Abstract
Rationale
In sinoatrial node cells (SANC), Ca2+ activates adenylate cyclase (AC) to generate a high basal level of cAMP-mediated/protein kinase A (PKA)-dependent phosphorylation of Ca2+ cycling proteins. These result in spontaneous sarcoplasmic-reticulum (SR) generated rhythmic Ca2+ oscillations during diastolic depolarization, that not only trigger the surface membrane to generate rhythmic action potentials (APs), but, in a feed-forward manner, also activate AC/PKA signaling. ATP is consumed to pump Ca2+ to the SR, to produce cAMP, to support contraction and to maintain cell ionic homeostasis.
Objective
Since a negative feedback mechanism links ATP-demand to ATP production, we hypothesized that (1) both basal ATP supply and demand in SANC would be Ca2+-cAMP/PKA dependent; and (2) due to its feed–forward nature, a decrease in flux through the Ca2+-cAMP/PKA signaling axis will reduce the basal ATP production rate.
Methods and Results
O2 consumption in spontaneous beating SANC was comparable to ventricular myocytes (VM) stimulated at 3 Hz. Graded reduction of basal Ca2+-cAMP/PKA signaling to reduce ATP demand in rabbit SANC produced graded ATP depletion (r2=0.96), and reduced O2 consumption and flavoprotein fluorescence. Neither inhibition of glycolysis, selectively blocking contraction nor specific inhibition of mitochondrial Ca2+ flux reduced the ATP level.
Conclusions
Feed-forward basal Ca2+-cAMP/PKA signaling both consumes ATP to drive spontaneous APs in SANC and is tightly linked to mitochondrial ATP production. Interfering with Ca2+-cAMP/PKA signaling not only slows the firing rate and reduces ATP consumption, but also appears to reduce ATP production so that ATP levels fall. This distinctly differs from VM, which lack this feed-forward basal cAMP/PKA signaling, and in which ATP level remains constant when the demand changes.
Keywords: Calcium-activated adenylyl cyclase, constitutive basal PKA-dependent phosphorylation, bioenergetics, pacemaker automaticity, respiration
Introduction
Cells within the sinoatrial node (SAN), the heart’s pacemaker, initiate each normal heart beat. SAN cells (SANC) generate a high basal (i.e., in the absence of β-adrenergic receptor stimulation) level of cAMP-mediated, protein kinase A (PKA)-dependent phosphorylation of Ca2+ cycling proteins. This enables the occurrence of spontaneous rhythmic, sarcoplasmic reticulum (SR)-generated, local subsarcolemmal Ca2+ releases (LCRs) via ryanodine receptors (Ca2+ clock) during diastolic depolarization, i.e., prior to the next action potential (AP). LCR’s activate an inward Na+-Ca2+ exchange current which depolarizes the surface membrane. LCRs prompt the ensemble of voltage-dependent surface membrane electrogenic proteins (membrane clock) to generate the next spontaneous AP, which then triggers a global SR Ca2+ release and contraction [1, 2].
We [3] and others [4] have shown that basal activation of adenylyl cyclase (AC) isoforms produce a high basal level of cAMP coupled to high basal levels of phosphodiesterase activity which creates high rates of cAMP synthesis and degradation [5]. In spite of the high rate of cAMP degradation, the basal cAMP level is higher in SANC than VM [6]. In SANC, cAMP activates PKA, resulting in a high basal rate of PKA-dependent phosphorylation of SAN proteins among which are Ca2+ cycling proteins [6]. This results in a high basal rate of SR Ca2+ pumping in SANC and spontaneous diastolic rhythmic LCR from SR via ryanodine receptors. Both the global Ca2+ release triggered by APs and LCRs may activate AC to perpetuate the feed-forward Ca2+-cAMP/PKA signaling cycle. To maintain rhythmic electrical activity, ATP must be supplied to activate AC to produce cAMP, and a significant part of ATP is consumed to pump Ca2+ into the SR and to maintain cell ionic homeostasis”. It has been shown that inhibition of basal cAMP/PKA signaling reduces the spontaneous AP firing rate [6], and on this basis the basal ATP demand should be reduced when cAMP/PKA signaling is inhibited. But whether and how Ca2+-cAMP/PKA signaling in SANC links to ATP supply is unknown.
We hypothesized that maintenance of the basal state of Ca2+-cAMP signaling requires ATP supply and that both ATP production and demand are tightly linked to the basal Ca2+-cAMP/PKA in SANC. If such link was to exist, interfering with ATP production by NaCN will reduce cAMP and Ca2+ [7], and graded reduction in basal PKA-dependent signaling (that results in graded reductions in ATP demand) will also result in graded reductions in ATP production, and thus to a reduction in the ATP level. If this type of ATP supply-demand regulation indeed were to occur in SANC, it would be very different from that in ventricular tissue or myocytes (VM) which maintain a nearly constant ATP level, regardless of changes in ATP demand [8]. To test our hypothesis, we employed a battery of pharmacological tools, documented in prior studies (for review see [9]) to concomitantly reduce SANC Ca2+-cAMP/PKA signaling and the spontaneous AP firing rate or to reduce ATP production [7]. We observed that graded inhibition of Ca2+-cAMP/PKA signaling in SANC to reduce ATP demand was accompanied by graded reduction in the ATP level, indicating that Ca2+-cAMP/PKA signaling is the core feed-forward link between basal utilization of ATP and the mitochondrial ATP production. We also observed that direct and specific inhibition of mitochondrial Ca2+ flux does not apparently affect the basal ATP level of SANC (again in stark contrast with VM). This suggests that basal cAMP-PKA signaling, rather than the Ca2+ component of Ca2+-cAMP/PKA signaling, directly regulates basal SANC ATP supply.
Methods
A complete description of the methods used in the study is provided in the on-line supplement.
SANC preparations, cell contraction, flavoprotein autofluorescence and Ca2+ recording
Single, spindle-shaped, spontaneously beating SANC or VM were isolated from the rabbit hearts as previously described [5], and bathed in Tyrode solution at 35±0.5°C (see on-line supplement for solution composition and drug concentrations). SANC were imaged by a Zeiss LSM-510 inverted confocal microscope (Carl Zeiss). Images were recorded in the linescan mode (0.8 ms/line) with a scan line oriented along the short axis of the cell using a 633 nm laser to quantify the spontaneous contraction frequency. Flavoprotein autofluorescence was recorded (20 s/frame) using a 488 nm laser. SANC were loaded with 5μmol/L Fluo-4 AM (Molecular probes) for Ca2+ imaging using a 488 nm laser in line scan mode (2 ms/line) as previously described [5].
