Summary
Background
Stromal interaction molecule 1 (STIM1) was recently identified as a critical component of store-operated calcium entry (SOCE) in platelets. We previously reported the Ca2+-sensing guanine nucleotide exchange factor CalDAG-GEFI as critical molecule in Ca2+ signaling in platelets.
Objective
To evaluate the contribution of STIM1/SOCE to Ca2+-dependent platelet activation and thrombosis, we here compared the activation response of platelets lacking STIM1 and platelets lacking CalDAG-GEFI.
Methods
The murine Stim1 gene was conditionally deleted in the megakaryocyte/platelet lineage. CalDAG-GEFI−/− and Stim1fl/fl PF4-Cre along with littermate control mice were used for in vitro and in vivo experiments under flow as well as static conditions.
Results
αIIbβ3-mediated aggregation was markedly impaired in CalDAG-GEFI- but not STIM1-deficient platelets, both under static or flow conditions. In contrast, deficiency in either STIM1 or CalDAG-GEFI significantly impaired the ability of platelets to express phosphatidyl serine on the cell surface. When subjected to a laser injury thrombosis model, mice lacking STIM1 in platelets were characterized by the formation of unstable platelet-rich thrombi and delayed and reduced fibrin generation in injured arterioles. In CalDAG-GEFI−/− mice, fibrin generation was also delayed and reduced, but platelet accumulation was virtually abolished.
Conclusions
Our studies suggest that 1) STIM1/SOCE is critical for the pro-coagulant activity but not the pro-adhesive function of platelets, and 2) at the site of vascular injury, STIM1 and CalDAG-GEFI are critical for the first wave of thrombin generation mediated by pro-coagulant platelets.
Keywords: Platelet, procoagulant, STIM1, SOCE, thrombosis
Introduction
Platelet activation is triggered by various agonists including subendothelial collagens, the second wave mediators adenosine diphosphate (ADP) and thromboxane A2 (TxA2), and the protease thrombin [1]. ADP, thrombin and TxA2 stimulate platelets via specific G protein-coupled receptors (GPCR), while collagen signals via the immunoglobulin-like receptor glycoprotein (GP) VI and an immunoreceptor tyrosine activation motif (ITAM) within the associated Fc receptor γ chain. Independent of the activating receptor, cellular stimulation leads to the activation of phospholipase C and the subsequent mobilization of the second messengers calcium (Ca2+) and diacylglycerol (DAG). DAG is critical for protein kinase C (PKC) activation, a key event in platelet granule release and integrin activation [2]. Ca2+ regulates various adhesive platelet responses such as integrin activation and the release of ADP and TxA2 [1, 3, 4]. Furthermore, sustained increases in the intracellular calcium concentration ([Ca2+]i) are critical for the ability of platelets to switch from a pro-adhesive to a pro-coagulant state [5, 6]. Crucial to this conversion is the scrambling of phosphatidyl serine (PS) from the inner to the outer membrane leaflet, a process that is mediated by transmembrane protein 16F [7]. The surface-exposed PS provides a negatively charged surface for the assembly of factors and cofactors of the prothrombinase (FXa and FVa) complex, resulting in a potent enhancement of thrombin/fibrin generation. Strong platelet activation also mobilizes FV/FVa from granular stores, making it available for incorporation into the prothrombinase complex [8].
In our recent work, we identified the guanine nucleotide exchange factor, CalDAG-GEFI, as a critical molecule in Ca2+ signaling in platelets [9–12]. CalDAG-GEFI activates the small GTPase Rap1, a central molecular switch that drives platelet activation by directly regulating integrin-mediated aggregation [9,12,13] and the release of autocrine agonists [11]. CalDAG-GEFI-independent activation of Rap1 requires signaling by PKC and the Gi-coupled receptor for ADP, P2Y12. Platelets lacking CalDAG-GEFI are severely impaired in their ability to aggregate and to adhere to sites of vascular injury [14].
An increase in the [Ca2+]i can derive from two main sources: release of Ca2+ from intracellular stores and Ca2+ entry through the plasma membrane (PM). Store release depends on the formation of inositol 1,4,5-triphosphate (IP3) by PLC, which triggers the activation of IP3 receptors and thus opening of the pore. Calcium entry through the PM is directly coupled to store release by a mechanism called store operated Ca2+ entry (SOCE). Stromal interaction molecule 1 (STIM1) and Orai1 were recently identified as two main players in SOCE [15–19]. STIM1 is a trans-membrane protein containing two EF hand domains that are situated in the lumen of the endoplasmic reticulum (ER). Upon store depletion, Ca2+ dissociates from the EF hand domain of STIM1, leading to co-localization of STIM1 and the PM membrane channel moiety, Orai1, and subsequent Ca2+ entry through the PM.
Recent studies in mice lacking functional STIM1 or Orai1 demonstrated a key role for both proteins in SOCE in platelets [20–23]. Observations common to these studies were the virtually abolished Ca2+ entry in response to physiological and non-physiological agonists, and the moderate defects in integrin activation and granule release observed in mutant platelets. Discrepant results, however, were observed with regard to the importance of SOCE for thrombus formation under physiological flow conditions [23]. While platelet adhesion to collagen was significantly reduced in blood from chimeric mice lacking STIM1 or Orai1 in blood cells only, normal thrombus formation was observed under similar experimental conditions for platelets expressing an inactive mutant of Orai1 [22]. In the latter studies, we further suggested that SOCE is critical for the pro-coagulant but not the pro-adhesive role of Ca2+ in platelet activation.
In the current study, we compared the platelet activation response in mice with a conditional deletion of STIM1 in platelets/megakaryocytes and mice lacking CalDAG-GEFI, both in vitro and in vivo. The main conclusions from our studies are: (1) STIM1/SOCE is not critical for the pro-adhesive role of Ca2+ in platelet activation, (2) PS exposure and fibrin generation at a site of vascular injury are dependent to a similar degree on STIM1 and CalDAG-GEFI, (3) the conversion from a pro-adhesive to a pro-coagulant platelet requires signaling via the small GTPase Rap1, and (4) platelet-dependent thrombin/fibrin generation is critical for thrombus stability and growth in vivo.
