SUMMARY
Background
In Drosophila the bHLH protein DIMM coordinates the molecular and cellular properties of all major neuroendocrine cells, irrespective of the secretory peptides they produce. When expressed by non-neuroendocrine neurons, DIMM confers the major properties of the Regulated Secretory Pathway and converts such cells away from fast neurotransmission and towards a neuroendocrine state.
Results
We first identified 134 transcripts upregulated by DIMM in embryos, then evaluated them systematically using diverse assays (including embryo in situ hybridization, in vivo ChIP, and cell-based transactivation assays). We conclude that of 11 strong candidates, six are strongly and directly controlled by DIMM in vivo. The six targets include several large dense-core vesicle (LDCV) proteins, but also proteins in non-LDCV compartments such as the RNA-associated protein MAELSTROM. In addition, a functional in vivo assay, combining transgenic RNAi with MS-based peptidomics, revealed that three DIMM targets are especially critical for its action: These include two well-established LDCV proteins, the amidation enzyme PHM and the ascorbate-regenerating electron transporter Cytochrome-b561-1. The third key DIMM target, CAT-4 (CG13248), has not previously been associated with peptide neurosecretion – it encodes a putative cationic amino acid transporter, closely related to the SLIMFAST Arginine transporter. Finally, we compared transcripts upregulated by DIMM with those normally enriched in DIMM neurons of the adult brain and found an intersection of 18 DIMM-regulated genes, which included all six direct DIMM targets.
Conclusions
The results provide a rigorous molecular framework with which to describe the fundamental regulatory organization of diverse neuroendocrine cells.
Keywords: Dimmed, peptidergic neuron, bHLH, Drosophila, CAT-4, Phm, Cytochrome b561
INTRODUCTION
Neuroendocrine (NE) cells embody highly dedicated secretory cell states. While different NE cells express unique arrays of neuropeptide/ neurohormone-encoding genes, all peptidergic NE cells nevertheless share many critical cellular functions. These functions reflect a common need for enzymes that conduct the post-translational processing of numerous neuropeptide precursors, and a need for the structural and regulatory components that execute large dense-core vesicle (LDCV) biogenesis, packaging, maturation and trafficking [1–2]. They also exhibit a choreographed capacity to modify their cell properties in response to changing physiological needs [3]. Many proteins are normally enriched in neuroendocrine tissues, and these may be coordinately regulated under different physiological states [4–5]. Hence, NE programs of cell differentiation must reflect the operations of complex regulatory circuits, the details of which are only beginning to emerge. Examining the intracellular regulatory pathways that organize and modulate these specialized properties is an important question for cell biology, and specifically is also critical to understanding NE cell physiology.
Important clues to understanding peptidergic cell biology may come from parallel studies of other neuronal secretory systems. Developmental programs of neurotransmitter expression are governed by dedicated transcriptional organizers. For example, serotonergic neuron differentiation (but not fate specification or survival) is substantially controlled by an ETS domain transcription factor called PET-1 [6]. Likewise, dopaminergic (DA) neuron maturation is promoted by the ETS transcription factor, AST-1, although it is not necessary for DA neuron generation or survival [7]. To what extent therefore, do peptidergic secretory cells rely on similar transcriptional regulatory controls?
In the Drosophila model system, the transcription factor DIMM operates in peptidergic NE cells in many ways similar to PET-1 and AST-1 in aminergic cells. DIMM is specifically expressed in peptidergic NE cells [8–12] – termed LEAP cells (Large, Episodically- Releasing, Amidating Peptide producing [13]. Notably, DIMM acts like a master cell regulator for professional secretory cell properties. It confers two cardinal features of the Regulated Secretory Pathway (RSP) [14] onto neurons that otherwise do not display such properties [15]. The first property is generation and accumulation of large dense core vesicles (LDCV), which can house neuropeptides if these are available. Second it activates the complete post-translational processing machinery, which is sufficient to produce biologically active peptides from neuropeptide precursors. These cell biological observations raise important questions as to how DIMM, a single transcription factor, can efficiently organize a complete and functional sub-cellular domain like the RSP. We reasoned that direct targets provide the most compelling basis to describe DIMM action mechanisms. Here we report a genome-wide screen to define candidate targets for DIMM regulation.
RESULTS
Identification of candidate genes as putative direct DIMM targets
To amass candidate DIMM targets in addition to Phm [12], we used genome-wide microarray profiling by over-expressing DIMM throughout the embryonic nervous system. To identify up-regulated transcripts, we compared profiles from experimental (elav>dimm) and control (elav-GAL4) embryos at 22–26 hr and 28–32 hr after egg laying (AEL). We identified 134 candidate DIMM targets that were up-regulated at least 1.5 fold following DIMM over-expression, at both time points (Table S3). Many genes were also down-regulated (Table S4), but we did not study these further.
