physically after stimulation, that Pyk2 is necessary for SFK/EGFR activation, and that Pyk2 function is required for promotion of motility by ATP. Together, these data suggest that ATP stimulates sequential activation of Pyk2, SFKs, and the EGFR to induce cell migration.
Pyk2 has been extensively described as a downstream effector of various hormones and growth factors in induction of cell motility by modulating the dynamics of the cytoskeleton [22, 23, 40–42]. A few reports have identified an upstream role for Pyk2 in activating the EGFR. Activation of the EGFR was shown to be blocked by PRNK in rat liver cells treated with the ciglitazone, a ligand for the nuclear proliferator-activated receptor γ [29], and Pyk2 deficient fibroblasts exhibit reduced EGFR activation after stimulation with various G-protein coupled receptor agonists, although this involves a direct phosphorylation of the EGFR rather than a triple-membrane-passing mechanism [43]. Activation of the SFKs is known to be a necessary element of the reactions that lead to transactivation of the EGFR by many G-coupled receptors [18–21]. It will be interesting to see whether G protein transactivation of the EGFR generally occurs through Pyk2 activation.
Hyperactive EGFR signaling drives the initiation and progression of many tumors, and extensive efforts are directed to develop drugs to inhibit this signaling system [44–46]. One mechanism for inappropriate EGFR activation is overproduction of ligands, and it is therefore important to understand how their shedding is regulated [47–49]. Pyk2 has been noted to be overexpressed in many types of cancer [50], which could result in overstimulation of the receptor through enhanced production of EGFR ligands by the pathway described here. Since Pyk2 is also activated downstream of the receptor, it is conceivable that they participate in a positive feed-back loop that accentuates activation of the EGFR. There is considerable interest in developing small molecular weight inhibitors of Pyk2 as anti-tumor agents [50]. The rationale given is that Pyk2 is a downstream effector of the EGFR and other receptors that regulates cell migration, survival, and growth. The data provided here suggest that inhibiting Pyk2 may also attenuate the transformed phenotype of tumor cells by decreasing production of EGFR ligands.
Supplementary Material
Highlights.
ATP activates the EGF receptor and cell motility through signaling by Src family kinases (SFKs)
Pyk2 is rapidly activated by stimulation with exogenous ATP
Blocking Pyk2 function inhibits SFKs and EGF receptor activation
Blocking Pyk2 function also inhibits ATP-stimulated healing of wounds in cell sheets
Pyk2 mediates ATP-induced activation of the SFKs, the EGF receptor, and the enhanced cell motility
Acknowledgments
This work was supported by the National Institutes of Health Grants EY08098 and T32 EY017271 and grants from Research to Prevent Blindness and The Eye and Ear Foundation (Pittsburgh, PA).
Abbreviations
- EGFR
epidermal growth factor receptor
- SFK
Src family kinase
- FAK
focal adhesion kinase
- Pyk2
proline-rich tyrosine kinase 2
- HCLE
human corneal limbal epithelial
- SKI
Src Kinase Inhibitor-I
- siRNA
small interfering RNA
- PRNK
Pyk2-related non-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 3.Schultz GS, Wysocki A. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 4.Carter RT. Vet Ophthalmol. 2009;12(Suppl 1):2–9. doi: 10.1111/j.1463-5224.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu FS, Yin J, Xu K, Huang J. Brain Res Bull. 81:229–235. doi: 10.1016/j.brainresbull.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block ER, Tolino MA, Lozano JS, Lathrop KL, Sullenberger RS, Mazie AR, Klarlund JK. Mol Biol Cell. 2010;21:2172–2181. doi: 10.1091/mbc.E09-12-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block ER, Klarlund JK. Mol Biol Cell. 2008;19:4909–4917. doi: 10.1091/mbc.E08-01-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kartha S, Toback FG. J Clin Invest. 1992;90:288–292. doi: 10.1172/JCI115851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sponsel HT, Breckon R, Anderson RJ. Kidney Int. 1995;48:85–92. doi: 10.1038/ki.1995.271. [DOI] [PubMed] [Google Scholar]