Material and Methods
Materials
Low molecular weight Lovenox (enoxaparin sodium; Sanofi-Aventis, Bridgewater, NJ), heparin-coated capillaries (VWR, West Chester, PA), bovine serum albumin (BSA, fraction V), prostacyclin (PGI2), and human fibrinogen (type I) (all from Sigma-Aldrich, St Louis, MO), 2-methylthio-AMP triethylammonium salt hydrate (2-MeSAMP, P2Y12 inhibitor, BioLog, Bremen, Germany), fibrillar collagen type I (Chronlog, Havertown, PA), polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA), annexin V (Invitrogen Corporation, Carlsbad, CA), and PAR4-activating peptide (Advanced Chemtech, Louisville, KY) were purchased. Convulxin and collagen-related peptides (CRP) were provided by Kenneth Clemetson (Theodor Kocher Institute, University of Berne, Switzerland) and Richard Farndale (University of Cambridge, Cambridge, UK), respectively. Antibodies directed against the activated form of murine αIIbβ3, JON/A-PE, GPIX (Emfret Analytics, Wuerzburg, Germany), β1 integrin and P-selectin (BD Biosciences, Chicago, IL), von Willebrand factor (VWF) and fibrinogen (Dako, Carpinteria, CA) were purchased.
Mice
Stim1fl/fl Pf4-Cre [24], CalDAG-GEFI−/− [9] and littermate control mice were bred in the mouse facility of Thomas Jefferson University. Experimental procedures were approved by the Animal Care and Use Committee of Thomas Jefferson University.
Blood collection and platelet preparation
Blood was drawn from the retroorbital plexus into heparinized tubes. Platelet rich plasma (PRP) was prepared by centrifugation of heparinized blood at 100g for 5 minutes. Platelets were washed twice with modified Tyrode’s buffer (137 mM NaCl, 0.3 mM Na2HPO4, 2mM KCl, 12 mM NaHCO3, 5 mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid, 5 mM glucose, pH 7.3) in the presence of PGI2 by centrifugation at 700g and re-suspended in Tyrode’s buffer at concentration of 4 × 108 platelets/ml.
Flow cytometry
Ca2+ flux measurement
Washed platelets diluted in Tyrode’s buffer were loaded with 10 µM Fluo-4 for 10 minutes. Platelets were activated with the indicated agonists in the presence of 0.5 mM CaCl2 and immediately analyzed by flow cytometry.
STIM1 expression in platelets and leukocytes
400 µl heparinized whole blood was lysed in 3,600 µl red cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2-EDTA pH 7.3) for 10 minutes at room temperature (RT). The lysed cells were centrifuged at 400g and the supernatant was discarded carefully. The pellet was re-suspended in 400 µl PBS and 44 µl of formaldehyde (37%) was added for 10 minutes. The cells were washed with PBS, the supernatant was discarded carefully and the pellet was incubated for 5 minutes with 0.5% Triton. After a washing step with PBS containing 1% BSA, the cells were incubated with rabbit anti-STIM1 (2 µg/ml) or rabbit anti-VWF (2 µg/ml) for 10 minutes, washed once in PBS/BSA, and stained with anti-Mac1-PE (2 µg/ml) or anti-CD41b-PE (2 µg/ml) along with anti-rabbit-Alexa488 (10’, RT). Cells were immediately analyzed by flow cytometry.
Expression levels of platelet surface receptors and intracellular proteins
Washed platelets were stained with FITC-labeled antibodies to GPVI, GPIX, αIIbβ3, or β1 integrin and immediately analyzed on a FACScalibur. For the detection of intracellular proteins, washed platelets were fixed and permeabilised as described above, stained with FITC-labeled antibodies to VWF, fibrinogen, or P-selectin, and immediately analyzed.
αIIbβ3 activation and P-selectin expression
Platelets were washed and diluted with Tyrode’s containing 1 mM CaCl2, activated with different doses of PAR4p or Cvx for 10 minutes in the presence or absence of the P2Y12 inhibitor 2-MeSAMP (100 µM), and stained for activated αIIbβ3 (JON/A-PE) and P-selectin expression (α-Pselectin-FITC).
Phosphatidyl-serine exposure
Platelets were stimulated in Tyrode’s buffer containing 2 mM CaCl2 with different doses of Cvx along with 100 µM of PAR4p in the presence or absence of 2-MeSAMP. Cells were stained with annexin V-Alexa647 (2 µg/ml) for 10 minutes at RT and immediately analyzed by flow cytometry.
Flow chamber studies
In vitro flow studies were performed in a microfluidic device fabricated in poly dimethylsiloxane (PDMS). Fabrication of microfluidic devices and microfluidic collagen patterning were performed as previously described [25]. Briefly, a 100 µm-strip of fibrillar collagen type I (200 µg/ml) was deposited and immobilized by microfluidic patterning along the length of a glass slide. A PDMS device with 10 flow channels (width: 250 µm, hight: 60 µm, length: 6 mm) was oriented perpendicular to the patterned collagen. Murine whole blood was drawn from the retro-orbital plexus into heparinized tubes (30 U/ml Lovenox), incubated with 1.5 µg/ml of anti-GPIX-Alexa488 and annexinV-Alexa647 (1 µg/ml), and infused at arterial (1700-S) or venous (400-S) wall shear rates for 5 minutes. Adhesion of platelets was monitored continuously with a Nikon Ti-U inverted microscope (Nikon Instruments Inc., Melville, NY) equipped with a Retiga EXL monochrome camera (QImaging, Surrey, Canada). Images were analyzed using Nikon NIS Elements software (NIS-Elements Advanced Research; Melville, NY, USA).
Aggregometry
Platelet rich plasma (PRP) was obtained from heparinized blood. PRP was washed twice and count was adjusted to 3 × 108 platelets/ml with modified tyrodes buffer. Indicated doses of various agonists were added and transmission was recorded over 10 minutes on a Chrono-log 4-channel optical aggregation system (Chrono-log, Havertown, PA).
Western blotting
Platelet lysates were separated by 4% to 12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes. STIM1 was detected with a rabbit polyclonal antibody raised against the C-terminal domain of STIM1 with a conserved 29-amino acid peptide [26]. After incubation with horseradish peroxidase-coupled anti–rabbit antibodies (Vector Laboratories), immunoreactivity was detected by Western Lightning enhanced chemiluminescence (G-Biosciences).
Laser-injury induced thrombosis in cremaster muscle
Laser-induced thrombosis in the cremaster muscle microcirculation was performed as described previously [27, 28]. Briefly, male mice (12–14 weeks of age) were intraperitoneally injected with sodium pentobarbital (11 mg/kg, Abbott Laboratories), and maintained under anesthesia with the same anesthetic delivered via the catheterized jugular vein as described previously. The cremaster muscle was isolated and the microvessels studied using an Olympus BX61WI microscope (Olympus, Center Valley, PA, USA) with a 40×/0.8 numeric aperture water-immersion objective lens. Laser injuries were done using an SRS NL100 Nitrogen Laser system (Photonic Instruments, Saint Charles, IL, USA) at 65% energy level. 5 min prior to injury, mice were injected intravenously with Alexa488-coupled Fab fragments of MWReg30, an antibody directed against murine αIIbβ3 (0.2 µg/g body weight, BD Pharmingen), and Alexa647 conjugated murine anti-fibrin antibody (0.45 µg/g). No more than 5 arterioles were studied per mouse. All data were analyzed using Slidebook 4.2 software (Intelligent Imaging Innovations Philadelphia, PA, USA).