We used quantitative real time PCR to confirm that 22 candidate targets were up-regulated at least 1.5-fold by DIMM overexpression and of these, 18 genes were up-regulated at least 2-fold (Table S5). Many of the 134 candidates were not tested based on low expression levels, or were tested but not detected by qPCR at this stage, or had primer sets that failed quality control tests. Finally, we focused on the 18 most highly-elevated transcripts and reasoned that as DIMM targets, they would be expressed in the central nervous system (CNS), and their RNA levels would decline in a dimm loss-of-function mutant. Using conventional PCR, we found that transcripts of all 18 genes were in fact expressed in CNS (Table S5). In addition, in 1st instar larvae of severe dimm hypomorphs (Rev4/Rev8: [8]), transcript levels for 11 of the 18 genes were significantly decreased. We therefore focused on these 11 genes: their identities, and GO terms, are listed in Table S6.
Two of the 11 candidates, Phm and CG1275, were previously associated with peptidergic signaling. Consistent with previous findings, the inclusion of Phm provides internal validation to the profiling method. CG1275 encodes a cytochrome (Cyt) trans-membrane electron transporter most related to the b561-1 protein [16]. The Cyt-b561-1 is a specific to dense-core secretory vesicles that contain catecholamines or amidated peptides, as are found in chromaffin cells and elsewhere. Cyt-b561-1 regenerates ascorbate which is an essential co-factor to support the biosynthetic functions of peptidylglycine-a-hydroxylating monooxygenase (PHM) [17–18] from within the vesicles.
Evaluating the 11 candidate DIMM targets
We performed three independent experiments to evaluate the degree to which the 11 candidate targets are strongly regulated by DIMM, and devised a functional screen to determine the potential contributions of the candidates to DIMM action mechanisms. Figure 1 presents an overview of the work plan. As summarized in Table 1 and argued in the Discussion, these experiments suggest the 11 targets are divisible into a “strongly-regulated” set of six genes (direct regulation), two additional ones that may be directly-targeted, and another set of three likely indirect targets.
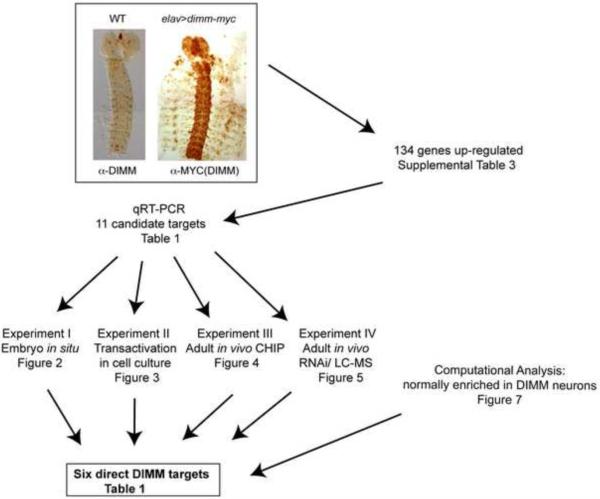
Figure 1.
A schematic overview of the workplan for this study.A genome wide search for dimm targets performed by Genechip, then evaluated by three downstream analyses of DIMM regulation, and further evaluated by a functional in vivo assay.
Table 1.
Summary of results to determine the validity of 11 candidate direct DIMM genes.
| Gene | Embryo in situ* | Trans activation in cell culture | Adult in vivo ChIP** | Adult in vivo RNAi/MS | Enriched in adult DIMM neurons*** | Conclusion | |
|---|---|---|---|---|---|---|---|
| Control | Dimm-OVX | ||||||
| CG3832 (Phm) | + | ++ | ++ | ++ | + | + | Direct |
| CGI275 (Cyt-b561-1) | − | ++ | ++ | ++ | + | + | Direct |
| CGI3248 (CAT-4) | + | ++ | ++ | + | + | + | Direct |
| CG11254 (mael) | − | ++ | ++ | + | − | + | Direct |
| CG17293 (wdr-82) | + | ++ | ++ | + | − | + | Direct |
| CG7785 (CLLD6) | + | ++ | − | + | − | + | Direct |
| CG6522 | + | +@ | ++ | ++ | − | − | Maybe Direct |
| CG32850 (RNF-11) | + | ++ | − | + | − | − | Maybe Direct |
| CG14621 (SIc35el) | − | − | − | + | − | − | Indirect |
| CG31346 | − | − | ND | + | ND | − | Indirect |
| CG8588 (pastrel) | − | − | − | − | − | − | Indirect |
(−) – no expression; (+) –moderate expression detected; (++) strong expression detected. @ no obvious change.
