Abstract
Elucidating the mechanisms underlying sex biases in the prevalence and severity of diseases can advance our understanding of their pathophysiological basis and serve as a guide for developing treatments. A well-established sex difference in psychiatry is the higher incidence of mood and anxiety disorders in females. These disorders share stress as a potential etiological contributor and hyperarousal as a core symptom, suggesting that the distinction between sexes lies at the intersection of stress and arousal systems. This review focuses on the link between the stress axis and the brain norepinephrine arousal system as a key point at which sex differences occur and are translated to differences in the expression of mood disorders. Evidence for a circuit designed to relay emotion-related information via the limbic corticotropin-releasing factor (CRF) system to the locus coeruleus (LC)–norepinephrine arousal system is reviewed. This is followed by recent novel findings of sex differences in CRF receptor signaling and trafficking that would result in an enhanced arousal response and a compromised ability to adapt to chronic stress in females. Finally, we discuss evidence for sex differences in LC dendritic structure that allow for increased receipt and processing of limbic information in females compared to males. Together these complementary sets of data suggest that in females, the LC arousal system is poised to process more limbic information and to respond to some of this information in an enhanced manner compared to males. The clinical and therapeutic considerations arising from this perspective are discussed.
Keywords: corticotropin-releasing factor, locus coeruleus, norepinephrine, beta arrestin, corticotropin-releasing hormone
Many psychiatric diseases exhibit a bias whereby their prevalence and/or severity are greater in one sex. For example, autism and attention deficit disorder are more prevalent in males (Gaub and Carlson, 1997; Ramtekkar et al., 2010). In contrast, affective disorders and many anxiety disorders are nearly twice as prevalent in females compared to males (Kessler, 2003; Kessler et al., 1994). Similarly, the incidence of posttraumatic stress disorder is greater in females despite males being exposed to more traumatic events (Breslau, 2001, 2002). Identifying the neurobiological bases for sex differences in psychiatric diseases can advance our understanding of their etiology and guide individualized therapies. This review focuses on understanding the prevalence of certain stress-related disorders (depression, post-traumatic stress disorder) in females. Using the framework that these disorders share stress as a mitigating factor and hyperarousal as a symptom, we review recent evidence in the rat for sex differences in a specific link between stress and arousal, that between corticotropin-releasing factor (CRF) and the brain norepinephrine system. It should be recognized that sex differences in behavior and animal models of stress-related psychiatric disorders depend on species and the endpoint being examined. For example, motor activity in many situations is higher for females compared to males in rats, but not mice (Johnston and File, 1991; Palanza, 2001) and the opposite pattern is seen in meadow voles (Perrot-Sinal et al., 2000). Because motor activity is often a component of endpoints in animal models of psychiatric disorders, an examination of sex differences across different animal models may yield different interpretations (Dalla et al., 2009; Johnston and File,1991). There are several reviews of sex differences in animal models of psychiatric disorders and that is not the intent of the present review (Dalla et al., 2009; Palanza, 2001). Here we center on the concept that there are sex differences in the interaction of stress with the locus coeruleus (LC)-norepinephrine arousal system and as a result, this component of the stress response and core symptom of stress-related psychiatric disorders is comparatively exaggerated in females.
1. Stress and Arousal as common features of female biased psychiatric disorders
Two common interrelated features of stress-related psychiatric disorders that exhibit a female bias, including depression and post-traumatic stress disorder, are an association with stress and the core symptom of hyperarousal. Stress is thought to be a precipitating or contributing factor to all of these disorders (Kendler et al., 1993; Kendler et al., 1995; Stein and Steckler, 2010). As a result, many investigations into the sex bias of affective disorders, anxiety and posttraumatic stress disorder have focused on differences in hypothalamic-pituitary-adrenal (HPA) axis responses to stressors and the role of sex hormones in regulating this axis (e.g., Binder et al., 2009; Kudielka and Kirschbaum, 2005; Young and Korszun, 2010). In contrast, there are few investigations of sex differences in arousal systems. The system that is generally attributed to the arousal features of mood disorders is the major brain norepinephrine system that arises from the nucleus locus coeruleus (LC) (Gold and Chrousos, 2002; Koob, 1999; Southwick et al., 1999). Convergent evidence links stress to the LC-norepinephrine system through the neuropeptide that orchestrates the stress response, corticotropin-releasing factor (CRF). This has led to the hypothesis that sex differences in some aspect of this link could account for the female bias in psychiatric disorders that are both etiologically associated with stress and that share hyperarousal as a core symptom.
2.1. CRF pathways linking stress to the LC-norepinephrine arousal system
CRF was initially characterized as the hypothalamic neurohormone that initiates adrenocorticotropin release from the anterior pituitary and thus, the cascade that leads to secretion of adrenal corticosteroids (Vale et al., 1981). The widespread distribution of CRF and its receptors throughout the brain, taken with the ability of centrally administered CRF to mimic many of the autonomic and behavioral aspects of the stress response, suggested that CRF acts as a neuromodulator on neurons outside of the HPA axis to initiate these other components of the stress response (Chalmers et al., 1996; Sakanaka et al., 1987; Swanson et al., 1983; Valentino and Van Bockstaele, 2002). The ability of centrally administered CRF antagonists to block behavioral and autonomic responses to stress gave further credence to the concept of CRF as an endogenous neurotransmitter/neuromodulator that is released in a coordinated manner in different brain regions to orchestrate the different limbs of the stress response (Bale and Vale, 2004; Owens and Nemeroff, 1991).