Statistics
Results are reported as mean ± SEM and statistical significance was assessed by unpaired 2-tailed Student t test. A P value less than 0.05 was considered significant.
Results
Generation of Stim1fl/fl Pf4-Cre mice
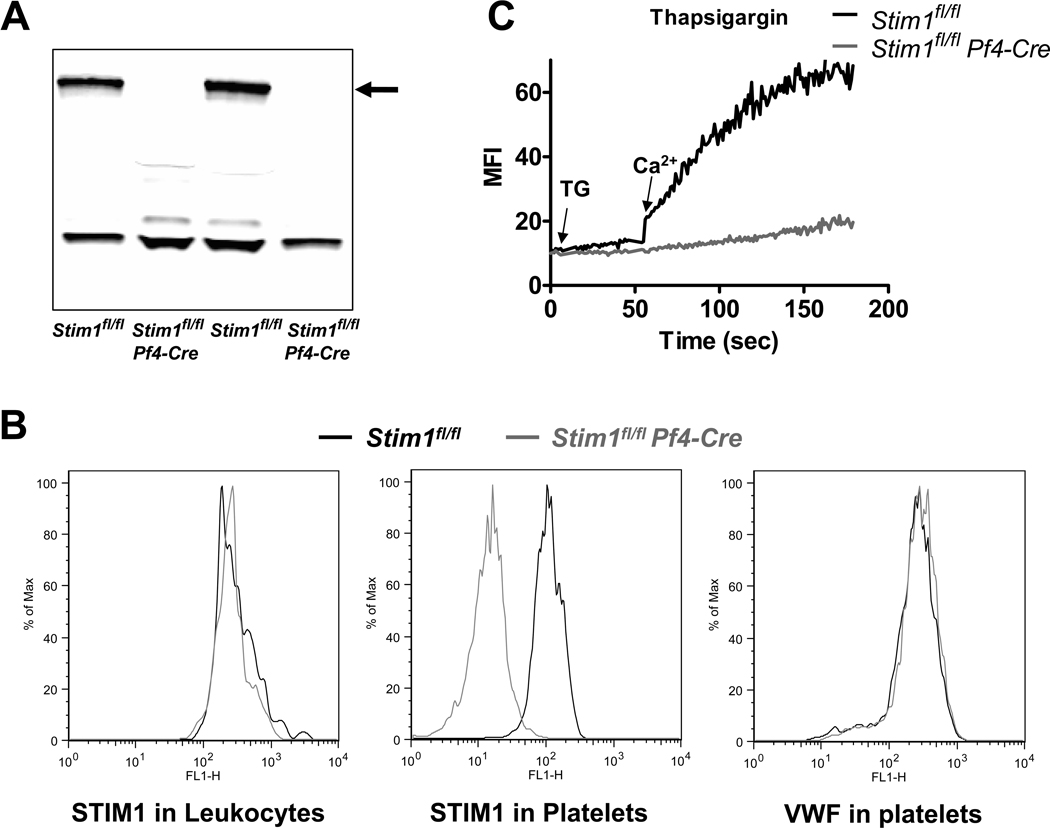
Stim1fl/fl mice were generated as described recently [24]. Stim1fl/fl mice were crossed with Pf4-Cre mice [29] to delete the Stim1 gene in the megakaryocyte/platelet lineage. Deficiency in platelet STIM1 was confirmed by western blotting and flow cytometry. STIM1 protein was detectable in lysates of Stim1fl/fl but not Stim1fl/fl Pf4-Cre platelets (Fig. 1A). Intracellular staining for STIM1 in platelets and Mac1-positive leukocytes demonstrated specific deletion in Stim1fl/fl Pf4-Cre platelets (Fig. 1B). Von Willebrand factor, a protein stored in alpha granules of platelets, was present in both Stim1fl/fl and Stim1fl/fl Pf4-Cre platelets. Specific deletion of STIM1 in platelets did not affect peripheral platelet counts or platelet size (not shown). No difference was observed between Stim1fl/fl and Stim1fl/fl Pf4-Cre platelets with regard to (a) the surface expression of platelet receptors GPVI, GPIX, αIIbβ3, and β1 integrins (Table 1) and (b) the cellular levels of important platelet proteins such as VWF, fibrinogen, or P-selectin (Table 2).
Figure 1. Specific deletion of STIM1 in platelets.
(A) Western blot for STIM1 in platelet lysates from Stim1fl/fl and Stim1fl/fl Pf4-Cre mice (two samples each). (B) Intracellular staining for STIM1 in Mac1-positive leukocytes (left panel) or platelets (middle panel) from Stim1fl/fl (black line) and Stim1fl/fl Pf4-Cre (grey line) mice. Platelets were stained for von Willebrand factor (VWF) as a control (right panel). (C) Stim1fl/fl (black) and Stim1fl/fl Pf4-Cre (grey) platelets were loaded with the calcium sensing dye Fluo-4 and treated with thapsigargin (TG) in the absence of extracellular Ca2+. Ca2+ influx was induced by addition of 0.5 mM CaCl2 to the buffer solution.
Table 1.
Surface expression of GPVI, GPIX, αIIbβ3 and β1 integrin in Stim1fl/fl and Stim1fl/fl Pf4-Cre platelets. Data shown are mean ± SD, n=5 for each group. No significant differences were observed between the two groups.
|
Stim1fl/fl (mean ± SD) |
Stim1fl/fl Pf4-Cre (mean ± SD) |
|
|---|---|---|
| IgG | 7.9 ± 0.2 | 8.1 ± 0.4 |
| GPVI | 185.3 ± 10.8 | 186.5 ± 3.8 |
| GPIX | 693.2 ± 82.3 | 706.2 ± 121.3 |
| αIIbβ3 | 2287.8 ± 207.9 | 2188 ± 194.5 |
| β1 | 575.2 ± 38.6 | 584.8 ± 45.9 |
Table 2.
