Abstract
Among the repertoire of motor functions, although hand movement and speech production tasks have been investigated widely by functional neuroimaging, paradigms combining both movements have been studied less so. Such paradigms are of particular interest in Parkinson’s disease (PD) in which patients have specific difficulties performing 2 movements simultaneously. In 9 unmedicated PD patients and fifteen healthy control subjects, externally-cued tasks (hand movement, speech production and combined hand movement and speech production) were performed twice in a random order and fMRI detected cerebral activations compared to rest. F-statistics tested within-group (significant activations at p-values < 0.05, FWE corrected), between-group and between-task comparisons (regional activations significant at p-values < 0.001, uncorrected, with cluster size > 10 voxels). For control subjects the combined task activations comprised the sum of those obtained during hand movement and speech production performed separately, reflecting the neural correlates of performing movements sharing similar programming modalities. In PD patients, only activations underlying HM were observed during the combined task. We interpreted this phenomenon as the patients’ potential inability to recruit facilitatory activations while performing2 movements simultaneously. This lost capacity could be related to a functional prioritization of one movement (hand movement) in comparison with the other (speech production). Our observation could also reflect the inability of PD patients to intrinsically engage the motor coordination necessary to perform a combined task.
Keywords: Parkinson’s disease, fMRI, Hand movement, Speech production, Dual task
INTRODUCTION
Functional neuroimaging has revealed changes in cerebral activation patterns caused by Parkinson’s disease (PD). An underactivation of the supplementary motor area (SMA), dorsolateral prefrontal cortex (DLPFC) and anterior cingulate gyrus has been reported during the execution of self-generated or self-initiated movements of the upper limb; these regions correspond respectively to the output projections of the motor, associative and limbic fronto-striatal circuits.1 The SMA underactivation involves more predominantly the anterior or pre-SMA, while the caudal part seems overactivated.2 The primary motor cortex (M1), both ipsi- and contralateral to the motor task, has been identified as either normally activated 1 or overactivated, 2 especially in early stages of the disease. During an externally-cued, sequential and repetitive motor task, the SMA was found normally activated in PD, while the M1 and the cerebellum were underactivated.3 Underactivation of the SMA in PD relative to controls was also observed during a task that involved greater engagement of attention and selection processes.4 More recent studies have obtained similar results providing increasing support for the hypothesis that the cerebello-cortical increased activation is a “compensation” for the impaired basal ganglia motor circuit,5,6 even if contralateral M1 activation may be more obviously associated with rigidity than part of a compensatory mechanism.6
In an early study Liotti and colleagues reported a bilateral overactivation of cortical motor/premotor regions in PD, including the SMA and inferior lateral premotor cortex during various speech production tasks.7 The patients were assessed twice, before and after voice therapy, in a similar manner to that in a more recent study 8 which also confirmed previous observations in the off-stimulation state during deep brain stimulation (DBS)9, i.e. i) decreased activation in the right orofacial area of the motor cortex and bilateral cerebellar hemispheres, ii) abnormally increased activation in the right superior premotor cortex and bilateral DLPFC and iii)an overactivation of the SMA. Overactivation of M1 was also reported in a recent case study.10 Recruitment of additional temporal activation regions compared with controls in PD during phonation and phoneme repetition has been noted11, another investigation reporting significantly higher right orofacial sensorimotor cortex activation in PD patients compared to controls, interpreted as a compensatory mechanism to preserve speech production in PD.12 Brain activation patterns observed during PD speech did not parallel the modification patterns associated with hand movement in any of the studies listed.
The aim of the present study was to explore within the same group of patients, and age-matched healthy controls, the neural correlates of hand movement (HM) and speech production (SP) tasks performed both alone and simultaneously in a combined (HM+SP) task. Our first a priori hypothesis was that PD patients would show compensatory cerebellar activation during the HM task alone; our second hypothesis posited the lack of such cerebellar activation during the SP task alone and the possibility of other functional reorganisation. No a priori hypothesis was proposed for the combined [HM+SP] task, which was very specifically formulated to avoid cognitive conflict, an advantage when assessing the neural correlates of concomitant task performance using the same input without cognitive overload, which is usually a confound in dual task paradigms.
MATERIALS AND METHODS
Patients and subjects
Nine patients, right-handed (Edinburgh Handedness Inventory > 80 %) and with a predominant a kinetic-rigid form of PD participated in this study (Table 1). They all fulfilled the UK Parkinson’s disease Brain Bank Criteria for diagnosis of idiopathic PD.13 All the patients were studied in the “off” medication state, at least twelve hours (an overnight) after all anti-Parkinsonian drugs had been withheld. Before the patients were scanned, their global motor disability was assessed using the motor part (part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS).14 Patients with mild to moderate symptoms and with no or little tremor were selected in order to ensure that they could perform the tasks “off” medication. The patients presented with moderate speech impairment, with no unintelligible dysarthric speech that would have impeded the speech production task performance.
Table 1.