Through its ability to regulate the locus coeruleus (LC)-norepinephrine (NE) system, CRF released during stress can initiate an arousal response to a stressor. CRF axon terminals arising from Barrington’s nucleus, the paraventricular hypothalamic nucleus and possibly the nucleus paragigantocellularis innervate the LC nuclear core (Reyes et al., 2005; Valentino et al., 1996; Valentino et al., 1992). A denser CRF terminal field arising from the central nucleus of the amygdala and bed nucleus of the stria terminalis is situated in the dorsolateral peri-LC into which LC dendrites extend (Van Bockstaele et al., 1996a; Van Bockstaele et al., 1998; Van Bockstaele et al., 1999). As this peri-LC CRF innervation derives from limbic regions that convey emotion-related information, this circuit may serve as an arousal limb of this emotional response. The relevance of this topographically organized CRF innervation for sex differences in affective arousal is discussed below.
CRF-immunoreactive axon terminals form synaptic specializations with LC dendrites in the core and peri-LC, the majority of which are asymmetric (excitatory type) (Van Bockstaele et al., 1996b). Many CRF axon terminals here co-localize glutamate and fewer co-localize enkephalin and GABA (Tjoumakaris et al., 2003; Valentino et al., 2001). CRF axon terminals are also found apposed to unlabeled terminals that form synaptic specializations with LC dendrites, providing a mechanism for indirect presynaptic modulation of LC activity (Van Bockstaele et al., 1996b).
2.2. CRF effects on the LC-norepinephrine arousal system
CRF, acting on CRF1 receptors, increases spontaneous LC discharge rate when locally administered into the LC either in vivo or in vitro (Curtis et al., 1997; Jedema and Grace, 2004). LC activation by CRF is associated with c-fos expression by LC neurons and norepinephrine release in terminal fields (although this has just been examined in male rats) (Page and Abercrombie, 1999; Rassnick et al., 1998). Importantly, LC activation by CRF is translated to activation of cortical electroencephalographic (EEG) activity, indicative of increased arousal (Curtis et al., 1997).
In addition to increasing spontaneous or tonic LC discharge rate, CRF attenuates phasic sensory-evoked LC activity (Valentino and Foote, 1987; Valentino and Foote, 1988). This may occur through presynaptic inhibition as suggested by the electron microscopy studies discussed above. Given the evidence for co-localization of CRF and glutamate in axon terminals in the LC and convergence onto common LC dendrites, there are multiple potential mechanisms for this interaction. The net effect of CRF on LC neurons is to shift the mode of LC discharge to a high tonic-low phasic state (Valentino and Foote, 1987; Valentino and Foote, 1988; Valentino and Van Bockstaele, 2008). This mode of firing has been associated with high arousal, decreased focused attention and increased behavioral flexibility or going off-task in a search for optimal outcomes (Aston-Jones and Cohen, 2005) (see below).
Generally, the LC-norepinephrine system is not under tonic regulation by endogenous CRF because CRF antagonists have no effect on either LC discharge rate or norepinephrine release in targets in unstressed rats (Curtis et al., 1994; Page and Abercrombie, 1999). However, there is substantial evidence that acute stressors release endogenous CRF within the LC to activate LC neurons during acute stress. For example, hypotensive challenge, which activates the hypothalamic-pituitary-adrenal axis, mimics the effects of CRF on tonic and phasic LC discharge (Valentino and Wehby, 1988). Increases in LC discharge rate during the stress are temporally correlated to, and necessary for, forebrain EEG activation, underscoring the arousal component of this response (Page et al., 1993). Sensory-evoked phasic discharge is inhibited during the stress, presumably keeping the LC in an elevated tonic mode that would promote heightened arousal and scanning attention (Valentino and Wehby, 1988). These effects are completely prevented by prior administration of a CRF antagonist into the LC (Curtis et al., 2001; Valentino et al., 1991). In addition to hypotensive stress, non-noxious visceral stimuli, such as colon distention, increase LC discharge rates and cortical EEG activity by a CRF-dependent mechanism (Lechner et al., 1997). Studies using other endpoints of LC activation, including tyrosine hydroxylase expression in LC neurons (Melia and Duman, 1991) and cortical norepinephrine extracellular levels (Kawahara et al., 2000; Smagin et al., 1996) also provide evidence for CRF neurotransmission in the LC during stress.