++: p < 0.05; +: positive but not surpassing statistical significance.
greatly enriched in DIMM-positive 1-LNv versus s-LNv (Kula-Eversole et al., 2010).
ND – not determined.
Experiment I. Embryonic RNA in situ hybridization
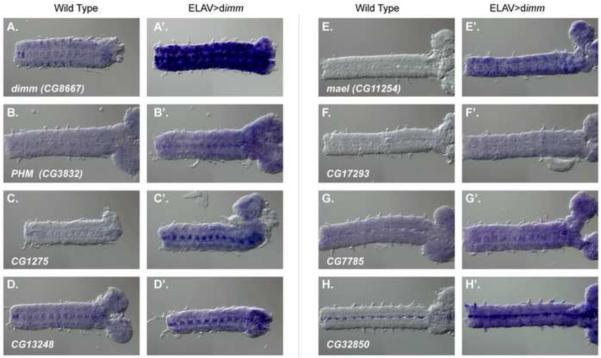
We first analyzed transcript distributions for each of the 11 candidates in embryos of diverse stages, and of two different genotypes (control and elav>dimm – see Methods for precise genotypes). Representative specimens are shown in Figure 2; control specimens are on the left and dimm over-expression specimens are on the right of each column. By Stage 16–17 in control embryos, we observed a clear cell-specific pattern of dimm transcripts in the CNS ([8]; Figure 2A). Up-regulation of dimm by elavGAL4 produced the expected pan-neuronal activation of dimm transcripts (Figure 2A'). Transcripts for the only previously known direct DIMM target, Phm, display a moderate level of expression in control embryonic tissues (Figure 2B) and clear up-regulation with DIMM over-expression (Figure 2B'), although the up-regulation was not as pronounced as the one shown by dimm transcripts.
Figure 2.
RNA in situ hybridization in control embryos and embryos that over-express dimm Left - control (w1118); Right - elav> dimm; Probes: A, B) dimm; C,D) Phm; E,F) CG13248; G,H) CG11254 (mael); I,J) CG17293; K,L) CG6522. See also Figure S1.
Seven other candidates (CG1275, CG13248, mael (CG11254), CG17293, CG7785, CG32850 and CG6522) displayed weak-to-moderate expression levels in control embryos (Figure 2C–H). In the case of CG6522, transcripts were moderately to broadly expressed in normal tissues, and were modestly increased with dimm over-expression (data not shown). None of these candidate targets displayed a normal expression pattern exactly matching that of dimm.CG13248 (Figure 2D) and CG32850 (Figure 2H) were broadly expressed with very prominent accumulation in paired mid-line cells of the ventral nerve cord. In the cases of Phm and CG13248, those patterns were present early (~Stage 13) but resolved to stronger staining in lateral CNS regions later in embryogenesis that resembled that of dimm (Figure S1). mael (CG112545) transcripts were not clearly detected in normal CNS (Figure 2E) although we clearly observed mael in normal germ cells (cf. [19]). CG17293 was weakly expressed throughout the CNS (Figure 2F), and CG7785 moderately expressed (Figure 2G).
DIMM over-expression resulted in heightened target RNA accumulation for seven of the 11 candidate targets. The dimm-stimulated transcript patterns varied, but did show some similarities: most prominently, many patterns included heavy accumulation in the paired midline cells (Figures 2B', C', D', E', G' and H'). Such mid-line cells do not normally express high levels of dimm (Figure 2A). Transcripts for the three other candidate targets (CG14621, CG31346, and pastrel (CG8588) were not detectable in control CNS (although some were evident in non-neuronal tissues). Likewise, we could not detect them following dimm over-expression (data not shown).
In summary, seven of the 11 candidate genes are regulated by DIMM according to embryonic stage in situ hybridization experiments – Phm, Cyt-b561-1, CG13248, mael, CG17293, CG7785, and CG32850.
Experiment II. In vitro trans-activation assay
Our second test for strength of regulation measured DIMM's ability to trans-activate regulatory fragments of the candidate gene targets using Luciferase levels as a readout. We generated test constructs containing the luciferase reporter downstream of a mini-SV promoter and the putative DIMM-binding locus of the gene. Putative DIMM binding sites were selected by the presence and locations of specific E-box sequences (CATATG or CAGCTG) based on our previous analysis [12]: therefore, we focused especially on E-boxes within the first intron. Most candidate genes contained either CATATG or CAGCTG E-box sequences throughout the gene locus (Figure S2). In addition, we also combined the data from ChIP analysis (see below). Although our earlier work on DIMM regulation of Phm utilized mammalian hEK-293 cells [12], we wished to approximate a more homologous cellular context, and so tested a Drosophila neuronal cell line, BG3-c2 [20]. First, we confirmed that the positive control (Phm-sv-Luc) displayed the expected responsiveness (~10 fold induction) when dimm is co-transfected, but not when a mutant dimm isoform was co-transfected, all such results consistent with our previous observations [12]. Next, we measured dimm-responsiveness for each of the candidate genes. We found that in addition to Phm, five candidate DIMM targets (CG1275, CG13248, mael, CG17293, and CG6522) all displayed significant transactivation responses to DIMM (Figure 3).