ATP measurements
The cell suspension was divided equally into 3 to 5 aliquots: the first aliquot was treated with specific pharmacological agents; the second with 100 μmol/L DNP for 15 min; and the third was used as a control. The other two aliquots were used if cAMP level was measured. SANC suspensions were centrifuged at 100 g for 5 min, and the supernatant removed. The ATP concentrations in spontaneously firing SANC and in VM in the absence and presence of electrical stimulation at 3 Hz were determined from the supernatant by a bioluminescence assay kit, HS II (Roche). The ATP concentration was normalized to the cell ATP level following a 15 min incubation with 2,4-dinitrophenol (mitochondrial uncoupler). A complete description of this method is provided in the on-line supplement.
cAMP measurements
The cAMP concentration was determined by a LANCE cAMP 384 kit (PerkinElmer).
O2 consumption measurements
Oxygen consumption was measured in cell suspensions using Clark-type electrodes (MT200, Strathkelvin Instruments Ltd.).
Western Blotting
Western-blot was performed in tissue lysates isolated from SAN, atria, ventricular and brain tissue.
Statistical Analysis
Data are presented as mean±SEM. When the means of paired samples are to be compared, a paired t-test was employed. In cases where the samples are independent, a two-sample t-test was applied. For multiple treatments a linear mixed-effects model with Dunnett’s method to adjust p-value was used. This model accounts for repeated measurements on the same animal while allowing for tests among the groups. P < 0.05 was taken to indicate statistical significance.
Experimental Protocol
In order to reveal the nature of the control mechanisms that manage the SANC ATP budget we used both isolated SANC and SANC suspensions. In cell suspensions, the number of healthy and functional cells varies from preparation to preparation. To ensure comparability of ATP, cAMP and O2 consumption responses to a given pharmacological intervention in different suspensions, ATP, cAMP and O2 consumption were measured in aliquots of a given suspension, containing an equal amount of protein and viable cells. Also the amount of viable cells in each aliquot of a given suspension was counted prior-to and during exposure to the drug. To reduce the background noise, ATP measurements were made prior-to and following addition of DNP (to kill all cells by uncoupling oxidative phosphorylation). Note that a comparison of ATP levels prior to and in the presence of DNP provides an additional estimate of the ratio of live to dead cells (since dead cells do not produce ATP). The pre and post drug ATP level within an aliquot of a given suspension was normalized to its ATP level in the presence of DNP. Cell suspensions in which the ATP level prior to and post DNP was less than 3 fold were omitted from further data analysis. The average ATP level pre to post DNP ratio for cells that were entered into statistical analysis was 4.1±0.2 (range 3.2 to 5.4). Note also that since DNP interferes with the cAMP detection method (for details about cAMP measurements see the on-line supplement), the cAMP level post DNP could not be used to normalize cAMP measurements. However, since cAMP and ATP were measured in aliquots of the same suspension the viability index described above was also applied for cAMP measurements. DNP was also used to quantify flavoprotein fluorescence (see supplement for quantification with both DNP and NaCN). Endogenous autofluorescence of mitochondrial NADH was too low to measure, due to the small size of the cells (higher laser power could not be employed due to the cell damage that it produced).
Finally, to compare results of different suspensions studied on different days, drug effects in aliquots from all suspensions were expressed as a % of their respective controls. Beating rate (which is the same as the spontaneous AP firing rate) was measured in single cells. To insure the validity of comparison between ATP, cAMP and spontaneous beating rate, the beating rates were measured in single SANC extracted from the same suspension in which ATP, cAMP and O2 were measured.
For VM studies, ATP and O2 consumption measurements were performed in suspensions that contained greater than 70% viable cells, and in which more than 80% of viable cells responded to electrical stimulation at 3Hz.
Results
SANC have a high density of mitochondria and a high respiration rate
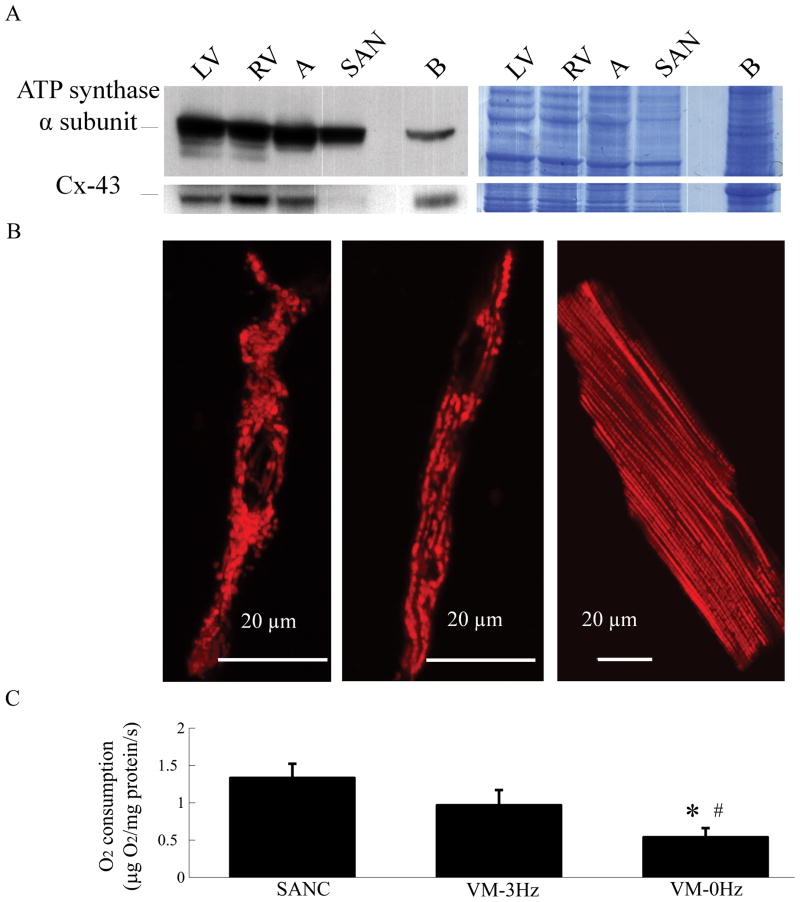
The expression of ATP synthase α subunit, the end-point reaction in mitochondrial ATP production, and immunolabeling of mitochondrial membrane with tetramethylrhodamine methyl ester were used as indices of mitochondrial density. Figure 1A–B show that SANC have a high density of mitochondria, i.e., similar to that of other heart and brain tissues. Oxygen consumption was used to index the mitochondrial metabolic rate. Figure 1C shows that SANC have a respiration rate (1.3±0.2 μg O2/mg protein/s) comparable to electrically stimulated, unloaded, VM (0.8±0.2 μg O2/mg protein/s). Note also that when resting VM were stimulated at a rate of 3Hz (i.e. a rate similar to the average spontaneous action potential firing rate in SANC), O2 consumption increased by 60%.