2.3. The LC-NE arousal system
The anatomical and physiological characteristics of LC neurons have been the topic of numerous reviews (Aston-Jones et al., 1995; Berridge and Waterhouse, 2003; Bouret and Sara, 2005; Foote et al., 1983; Waterhouse et al., 1998). A salient anatomical feature of LC neurons is the divergent efferent system that allows for a global influence on neuronal activity at multiple levels of the neuraxis (Swanson and Hartman, 1976). The LC-norepinephrine system was initially implicated in arousal and vigilance based on certain physiological attributes of LC neurons. For example, LC neurons discharge spontaneously and their frequency is positively correlated to behavioral and electroencephalographic (EEG) indices of arousal (Aston-Jones and Bloom, 1981a). Moreover, regionally selective pharmacological activation of LC neurons is temporally correlated to cortical and hippocampal EEG activation indicating that increases in LC discharge are sufficient to produce arousal (Berridge and Foote, 1991; Berridge et al., 1993). LC neurons are also phasically activated by salient sensory stimuli and this activation precedes behavioral responses to the stimuli, implying a role for the LC in shifting attention (Aston-Jones and Bloom, 1981b; Foote et al., 1980). It has recently been proposed that by shifting between tonic and phasic modes of discharge, the LC facilitates different behavioral outcomes (Aston-Jones and Cohen, 2005). The phasically-activated LC, characterized by synchronous driven discharge, has been associated with focusing attention and maintaining on-going behavioral tasks. In contrast, high tonic discharge, which is spontaneous and asynchronous, is associated with scanning the environment and going off-task. The ability of LC neurons to switch between tonic and phasic modes of discharge would facilitate rapid behavioral adjustments in response to environmental challenges. As discussed above, exposure to CRF or stressors biases LC discharge toward the high tonic mode, favoring increased arousal and behavioral flexibility.
Together the anatomical and electrophysiological findings summarized above support a scheme whereby stressors elicit CRF release in the LC region, which acts as a neurotransmitter to shift the mode of discharge towards a high tonic state that would favor heightened arousal and behavioral flexibility. Other data not reviewed here provide evidence that endogenous opioids serve as a counterregulatory mechanism to curb CRF effects during stress and to help return the system to baseline activity when the stressor is terminated (Curtis et al., 2001).
3.1. Sex differences in CRF1 signaling
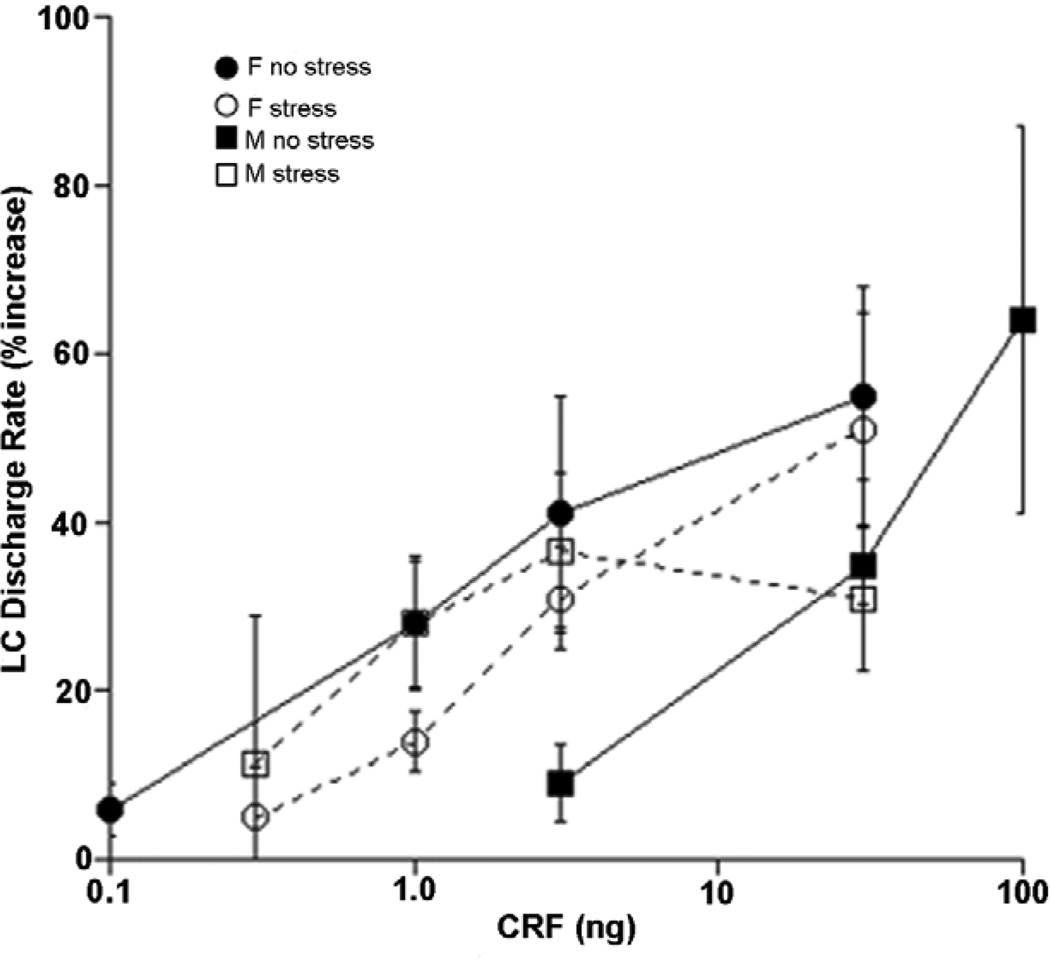
Heightened arousal and a shift from focused to scanning attention would be adaptive for dealing with an acute stressor. However, if this mode of activity persisted beyond the duration of the stressor or if it were initiated in the absence of stress, the same effects would be expressed as hyperarousal, sleep disturbances and inability to concentrate, symptoms of depression, anxiety and PTSD. Consistent with this, excessive activity of the LC-NE system has been implicated in each of these disorders (Gold and Chrousos, 2002; Koob, 1999; Southwick et al., 1999). Therefore, sex differences in the activity or response of the LC-NE system could contribute to the sex bias of these disorders. Initial studies showed no sex difference in LC discharge rates regardless of levels of circulating hormones (Curtis et al., 2006). In contrast, exposure to acute hypotensive stress, which increases LC discharge through CRF release in the LC, produced a greater activation of LC neurons of female compared to male rats, regardless of circulating sex hormone levels (Curtis et al., 2006). Moreover, enhanced LC activation by stress was found to be the result of an increased postsynaptic sensitivity to CRF in females. Thus, the CRF dose-response curve for LC activation was shifted to the left in female compared to male rats, and certain doses of CRF that were completely ineffective in males produce a substantial increase of LC discharge rate in females (Fig. 1).