Figure 3.
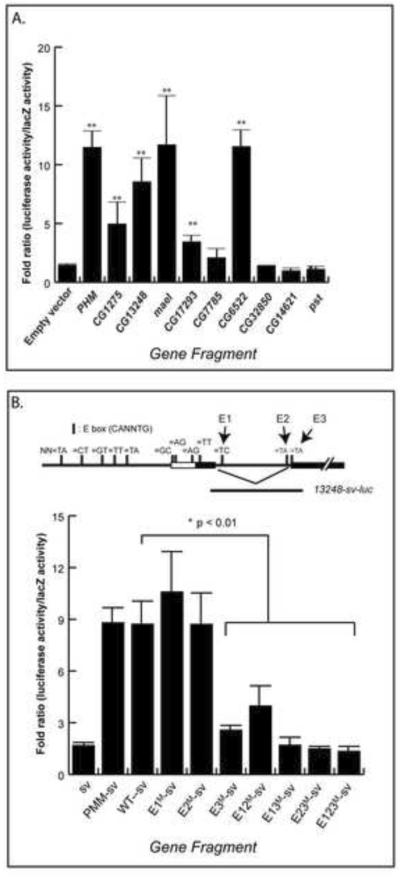
Trans-activation by DIMM of genomic fragments of candidate genes in Drosophila BG3-c2 neuronal cell lines.Fold ratios represent Luciferase levels with dimm co-transfection divided by those without. Histograms represents means and SEMs of at least three independent replicate assays. (A). Results of testing ten DIMM targets. (B) Results from analysis of E box sequence requirements within the CG13248 regulatory region. E1, E2 and E3 indicate three separate E boxes, which were mutated singly, doubly or in triple-format. In (A), * p<0.05; ** p< 0.01 vs empty vector, by student's T-test. In (B), * p< 0.01 vs CG13248 WT sequence, by student's T-test. See also Figure S3 which illustrates E box positions in and around these candidate targets.
To evaluate DIMM transactivation in more detail, we constructed a series of sequence variants of the CG13248 responsive fragment – the ~1 kB fragment that comprises the 1st intron and which contain three E boxes. We mutated three of the six consensus bases of the E boxes: we tested each mutated E box in single, double and triple format: the positions of the E boxes and the results obtained are shown in Figure 3B. Once again, the wild type CG13248 sequence produced an activity level comparable to that of Phm. Of the three single E box mutations, only E3 proved necessary for full DIMM transactivation. Furthermore, all mutant combinations containing the E3 mutation were not at all transactivated. Finally, the E1/E2 double mutant showed a diminished level of transactivation. In summary, the evidence points to the involvement of multiple E boxes in the transactivation of CG13248, with the E3 sequence playing the largest role.
Experiment III. In vivo Chromatin immunopreciptation (ChIP)
To determine the potential occupancy by DIMM at candidate target gene binding sites in vivo, we used an epitope-tagged dimm transgene and ChIP methods. The transgene - UAS-dimm-MYC (II) - was selectively expressed in dimm-containing neurons by using the c929-GAL4 driver. Furthermore, we restricted GAL4: UAS activities to adult stages using temperature-sensitive GAL80 (tub-gal80ts) to avoid the lethality that results from dimm over-expression at earlier stages (T Hadzic, unpublished). After raising the flies at the restrictive temperature (18°C), 1–3 day old adults were transferred to 29°C for a three-day period to permit expression of DIMM-MYC protein. We confirmed DIMM-MYC expression under these conditions by Western blot analysis of fly head extracts, and an absence of such without transfer to 29°C (data not shown).