Figure 1.
Sinoatrial node cells (SANC) have a high density of mitochondrial proteins and a high basal respiratory rate: (A) Immunoblotting of the mitochondrial complex V and connexin 43 in rabbit tissues; LV-left ventricle, RV-right ventricle, SAN-sinoatrial node and B-brain. RV, LV and SAN have mitochondrial protein densities similar to other tissues. (Lack of expression of gap junction protein, connexin 43 verifies that the SAN tissue is truly SAN, and not atrial tissue). Colloidal blue staining of the gel in the right panel confirmed comparable protein amounts among all the samples loaded on the gel. (B) SANC (left and middle) and VM (right) mitochondria visualized by tetramethylrhodamine methyl ester staining. Colloidal blue staining of the gel in the right panel confirmed comparable protein amounts among all the samples loaded on the gel. (C) Respiration rates of isolated spontaneously beating SANC suspension (n=17) and quiescent or electrically stimulated (3Hz) ventricular myocytes (VM) suspension (n=8). *p<0.05 vs. SANC, #p<0.05 vs. VM-3Hz.
Blocking ATP production reduces Ca2+ and cAMP
In SANC, Ca2+ is required for AC activation [3] and ATP is consumed in the synthesis of cAMP. Short-term metabolic inhibition is known to reduce the size of intracellular Ca2+ transients (in toad pacemaker cells) [7]. To determine the extent to which short-term metabolic inhibition affects intracellular Ca2+ or reduces cAMP we used NaCN to block oxidative phosphorylation. A 5-min exposure to 1 mmol/L of NaCN decreased the peak cytosolic Ca2+ by 29±2% (n=9). A 5-min exposure to 5 mmol/L of NaCN reduced cAMP by 44±8% (n=3). That the spontaneous AP firing rate concomitantly decreased by 94±5% (n=9, Fig. 3S) suggests that reduction in cAMP is attributable to a reduction in ATP supply.
The steady ATP levels, spontaneous AP firing rate, and cAMP in SANC are reduced in response to a reduction in [Ca2+], PKA inhibition or muscarinic receptor stimulation
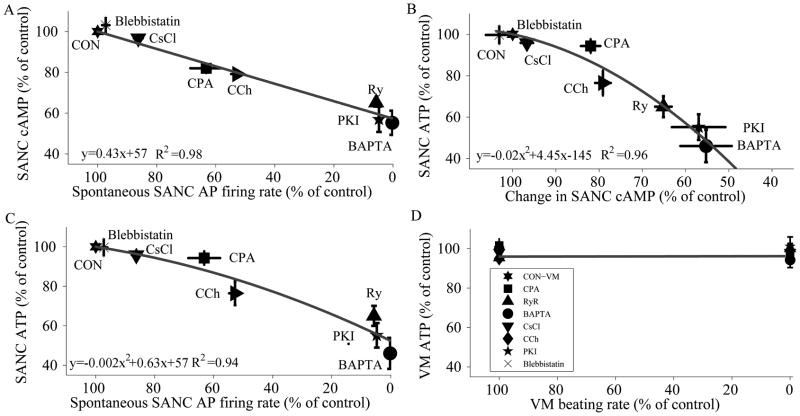
Numerous studies ([10, 11] also reviewed in [9]) have shown that interfering with sarcoplasmic reticulum Ca2+ cycling using CPA to inhibit SERCA, or ryanodine to disable RyR function, decreases the spontaneous AP firing rate. Figure 2A and Table 1S (on-line supplement) show that interfering with [Ca2+] cycling by inhibiting the sarcoplasmic reticulum Ca2+ pump (CPA, 5 μmol/L) significantly diminishes cell contraction rate (and therefore the spontaneous AP firing rate) by 36.8±5.4%. Thapsigargin (200 nmol/L) has a similar effect (Table 1S). Disabling Ry receptors (Ry, 30 μmol/L), or buffering intracellular Ca2+ (BAPTA-AM, 25 μmol/L) each markedly reduce spontaneous AP firing rate by 94.3±0.2% and 99.8±0.15%, respectively (Fig. 2A, table 1S). Note that application of CsCl (2 mmol/L), which blocks If current in spontaneously contracting SANC has a modest effect on spontaneous AP firing rate (−14±2%) (Fig. 2A, table 1S). The effect of these pharmacologic interventions on the spontaneous AP firing rates for these drugs in this study were identical to those measured in previous studies [9] which also demonstrate that cAMP is an important modulator of spontaneous AP firing rate [9, 12, 13].
Figure 2.
The relationship between ATP, cAMP and the spontaneous beating rate in SANC. (A) Decreasing the spontaneous beating rate is associated with a decrease in the cAMP level (n=3 for each drug). (B) The decrease in the cAMP level is linked to a decrease in the ATP level (n=5 for each drug) in SANC. Thus a decrease in spontaneous beating rate is associated with reductions in both cAMP and ATP (B and C) and a reduction in ATP is linked to the reduction in cAMP. (D) In contrast, to SANC, a decrease in the electrical stimulation rate (3Hz vs. quiescent) of VM does not decrease the ATP level.