Expression levels of fibrinogen, von Willebrand factor (VWF) and P-selectin in Stim1fl/fl and Stim1fl/fl Pf4-Cre platelets. Data shown are mean ± SD, n=5 for each group. No significant differences were observed between the two groups.
|
Stim1fl/fl (mean ± SD) |
Stim1fl/fl Pf4-Cre (mean ± SD) |
|
|---|---|---|
| IgG | 2.0 ± 0.1 | 2.3 ± 0.2 |
| fibrinogen | 100.9 ± 7.1 | 118.8 ± 14.3 |
| VWF | 55.5 ± 4.2 | 60.7 ± 6 |
| P-selectin | 11.3 ± 0.4 | 12.6 ± 1.1 |
Impaired store operated calcium entry (SOCE) in STIM1 deficient platelets
First, we determined the effect of STIM1 deficiency on SOCE in platelets. Stim1fl/fl and Stim1fl/fl Pf4-Cre platelets were loaded with the calcium sensing dye Fluo-4 and treated with the sarcoplasmic reticulum Ca2+ATPase (SERCA) pump inhibitor thapsigargin (TG, 5 µM) in the absence of extracellular calcium (Fig. 1C). Upon addition of extracellular calcium, rapid influx of Ca2+ was observed in Stim1fl/fl but not in Stim1fl/fl Pf4-Cre platelets.
Calcium flux was also studied in platelets activated via the thrombin receptor, PAR4, or the collagen receptor, GPVI. Cellular stimulation with high doses of PAR4-activating peptide (PAR4p, supplemental Fig. 1A) or the GPVI agonist convulxin (Cvx, supplemental Fig. 1B) led to a rapid, sustained increase in [Ca2+]i in Stim1fl/fl platelets. In stimulated Stim1fl/fl Pf4-Cre platelets, however, [Ca2+]i rose rapidly but dropped back to baseline within a few minutes, presumably due to impaired Ca2+ entry into the cell. A more prominent defect in Ca2+ mobilization was observed in Stim1fl/fl Pf4-Cre platelets activated with low doses of PAR4p or Cvx, suggesting that STIM1-mediated SOCE is crucial for calcium flux under conditions of weak platelet activation. Confirming this hypothesis, we observed the strongest defect in calcium mobilization in Stim1fl/fl Pf4-Cre platelets activated with collagen-related peptides (supplemental Fig. 1C), a GPVI-specific agonist with weaker activating activity than Cvx.
Contribution of STIM1 and CalDAG-GEFI to Ca2+-dependent platelet activation, aggregation, and thrombus formation in vitro
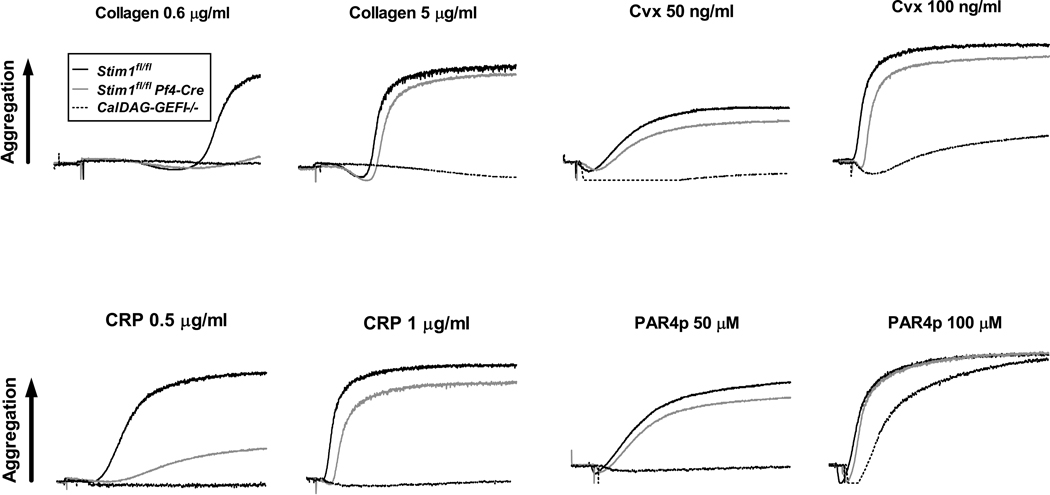
To evaluate the contribution of STIM1 to Ca2+-dependent platelet activation, we compared αIIbβ3 integrin activation and alpha granule release in stimulated Stim1fl/fl Pf4-Cre and CalDAG-GEFI−/− platelets. Stim1fl/fl Pf4-Cre platelets activated with PAR4p (supplemental Fig. 2A) or Cvx (supplemental Fig. 2B) showed a significant reduction in αIIbβ3 activation and P-selectin expression (a measure for alpha granule release) at low and high doses of the agonists when compared to controls. Stim1fl/fl Pf4-Cre platelets showed a normal aggregation response when activated with PAR4p or Cvx and a mild aggregation defect upon stimulation with type-I fibrillar collagen or CRP (Fig. 2). In contrast, CalDAG-GEFI−/− platelets have a documented aggregation defect when activated with low doses of PAR4p [10] or Cvx [11], and they failed to aggregate in response to all tested concentrations of collagen or CRP. As we recently demonstrated, platelet aggregation in the absence of Ca2+/CalDAG-GEFI requires signaling via the Gi-coupled receptor for ADP, P2Y12 [10]. To evaluate the contribution of SOCE to CalDAG-GEFI-dependent platelet activation, we stimulated Stim1fl/fl Pf4-Cre platelets in the presence of a P2Y12 inhibitor, 2-MeSAMP. Compared to 2-MeSAMP-treated control platelets, PAR4p or Cvx-induced αIIbβ3 activation of P2Y12-treated Stim1fl/fl Pf4-Cre platelets was significantly reduced at all tested agonist concentrations (supplemental Fig. 3A, C). Similarly, degranulation as measured by surface expression of P-selectin was significantly reduced in 2-MeSAMP-treated Stim1fl/fl Pf4-Cre platelets (supplemental Fig. 3B).
Figure 2. Role of CalDAG-GEFI and STIM1 for integrin-mediated platelet aggregation.
Integrin-mediated aggregation of Stim1fl/fl (black line), Stim1fl/fl Pf4-Cre (grey line), and CalDAG-GEFI−/− (dotted line) platelets in response to threshold concentrations of the indicated agonists. Traces are representative of 5 independent experiments.