In a previous report, we demonstrated that DIMM activates Phm directly via three palindromic E-boxes located in the Phm 1st intron (having sequences – CATATG and CAGCTG) [12]. Therefore, as a positive control, we asked whether DIMM is resident at this Phm intron and took a comparative approach by testing each of two sites in the Phm gene locus for DIMM occupancy – the E-box-containing test site within the 1st intron, and a control site located about 6 kb upstream (See Figure S2; control sites listed in Table S2). Across two biological replicates, DIMM-MYC CHIP'ed samples for the Phm gene showed a strong (~32 fold) average enrichment over samples from the negative control genotype (c929-GAL4; tub-gal80ts). This difference was significantly different (p < 0.05) from the average enrichment found at its negative control site. We then asked whether DIMM is resident at the other target genes in vivo and selected test sites based on inclusion of potential DIMM-binding E-boxes in the immediate 5' upstream or 1st intronic regions. Two of these candidates displayed a statistically higher level of enrichment compared to controls – CG1275 and CG6522. For seven other genes - CG13248, CG11254 (mael), CG17293, CG7785, CG32850, CG14621 and CG31436 - we observed a trend for DIMM enrichment at the E-box containing site (Figure 4; Table 1). However, these seven other examples did not achieve statistical significance.
Figure 4.
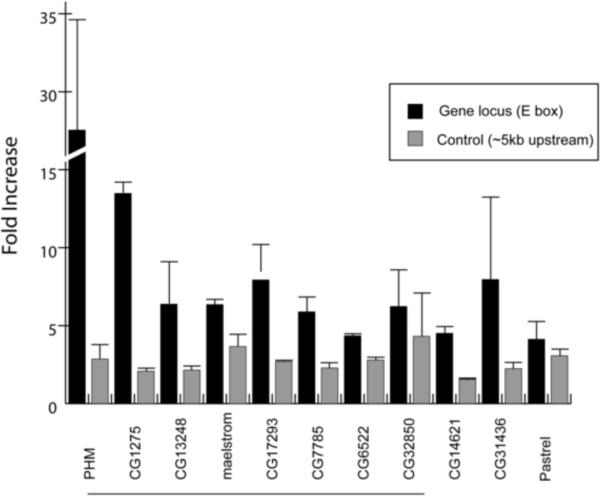
ChIP analysis in vivo of putative DIMM binding at E-boxes within dimm-dependent candidates genes.Darker histograms show the level of enrichment of the putative DIMM binding sites defined by the value in experimental versus control genotypes (see Methods). Lighter histograms show the level of enrichment of arbitrarily chosen sites ~6 kB upstream of the putative DIMM-binding sites, in the same experimental versus control genotypes. Histograms represents average and SEMs at least two independent assays (two biological replicates).
In summary, ChIP analysis suggests that DIMM protein is normally resident in adult head DIMM cells in vivo in the regulatory DNA of at least three (Phm, CG1275, CG6522) and likely as many as ten, of the 11 candidate targets. Furthermore, through all three independent tests of validation, ten of 11 candidate target genes subsequently scored positive in at least one independent assay for direct DIMM regulation (Summarized in Table 1). Five genes were responsive in all three (Phm, CG1275, CG13248, mael, and CG17239) and two others, CG7785 and CG6522, were responsive in two of the assays. CG32850, CG14621, CG31436 all showed DIMM residency in vivo by ChIP, but of these three candidates, only CG32850 also displayed mRNA induction by DIMM in embryos. Only the eleventh candidate, pst, failed to show significant responsiveness in any test.
Experiment IV. Adult in vivo RNAi and LC-MS
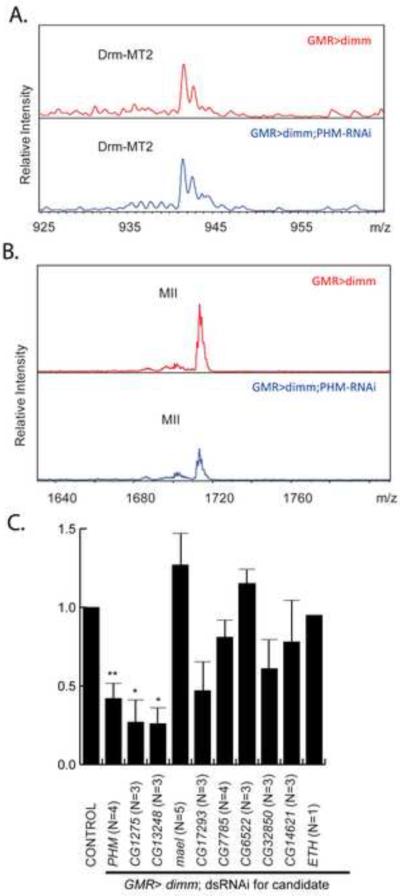
To determine if DIMM target genes contribute to its action mechanisms, we devised a functional screen. We previously demonstrated that ectopic expression of DIMM in photoreceptor neurons confers peptidergic neuroendocrine properties onto them – photoreceptor neurons do not normally display such properties of the Regulated Secretory Pathway. When such cells are also forced to express a heterologous neuropeptide precursor (ppMII), the MII peptide is fully processed [15]. We therefore used the DIMM-dependent accumulation of processed MII peptide as an end-point assay to measure potential contributions of single candidate targets to the DIMM-generated secretory pathway. We used transgenic RNAi methods to knock down individual DIMM targets exclusively within photoreceptor cells and employed quantitative mass spectrometry to analyze the processing of MII. In the control condition (GMR> UAS-dimm, UAS-ppMII), fully-processed MII peptide is detected at 1710.69 m/z ratio [15]. To normalize the results of MII peptide accumulation across conditions, we also measured an endogenous Drosophila brain peptide, not found in photoreceptors, as an internal standard. For this, we chose the Drm-MT2 peptide derived from the HUGIN neuropeptide precursor (SVPFKPRLamide, m/z 942.59: [21], because hugin-expressing neurons do not express the RNAi transgene in this experimental design. An additional negative control in this design tested the effect of an RNAi transgene for a neuropeptide precursor never found in photoreceptors – ecdysis triggering hormone (eth) [8].