Figure 2A shows that interfering with cell Ca2+ cycling by buffering with BAPTA reduced cAMP. To determine factors that link the reduction in AP firing rate to a reduction in cAMP without directly inhibiting intracellular Ca2+ cycling, we inhibited cAMP production by inhibiting AC activity. A low dose of CCh (200 μmol/L), which in previous studies inhibited cAMP/PKA signaling in rabbit SANC by inhibition of AC with minor effects on IkAch [14], reduced the spontaneous AP firing rate by 47.7±2.1%, while reducing cAMP by 21±2% (Fig. 2A). To test whether the concomitant reductions in cAMP and spontaneous AP firing rate in response to CCh were directly due to a reduction in cAMP-mediated, PKA-dependent phosphorylation, we inhibited PKA signaling using two different inhibitors, specific PKA peptide inhibitor (PKI 14–22 Amide) (25 μmol/L) and H-89 (6 μmol/L). These not only markedly inhibited beating by 95.2±1 (TableS1 and Fig. 2A) and 99.5±0.2%, (TableS1) respectively, but surprisingly (Fig 2A) specific PKA peptide inhibitor (PKI 14–22 Amide) also markedly reduced cAMP by 43±6%, suggesting that a reduction in PKA-dependent phosphorylation induces a feedback inhibition on cAMP production. Thus, regardless of whether the SANC AP firing rate is suppressed by targeting intracellular Ca2+ cycling or by inhibiting cAMP-PKA signaling, cAMP levels are reduced and the graded reductions in the spontaneous AP firing rate was highly and linearly correlated with the concomitant reduction in cAMP (R2=0.98). It is noteworthy that while If is regulated directly by cAMP [12], blocking If current by CsCl has only a moderate effect on the spontaneous AP firing rate or on the cAMP level (Fig. 2). Therefore, If is not a major player in the SANC energy budget.
cAMP/PKA signaling links the spontaneous action potential firing rate to the ATP level in SANC
The next obvious question, and the main question of our study, is how do the interventions in Fig. 2A, which reduce AP firing rate and cAMP, affect ATP? Figure 2B shows that ATP declines as cAMP declines, and that there is a tight parabolic relationship (R2=0.96) between the cAMP reduction and ATP depletion. To verify that longer exposure to the short-term drug treatments would not lead to a further decrease in the ATP level, we exposed the SANC to a specific PKA peptide inhibitor (PKI 14–22 Amide) for different times. Application of a specific PKA peptide inhibitor (PKI 14–22 Amide) for 3-min reduced the ATP by 44.8±6% and this was not significantly increased by a longer (10-min) exposure to the specific PKA peptide inhibitor (PKI 14–22 Amide) (48.5±8%). The effect of H-89 was similar to specific PKA peptide inhibitor (PKI 14–22 Amide), i.e. ATP was reduced by 43.5±5% after a 3min exposure. These data therefore suggest that Ca2+ -regulated-cAMP/PKA signaling in SANC modulates ATP supply to meet ATP demand. When CPA, CCh or a specific PKA peptide inhibitor (PKI 14–22 Amide) was applied to single SANC we validated that the drug effects were reversible. Washing the specific PKA peptide inhibitor (PKI 14–22 Amide) or CPA in cell suspensions reversed the decline in ATP by 69±23% and 80±11%, respectively (n=3).
Blocking only Ca2+-myofilament interactions in SANC, without decreasing the spontaneous action potential firing rate, does not affect SANC ATP level
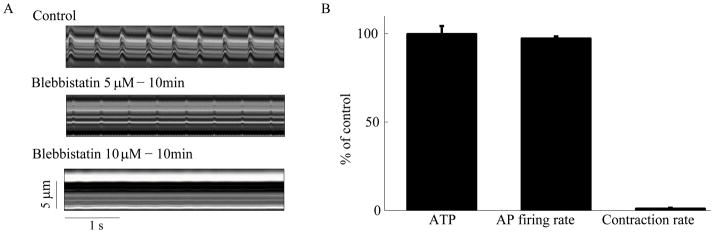
The AP initiated cytosolic Ca2+ transient presents Ca2+ to the contractile proteins to induce myofilament force production and displacement, the major ATP consumers in VM. Although SANC myofilament density is relatively low [15], to determine the impact of ATP consumption by the contractile machinery on ATP production in SANC we used blebbistatin (10 μmol/L) to block spontaneous contractions while preserving the AP firing rate. The lowest concentration of blebbistatin (Fig. 3A) that stopped SANC contraction has insignificant effects on spontaneous AP firing rate (from 195±9 to 190±10 beats/min) (Fig. 3B). Blebbistatin did not significantly alter either ATP (Fig. 3B) or cAMP levels (not shown). These results, therefore, demonstrate that blocking only the Ca2+-myofilament interactions in SANC, without decreasing the spontaneous AP firing rate, does not affect ATP production, i.e. ATP supply to demand matching. Changes in ATP consumption caused by interfering with the myofilament-Ca2+ interaction in SANC that are too small to detect might explain the lack of effect of blebistatin on ATP levels.
Figure 3.
Contractions (from linescan images), AP firing rate and ATP in SANC. Blebbistatin, which prevents normal myofilament interaction blocks cell contraction (n=12) (A), but has only minor effect on spontaneous action potential firing rate (n=7) (B) and no significant effect on the ATP levels (n=5) (B).