Figure 1.
Sex differences in LC responses to CRF and regulation by prior swim stress. Shown are CRF dose-response curves generated for female (circles) and male (squares) rats with no prior history of stress (no stress, solid symbols) and 24 h after swim stress (stress, open symbols). The abscissa indicates the CRF dose (ng) injected into the LC and the ordinate indicates the magnitude of increase in LC discharge rate expressed as a percentage increase above the pre-injection rate. In the unstressed condition, LC neurons of females are more sensitive to CRF as indicated by a leftward shift in their dose-response curve. When CRF dose-response curves were generated 24 h after exposure to swim stress, the CRF dose-response curve generated in males was shifted to the left and had a lower maximum response. Swim stress did not significantly alter the dose-response curve of females. Reproduced from Curtis et al., 2006.
A second observed sex distinction in the CRF-LC interaction was a differential regulation by a history of prior stress. In male rats, prior stress (shock or swim history) changes the CRF dose-response curve such that it shifts to the left in the low dose range and the maximum response decreases (Curtis et al., 2006; Curtis et al., 1995, 1999). In female rats, prior stress has little effect on the CRF dose-response curve for LC activation (Fig. 1).
Differences in the postsynaptic response to CRF and its regulation by stress could be attributed to differences in CRF receptor (CRF1) signaling. LC activation by CRF is mediated by the CRF1 receptor subtype, a G-protein coupled receptor that preferentially binds the Gs protein, leading to activation of adenylyl cyclase, formation of cyclic adenosine monophosphate (cAMP), and protein kinase A (PKA) activation (Chalmers et al., 1996; Grammatopoulos and Chrousos, 2002). Phosphorylation of a potassium channel through cAMP is thought to mediate the CRF-induced increase in LC firing (Jedema and Grace, 2004). Notably, electrophysiological studies using a cAMP antagonist revealed that in unstressed female rats, the LC response to CRF is predominantly cAMP-mediated, whereas in unstressed males only about 50% of the response is cAMP-dependent (Bangasser et al., 2010). The stress-induced sensitized response of LC neurons to CRF that occurs only in males was completely cAMP-mediated. These data suggested a cAMP signaling bias of the CRF1 in unstressed females compared to males and a change in males following stress.
Consistent with a greater cAMP component of the LC response to CRF in females, receptor immunoprecipitation studies revealed enhanced CRF1- Gs coupling in unstressed females compared to males (Bangasser et al., 2010). Furthermore, swim stress increased CRF1-Gs coupling in males only, to a level comparable to that observed in females, reflecting the sensitized electrophysiological response. As was observed for the electrophysiological responses, the higher levels of CRF1-Gs coupling were similar in intact and ovariectomized females, suggesting no contribution of circulating ovarian hormones to the sex difference. Taken together, these results suggest that the increased neuronal sensitivity to CRF observed in females in the unstressed state is attributable to greater Gs-CRF1 coupling and subsequent activation of the cAMP-PKA signaling cascade. Additionally, the stress-induced sensitization of LC neurons of male rats to low doses of CRF is due to increased CRF1-Gs coupling. To our knowledge this is the first report of sex differences in receptor G-protein coupling that has functional consequences.
It should be noted that there were no sex differences in coupling of the CRF1 to Go or Gq/11, G-proteins that do not increase cAMP-PKA signaling (Bangasser et al., 2010). These findings underscore the important contribution of Gs-CRF1 coupling to sex differences in CRF1 function.
3.2. Sex differences in CRF1 trafficking
Agonist activation of many G-protein coupled receptors often results in internalization or trafficking of receptors from the plasma membrane to the cytoplasm (Krupnick and Benovic, 1998; Lefkowitz, 1998; Premont, 2005). Internalized receptors can be recycled to the plasma membrane or targeted for degradation. The latter pathway is adaptive in conditions of excess receptor ligand. CRF1 internalization is initiated by the phosphorylation of a serine or threonine residue on the carboxy terminus of the receptor (Oakley et al., 2007; Teli et al., 2005). This leads to the recruitment of β-arrestin2, which targets the receptor for internalization (Hauger et al., 2009; Holmes et al., 2006; Oakley et al., 2007). There is in vivo evidence for agonist- and swim stress-induced CRF1 internalization in LC dendrites of male rats (Reyes et al., 2006; Reyes et al., 2008). A significant proportion of the internalized receptors are incorporated into multivesicular bodies, suggesting that they are targeted for degradation (Reyes et al., 2008).