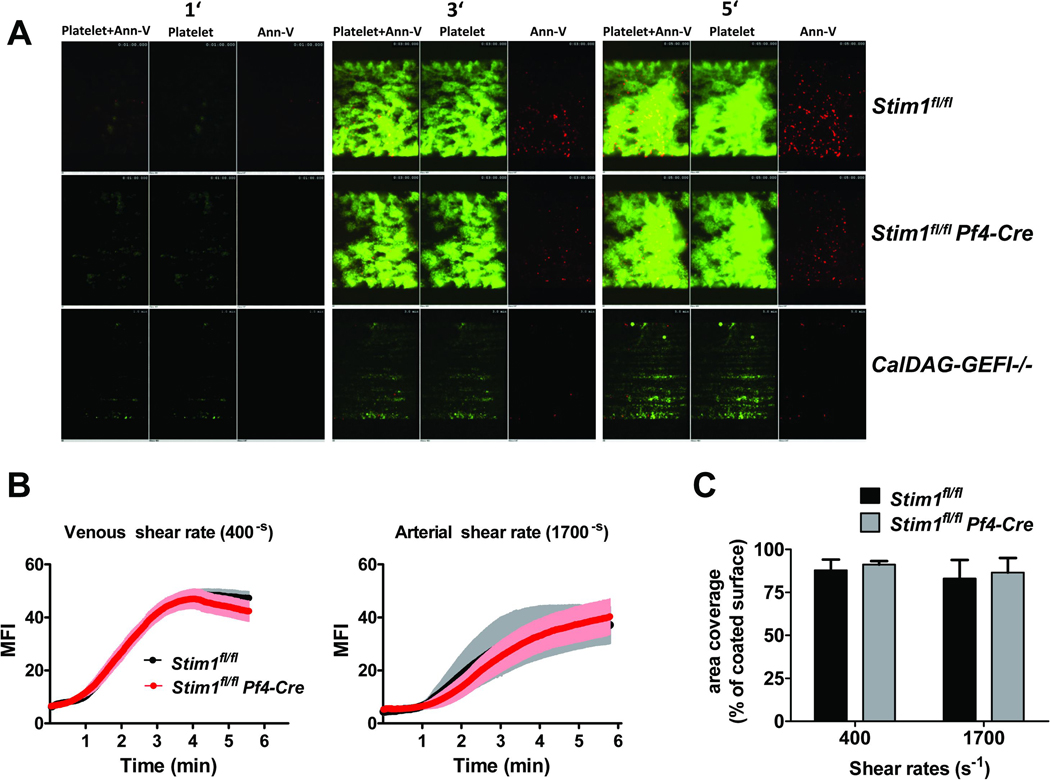
Consistent with the activation/aggregation studies, thrombus formation in Stim1fl/fl Pf4-Cre blood perfused over collagen at arterial (1700-S) or venous (400-S) shear rates was normal when compared to controls (Fig. 3A,B,C). In contrast, CalDAG-GEFI−/− platelets were unable to form three-dimensional thrombi under the same experimental conditions (Fig. 3A, lower panel), confirming recent results [14]. Thus, STIM1-mediated SOCE contributes little to the adhesive function of platelets, both under static and flow conditions.
Figure 3. Role of CalDAG-GEFI and STIM1 for thrombus formation on collagen under flow conditions.
Anticoagulated whole blood from Stim1fl/fl, Stim1fl/fl Pf4-Cre, or CalDAG-GEFI−/− mice was perfused over collagen at venous or arterial shear rates. Platelet adhesion and surface PS exposure were detected by staining with anti-GpIX-Alexa488 (green) and annexin V-Alexa647 (red). (A) Representative images taken at the indicated time points during the perfusion at arterial shear rates. (B) Mean fluorescence intensity measured over time ± SEM; n = 10. Stim1fl/fl: black line; Stim1fl/fl Pf4-Cre: red line (C) Surface area covered by platelets at the end of the perfusion period (presented as % of surface area covered by collagen ± SEM; n = 10). Stim1fl/fl: black bars; Stim1fl/fl Pf4-Cre: grey bars
STIM1 and CalDAG-GEFI are critical for platelet phosphatidylserine exposure and fibrin generation in vivo
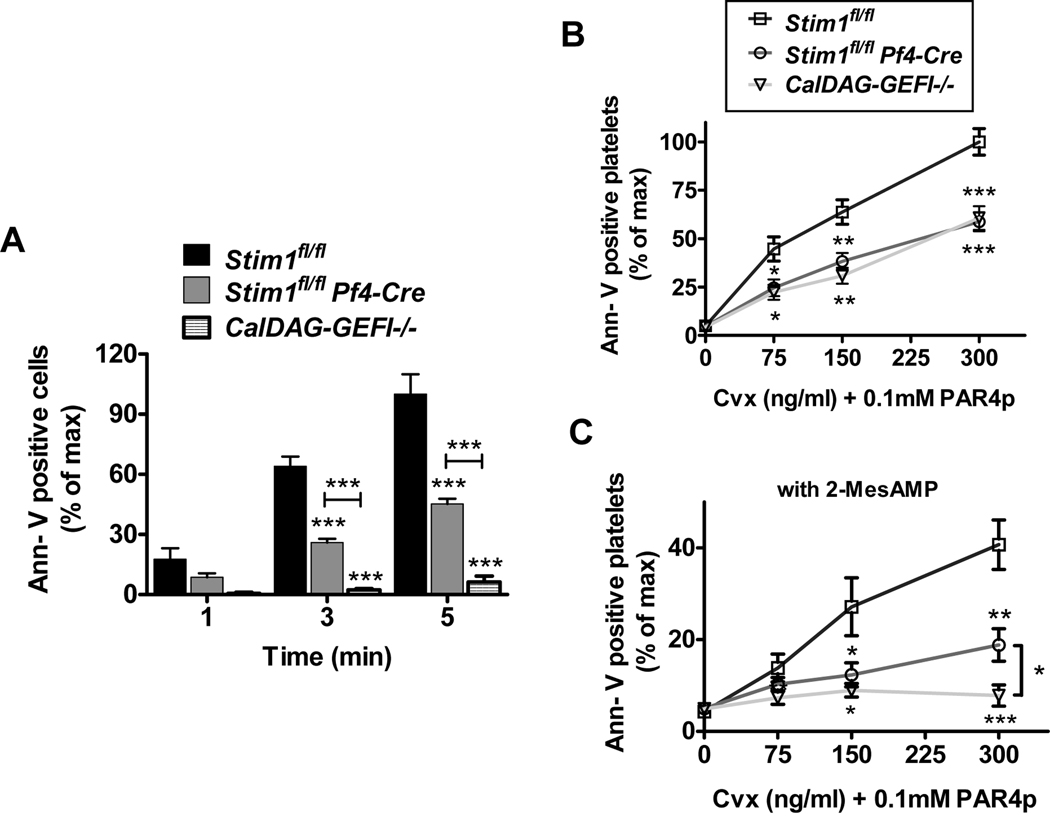
Aside of integrin activation and granule release, calcium plays a crucial role for phosphatidyl serine (PS) exposure and the pro-coagulant response in activated platelets [7, 22, 23]. To test the ability of Stim1fl/fl Pf4-Cre or CalDAG-GEFI−/− platelets to express PS upon stimulation, we quantified PS exposure during perfusion of Stim1fl/fl Pf4-Cre blood over collagen (Fig. 4A) and by flow cytometry after stimulation with PAR4p and Cvx (Fig. 4B). In both assays, significantly less PS-positive Stim1fl/fl Pf4-Cre platelets were observed when compared to controls. Interestingly, the defect in PS exposure in stimulated CalDAG-GEFI−/− platelets was similar to that of Stim1fl/fl Pf4-Cre platelets, suggesting that sustained increased [Ca2+]i leads to PS exposure via signaling through CalDAG-GEFI/Rap1. Furthermore, we observed that PS exposure was completely inhibited in CalDAG-GEFI−/− platelets pretreated with a P2Y12 inhibitor (Fig. 4C), i.e. platelets with abolished Rap1 signaling [10, 11]. Similarly, P2Y12 inhibition further reduced PS exposure in Stim1fl/fl Pf4-Cre platelets. Together these studies suggested that the previously described anti-thrombotic phenotype of mice lacking functional platelet STIM1 may be due to an impaired contribution of platelets to thrombin generation at the site of injury rather than an impaired adhesive ability of these cells.