Patient demographics and clinical motor assessments
| Patients | Gender | Age (years) | Disease duration (years) | Worst side | Total motor score items 18–31 (score/108) | Speech item 18 (score/4) | Tremor items 20–21 (score/28) | Rigidity item 22 (score/20) | Axial signs items 18, 22 (neck), 27–30 (score/24) | Akinesia items 23–26 (score/32) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | 6 | left | 28 | 1 | 0 | 5 | 12 | 10 |
| 2 | M | 67 | 21 | right | 26 | 2 | 0 | 7 | 10 | 5 |
| 3 | F | 62 | 24 | right | 25 | 1 | 0 | 5 | 9 | 9 |
| 4 | M | 58 | 20 | right | 29 | 1 | 2 | 8 | 6 | 10 |
| 5 | M | 61 | 13 | left | 21 | 1 | 3 | 7 | 5 | 7 |
| 6 | M | 57 | 7 | left | 31 | 1 | 2 | 8 | 10 | 11 |
| 7 | M | 42 | 8 | left | 33 | 1 | 4 | 10 | 6 | 13 |
| 8 | M | 68 | 21 | left | 40 | 2 | 1 | 10 | 15 | 13 |
| 9 | M | 49 | 8 | left | 64 | 1 | 10 | 11 | 11 | 28 |
|
| ||||||||||
| Mean ± SD | 59 ± 9 | 14 ± 7 | 33 ± 13 | 1 ± 1 | 2 ± 3 | 8 ± 2 | 9 ± 3 | 12 ± 7 | ||
All motor scores (part III of the scale) of the UPDRS (Unified Parkinson’s Disease Rating Scale; Fahn, 1987) are off-medication; M: male; F: female; SD: standard deviation.
Fifteen healthy control subjects (5 females, 10 males), with no history of physical, neurological or psychiatric illness, head injury or alcohol or drug abuse also participated. They were reasonably age-matched with the patient group (mean age: 55 ± 11 years; range of years from 37 to 75) and were all right-handed (Edinburgh Handedness Inventory > 80 %). The study (project number 04-Q0512-42) was performed with the approval of the Joint Research Ethics Committee (REC) of the Institute of Neurology and National Hospital for Neurology and Neurosurgery (London, United Kingdom).
In accordance with the Declaration of Helsinki15 all controls and patients participated after the nature of the procedure had been fully explained and they had given written informed consent.
Activation paradigms
Prior to the fMRI measurements, participants were provided with an opportunity to practice the tasks. The following paradigms were used:
Hand movement (HM) – Participants were asked to perform a freely chosen sequence of movements with the right hand, moving a joystick in 4 possible directions (forward, backward, right and left). To ensure that all individuals performed the same number of movements during the task period, movements were paced with synchronized audio-visual signals presented every 2 s.
Speech production (SP) – Participants were asked to produce a freely chosen speech sequence, using 4 possible words (“Up”, “Down”, “Right” and “Left”). Speech production was paced with the same synchronized audio-visual signals.
Combined task (HM+SP) – Participants were asked to perform a freely chosen sequence of joystick movements with the right hand and to simultaneously say out loud the associated directions (the word “Up” for the forward direction and “Down” for backward). This task was paced as for the previous tasks.
Participants were free to perform a joystick movement in the same direction and/or produce the same word several times in a row.
fMRI data acquisition
The experimental protocol comprised a block-design. Separate fMRI volume-series were acquired twice for each of the 3 conditions, in a randomized order, such that a total of 6 fMRI runs were acquired. Within each fMRI run, the experimental condition (tasks cued at 2s intervals) was maintained for 16.8s followed by a rest period of the same duration; such alternations were carried out 8 times, a total of 64 movements and/or word articulations expected to be generated by the end of the run. During the rest periods, participants were required to remain still, making no movement and pronouncing no words, although the audio-visual pacing signals were still presented. For conditions HM and HM+SP, the joystick movements were recorded using a computer monitoring system (Spike 2, Science Products GmbH, Germany). For condition SP, an experimenter inside the scanner room, close to the patient, wrote down all the words produced by the subject. A posteriori analyses of the directions of joystick movement, and/or directions spoken confirmed their random distribution.
Imaging was performed with a 1.5 Tesla Signa Excite whole-body MRI System (GE Medical System, Milwaukee WI, USA) equipped with an 8-channel head coil. All controls and patients lay supine on the MRI couch with the head inside the head coil. A dedicated hardware and software paradigm presentation package (Eloquence system, Invivo, USA) was used. For each fMRI run, 64 volumes covering the whole brain with 41 axial slices of 2 mm thickness and 1 mm gap were acquired using a BOLD-contrast multi-slice T2*-weighted echo-planar imaging (EPI) sequence (echo time (TE) = 50 ms, repetition time (TR) = 4.2 s, flip angle = 90°, field of view (FOV) = 200 mm, matrix size 64 × 64 giving 3 mm × 3 mm in plane resolution). Three-dimensional T1-weighted images of the whole brain were also acquired, using an inversion recovery-prepared spoilt gradient echo sequence (inversion recovery preparation time = 450 ms, TE = 2.4 ms, flip angle = 12°, voxel size = 1.2 mm).