Functionally, CRF1 downregulation is seen as an earlier plateau of the CRF dose-response curve for LC activation observed in stressed males (Curtis et al., 2006; Curtis et al., 1995, 1999). The lack of this plateau in females suggests that the adaptive process of receptor internalization may be compromised. Consistent with this, CRF1 was associated with β-arrestin2 after stress in male, but not female rats (Bangasser etal., 2010). Like the sex difference in CRF1-Gs coupling, sex differences in CRF1 association with β-arrestin2 were not dependent on circulating ovarian hormones. Immunoelectron microscopy revealed a differential cellular localization and trafficking of CRF1 in LC dendrites of male and female rats (Bangasser et al., 2010). For males, in the unstressed state a greater portion of CRF1 is on the plasma membrane and this internalizes following stress, whereas for females a large proportion of CRF1 are cytoplasmic before stress and become more prominent on the plasma membrane following stress. The greater number of plasma membrane bound CRF1 in stressed females could be due to either recruitment of CRF1 to the plasma membrane, or decreased production of cytoplasmic receptors. Importantly, these data suggest that the cellular adaptation of internalization is compromised in females. This difference would translate to an inability of LC neurons of females to adapt to conditions of CRF hypersecretion, as has been hypothesized to occur with chronic stress or depression (Nemeroff, 1996), with the consequence of persistent activation of this arousal system.
3.3. Cellular implications of sex differences in signaling and trafficking
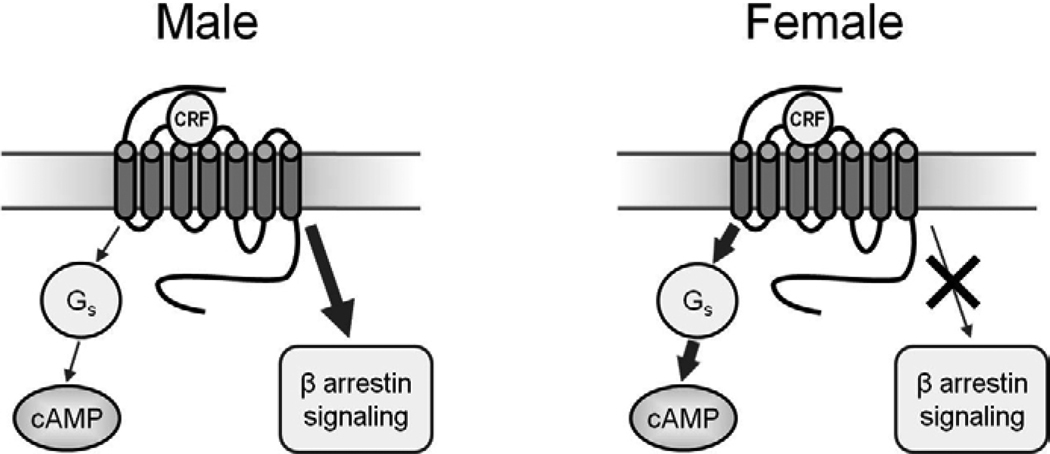
The relative inability of CRF1 in females to associate with β-arrestin2 is consistent with its greater association with Gs, as β-arrestin2 inhibits Gs-receptor association (Violin and Lefkowitz, 2007). In addition to their role in receptor internalization, β-arrestins can also engage cellular signaling cascades that may be unique and potentially opposed to those engaged by G-proteins, (Ahn et al., 2004; DeWire et al., 2007; Lefkowitz and Shenoy, 2005; Violin and Lefkowitz, 2007; Wei et al., 2003). The findings reviewed above predict that CRF1 signaling in female LC neurons will be biased towards the engagement of the Gs-cAMP-PKA pathway (Fig. 2). In contrast, in males CRF is able to initiate β-arrestin2 signaling resulting in unique cellular functions. Although β-arrestin2 signaling is not well characterized in rat LC neurons, in other cells this has been associated with the activation specific kinases such as JNK3 and AKT (Violin and Lefkowitz, 2007). Importantly, the differential activation of Gs- and β-arrestin2-linked signaling cascades may contribute to sex specific cellular responses in LC neurons that lead to an increased arousal response in females. This concept has important therapeutic implications for novel compounds that can shift the bias of CRF1 signaling from Gs- to β-arrestin2-related pathways.
Figure 2.
The schematic depicts CRF1 coupling and signaling in male and female LC neurons. In males, CRF1 binds β-arrestin2, which can activate unique signaling cascades and cellular events. In females, the CRF1 is highly coupled to Gs and association with β-arrestin2 following stress is relatively poor, resulting in a bias towards Gs-related cAMP signaling and cellular effects.
Presently, the basis for sex differences in CRF1 association with Gs and β-arrestin2 is unknown. The most likely explanation is a difference in post-translational modification such as phosphorylation or sulfation of an amino acid. Differences in CRF1 glycosylation are not likely because this would substantially affect molecular weight and this was not apparent in Western blots. Proteomic studies designed to compare structural features and post-translational modifications of CRF1 in males and females are necessary to address this question.
The finding that sex differences in LC sensitivity to the electrophysiological effects of CRF, coupling of CRF1 to Gs and coupling of CRF1 to β-arrestin2 were all independent of adult circulating ovarian hormones was of interest. We speculate that these differences may be organized by sex hormones at earlier developmental periods. Alternatively, this may reflect differential representation of genes on the X and Y chromosomes.