Figure 4. STIM1 and CalDAG-GEFI are critical for Ca2+-dependent PS exposure in activated platelets.
(A) Number of annexin V-positive cells detected at the end of the perfusion over collagen at arterial shear rates (% ± SEM). The number of annexin V-positive platelets in Stim1fl/fl blood after 5 min of perfusion was defined as 100%; n = 10. (B, C) PS exposure on platelets activated under static conditions. Stim1fl/fl (squares), Stim1fl/fl Pf4-Cre (circles), and CalDAG-GEFI−/− (triangles) platelets were stimulated with the combination of PAR4p (0.1 mM) and Cvx (indicated concentrations) for 10 minutes under static conditions, stained with annexin V-Alexa647, and immediately analyzed. The studies were done in the presence and absence of 100 µM 2-MeSAMP, an inhibitor of P2Y12. Results are expressed as % ± SEM with the number of annexin V-positive Stim1fl/fl platelets activated with 300 ng/ml Cvx defined as 100%; n = 10. *p<.05, **p<0.005, ***p<0.0001.
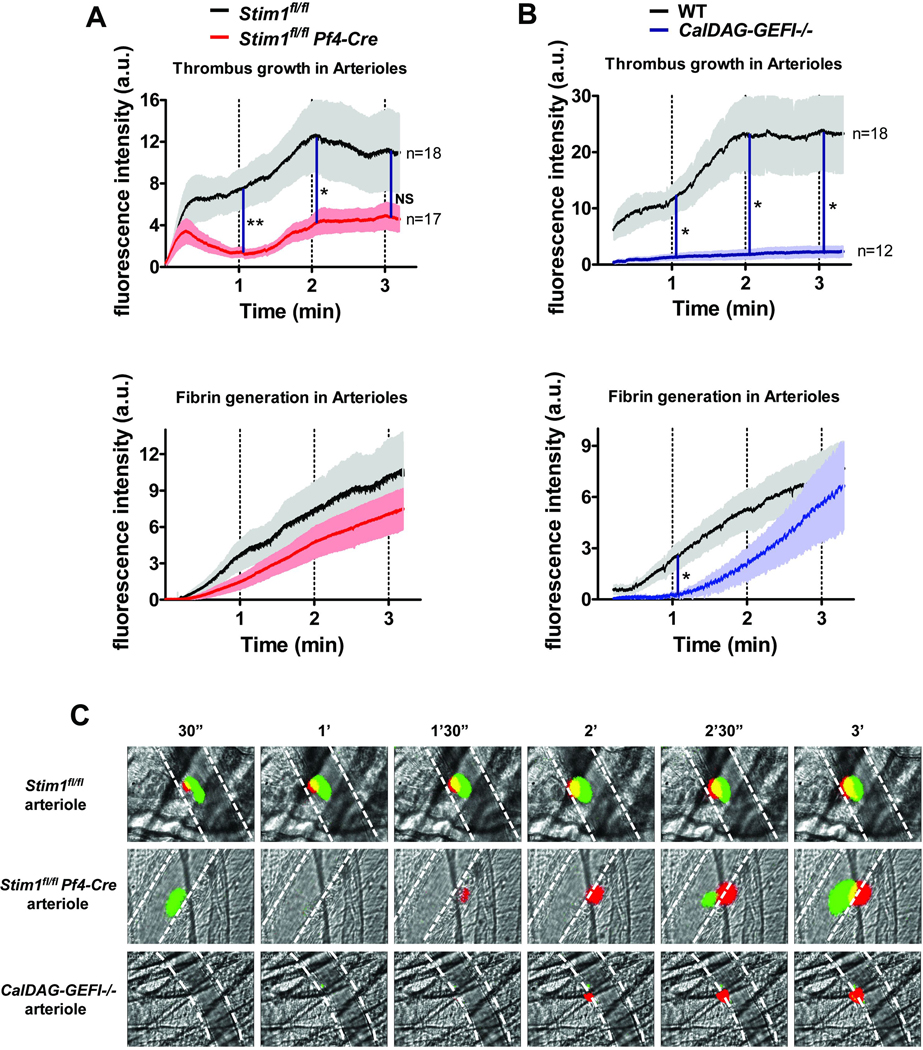
To compare the contribution of STIM1 and CalDAG-GEFI to thrombin generation and thrombus formation in vivo, we measured fibrin generation and platelet accumulation in arterioles damaged by laser injury. Compared to controls, accumulation of Stim1fl/fl Pf4-Cre platelets was significantly reduced, confirming previous results [21]. When looking at the kinetics of platelet accumulation, we observed that the initial phase of platelet accumulation was not affected in Stim1fl/fl Pf4-Cre mice. Early thrombi, however, were reversible and in most cases disappeared within one minute after laser injury (Fig. 5A,C). Interestingly, we also observed a marked delay and reduction in fibrin generation in injured arterioles of Stim1fl/fl Pf4-Cre mice, suggesting that STIM1 plays an important role for platelet-dependent thrombin/fibrin generation in the early phase of thrombus formation. A similar delay and reduction in fibrin generation was observed in CalDAG-GEFI−/− mice. Platelet adhesion, however, was almost completely inhibited in the absence of CalDAG-GEFI (Fig. 5B,C), confirming the key role of this molecule in the regulation of the adhesive properties of platelets.
Figure 5. Impaired platelet adhesion and fibrin accumulation in arterioles of Stim1fl/fl Pf4-Cre and CalDAG-GEFI−/− mice.
Mice were injected with Alexa488-labeled Fab fragments to αIIbβ3 (MWReg30) and Alexa647-labeled antibodies to fibrin. (A, B) Changes in fluorescence intensity over time measured after laser injury in cremaster muscle arterioles of Stim1fl/fl Pf4-Cre (A) and CalDAG-GEFI−/− (B) mice. Results represent the mean fluorescence intensity ± SEM measured in 3 independent experiments (n = 12–18 vessels for each group). (C) Representative images (Green-platelets; Red-Fibrin). See supplemental Videos 1–3 for a better visualization of the differences in thrombus growth and stability observed in the respective study groups. *p<0.05, **p<0.005.