Data analysis
Analysis of fMRI data was performed in MATLAB (Math Works, Natick, Massachusetts, USA) using SPM5 (Wellcome Department for Cognitive Neuroscience, London, UK) for statistical parametric mapping.16 First-level analyses were carried out for each subject. Contrasts for each experimental paradigm were introduced into a second-level analysis, in a two-factor ANOVA model using the experimental tasks (HM, SP and HM+SP) and groups (controls and patients) as factors. F-statistics were then used to test within-group (p-values < 0.05, FWE corrected), between-group and between-task comparisons (p-values < 0.001, uncorrected, cluster size > 10 voxels). The rationale for using F-tests rested in the exploratory nature of our study. For these comparisons, the signal intensity of cerebral activation was examined in order to identify the group (patients or controls) or the task (HM + SP or HMS]) with which it was associated. The activation region coordinates were transformed into the Talairach standard stereotactic space. 17
RESULTS
Behavioural data
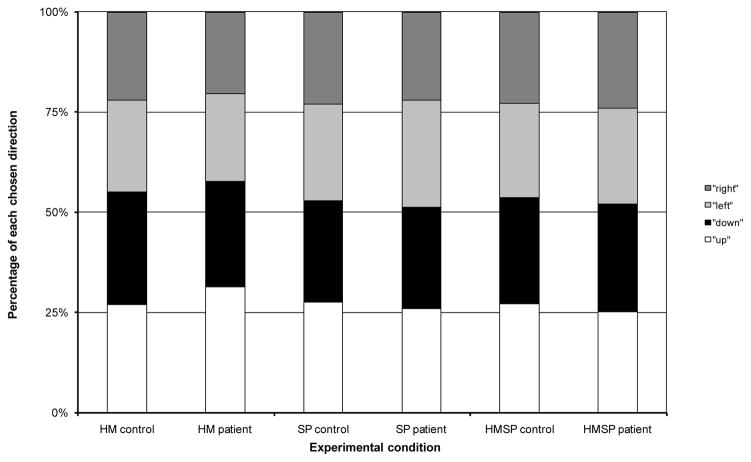
Eight out of 9 patients and 14 out of 15 healthy controls generated the expected number (64) of hand movements and word articulations during the experimental tasks. The remaining patient and control subject performed respectively 59/64 and 55/64 hand movements and word articulations during one HM+SP fMRI run. Patients included in our study did not report any additional effort while performing the combined task. In terms of the random nature of the directions chosen or articulated, the patients’ behaviour did not exhibit any significant difference in comparison with the controls (Fig. 1).
Fig. 1. Summary of the direction chosen during the 3 experimental tasks: hand movement (HM), speech production (SP)and the combining [HM+SP] task.
There seems to be a preference for the “up” direction and avoidance of the “right” direction. This may reflect the physical ease of moving the joystick in certain directions inside the scanner with our experimental arrangement.
fMRI within-group comparisons
Brain areas activated during the Task versus Rest contrast are summarized in Table 2 for the controls and in Table 3 for the PD patients.
Table 2.
Cerebral sites of hemodynamic response during hand movement, speech production and combined tasks in control subjects
| Cerebral areas | Hand movement | Speech production | Combined [hand + speech] task | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Localisation | L/R | BA | x, y, z | Z-score | k | x, y, z | Z-score | k | x, y, z | Z-score | k |
| Frontal lobe | |||||||||||
| Primary motor cortex (M1) | Left | 4 | −31, −25, 42 | Inf | 2692 | −40, −13, 27 | Inf | 968* | −31, −25, 41 | Inf | 2143* |
| Right | 40, −11, 26 | Inf | 689* | 48, −5, 15 | 6.69 | 239* | |||||
|
| |||||||||||
| Lateral premotor cortex | Left | 6 | −50, 1, 28 | 7.14 | 74 | ||||||
|
| |||||||||||
| Medial premotor cortex (SMA) | 6 | −4, −1, 47 | 5.84 | 59 | −8, −3, 56 | 5.18 | 22 | −10, −1, 56 | 5.94 | 121 | |
|
| |||||||||||
| Dorsolateral prefrontal cortex | Left | 8(9/46) | −18, 26, 47 | 5.88 | 39 | −4, 39, 39 | 5.65 | 24 | |||
|
| |||||||||||
| Prefrontal cortex | Left | 47 | −27, 28, −11 | 7.24 | 88 | −29, 28, −11 | 6.47 | 77 | |||
| Parietal lobe | |||||||||||
| Primary somatosensory cortex | Right | 3/2/1 | 47, −23, 34 | 5.04 | 6 | ||||||
|
| |||||||||||
| Secondary somatosensory cortex | Left | 5/7 | −8, −81, 40 | 5.76 | 94 | −22, −63, 35 | 5.26 | 6 | |||
|
| |||||||||||
| Other associative somatosensory cortex | Left | 43 | −52, −19, 16 | 5.35 | 18 | ||||||
| Right | 52, −17, 16 | 5.65 | 17 | ||||||||