We used both labeling and label-free quantitative mass spectrometry approaches CapLCMALDI-TOF/TOF MS. The MS-based labeling method is well validated [22–23] but involves multiple, sample-handling steps and is less effective for samples with low concentrations and small volumes. While we were able to quantify MT2 with the labeling approach (Figure S3), MII was not observed after labeling. We therefore turned to label-free quantitation because isolated peak heights can be directly compared across conditions.
Using the label-free quantitation, MT2 levels showed equal intensities in the control (GMR>dimm;>ppMII) and experimental (GMR>dimm; >ppMII; Phm-RNAi) samples (Figure 5A), consistent with labeling results (Figure S3)M II levels in the experimental sample were significantly decreased from those in the control sample (Figure 5B). MII levels were significantly lower following knockdown of Phm, CG1275 and CG13248 (Figure 5C).CG17293 RNAi displayed less fully-processed peptide and CG11254 (mael) RNAi showed a trend of up-regulation, but their final values did not exceed statistical significance. MII levels in the other six RNAi tests were unchanged.
Figure 5.
MS-based label-free quantitative analysis of alterations in MII peptide accumulation in photoreceptors following RNA interference of candidate DIMM targets.(A) Mass spectra of MII in the control (GMR>UAS-ppMII; UAS-dimm, red) and experimental (GMR>UAS-ppMII; UAS-dimm; UAS-PHM-RNAi, blue) samples. The intensity ratio of MII in experimental versus control samples in these spectra is 0.54. B) Mass spectra of MT2 in the control and experimental samples. The intensity ratio of MT2 in experimental to control in these spectra is 1.02. C) Exogenous MII level in the experimental samples with RNAi compared to that in the control samples. Histograms represents means and SEMs, * p<0.05; vs control (GMR>UAS-dimm), by student's T-test. N, biological replicates. See also Figure S3 for analysis of MS-based quantitation of the endogenous peptide Drm-MT2.
In summary, three of ten candidate DIMM targets (Phm, CG1275 and CG13248) make critical contributions to DIMM mechanisms. Two candidates (Phm and CG1275) are clearly involved in processing, while CG13248 may as well be involved in overall organization of the secretory pathway (see Discussion). The functional assay we employed was sensitive to disturbances of processing, but that is not the only interpretation for a reduction in the level of the secretory peptide. A reduced level could also result from an inability to accumulate, properly traffic or retain secretory peptides. We did not detect a build up of intermediates with CG13248 RNAi and this likely indicates a defect at a stage different from processing.
The CG13248 protein is enriched in DIMM-neurons and dependent on dimm
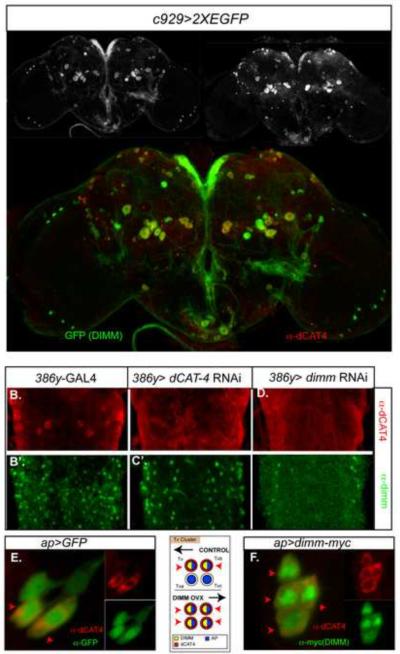
CG13248 encodes a putative cationic amino acid transporter, orthologous to mammalian cationic amino acid transporter 4 (CAT-4; SLC7a4). We analyzed CG13248 protein expression using anti-CAT-4 antibodies in both the adult brain in the 3rd instar larval brain and evaluated it in the context of DIMM expression.