Discussion
In this study, we compared platelet activation in mice with a conditional deletion of STIM1 in platelets/megakaryocytes and mice lacking CalDAG-GEFI. CalDAG-GEFI senses increased intracellular Ca2+ concentrations and triggers various cellular responses via the activation of the small GTPase Rap1. STIM1 was shown to be important for store-operated calcium entry in platelets and its deletion in mice led to protection from injury-induced thrombosis, presumably due to a defect in the adhesive function of STIM1-deficient platelets [21]. By contrast, our studies demonstrate that STIM1 is not critical for platelet aggregation both under static and flow conditions, while it plays an important role for the platelet pro-coagulant response.
Our studies confirm STIM1 as a crucial molecule in SOCE in platelets. When looking at aggregation and thrombus formation of STIM1-deficient platelets, we observed similarities and discrepancies to previous studies. In accordance with Varga-Szabo et al. [21], we found the most prominent defects in integrin αIIbβ3 activation upon stimulation of STIM1-deficient cells via GPVI. On a molecular level, GPVI-mediated αIIbβ3 activation in Stim1fl/fl Pf4-Cre platelets was reduced by ~50% when compared to controls. Given the high number of αIIbβ3 receptors on the platelet surface, however, this defect in activation had no effect on the ability of these cells to aggregate in vitro. Defects in aggregation were only observed in Stim1fl/fl Pf4-Cre platelets activated with threshold concentrations of collagen or collagen-related peptides, but not the more potent GPVI agonist, convulxin. Consistent with this minor defect in aggregation, we observed normal adhesion and thrombus formation of Stim1fl/fl Pf4-Cre platelets perfused over a collagen surface at arterial or venous shear rates. In contrast, platelets lacking CalDAG-GEFI showed marked defects in aggregation and thrombus formation under flow. Thus, in contrast to previous studies [21, 23], our studies suggest only a minor role for STIM1 in platelet aggregation. One potential explanation for the different phenotypes comes from methodological differences between the studies. For example, the microfluidic devices used for the respective studies are custom-made and thus may generate slightly different flow patterns. Variation may also derive from differences between the collagen preparations and the collagen coating procedures used to generate the pro-thrombotic surface. Arguably the most obvious difference between these studies, however, is the difference in strategies to delete STIM1 in platelets/mice. Both Varga-Szabo et al. and Gilio et al. used chimeric mice with a complete knockout of STIM1 in all blood cells. In our study, we used mice with a conditional deletion of Stim1 in the platelet/megakaryocyte lineage. There are drawbacks to both systems: the chimeric mice lack STIM1 in all blood cells, a potential limitation in assays monitoring thrombosis in whole blood. Furthermore, lethal irradiation required for the generation of chimeric mice causes the systemic release of inflammatory mediators [30], which may adversely affect circulating platelets [31]. The platelet-specific, conditional deletion of STIM1 described here eliminates the above-mentioned complications. While deletion by the Cre recombinase can be incomplete, we did not detect significant amounts of STIM1 in lysates from Stim1fl/fl Pf4-Cre platelets, and intracellular staining for STIM1 was absent in >95% of circulating platelets, confirming the virtually complete deletion of β1 integrin in the initial description of the Pf4-Cre transgenic mice [29].
In contrast to the retained pro-adhesive function of STIM1-deficient platelets, PS exposure was markedly impaired in Stim1fl/fl Pf4-Cre platelets. In our previous work, we found a similar defect in platelet PS exposure for cells isolated from mice expressing an inactive mutant of the STIM1-activated Ca2+ channel Orai1 [22], a finding that was confirmed for STIM1-deficient platelets by Gilio et al. [23]. When perfused over collagen in the presence of thrombin, however, PS exposure and fibrin generation were normal in blood from Stim1−/− chimeras. Thus, the authors suggested that platelet SOCE could be redundant for the formation of fibrin-rich thrombi at injury sites where thrombin is present as a co-agonist. Our studies argue against this conclusion. In a model of thrombin-dependent, localized thrombosis (laser injury, Fig. 5), we observed impaired formation of fibrin-rich thrombi at sites of vascular injury. While the initial adhesion and aggregation of platelets was not affected in arterioles of Stim1fl/fl Pf4-Cre mice, thrombi were instable and substantially smaller than in WT controls, and fibrin formation was delayed and reduced. Thus, our studies argue for a critical role of STIM1 and SOCE for platelet-dependent, early thrombin generation at the site of vascular injury.
Our results are also in line with studies on the adhesive and pro-coagulant properties of platelets isolated from patients with Scott syndrome [32–33]. Like STIM1-deficient platelets, “Scott” platelets adhere, spread, and capture free-flowing platelets equally well as platelets from a control subject. However, platelet PS exposure and platelet-dependent fibrin formation is strongly impaired. Platelets from Scott patients are severely impaired in their ability to express PS in response to elevated [Ca2+]i, probably due to a defect in calcium-dependent phospholipids scrambling by transmembrane protein 16F (TMEM16F) [7]. The identification of calcium-dependent phospholipid scramblases like TMEM16F may suggest that PS exposure simply requires sustained elevated [Ca2+]i but is independent of intracellular signaling. Our studies, however, do not support this hypothesis as PS exposure in activated platelets was dependent on signaling via CalDAG-GEFI and P2Y12 (Fig. 4C), confirming studies in human platelets, which also identified a role for P2Y12 in the pro-coagulant response of platelets [34]. The complete inhibition of PS exposure observed in P2Y12 inhibitor-treated CalDAG-GEFI−/− platelets suggests that PS exposure in platelets depends on signaling by Rap1. It is currently not clear how one molecule, Rap1, can regulate diverse cellular functions such as integrin-mediated adhesion and pro-coagulant response. Interestingly, we observed similar defects in PS exposure in STIM1-deficient and CalDAG-GEFI-deficient platelets, suggesting that sustained elevated [Ca2+]i in platelets is particularly important for the pro-coagulant role of the CalDAG-GEFI/Rap1 signaling module. Furthermore, our studies with platelets activated in the presence of the P2Y12 inhibitor 2-MeSAMP provide an explanation for the limited contribution of STIM1/SOCE to platelet aggregation. Compared to cells activated in the absence of the P2Y12 inhibitor, Stim1fl/fl Pf4-Cre platelets activated in the presence of 2-MeSAMP showed a marked defect in integrin activation and aggregation. Thus, sustained calcium signaling via SOCE is critical for integrin-mediated aggregation in the absence of P2Y12 signaling, mediated via CalDAG-GEFI. Under normal conditions, however, SOCE is not critical for platelet aggregation as Ca2+ from intracellular stores triggers CalDAG-GEFI-dependent Rap1/integrin activation, which is sustained via signaling by P2Y12.