| Temporal lobe
| |||||||||||
| Associative auditory cortices | Left | 42/22/21 | −45, −11, −4 | 4.89 | 4 | −41, −40, 12 | 6.43 | 96 | −40, −38, 12 | 6.60 | 322 |
| Right | 52, −25, −7 | 5.22 | 11 | 43, −27, −9 | 5.90 | 60 | |||||
|
| |||||||||||
| Superior temporal gyrus | Left | 38 | −31, 8, −21 | 5.71 | 12 | ||||||
|
| |||||||||||
| Parahippocampic gyrus | Left | 36 | −22, −23, −21 | 6.21 | 57 | ||||||
| Occipital lobe | |||||||||||
| Associative visual cortices | Left | 18/19/20 | −3, −85, 20 | 5.32 | 11 | ||||||
| Right | 10, −87, 32 | 5.11 | 11 | ||||||||
| Cingulate cortex | |||||||||||
| Posterior cingulate gyrus (dorsal part) | Left | 31 | −3, −46, 27 | 6.93 | 197 | −1, −46, 27 | 6.6 | 199 | −3, −46, 25 | 5.27 | 12 |
| Basal ganglia | |||||||||||
| Putamen | Left | −25, 12, 7 | 5.27 | 29 | |||||||
| Right | 22, 2, −2 | 5.03 | 7 | ||||||||
|
| |||||||||||
| Caudate nucleus | Left | −8, 4, 14 | 5.13 | 3 | −8, −5, 17 | 4.92 | 2 | ||||
|
| |||||||||||
| Thalamus (VPl) | Left | −15, −21, 2 | 6.33 | 62 | −13, −21, 2 | 5.38 | 8 | ||||
| Cerebellum | Left | −27, −54, −28 | Inf | 395 | −18, −65, −23 | 5.89 | 144 | −20, −65, −21 | 6.66 | 155 | |
| Right | 13, −54, −16 | Inf | 1269 | 31, −58, −25 | 6.05 | 31 | 13, −54, −21 | Inf | 631 | ||
Cerebral activation locations refer to maximal hemodynamic response sites. L/R: left/right; BA: Brodmann’s area; x, y, z: mediolateral, rostrocaudal and dorsoventral Talairach coordinates; Z scores > 4.88 correspond to corrected pFWE-corr values < 0.05; Inf: infinite; k: cluster size (number of voxels); M1: primary motor cortex; SMA: supplementary motor area.
including also lateral premotor cortex (BA 6).
Table 3.
Cerebral sites of hemodynamic response during hand movement, speech production and combined tasks in PD patients
| Cerebral areas | Hand movement | Speech production | Combined [hand + speech] task | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Localisation | L/R | BA | x, y, z | Z-score | k | x, y, z | Z-score | k | x, y, z | Z-score | k |
| Frontal lobe | |||||||||||
| Primary motor cortex (M1) | Left | 4 | −29, −27, 46 | Inf | 1517* | −29, −27, 44 | 7.35 | 402* | |||
|
| |||||||||||
| Lateral premotor cortex | Left | 6 | −40, −17, 29 | 7.43 | 263** | −20, −15, 48 | 5.18 | 7 | |||
| Right | 40, −13, 29 | 6.28 | 78** | ||||||||
|
| |||||||||||
| Medial premotor cortex (SMA) | Medial | 6 | −4, −9, 49 | 5.20 | 5 | ||||||
| Parietal lobe | |||||||||||
| Secondary somatosensory cortex | Left | 5/7 | −17, −73, 43 | 4.91 | 2 | ||||||
|
| |||||||||||
| Temporal lobe
| |||||||||||
| Wernicke’s area | Left | 39/40 | −27, −52, 34 | 5.04 | 6 | −34, −42, 35 | 5.73 | 62 | |||
| Secondary auditory cortex | Left | 21 | −59, −42, 1 | 5.51 | 7 | ||||||
| Right | 45, −25, −3 | 5.20 | 3 | ||||||||
| Occipital lobe | |||||||||||
| Secondary and tertiary visual cortices | Left | 18/19/20 | 4, −89, 22 | 5.76 | 24 | ||||||
| Right | −20, −96, 12 | 5.21 | 5 | ||||||||
| Cerebellum | Left | −22, −48, −27 | 5.19 | 19 | −36, −67, −28 | 5.66 | 22 | −34, −63, −30 | 5.19 | 9 | |
| Right | 20, −52, −28 | Inf | 720 | 34, −67, −30 | 4.91 | 1 | 13, −52, −22 | 7.14 | 537 | ||
Cerebral activation locations refer to maximal hemodynamic response sites. PD: Parkinson’s disease; L/R: left/right; BA: Brodmann’s area; x, y, z: mediolateral, rostrocaudal and dorsoventral Talairach coordinates; Z scores > 4.88 correspond to corrected pFWE-corr values < 0.05; Inf: infinite; k: cluster size (number of voxels); M1: primary motor cortex; SMA: supplementary motor area.
these clusters also included the primary somatosensory cortex (BA 3/2/1);
these clusters also implicated the primary motor cortex (BA 4).