First, we performed double immunostaining with dimm neurons labeled by anti-GFP (in c929-GAL4>GFP brains). In both developmental stages, CAT-4-like IR is heavily enriched in DIMM-positive neurons; Figure 6A shows an example of an adult brain. We also noted minorities of DIMM-only and CAT-4-only stained neurons. CAT-4-like IR was found mainly in cell bodies and terminals (not shown), and very little within axonal tracts. CAT-4-like IR was specific as shown by the action of a CG13248 RNAi transgenic construct: when driven by 386y-GAL4 CAT-4-like IR was lost, while DIMM-like-IR was unaffected (Figure 6C and C'). 386y-GAL4 is an insertion in the prohomone convertase gene dPC2 and its drives expression in most or all of dimm neurons, as well as in other cells [24].
Figure 6.
Enrichment of CG13248 (CAT-4) in DIMM neurons and its regulation by dimm in vivo A) DIMM-like and CAT-4-like IR are extensively co-localized in the adult brain. c929>UAS-GFP (anti-GFP, green), anti-CAT-4 (red); bottom: merged image. B–D) CAT-4-like IR following RNAi knock-down of dimm. B, B') parental control (386Y-GAL4); C, C') 386Y>UAS-DCR2/UAS-CAT-4-RNAi; D, D') 386Y>DCR2/ dimm-RNAi; (B–D) anti-DIMM (green), (B'–D') anti-CAT-4 (red). (E–F) CAT-4-like IR is normally present in the two DIMM-positive neurons of the four-cell Tv-cluster; following DIMM mis-expression throughout the cluster, CAT-4 appears in all four cells. E) ap> GFP, anti-GFP (green), anti-CAT-4 (red), F) ap> dimm, anti-MYC (=DIMM) (green); anti-CAT-4 (red). See also Figure S4 for high power images of cDAT-4-like immunoreactivity in different adult brain DIMM neurons.
We next asked whether CAT-4 expression is dependent on DIMM in loss-of-function and gain-of-function dimm states. We generated UAS-dimm RNAi flies, confirmed the ability of these transgenes to produce a large-scale reduction of DIMM-like IR (Figure 6D and D') and observed a concomitant reduction of CAT-4-like IR (Figure 6D–E). This result indicates that high level CAT-4 expression in dimm-positive neurons depends on DIMM. To test the effects of DIMM over-expression, we turned to the four-cell Tv cluster of the larval CNS. CAT-4-like IR is normally found in the two of the four Tv cluster neurons – the peptidergic Tv and Tvb, but not in the Tva or Tvc neurons (Figure 6E, cf., [25]). We used an ap-GAL4 driver to misexpress DIMM in all four Tv cells and observed ectopic CAT-4-like expression within the Tva and Tvc cells as well (Figure 6F). These results confirm that in vivo CAT-4 is specifically enriched in DIMM-positive cells and that it is regulated by dimm. The distribution of CAT-4-like immunoreactivity within DIMM neurons was studied in various identified peptidergic neurons of the adult brain (Figure S4), including in diverse neurons of the Pars Intercerebralis, HUG-positive neurons of the sub-esophageal neuromeres, and PDF-positive large LNv. In these neurons, CAT-4-like IR was strongly expressed by many DIMM neurons and weakly by others. It appeared principally cytoplasmic, and displayed heterogeneous accumulations.
A computational analysis afforded by prior transcript profiling of mature DIMM neurons
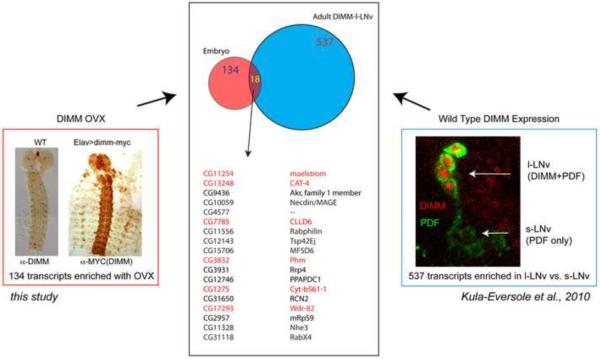
A recent microarray analysis of identified neurons from the adult Drosophila brain provided a fortuitous means to independently assess the authenticity of the original 134-gene list [26]. Importantly it was conducted on wild type brains containing normal DIMM levels, and so it serves as a useful counterpoint to our study of DIMM over-expression. Kula-Eversole et al. [26] profiled three types of neurons – the large lateral neuron ventral (l-LNv) and the small lateral neuron ventral (s-LNv). These two identified neuron groups are similar in that both are circadian pacemakers and both are neuropeptide PDF-expressing cells [27]. However they are different in that only the large LNv are DIMM-positive, while the small-LNv are not ([24]; Figure 7, and see [13]). Kula-Eversole et al. [26] compared l-LNv and s-LNv with a generic (ELAV-positive) brain neuron type for ~19,000 transcripts. Using that primary data set, we identified 579 genes enriched in large over small LNv. By comparing our 134-gene list derived from DIMM over-expression in embryonic stages to those ~579 transcripts normally enriched in DIMM-positive cells from adult stages, we find an intersection of 18 putative DIMM direct target genes specifically enriched in adult l-LNv versus s-LNv (Figure 7; Table S3). Significantly, the six DIMM targets we identified by experimental analysis are all included in this intersection – Phm, CAT-4, mael, CG17293, CG7785, and Cyt-b561-1.