4.1. Sex differences in LC structure
In addition to its acute effects on LC neuronal activity, CRF alters LC dendritic structure, increasing the length of LC dendrites in slice cultures and neurite outgrowth in LC-like CATHa cells (Cibelli et al., 2001; Swinny and Valentino, 2006). Similarly, early rearing conditions alter LC dendritic length, with mild handling during the neonatal period resulting in decreased total dendritic length and branching (Swinny et al., 2010). Because much communication with the LC, particularly between the LC and limbic system occurs some distance from the nucleus, in the peri-LC, these effects on dendritic structure and length can determine the extent to which the LC is regulated by limbic afferents relaying emotion-related information.
A comparison of the LC dendritic tree between males and females based on analysis of immunoreactivity for tyrosine hydroxylase (TH), the norepinephrine synthetic enzyme, revealed that LC dendrites of female rats were denser and extended further into the peri-LC region than those of males (Bangasser et al., 2011). Juxtacellular labeling of LC neurons in vivo demonstrated that LC dendrites of females were longer with more branch points and ends compared to those of male rats, indicative of increased complexity. This was corroborated with Sholl analysis. Finally, immunoreactive-labeling for synaptophysin, a synaptic vesicle protein, was significantly greater in the LC core and peri-LC region of female rats, providing evidence that the longer and more complex dendritic tree actually makes more synaptic contacts and suggesting that the female LC is processing more information.
It is possible that the sex differences in LC dendritic structure are due to sex differences in CRF1 signaling. CRF effects on neurite outgrowth of CATHa cells and dendritic structure of LC neurons in slice cultures are mediated by the PKA signaling cascade (Cibelli et al., 2001; Swinny and Valentino, 2006). Therefore, one downstream consequence of the greater CRF1-Gs signaling in females may be a more extensive LC dendritic tree. Nonetheless, the role of CRF in this sex difference has yet to be determined and alternative mechanisms have not been ruled out.
4.2. Network implications of sex differences in LC dendritic structure
The relevance of sex differences in the length and extent of the LC dendritic tree relates to the topographical organization of LC afferents. Given the extensive efferent system arising from LC neurons, it was initially surprising when retrograde tracing studies revealed a limited set of afferents to the LC nucleus (Aston-Jones et al., 1986). These derived from nucleus paragigantocellularis, nucleus prepositus, dorsal cap of the paraventricular hypothalamic nucleus, and Barrington’s nucleus (Aston-Jones et al., 1986; Valentino et al., 1996). Many of these nuclei are involved in central control of autonomic function and their projections to the LC provide a mechanism for coordinating autonomic activity with behavior and cognitive functions. In contrast to the nuclear LC, which receives a relatively limited number of afferents, the peri-LC is a site for the termination of axons from numerous and diverse regions, many of which are synaptically linked with LC neurons whose dendrites extend into this outlying area (Luppi et al., 1995; Reyes et al., 2005; Van Bockstaele et al., 2001; Van Bockstaele et al., 1996a; Van Bockstaele et al., 1998; Van Bockstaele et al., 1999). For example, axon terminals from the nucleus of the solitary tract and periaqueductal gray can convey autonomic and nociceptive information through synapses with LC dendrites in the ventromedial peri-LC, respectively (Van Bockstaele et al., 2001). Importantly, the peri-LC is a major site of termination of limbic projections including the central nucleus of the amygdala, bed nucleus of the stria terminalis and paraventricular hypothalamic nucleus (Reyes et al., 2005; Van Bockstaele et al., 2001; Van Bockstaele et al., 1998; Van Bockstaele et al., 1999). Axon terminals from these regions synapse with LC dendrites here (Van Bockstaele et al., 1996a; Van Bockstaele et al., 1998). Given the role of the limbic system in the expression of emotion and the role of the LC in initiating forebrain arousal in response to salient stimuli, the limbic-LC link in the peri-LC may form the structure of an arousal limb of the emotional response. Notably, most of the CRF innervating this region that synapses with LC dendrites derives from the central nucleus of the amygdala, which coordinates behavioral and autonomic aspects of emotional responses through connections to the central gray and lateral hypothalamus, respectively (LeDoux et al., 1988). CRF inputs from the central nucleus of the amygdala to LC dendrites in the peri-LC would integrate forebrain arousal and cognitive responses with these other aspects of the emotional response. Because of the functional distinctions between afferents to the nuclear LC and afferents to the peri-LC, the length and complexity of branching of LC dendrites will be an important determinant of how the nucleus is regulated. The further LC dendrites extend into the peri-LC, particularly the dorsolateral peri-LC, and the more highly branched LC dendrites are, the greater the probability that they will contact limbic afferents conveying emotion-related information. Extensions of LC dendrites, particularly into the dorsolateral peri-LC where axons from the central nucleus of the amygdala terminate, form a structural basis for affective arousal. The greater complexity of LC dendrites and further extension into this region in females would support a greater arousal response to emotion-related information conveyed by these limbic afferents. Evidence for more synaptic contacts in this region is also consistent with more processing of emotion-related information. Together, the anatomical findings suggest that the LC of female rats is structurally designed to be under greater limbic regulation and to receive and process more emotion-related information conveyed by limbic afferents. Interestingly, a recent imaging study in females suggests that stress exposure enhances amygdala-LC connectivity although it is not known whether this effect is exclusive to females (van Marle et al., 2010).