ATP level in SANC under basal conditions is not supported by glycolysis
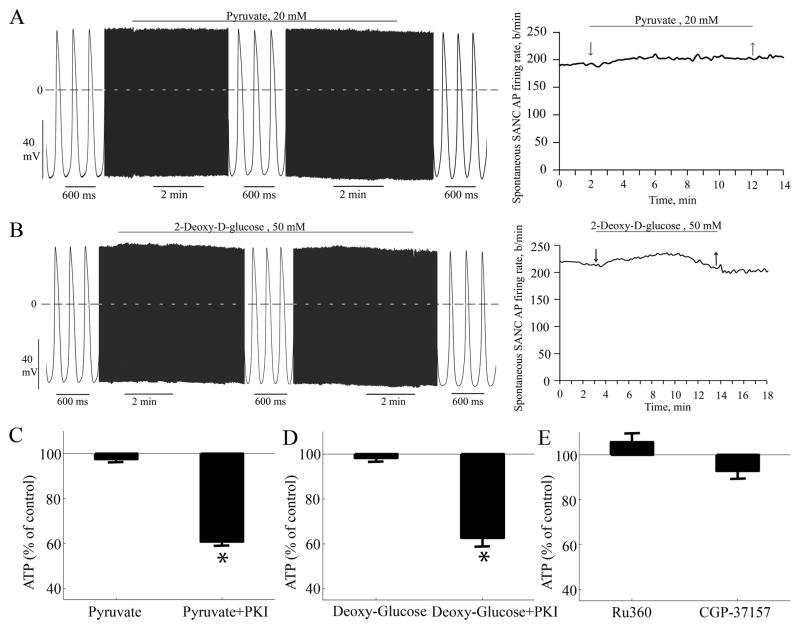
To demonstrate whether the mitochondrial, rather than glycolytic, ATP production, provides sufficient ATP in SANC to generate spontaneous AP, we bathed SANC separately in 2 mmol/L pyruvate or 50 mmol/L 2-deoxy-d-glucose to out-compete and/or inhibit glycolysis. Application of pyruvate or 2-deoxy-d-glucose alters neither the spontaneous AP firing rate (n=9), nor the ATP level (n=3), nor O2 consumption (n=3) (Fig. 4). Actually, this result is in accord with a prior study [16].
Figure 4.
Glycolysis and mitochondrial Ca2+ roles in ATP production. (A) Pyruvate, and (B) deoxyglucose (glycolysis inhibitor) has only minor effect on AP. Pyruvate (C) and deoxyglucose (D) has no significant effect on the ATP levels (n=3) or on the ATP reduction in response to a PKA peptide inhibitor (PKI 14–22 Amide) (n=3). (E) Blocking Ca2+ influx (Ru360; n=5) or increasing mitochondrial Ca2+ by blocking its Na+-Ca2+ exchanger (CGP-37157; n=5) has no significant effect on SANC ATP levels.*p<0.05 vs. each drug control.
Reduction in Ca2+-cAMP/PKA signaling in SANC impacts mitochondrial redox state and electron transport flux
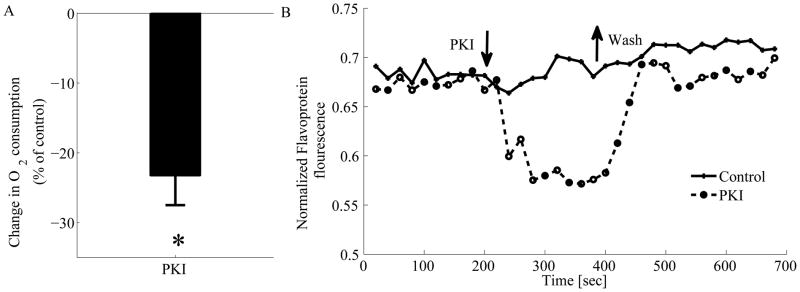
We next examined the effect of a reduction in Ca2+-cAMP/PKA signaling on flavoprotein fluorescence level and O2 consumption. To quantify the range of O2 in SANC we treated the cells with different concentrations of DNP (online-supplement Fig. 1S). We used specific PKA peptide inhibitor (PKI 14–22 Amide) to demonstrate that direct inhibition of Ca2+-cAMP/PKA signaling can decrease ATP production by the mitochondria. Application of the specific PKA peptide inhibitor (PKI 14–22 Amide) decreases O2 consumption (n=5) by 23±4% (Fig. 5A) and flavoprotein fluorescence (n=10) by 17±3% (Fig. 5B). In steady state Ry and BAPTA each, indirectly, lead to a reduction in cAMP-PKA and both these interventions reduce the cAMP to the same level as the specific PKA peptide inhibitor (PKI 14–22 Amide). Thus, we did not appreciate a need to repeat these experiments in the presence of Ry and BAPTA. (Note that the effect of the specific PKA peptide inhibitor (PKI 14–22 Amide) on ATP reduction is similar in the presence of pyruvate or 2-deoxy-d-glucose (Fig. 4C–E), further supporting the idea that Ca2+-cAMP/PKA signaling regulates the mitochondrial ATP production rather than glycolysis in SANC (Fig. 4)).
Figure 5.
PKA inhibition by a specific PKA peptide inhibitor (PKI 14–22 Amide) decreases SANC O2 consumption (n=5) (A) and representative example of mitochondrial flavoprotein fluorescence trace (B) indicative of net reduction of flavoprotein pool. Washout of PKI reverse this effect. *p<0.05 vs. control.
Perturbing mitochondrial Ca2+ directly does not affect SANC ATP level
To inquire whether the Ca2+-cAMP/PKA signaling to the mitochondria that links ATP demand and supply is a direct effect of Ca2+ or cAMP/PKA component we used Ru360 (Ca2+ uniporter blocker) to decrease mitochondrial Ca2+, or CGP-37157 (mitochondrial Na+Ca2+ exchanger blocker) to increase the mitochondrial Ca2+ (results not shown). Neither reducing Ca2+ influx into nor inhibiting Ca2+ efflux from the mitochondria significantly altered the SANC ATP level (Fig. 4E). The effects of Ru360 and CGP37157 on mitochondrial Ca2+ in SANC (data not shown) are similar to the drug’s effects in other heart tissue [17]. These results strongly suggest that mitochondrial Ca2+ does not directly regulate ATP supply to demand. That neither inhibition of the Ca2+ myofilament interaction (Fig. 3) nor mitochondrial Ca2+ cycling (Fig. 4) can cause a reduction in ATP levels further supports the idea that cAMP-PKA signaling to mitochondria is a direct and key link between ATP demand and supply (Fig 2B).