Hand movement (HM)
In controls the network of cerebral activation elicited by the HM task involved strongly and consistently the right cerebellum and the left M1 cortex; further activations were seen in the left cerebellum, medial (left) SMA and lateral (left) premotor cortices(Fig.2a). Additional activations were observed in the somatosensory, auditory and posterior cingulate cortices. Subcortically, involvement of the putamen (bilaterally) and the thalamus (left) were also revealed. In PD patients, brain activation was elicited by the HM task in the left M1 and right cerebellum, as well as in the left cerebellum and SMA, although a decreased number of voxels were recruited for all these regions compared to controls. Neither temporo-parietal activations nor basal ganglia activations reached statistical significance. Associative visual cortex was bilaterally recruited (Fig. 2b).
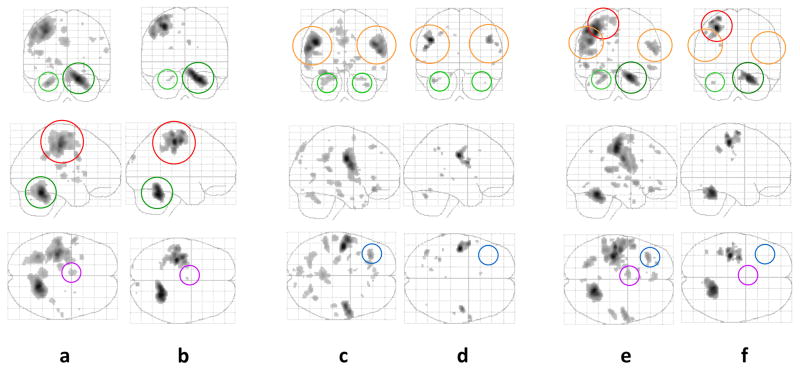
Fig. 2. Patterns of brain activation during the hand movement task (a – controls; b – patients with PD), speech production task (c – controls; d – patients with PD) and the combined [hand movement + speech production] task (e – controls; f – patients with PD). Activation thresholds correspond to corrected (family wise error, FWE) p-values< 0.05.
a-b: In controls, activations were found notably in the left primary motor cortex (right hand somatotopy; in red), bilateral cerebellum (right > left; in green), basal ganglia (bilateral putamen, left thalamus) and SMA (in purple) for the hand movement task. PD patients exhibited decreased activations in the motor and premotor regions and no activation in the basal ganglia.
c-d: Prominent activations were demonstrated in the bilateral primary motor cortex (orofacial somatotopy; in orange) as well as the bilateral cerebellum, left caudate nucleus, SMA, DLPFC (in blue) and temporal regions in control subjects during speech production. Smaller activations were found in PD patients, corresponding to motor (M1 and cerebellum) regions.
e-f: The brain activation profile in controls appeared as a summation of the patterns of activation associated with each type of movement separately. This addition was not as evident in PD patients, whose activation profile was closer to that displayed by the hand movement task alone.
Speech production (SP)
In controls, significant activations were present bilaterally in the cerebellum and orofacial M1 cortex, extending to the lateral premotor cortex (Fig. 2c). Bilateral associative audio-visual cortices were also activated. Significant left-sided activation of the DLPFC, prefrontal, somatosensory, parahippocampal and posterior cingulate cortices, as well as the caudate nucleus, were also observed. In PD patients, significant bilateral activations in the cerebellum, M1 and associative auditory cortex were found (Fig. 2d). Left parietal activations, additional to those found in the controls’ profile, were also observed.
Combined (HM+SP) task
In controls, the activation pattern elicited by the combined (HM+SP) task was essentially the sum of those elicited by the separate HM and SP tasks (Fig. 2e); associative auditory cortices, left caudate nucleus and thalamic activations were also displayed. In PD patients, the (HM+SP) task yielded an activation profile restricted principally to the right cerebellum and the left M1 hand somatotopy (Fig. 2f). Orofacial M1 and left cerebellum activations reached respectively no or poor significance for the PD patients.
fMRI between-group comparisons (control group vs. patient group contrast)
For HM, controls displayed significantly greater activations than patients in the left cerebellum/right precentral gyrus motor network, left insula and bilateral putamina (Table 4. A). Patients did not exhibit additional areas of activation to those seen in controls (Table 4. B).
Table 4.