5. Overview and Considerations
Many of the psychiatric disorders that are more prevalent in females have in common an etiological association with stress and hyperarousal as part of the symptom complex. Here we reviewed evidence for sex differences in these disorders that lie at the intersection of stress and arousal systems, specifically at the level of CRF regulation of the LC-norepinephrine arousal system. CRF released during stress shifts LC neuronal activity to a high tonic mode that favors increased arousal, scanning attention and behavioral flexibility. These responses would be adaptive in a dynamic life-threatening environment, but would be considered pathological in a more stable or less challenging environment. At a molecular level, CRF1 preferentially couples Gs in females compared to males resulting in a greater magnitude of LC activation for the same level of CRF (or stress). Moreover, a relatively low association of CRF1 with β-arrestin2 in females compromises CRF1 internalization and therefore the ability to adapt to high or persistent levels of CRF, as have been hypothesized to occur in stress-related psychiatric disorders. Increased CRF1-Gs association and decreased CRF1-β-arrestin2 association (probably interrelated effects) would render this arousal system more responsive to CRF (and stressors) in females. Although prior work has focused on the LC system and cortical CRF1, similar differences in CRF1 may be expressed in other brain regions and contribute to sex differences in the expression of other aspects of the stress response. In addition to an increased and prolonged response to CRF, the response may be qualitatively distinct in males and females as a result of engaging different cell signaling pathways.
Complementing the molecular sex differences, are sex differences in LC neuronal structure. Because of their more complex and longer dendrites, LC neurons of females are structurally designed to receive and process more information, particularly emotion-related information relayed by the limbic system. Notably, CRF is a major neurotransmitter in these afferents. Together, these molecular and cellular distinctions would create an LC system that receives and processes more emotion-related information and that is more responsive to that input. Consistent with this, human studies measuring event-related potentials in response to positive or negative valence stimuli indicate that negative stimuli elicit a larger response in females and that females are more sensitive to stimuli of lesser salience (Lithari et al., 2010; Yuan et al., 2009). These sex differences would bias females towards an enhanced level of affective arousal and behavioral flexibility. These may be adaptive features that evolved as part of the maternal role of females. However, these same features outside of the appropriate context, are expressed as pathology that characterizes stress-related psychiatric disorders.
Research Highlights.
Sex differences in CRF receptor-Gs coupling make female cells more sensitive to CRF
Sex differences in β-arrestin2 binding result in distinct CRF receptor trafficking
Female locus coeruleus dendrites are designed to receive more limbic information
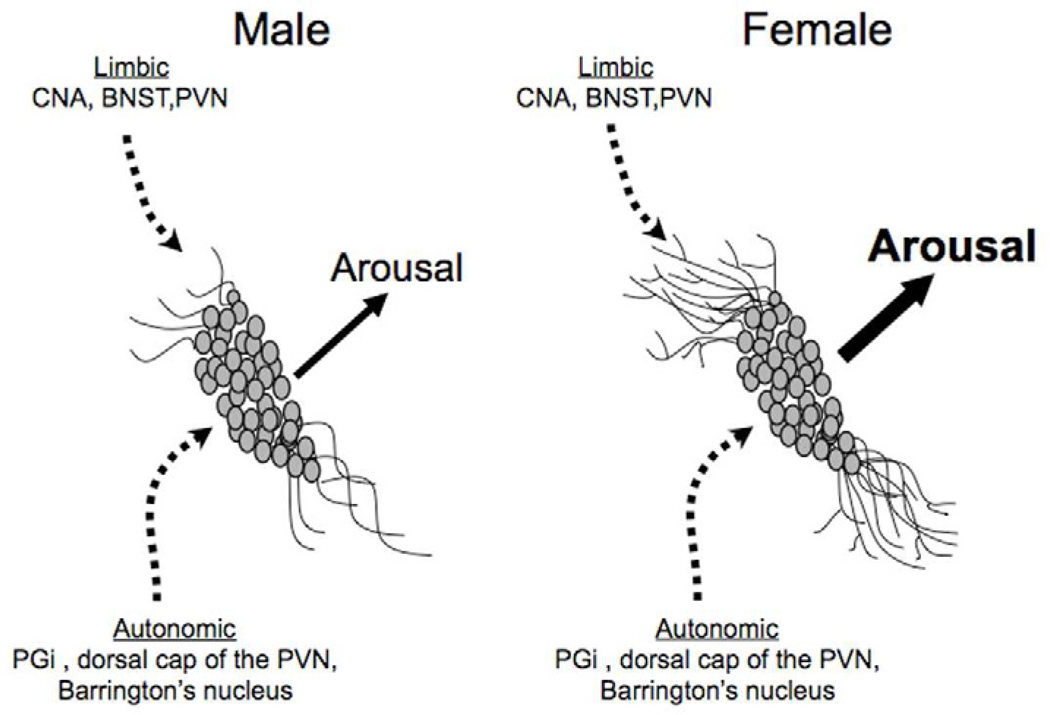
Figure 3.