ATP supply demand regulation in VM differs markedly from SANC
Prior reports in VM indicate that the ATP level remains constant when ATP demand changes [8]. This distinctly differs from the management of SANC ATP budget presented here. To directly compare ATP management in the two cell types we applied the same interventions in VM as in SANC. Electrical stimulation of VM at 3 Hz (to mimic SANC spontaneous AP firing at this rate) increases O2 consumption by 60% (Fig. 1C), but does not significantly alter (<6% reduction) the steady-state ATP level compared to quiescent cell suspensions (Fig. 2D). This indicates that, in response to stimulation from rest to 3 Hz, VM adjust their ATP supply (indicated by increased O2 consumption) to the increased ATP demand of pacing, consistent with numerous prior reports (e.g. [8]). In SANC, in contrast to VM, when the spontaneous AP firing rate is reduced, O2 consumption is reduced, but ATP levels are reduced too. Prior studies have demonstrated that a reduction in the beating frequency in VM leads to a reduction in mitochondrial Ca2+ [18] that directly affects dehydrogenase activity to moderate the Krebs cycle rate and ATP generation [19]. We confirmed this result in the present study. In VM stimulated at 3Hz, inhibition of mitochondrial Ca2+ influx reduced ATP (to 83.5±1.5% of control). Our VM data are in accord with recent evidence showing that application of Ru360 to VM reduces the NADH production at a stimulation rate of 4Hz vs. the quiescent mode while CGP-37157 does not alter it [17]. This response to Ru360 in VM contrasts with the response of SANC, in which ATP levels do not change in response to Ru360 (Fig. 4). Finally, in VM, in contrast to SANC, the steady state ATP level is not reduced in response to inhibition of Ca2+-cAMP/PKA (Fig. 2D).
Discussion
The present study is the first to describe the control mechanisms that match ATP production to ATP demand in SANC. The first novel finding of our study is that SANC possess a high mitochondrial density, similar to other heart tissues (Fig. 1A). This finding was not expected, since, in the older literature, SANC and specifically rabbit SANC have been referred to as ‘empty’ cells [15] with sparse myofilaments and mitochondrial densities compared to atrial and ventricular cells [20, 21]. In the present study TMRM staining revealed a relatively abundant presence of mitochondria in SANC. This observation was confirmed by immunoblots of heart tissue lysates and subsequent probing with antibodies raised against mitochondrial proteins. Another novel finding of our study is that SANC have a high basal cell respiration rate (an indicator of ATP production rate under normal coupling conditions). Even though SANC have a sparse myofilament density [22] their respiration rate is comparable to that in VM under comparable experimental conditions (Fig. 1C).
The second and most important novel finding of our study is that when ATP demand in SANC is reduced by interfering with basal Ca2+-cAMP/PKA signaling, these cells become depleted of ATP (Fig. 2). The present results also show that metabolic inhibition by NaCN, which reduces ATP production, also reduces the basal cAMP level in the context of a reduction in peak Ca2+ and in AP firing rate. A prior study [7] had also demonstrated a reduction in the peak Ca2+ and the spontaneous AP firing rate after 5-min exposure to 2mM NaCN, and also predicted a concomitant depletion in cAMP level, but the latter was not measured. Thus, taken together the effects of graded reduction in cAMP/PKA signaling, and of NaCN, suggest that a Ca2+-regulated-cAMP/PKA signaling cascade that drives spontaneous AP firing of SANC is a unique core feed-forward system, which not only drives the basal ATP consumption but also the ATP production (Fig. 2). In other words, Ca2+-cAMP/PKA signaling in SANC both consumes ATP and stimulates its production by mitochondria (Fig. 2B).
In contrast to SANC, reductions in ATP demand in VM does not appreciably change the steady ATP levels (Fig. 2D). This finding is in accordance with prior studies [8, 23] which have demonstrated that, over a wide range of workloads and ATP demand, the steady levels of ATP, CrP and other energy metabolites are maintained at essentially constant levels.
Since glycolysis does not support basal ATP production (Fig. 4), the high oxygen consumption in SANC documented in the present study (Fig. 1) is likely partially related to maintaining cytosolic Ca2+ concentration, due to ATP utilization required for (1) Ca2+ activation of Ca2+/calmodulin stimulated basal AC activity to generate cAMP [5, 6], (2) Ca2+ pumping into SR and (3) Ca2+ activation of myofilaments to cause contraction.
To further elucidate a link between cytosolic Ca2+ and ATP levels, we performed these types of experiments. Firstly, we observed that a reduction in cytosolic Ca2+ by BAPTA, which blocks all Ca2+-dependent cell function, depletes cAMP and ATP in SANC. Secondly, blocking only the Ca2+-myofilament interactions in SANC (with blebbistatin) which blocks contraction but preserves AP firing rate and Ca2+ cycling that drives AP, i.e. Ca2+-cAMP/PKA signaling, does not decrease cAMP and does not reduce the ATP level (Fig. 3). Thirdly, directly disabling mitochondrial Ca2+ influx or efflux, which increases or decreases the spontaneous AP firing rate, respectively (data not shown), does not affect SANC ATP levels (Fig. 4C).
While no prior study has identified ATP supply-demand mechanisms in SANC, three previously identified major regulators in VM that couple ATP demand to ATP supply by the mitochondria are as follows (for review cf [24]): (1) Pi and ADP, direct products of energy utilization, particularly at low workloads [24, 25], (2) Cytosolic Ca2+ is coupled to ATP hydrolysis and via mitochondrial Ca2+ flux an increase in mitochondrial Ca2+ activates mitochondrial enzymes that take part in ATP production, (3) cAMP/PKA signaling, which phosphorylates several mitochondrial proteins and complexes I–V [26–29]. Several mitochondrial proteins are known to be phosphorylated by cAMP-PKA signaling: VDAC [26], the 18-KDa subunit of complex I [27], a serine/threonine protein kinase of cytochrome c oxidase (complex IV) [30], and the c-subunit of the F1Fo-ATP synthase (complex V) [29]. An increase in phosphorylation of these complexes in the electron transport chain by cAMP/PKA enhances the flux of protons and drives it to complex V to increase the rate of ATP production. Phosphorylation of VDAC can also increase the delivery rate of ATP from the mitochondria to the cytosol. Therefore, a decrease in cAMP/PKA signaling can decrease the amount of ATP production by the mitochondria to reduce measured ATP level. The present study suggests therefore a unique control mechanism in SANC, which is different than VM. In VM basal cAMP and PKA dependent protein phosphorylation levels are lower than SANC [6] and during β-stimulation of VM the increase in cAMP-dependent phosphorylation may play a role to match ATP production to the increase in ATP demand.