Comparisons between control subjects and PD patients: cerebral sites of hemodynamic response during hand movement, speech production and combined tasks
|
A. Control subjects vs. PD patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral areas | Hand movement | Speech production | Combined [hand + speech] task | ||||||||
| Localisation | L/R | BA | x, y, z | Z-score | k | x, y, z | Z-score | k | x, y, z | Z-score | k |
| Pre-central gyrus | Left | 4/6 | −50, 1, 28 | 3.90 | 14 | ||||||
|
| |||||||||||
| Post-central gyrus | Left | 2 | −32, −27, 34 | 4.12 | 14 | −32, −27, 35 | 3.94 | 16 | |||
|
| |||||||||||
| Insula | Left | 13 | −41, 3, −7 | 3.64 | 23 | −41, 3, −5 | 4.06 | 36 | |||
|
| |||||||||||
| Anterior cingulate gyrus | Right | 25 | 15, 28, 12 | 3.57 | 16 | ||||||
|
| |||||||||||
| Lingual gyrus | Right | 18 | 19, −63, 5 | 3.59 | 10 | ||||||
|
| |||||||||||
| Putamen | Left | −24, 14, 6 | 3.81 | 35 | |||||||
| Right | 24, −3, −4 | 4.26 | 80 | ||||||||
|
| |||||||||||
| Caudate nucleus | Left | −13, 26, 6 | 3.68 | 13 | −11, 26, 8 | 3.81 | 41 | ||||
| Right | 8, 24, 6 | 3.52 | 13 | ||||||||
|
| |||||||||||
| Cerebellum | Right | 15, −58, −23 | 3.56 | 12 | |||||||
|
| |||||||||||
| Pons | Left | −10, −30, −30 | 4.09 | 81 | |||||||
|
B. PD patients vs. control subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral areas | Hand movement | Speech production | Combined [hand + speech] task | ||||||||
| Localisation | L/R | BA | x, y, z | Z-score | k | x, y, z | Z-score | k | x, y, z | Z-score | k |
| Superior frontal gyrus | Left | 8 | −18, 28, 47 | 4.06 | 63 | ||||||
|
| |||||||||||
| Mid-frontal gyrus | Left | 10/11 | −27, 43, 0 | 4.82 | 120 | −25, 43, −2 | 3.84 | 32 | |||
|
| |||||||||||
| Inferior frontal gyrus | Left | 47 | −27, 28, −11 | 4.46 | 37 | ||||||
|
| |||||||||||
| Medial frontal gyrus | Right | 9 | 4, 47, 30 | 3.81 | 54 | ||||||
|
| |||||||||||
| Pre-central gyrus | Left | 6 | −31, 1, 28 | 4.07 | 19 | ||||||
|
| |||||||||||
| Inferior temporal gyrus | Left | 20/37 | −55, −52, −14 | 3.77 | 44 | −55, −46, −13 | 3.90 | 23 | |||
|
| |||||||||||
| Parahippocampic gyrus | Left | 35 | −22, −23, −21 | 4.66 | 45 | ||||||
|
| |||||||||||
| Anterior cingulate gyrus | Right | 24/32 | 10, 28, 15 | 4.36 | 39 | 17, 43, 0 | 3.80 | 10 | |||
|
| |||||||||||
| Posterior cingulate gyrus (ventral part) | Left | 29 | −4, −38, 10 | 4.82 | 83 | ||||||
| Right | 6, −38, 10 | 3.83 | |||||||||
|
| |||||||||||
| Posterior cingulate gyrus (dorsal part) | Left | 31 | −3, −44, 26 | 4.08 | 68 | ||||||
|
| |||||||||||
| Lingual gyrus | Left | 18 | −13, −83, −5 | 3.31 | 12 | ||||||
|
| |||||||||||
| Cerebellum | Right | 36, −56, −21 | 3.73 | 17 | |||||||
Cerebral activation locations refer to maximal hemodynamic response sites. PD: Parkinson’s disease; L/R: left/right; BA: Brodmann’s area; x, y, z: mediolateral, rostrocaudal and dorsoventral Talairach coordinates; Z scores > 3.10 correspond to uncorrected p values < 0.001; Inf: infinite; k: cluster size (number of voxels); M1: primary motor cortex
During SP, controls showed increased bilateral activation in the pons (left) and in the caudate nucleus compared with patients (Table 4. A). PD patients exhibited additional areas of activation not seen in controls in the left frontal areas including the lateral premotor cortex, anterior and posterior cingulate cortices as well as the temporal and parahippocampal gyri (Table 4. B).
Comparison between controls and patients for the (HM+SP) combined task highlighted a greater involvement of the left caudate and insula (Table 4. A) in controls. PD patients on the other hand recruited additional temporal and cingulate gyri as expected (Table 4. B).
fMRI between-task comparison (simultaneous(HM+SP) vs. co-added HM and SP contrasts)
Comparison of the co-added HM and SP activation profiles compared with the combined simultaneous HM+SP task activation pattern revealed one significant cluster (k = 43 voxels) located within the deep mid-temporal gyrus (Talairach coordinates: x= −27, y= −46, z= 27) in control subjects. was Activation was higher in this region for the simultaneous HM+SP combined task. In PD patients this contrast did not yield any suprathreshold clusters.