Schematics depict how sex differences in LC dendritic morphology could affect emotional arousal. LC neurons of female rats have longer and more complex dendrites than neurons of males. Thus, the probability that LC dendrites will contact limbic afferents that terminate in the peri-LC is greater in females compared to males. This would be predicted to result in a greater magnitude of arousal in response to emotion-related stimuli. Abbreviations: BNST, bed nucleus of the stria terminalis; CNA, central nucleus of the amygdala; PGi, paragigantocellularis; PVN, paraventricular nucleus of the hypothalamus
Acknowledgements
The authors acknowledge the support of PHS grants MH40008, MH014654, MH084423 and MH092438.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleusnorepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxions G, editor. The Rat Brain. Academic Press; 1995. pp. 183–213. [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: A structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103:342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J. Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–383. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kunzel HE, Nickel T, Kern N, Pfennig A, Majer M, Uhr M, Ising M, Holsboer F. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology. 2009;34:99–109. doi: 10.1016/j.psyneuen.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry 62. 2001;17 Suppl 17:16–22. [PubMed] [Google Scholar]
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5:34–40. [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends in Pharmacological Science. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- Cibelli G, Corsi P, Diana G, Vitiello F, Thiel G. Corticotropin-releasing factor triggers neurite outgrowth of a catecholaminergic immortalized neuron via cAMP and MAP kinase signalling pathways. Eur J Neurosci. 2001;13:1339–1348. doi: 10.1046/j.0953-816x.2001.01510.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ. Endogenous opioids in the locus coeruleus function to limit the noradrenergic response to stress. J. Neurosci. 2001;21:RC152. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually Dimorphic Responses of the Brain Norepinephrine System to Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J. Pharmacol. Exp. Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Curtis AL, Grigoradis D, Page ME, Rivier J, Valentino RJ. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J. Pharmacol. Exp. Ther. 1994;268:359–365. [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Long term regulation of locus coeruleus sensitivity to corticotropin-releasing factor by swim stress. J. Pharmacol. Exp. Ther. 1999;289:1211–1219. [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2009;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36:1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psych. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur. J. Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psych. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lechner S, Curtis A, Brons R, Valentino R. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lithari C, Frantzidis CA, Papadelis C, Vivas AB, Klados MA, Kourtidou-Papadeli C, Pappas C, Ioannides AA, Bamidis PD. Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topogr. 2010;23:27–40. doi: 10.1007/s10548-009-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc. Natl. Acad. Sci. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol. Psych. 1996;1:336–342. [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Revs. 1991;43:425–474. [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci. Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal T, Ossenkopp KP, Kavaliers M. Influence of a natural stressor (predator odor) on locomotor activity in the meadow vole (Microtus pennsylvanicus): modulation by sex, reproductive condition and gonadal hormones. Psychoneuroendocrinology. 2000;25:259–276. doi: 10.1016/s0306-4530(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Premont RT. Once and future signaling: G protein-coupled receptor kinase control of neuronal sensitivity. Neuromolecular Med. 2005;7:129–147. doi: 10.1385/NMM:7:1-2:129. [DOI] [PubMed] [Google Scholar]
- Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49:217–228. e211–213. [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Hoffman GE, Rabin BS, Sved AF. Injection of corticotropin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience. 1998;85:259–268. doi: 10.1016/s0306-4522(97)00574-5. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederes K. Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidediaminobenzidene method. J. Comp. Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Harris RB, Ryan DH. Corticotropin-releasing factor antagonist infused into the locus coeruleus attenuates immobilization stress-induced defensive withdrawal in rats. Neurosci. Lett. 1996;220:167–170. doi: 10.1016/s0304-3940(96)13254-7. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Steckler T. Behavioral neurobiology of anxiety and its treatment. Preface. Curr Top Behav Neurosci. 2010;2:v–vii. [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat using dopamine-B-hydroxylase as a marker. J. Comp. Neurol. 1976;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swinny JD, O'Farrell E, Bingham BC, Piel DA, Valentino RJ, Beck SG. Neonatal rearing conditions distinctly shape locus coeruleus neuronal activity, dendritic arborization, and sensitivity to corticotrophin-releasing factor. Int J Neuropsychopharmacol. 2010;13:515–525. doi: 10.1017/S146114570999037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur J Neurosci. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ, Van Bockstaele EJ. Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J Comp Neurol. 2003;466:445–456. doi: 10.1002/cne.10893. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington's nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J. Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Page M, Van Bockstaele E, Aston-Jones G. Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience. 1992;48:689–705. doi: 10.1016/0306-4522(92)90412-u. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropinreleasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ. Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience. 2001;106:375–384. doi: 10.1016/s0306-4522(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ. Corticotropin-releasing factor: putative neurotransmitter actions of a neurohormone. In: Pfaff D, A A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 81–102. [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: Evidence for a neurotransmitter role in the locus coeruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Chan J, Pickel VM. Input from central nucleus of the amygdala efferents to pericoerulear dendrites, some of which contain tyrosine hydroxylase immunoreactivity. J. Neurosci. Res. 1996a;45:289–302. doi: 10.1002/(SICI)1097-4547(19960801)45:3<289::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996b;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Valentino RJ. Differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol. Psych. 1999;46:1352–1363. doi: 10.1016/s0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Volin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Devilbiss D, Fleischer D, Sessler FM, Simpson KL. New perspectives on the functional organization and postsynaptic influences of the locus ceruleus efferent projection system. Adv. Pharmacol. 1998;42:749–754. doi: 10.1016/s1054-3589(08)60856-x. [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]
- Yuan J, Luo Y, Yan JH, Meng X, Yu F, Li H. Neural correlates of the females' susceptibility to negative emotions: an insight into gender-related prevalence of affective disturbances. Hum Brain Mapp. 2009;30:3676–3686. doi: 10.1002/hbm.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]