A constant amount of total adenine nucleotide— in both mitochondria and in the cytosol compartments— and the equilibrium of the creatine kinase reaction, was demonstrated in isolated heart [23]. Assuming this biochemical balance exists in SANC the decline in ATP must be associated with increase in ADP and probably AMP. AMP-activated protein kinase can increase ATP production since it is activated by an increase in AMP/ATP ratio. If such a mechanism would occur it obviously does not prevent the measured depletion in ATP (present results) but, might serve as a fail-safe mechanism where it can prevent further reduction in ATP (below a certain level) that could be fatal in SANC. Therefore it is possible that AMP kinase activity is the “brake” that limits further reduction in ATP.
When the ATP level falls in response to the drug intervention that reduced Ca2+-cAMP/PKA, signaling Pi and ADP levels in the mitochondria must have become elevated. Nevertheless, there is no increase in basal ATP production, suggesting that in SANC the basal level of Pi/ADP is not a major regulator of mitochondrial ATP production. Since neither ADP/Pi nor a direct Ca2+ effect on mitochondrial ATP production appear to couple ATP supply to demand in SANC, the third mechanism, cAMP/PKA effects on mitochondrial proteins, emerges as the most likely candidate to signal mitochondria to modulate ATP production in order to couple ATP supply to demand. Our studies (Fig. 5) provide direct evidence that a decrease in cAMP/PKA signaling is affected by a specific PKA peptide inhibitor (PKI 14–22 Amide) which decreases cell respiration and flavoprotein oxidation. Due to the low number of SANC in SAN tissue we have not been able to isolate sufficient numbers of healthy mitochondria to assess directly the rate of ATP turnover by complex V. The only practical way for us to correlate ATP turnover rate and ATP levels is to measure steady ATP at two discrete points: before and after drug intervention and compare this to the change in the rate of O2 consumption at these two points. Note that to asses this correlation we have to assume no change in ox-phos coupling before and after drug intervention. PKI decreased both the steady ATP level (Fig. 2C) and O2 consumption rate (Fig. 5A). Therefore, if the changes in O2 indicate the changes in ATP turnover in a coupled system, they are correlated with the decrease in ATP levels. It remains to be determined whether cytosolic cAMP-activated PKA binding to PKA anchor proteins (e.g., S-AKSP84 and AKAP121) transmits cAMP-dependent signaling to the mitochondria, [28, 31, 32] or whether cAMP-dependent second messenger production within mitochondria regulates oxidative phosphorylation [33]. As described above we have not been able to isolate the mitochondrial complexes I–V in order to assess these phosphorylation sites. Therefore, this approach is beyond the scope of the present paper. Future studies to measure cAMP and ATP signals in real time are required to advance our understanding of in-vivo localization of the PKA-dependent phosphorylation of mitochondrial complex subunit proteins.
Summary
In summary, a high level of cAMP-PKA dependent signaling is present within SANC in the absence of β-adrenergic stimulation and this basal cAMP-PKA signaling is crucial for SANC to generate spontaneous rhythmic AP’s. This high basal level of PKA-dependent phosphorylation in SANC is maintained by Ca2+ activation of AC [3, 4]. In contrast to VM, in which Ca2+ and ADP/Pi directly signal the mitochondria to regulate basal state ATP supply, Ca2+-activation of cAMP/PKA signaling in SANC is the core feed-forward signaling link between basal utilization of ATP and the mitochondrial ATP production. In other words, in SANC the same signaling system that regulates the rate of rhythmic spontaneous AP firing, also directly regulates the required energy to accomplish a given spontaneous AP firing. Reducing the SANC AP firing rate decreases SANC energy supply. The regulation of the energy budget in SANC distinctly differs from that of VM, but since the major purpose of SANC is to initiate and propagate rhythmic action potentials to the atria to initiate contraction of VM, it is not surprising that differences exist in ATP supply-demand matching to accomplish the different tasks of these cell types.
Supplementary Material
Figure 6.
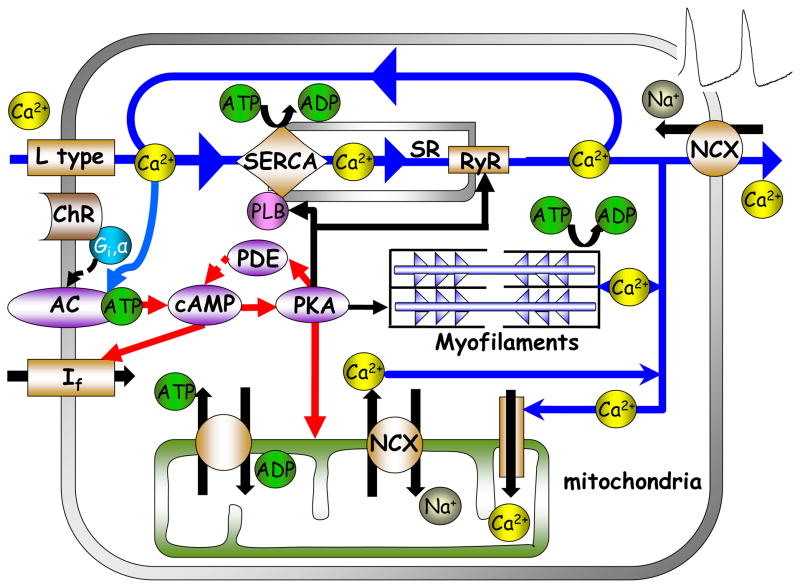
Schematic illustration of the interplay of adenylyl-cyclases (AC), PDE activity and cAMP/protein kinase A (PKA)-dependent signaling to the myofilaments, sarcoplasmic reticulum Ca2+ cycling proteins, ion channels and basal state ATP demand and supply in SANC.
Highlights.
We explored the mechanisms that match ATP supply and demand in pacemaker cells.
O2 consumption in pacemaker cells is comparable to stimulated ventricular myocytes.
Reduction in Ca2+-cAMP/PKA signaling in pacemaker cells reduced ATP production.
Ca2+-cAMP/PKA signaling in pacemaker cells links to ATP consumption and production.
Matching ATP supply to demand in pacemaker cells differs from ventricular myocytes.
Acknowledgments
We are deeply grateful to Mr. Bruce D. Ziman and Dr. Yue Li for preparing the isolated cells, to Dr. Tatiana Vinogradova for helpful discussion about drug concentrations and applications to Dr. Kenneth W. Fishbein for assistance in designing a custom sealed plunger for the oxygen chamber, and to Dr. Chris Morrell and Mrs. Veena Shetty for helpful suggestions for the statistic analysis.