DISCUSSION
In our study, for the HM task, cerebral activation across the brain, including the cerebellum and the primary motor cortex, was globally reduced in PD patients compared to controls; our first a priori hypothesis was therefore rejected, since we had expected a compensatory cerebello-cortical activation in PD. 2 We thus conclude that such a compensatory mechanism is not universally demonstrable3,18–19. This form of compensation was not expected for the SP task7,9, and since our data provided no evidence to support such a mechanism, our second a priori hypothesis was accepted; this lack of cerebellar activation during PD speech requires further investigation. It has been suggested that cerebellar activation has played an evolutionary key-role in the anatomical-functional substrate of vocal communication20. Furthermore the importance of the cerebellar-motor cortex pathway in speech motor control has been recently highlighted in PD patients undergoing bilateral DBS in the subthalamic nucleus: current spreading in pathways other than the corticobulbar tract, the pallidofugal and the cerebellothalamic fibres was hypothesised to result in dysarthria exacerbation.21,22
Between-group comparisons did reveal greater DLPFC (prefontal loop) and cingulate cortex (limbic loop) activations in PD patients when compared to control subjects for the SP and (HM+SP) tasks. Based upon the classical cortico-subcortical circuit models first defined in the early ninety-nineties,23 and further developed since,24,25 the concept of closed and open circuits has allowed for the possibility of cross-communication between circuits.26 Thus, it is possible that alteration of the motor loop may be compensated by the recruitment of a non-motor circuit. Temporal cortex activations are consistent with the probable increased involvement of the perception/action interface during SP in PD patients, as an indicator of an associative auditory cortex compensation for motor deficits.11 This might also be the case for the combined (HM+SP) task. However, between-group comparisons failed to show significantly higher activation in controls than patients in language regions for the combined task, suggesting a need for caution in interpreting these results. The controlsal ways displayed significant activation compared to rest in the auditory cortex during all the tasks, possibly due to the fact that these subjects rely on auditory information to guide their movement production. They demonstrated significant activation compared to rest in the visual cortex only during the isolated SP task, possibly due to the use of additional sensory information to maintain the pace of word articulation. The patients never displayed activation of both auditory and visual areas simultaneously; activation was seen either in one or the other, or none for the combined task. This difference between patients and controls for the combined task suggests that the patients did not use paced sensory input to drive their movements and speech production. Some PD patients may process auditory signals differently from controls, as deficient timing of speech production motor events,27 regardless of the quality of the behavioural performance. This possible interpretation must be confirmed with a larger number of subjects to be sure whether this is a difference specific to the context of speech production or merely due to a lack of statistical power.
The experimental tasks used in our study were both externally-cued and self-generated, with the expectation of generating both basal ganglia and cerebellar activations.28 It may be postulated that these two regions participate in separate components of the same internal timing device.27 Consistent with this idea, basal ganglia and cerebellar recruitment was indeed observed in controls, while PD patient activations failed to reach significance. However, the lack of striatal and cerebellar activation could be initially due to differences of behavioural performance. Patients could present more variability of motor performance in executing paced movements and/or speech, especially when they are off medication: this argument may be resolved in future experiments.
An often cited example of the functional challenge to performing simultaneous movements in PD is the difficulty in walking and talking at the same time 29; although no links between the “stop walking when talking” occurrences and falls were found, dual motor tasks are of specific interest because they are particularly difficult for patients with PD.30 A recent functional imaging study explored the neural correlates of dual task performance using finger tapping and letter counting as concurrent paradigms in PD: the patients displayed difficulty in performing the complex dual task, a consequence of restricted attentional resources, altered executive function and diminished automatic performance.31 Our “combined task” paradigm was not a dual-task comprising strictly independent tasks, as it did not involve cognitive conflict in response selection between the HM and the SP tasks.32 Motor programming was identical for the 2 modalities, (i.e. selection of the same response among 4 possibilities); only the motor execution differed. “In principle, it could be easier to perform two tasks concurrently when they involve similar inputs if this meant that the same set of processing machinery could be “turned on” and used for both” (page 221).33 This may be consistent with the controls while the PD patients’ activation suggested a different mechanism: a capacity sharing interference would ensure a preferentially good performance of one movement at the cost of a minimal realization of the other, therefore associated with sub-threshold cerebral brain activations.
The present data show cerebral activation differences between PD patients and controls, even though the recruited patients were in a relatively mild stage of the disease. As they progress to more severe levels of involvement, one might expect that the observed brain activation reorganisations may intensify. This conclusion may be confirmed in future studies with larger patient groups representing a wider spectrum of PD severity.
Acknowledgments
The authors wish to thank the following sources of funding: Fondation Simone et Cino Del Duca, The Wellcome Trust, Medtronic, National Institutes of Health (USA) contracts RO1-NS40902 (PI: Daniel Corcos) and RO1-NS40856-02 (PI: Marjan Jahanshahi), Parkinson’s disease Society UK, Parkinson’s Appeal. This work was undertaken at UCLH/UCL who received a proportion of funding from the department of health’s NIHR Biomedical Research Centres funding scheme. The authors wish also to thank Mr. Peter Asselman, Pr. John Rothwell and all the Queen Square Imaging Centre team (Mr. Robert Brehmer, Mr. Jacob Cameron, Mrs. Ellen-Marie Collins, Mrs. Jodee Cooper, Mrs. Trina Dewar, Mr. Darren Field, Mrs. Fiona Vicary) for their support during this study, as well as all the patients and the control participants.
Footnotes
Financial Disclosure/Conflict of Interest: The authors declare that they have no competing financial interests.