- 10.Dignass AU, Becker A, Spiegler S, Goebell H. Eur J Clin Invest. 1998;28:554–561. doi: 10.1046/j.1365-2362.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- 11.Yin J, Xu K, Zhang J, Kumar A, Yu FS. J Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Faseb J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 13.Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Invest Ophthalmol Vis Sci. 2000;41:1346–1355. [PubMed] [Google Scholar]
- 14.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 15.Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. J Invest Dermatol. 2004;123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Exp Eye Res. 2007;85:130–141. doi: 10.1016/j.exer.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer OM, Hart S, Gschwind A, Ullrich A. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 19.Blobel CP. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 20.Ohtsu H, Dempsey PJ, Eguchi S. Am J Physiol Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 21.Sanderson MP, Dempsey PJ, Dunbar AJ. Growth Factors. 2006;24:121–136. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- 22.Avraham H, Park SY, Schinkmann K, Avraham S. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 23.Loftus JC, Yang Z, Tran NL, Kloss J, Viso C, Berens ME, Lipinski CA. Mol Cancer Ther. 2009;8:1505–1514. doi: 10.1158/1535-7163.MCT-08-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Block ER, Tolino MA, Klarlund JK. J Biol Chem. 2010;285:13372–13379. doi: 10.1074/jbc.M109.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 26.Schauwienold D, Sastre AP, Genzel N, Schaefer M, Reusch HP. J Biol Chem. 2008;283:27748–27756. doi: 10.1074/jbc.M801431200. [DOI] [PubMed] [Google Scholar]
- 27.Duong LT, Lakkakorpi PT, Nakamura I, Machwate M, Nagy RM, Rodan GA. J Clin Invest. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewar BJ, Gardner OS, Chen CS, Earp HS, Samet JM, Graves LM. Mol Pharmacol. 2007;72:1146–1156. doi: 10.1124/mol.107.037549. [DOI] [PubMed] [Google Scholar]
- 30.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W, Graves LM, Gill GN, Parsons SJ, Samet JM. J Biol Chem. 2002;277:24252–24257. doi: 10.1074/jbc.M200437200. [DOI] [PubMed] [Google Scholar]
- 32.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 33.Carini R, Alchera E, De Cesaris MG, Splendore R, Piranda D, Baldanzi G, Albano E. J Hepatol. 2006;45:236–245. doi: 10.1016/j.jhep.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmi S, Joshi PG. Neuroscience. 2006;141:179–189. doi: 10.1016/j.neuroscience.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 35.Tamiya S, Okafor MC, Delamere NA. Am J Physiol Cell Physiol. 2007;293:C790–796. doi: 10.1152/ajpcell.00579.2006. [DOI] [PubMed] [Google Scholar]
- 36.Xiong WC, Macklem M, Parsons JT. J Cell Sci. 1998;111(Pt 14):1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]
- 37.Roskoski R., Jr Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Engen JR, Wales TE, Hochrein JM, Meyn MA, 3rd, Banu Ozkan S, Bahar I, Smithgall TE. Cell Mol Life Sci. 2008;65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingley E. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Rudge SA, Wakelam MJ. Biochim Biophys Acta. 2009;1791:856–861. doi: 10.1016/j.bbalip.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, Tanaka Y, Shibasaki M, Kanaho Y, Sasaki T, Frohman MA, Fukui Y. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Du G. Biochim Biophys Acta. 2009;1791:850–855. doi: 10.1016/j.bbalip.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. J Biol Chem. 2001;276:20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida T, Zhang G, Haura EB. Biochem Pharmacol. 2010;80:613–623. doi: 10.1016/j.bcp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Cassell A, Grandis JR. Expert Opin Investig Drugs. 19:709–722. doi: 10.1517/13543781003769844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pines G, Kostler WJ, Yarden Y. FEBS Lett. 2010;584:2699–2706. doi: 10.1016/j.febslet.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Cancer Sci. 2008;99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kataoka H. J Dermatol Sci. 2009;56:148–153. doi: 10.1016/j.jdermsci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto S, Fukami T, Yagi H, Kuroki M, Yotsumoto F. Anticancer Res. 2009;29:823–830. [PubMed] [Google Scholar]
- 50.Lipinski CA, Loftus JC. Expert Opin Ther Targets. 2010;14:95–108. doi: 10.1517/14728220903473194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