Sources of Funding
The work was supported entirely by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Glossary
- AC
adenylyl cyclase
- AP
action potential
- CCh
carbachol
- CPA
cyclopiazonic acid
- DNP
2,4-dinitrophenol
- IBMX
3-Isobutyl-1-methylxanthine
- PKA
protein kinase A
- Ry
ryanodine
- SAN
sinoatrial node
- SANC
sinoatrial node cells
- SR
sarcoplasmic reticulum
- VM
ventricular myocytes
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: molecular partners in pacemaker regulation. Circ Res. 2001;88(12):1254–8. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 2.Gao Z, Chen B, Joiner ML, Wu Y, Guan X, Koval OM, et al. I(f) and SR Ca(2+) release both contribute to pacemaker activity in canine sinoatrial node cells. J Mol Cell Cardiol. 2010;49(1):33–40. doi: 10.1016/j.yjmcc.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, et al. Ca(2+)-stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283(21):14461–8. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol. 2007;582(Pt 3):1195–203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circ Res. 2008;102(7):761–9. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 6.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98(4):505–14. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 7.Ju YK, Allen DG. Early effects of metabolic inhibition on intracellular Ca2+ in toad pacemaker cells: involvement of Ca2+ stores. Am J Physiol Heart Circ Physiol. 2003;284(4):H1087–94. doi: 10.1152/ajpheart.00755.2002. [DOI] [PubMed] [Google Scholar]
- 8.Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986;232(4754):1121–3. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- 9.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010;106(4):659–73. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nett MP, Vassalle M. Obligatory role of diastolic voltage oscillations in sino-atrial node discharge. J Mol Cell Cardiol. 2003;35(10):1257–76. doi: 10.1016/s0022-2828(03)00236-0. [DOI] [PubMed] [Google Scholar]
- 11.Ju YK, Allen DG. How does beta-adrenergic stimulation increase the heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. J Physiol. 1999;516 (Pt 3):793–804. doi: 10.1111/j.1469-7793.1999.0793u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351(6322):145–7. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 13.van Borren MM, Verkerk AO, Wilders R, Hajji N, Zegers JG, Bourier J, et al. Effects of muscarinic receptor stimulation on Ca2+ transient, cAMP production and pacemaker frequency of rabbit sinoatrial node cells. Basic Res Cardiol. 2010;105(1):73–87. doi: 10.1007/s00395-009-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyashkov AE, Vinogradova TM, Zahanich I, Li Y, Younes A, Nuss HB, et al. Cholinergic receptor signaling modulates spontaneous firing of sinoatrial nodal cells via integrated effects on PKA-dependent Ca(2+) cycling and I(KACh) Am J Physiol Heart Circ Physiol. 2009;297(3):H949–59. doi: 10.1152/ajpheart.01340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47(4):658–87. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 16.Senges J, Brachmann J, Pelzer D, Rizos I, Kubler W. Effect of glycolytic inhibitors on the sinoatrial node, atrium and atrioventricular node in the rabbit heart. J Mol Cell Cardiol. 1981;13(9):811–21. doi: 10.1016/0022-2828(81)90238-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103(3):279–88. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261(4 Pt 2):H1123–34. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 19.Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J. 1991;280 (Pt 3):561–73. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- 21.Bouman LN, Jongsma HJ. Structure and function of the sino-atrial node: a review. Eur Heart J. 1986;7(2):94–104. doi: 10.1093/oxfordjournals.eurheartj.a062047. [DOI] [PubMed] [Google Scholar]
- 22.Masson-Pevet M, Bleeker WK, Mackaay AJ, Bouman LN, Houtkooper JM. Sinus node and atrium cells from the rabbit heart: a quantitative electron microscopic description after electrophysiological localization. J Mol Cell Cardiol. 1979;11(6):555–68. doi: 10.1016/0022-2828(79)90430-9. [DOI] [PubMed] [Google Scholar]
- 23.Katz LA, Swain JA, Portman MA, Balaban RS. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989;256(1 Pt 2):H265–74. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- 24.Yaniv Y, Juhaszova M, Nuss HB, Wang S, Zorov DB, Lakatta EG, et al. Matching ATP supply and demand in mammalian heart. Ann N Y Acad Sci. 2010;1188:133–42. doi: 10.1111/j.1749-6632.2009.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortassa S, O’Rourke B, Winslow RL, Aon MA. Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys J. 2009;96(6):2466–78. doi: 10.1016/j.bpj.2008.12.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bera AK, Ghosh S, Das S. Mitochondrial VDAC can be phosphorylated by cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun. 1995;209(1):213–7. doi: 10.1006/bbrc.1995.1491. [DOI] [PubMed] [Google Scholar]
- 27.Sardanelli AM, Technikova-Dobrova Z, Speranza F, Mazzocca A, Scacco S, Papa S. Topology of the mitochondrial cAMP-dependent protein kinase and its substrates. FEBS Lett. 1996;396(2–3):276–8. doi: 10.1016/0014-5793(96)01112-x. [DOI] [PubMed] [Google Scholar]
- 28.Papa S, Sardanelli AM, Scacco S, Technikova-Dobrova Z. cAMP-dependent protein kinase and phosphoproteins in mammalian mitochondria. An extension of the cAMP-mediated intracellular signal transduction. FEBS Lett. 1999;444(2–3):245–9. doi: 10.1016/s0014-5793(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 29.Signorile A, Sardanelli AM, Nuzzi R, Papa S. Serine (threonine) phosphatase(s) acting on cAMP-dependent phosphoproteins in mammalian mitochondria. FEBS Lett. 2002;512(1–3):91–4. doi: 10.1016/s0014-5793(02)02226-3. [DOI] [PubMed] [Google Scholar]
- 30.Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, Marcus K, et al. Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics. 2008;7(9):1714–24. doi: 10.1074/mcp.M800137-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17(3):279–87. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Martin MC, Allan LA, Lickrish M, Sampson C, Morrice N, Clarke PR. Protein kinase A regulates caspase-9 activation by Apaf-1 downstream of cytochrome c. J Biol Chem. 2005;280(15):15449–55. doi: 10.1074/jbc.M414325200. [DOI] [PubMed] [Google Scholar]
- 33.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9(3):265–76. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.