AUTHOR ROLES
Serge Pinto: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Laura Mancini: 1C, 2A, 2B, 2C, 3A, 3B
Marjan Jahanshahi: 1A, 1B, 1C, 2C, 3A, 3B
John S. Thornton: 2A, 2C, 3A, 3B
Elina Tripoliti: 1C, 3A, 3B
Tarek A. Yousry: 1A, 1B, 2C, 3A, 3B
Patricia Limousin-Dowsey: 1A, 1B, 2C, 3A, 3B
References
- 1.Jahanshahi M, Jenkins HI, Brown RG, Marsden CD, Pasingham RE, Brooks D. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 2.Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;23:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- 3.Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. Neuroimage. 2003;19:163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 4.Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson’s disease. Impaired effective connectivity among frontal cortical regions. Brain. 2002;125:276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;28:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35:222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, Ingham JC, Fox PT. Hypophonia in Parkinson’s disease: Neural correlates of voice treatment revealed by PET. Neurology. 2003;60:432–440. doi: 10.1212/wnl.60.3.432. [DOI] [PubMed] [Google Scholar]
- 8.Narayana S, Fox PT, Zhang W, Franklin C, Robin DA, Vogel D, Ramig LO. Neural correlates of efficacy of voice therapy in Parkinson’s disease identified by performance-correlation analysis. Hum Brain Map. 2010;31:222–236. doi: 10.1002/hbm.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto S, Thobois S, Costes N, Le Bars D, Benabid AL, Broussolle E, Pollak P, Gentil M. Subthalamic nucleus stimulation and dysarthria in Parkinson’s disease: a PET study. Brain. 2004;127:602–615. doi: 10.1093/brain/awh074. [DOI] [PubMed] [Google Scholar]
- 10.Narayana S, Jacks A, Robin DA, Poizner H, Zhang W, Franklin C, Liotti M, Vogel D, Fox PT. A noninvasive imaging approach to understanding speech changes following deep brain stimulation in Parkinson’s disease. Am J Speech Lang Pathol. 2009;18:146–161. doi: 10.1044/1058-0360(2008/08-0004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachin S, Senthil Kumaran S, Singh S, Goyal V, Shukla G, Mahajan H, Behari M. Functional mapping in PD and PSP for sustained phonation and phoneme tasks. J Neurol Sci. 2008;273:51–56. doi: 10.1016/j.jns.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Rektorova I, Barrett J, Mikl M, Rektor I, Paus T. Functional abnormalities in the primary orofacial sensorimotor cortex during speech in Parkinson’s disease. Mov Disord. 2007;22:2043–2051. doi: 10.1002/mds.21548. [DOI] [PubMed] [Google Scholar]
- 13.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahn S, Elton RL. committee motUd. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, editors. Health Care Information. Recent developments in Parkinson’s disease. Florham Park, NJ: MacMillan; 1987. pp. 153–164. [Google Scholar]
- 15.World Medical Association General Assembly. Declaration of Helsinki. Amemdment; Tokyo: 2004. [Google Scholar]
- 16.Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Map. 1995;2:189–210. [Google Scholar]
- 17.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New-York, NY: Thième Medical Publishers; 1988. [Google Scholar]
- 18.Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson’s disease. Brain. 2006:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, Ma Y, Eidelberg D. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–272. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, Hariz MI, Limousin P. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord. 2008;23:2377–2383. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]
- 22.Aström M, Tripoliti E, Hariz MI, Zrinzo LU, Martinez-Torres I, Limousin P, Wårdell K. Patient-Specific Model-Based Investigation of Speech Intelligibility and Movement during Deep Brain Stimulation. Stereotact Funct Neurosurg. 2010;88:224–233. doi: 10.1159/000314357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 24.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000;23:S8–19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 25.Kopell BH, Rezai AR, Chang JW, Vitek JL. Anatomy and physiology of the basal ganglia: Implications for deep brain stimulation for Parkinson’s disease. Mov Disord. 2006;21:S238–246. doi: 10.1002/mds.20958. [DOI] [PubMed] [Google Scholar]
- 26.Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 27.Graber S, Hertrich I, Daum I, Spieker S, Ackermann H. Speech perception deficits in Parkinson’s disease: underestimation of time intervals compromises identification of durational phonetic contrasts. Brain Lang. 2002;82:65–74. doi: 10.1016/s0093-934x(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 28.Purzner J, Paradiso GO, Cunic D, Saint-Cyr JA, Hoque T, Lozano AM, Lang AE, Moro E, Hodaie M, Mazzella F, Chen R. Involvement of the basal ganglia and cerebellar motor pathways in the preparation of self-initiated and externally triggered movements in humans. J Neurosci. 2007;27:6029–6036. doi: 10.1523/JNEUROSCI.5441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beauchet O, Annweiler C, Dubost V, Allali G, Kressing RW, Bridenbaugh S, Berrut G, Assal F, Hermann FR. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16:786–95. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 30.Bloem BR, Grimbergen YA, Cramer M, Valkenburg VV. “Stops walking when talking” does not predict falls in Parkinson’s disease. Ann Neurol. 2000;48:268. doi: 10.1002/1531-8249(200008)48:2<268::aid-ana21>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2008;79:760–766. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- 32.Rochester L, Nieuwboer A, Baker K, Hetherington V, Willems AM, Kwakkel G, Van Wegen E, Lim I, Jones D. Walking speed during single and dual tasks in Parkinson’s disease: which characteristics are important? Mov Disord. 2008;23:2312–2318. doi: 10.1002/mds.22219. [DOI] [PubMed] [Google Scholar]
- 33.Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116:101–132. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]