In summary, our studies in mice with a conditional deletion of STIM1 in platelets/megakaryocytes demonstrate that STIM1/SOCE plays a crucial role for the conversion of platelets from a pro-adhesive to a pro-coagulant state. Our studies demonstrate that STIM1 is not critical for the pro-adhesive function of platelets, while it is required for platelet PS exposure in vitro and platelet-dependent thrombin/fibrin formation at sites of vascular injury. In contrast, CalDAG-GEFI is critical for both platelet accumulation at sites of vascular injury and the conversion to a pro-coagulant state.
Supplementary Material
Acknowledgement
This work was supported by the American Heart Association (10IRG4100001, W.B.), the American Society of Hematology (W.B.), and National Institutes of Health grants R01 HL094594 (W.B.) and AI066128 (to S.F.).
Footnotes
Conflict of interest disclosure: S.F. is a scientific co-founder of Calcimedia, a biotechnology company which develops CRAC channel inhibitors.
Reference
- 1.Worner P, Brossmer R. Platelet aggregation and the release induced by inophores for divalent cations. Thromb Res. 1975;6:295–305. doi: 10.1016/0049-3848(75)90079-1. [DOI] [PubMed] [Google Scholar]
- 2.Harper MT, Poole AW. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. J Thromb Haemost. 2010;8:454–462. doi: 10.1111/j.1538-7836.2009.03722.x. [DOI] [PubMed] [Google Scholar]
- 3.Feinstein MB, Becker EL, Fraser C. Thrombin, collagen and A23187 stimulated endogenous platelet arachidonate metabolism: differential inhibition by PGE1, local anesthetics and a serine-protease inhibitor. Prostaglandins. 1977;14:1075–1093. doi: 10.1016/0090-6980(77)90286-6. [DOI] [PubMed] [Google Scholar]
- 4.Feinstein MB, Fraser C. Human platelet secretion and aggregation induced by calcium ionophores. Inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol. 1975;66:561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevers EM, Comfurius P, van Rijn JL, Hemker HC, Zwaal RF. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur J Biochem. 1982;122:429–436. doi: 10.1111/j.1432-1033.1982.tb05898.x. [DOI] [PubMed] [Google Scholar]
- 6.Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–193. [PubMed] [Google Scholar]
- 7.Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 8.Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: effects of ionophore A23187, thrombin, collagen, and convulxin. Blood. 2000;95:1694–1702. [PubMed] [Google Scholar]
- 9.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 10.Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008;112:1696–1703. doi: 10.1182/blood-2008-02-139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefanini L, Roden RC, Bergmeier W. CalDAG-GEFI is at the nexus of calcium-dependent platelet activation. Blood. 2009;114:2506–2514. doi: 10.1182/blood-2009-04-218768. [DOI] [PubMed] [Google Scholar]
- 12.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Stolla M, Stefanini L, Roden RC, Chavez M, Hirsch J, Greene T, Ouellette TD, Maloney SF, Diamond SL, Poncz M, Woulfe DS, Bergmeier W. The kinetics of {alpha}IIb{beta}3 activation determines the size and stability of thrombi in mice: implications for antiplatelet therapy. Blood. 2011;117:1005–1013. doi: 10.1182/blood-2010-07-297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+ store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of storeoperated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 18.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for storeoperated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, Hanke P, Schropp P, Muhlstedt S, Zorn C, Huber M, Schmittwolf C, Jagla W, Yu P, Kerkau T, Schulze H, Nehls M, Nieswandt B. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest. 2007;117:3540–3550. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, Renné T, Stoll G, Nieswandt B. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med. 2008;205:1583–1591. doi: 10.1084/jem.20080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–678. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilio K, van Kruchten R, Braun A, Berna-Erro A, Feijge MA, Stegner D, van der Meijden PE, Kuijpers MJ, Varga-Szabo D, Heemskerk JW, Nieswandt B. Roles of platelet STIM1 and Orai1 in glycoprotein VI- and thrombin-dependent procoagulant activity and thrombus formation. J Biol Chem. 2010;285:23629–23638. doi: 10.1074/jbc.M110.108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neeves KB, Maloney SF, Fong KP, Schmaier AA, Kahn ML, Brass LF, Diamond SL. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shearresistance of platelet aggregates. J Thromb Haemost. 2008;6:2193–2201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 26.Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neyman M, Gewirtz J, Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood. 2008;112:1101–1108. doi: 10.1182/blood-2008-04-152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falati S, Gross PL, Merrill-Skoloff G, Sim D, Flaumenhaft R, Celi A, Furie BC, Furie B. In vivo models of platelet function and thrombosis: study of real-time thrombus formation. Methods Mol Biol. 2004;272:187–197. doi: 10.1385/1-59259-782-3:187. [DOI] [PubMed] [Google Scholar]
- 29.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 30.Van der Meeren A, Monti P, Lebaron-Jacobs L, Marquette C, Gourmelon P. Characterization of the acute inflammatory response after irradiation in mice and its regulation by interleukin 4 (Il4) Radiat Res. 2001;155:858–865. doi: 10.1667/0033-7587(2001)155[0858:cotair]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Oleksowicz L, Mrowiec Z, Zuckerman D, Isaacs R, Dutcher J, Puszkin E. Platelet activation induced by interleukin-6: evidence for a mechanism involving arachidonic acid metabolism. Thromb Haemost. 1994;72:302–308. [PubMed] [Google Scholar]
- 32.Weiss HJ, Turitto VT, Baumgartner HR. Role of shear rate and platelets in promoting fibrin formation on rabbit subendothelium. Studies utilizing patients with quantitative and qualitative platelet defects. J Clin Invest. 1986;78:1072–1082. doi: 10.1172/JCI112663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wielders SJ, Broers J, ten Cate H, Collins PW, Bevers EM, Lindhout T. Absence of platelet-dependent fibrin formation in a patient with Scott syndrome. Thromb Haemost. 2009;102:76–82. doi: 10.1160/TH08-11-0719. [DOI] [PubMed] [Google Scholar]
- 34.van der Meijden PE, Feijge MA, Giesen PL, Huijberts M, van Raak LP, Heemskerk JW. Platelet P2Y12 receptors enhance signalling towards procoagulant activity and thrombin generation. A study with healthy subjects and patients at thrombotic risk. Thromb Haemost. 2005;93:1128–1136. doi: 10.1160/TH04-09-0597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.