Figure 7.
Comparison of transcripts upregulated by DIMM in embryos with transcripts enriched in DIMM-positive peptidergic neurons of the adult brain Top: A Venn diagram illustrating the identification of 18 genes (13% of 134) of those up-regulated by DIMM over-expression among the 537 normally enriched in DIMM-positive large LNv [29]. Bottom lists the 18-gene intersection, asterisks mark those genes that were shown to be direct DIMM targets by current experiments.
DISCUSSION
The experiments reported here address the mechanisms underlying DIMM's regulatory functions within peptidergic neuroendocrine cells in Drosophila. The results from a genome-wide screening revealed a diverse array of potential DIMM targets and illustrated that the scope of DIMM actions is likely broad. The actions of its direct targets appear to extend from the nucleus (CG17293) to regulation of mRNAs (CG11254) to the ER and Golgi (CG13248) to peptide-containing LDCVs (Phm and CG1254). We found no neuropeptide–encoding genes on any of our lists, even the larger 134-gene list of transcripts exhibiting up-regulation with DIMM over-expression. We showed previously that DIMM is very inefficient by itself at driving ectopic neuropeptide gene expression [8–10]. Together these findings are consistent with our previous speculation that in Drosophila, specific neuropeptide expression is controlled by differing sets of transcription factors working within complex combinatorial codes [9, 13]. In contrast, DIMM provides parallel instructions for the cell biological machinery within which neuropeptides can be made, stored and trafficked [9, 13, 15].
Because we used an over-expression screen to generate a primary list of candidate targets, it was important to authenticate those results by reference to genes enriched in “normal” DIMM cells (i.e., cells in which DIMM levels were not artificially manipulated). We were fortunate to have access such information from the recently published Gene array study of Kula-Eversole et al. [26], from which we found that 13% of the 134 gene candidates were in fact highly-enriched in DIMM-positive neurons (versus DIMM-negative peptidergic neurons). While several candidates performed well in many of these tests and exhibit properties of direct DIMM targets, most did not score positive in all tests employed (only Phm, CG1275, CG13248, CG11254 and CG17293 did). The results emphasize the importance of employing multiple tests to fully evaluate and properly interpret lists of regulated transcripts. Of the 11 genes passing the first test, we then used diverse experimental criteria to divide them into sets of six direct targets, two likely-direct targets, and three indirect targets (Table 1). We emphasize that our categorization of direct targets is based on highly stringent criteria and here discuss the significance of the findings for neuroendocrine cell biology.
Phm, Cyt-b561-1 and CG13248 are key and direct DIMM mediators
The inclusion of Phm and Cyt-b561-1 genes in the original list of 11 candidates increased our confidence in the list's authenticity because both play well-established roles in LDCVs [18]. Furthermore, we had previously demonstrated that Phm is a true transcriptional DIMM target both in heterologous cells and in vivo [12]. Likewise the subsequent strong performance of Phm and Cyt-b561-1 in all four downstream assays provided further support for the validity of the experimental design to identify authentic DIMM targets.
CG13248 is a direct DIMM target and encodes a putative arginine transporter, CAT-4
In addition to Phm and Cyt-b561-1, these studies show that a third bona fide DIMM target gene, CG13248 is critical to normal regulation of neuroendocrine cell properties. Notably, in results described by Kula-Eversole et al. [26], Phm, Cyt-b561-1 and CG13248 all ranked near the top for absolute transcript abundance in DIMM-positive neurons. The identification of CG13248 as an integral component of neuroendocrine physiology is a significant new finding, but its specific contribution is a mystery because its precise molecular functions are not known. It is the clear sequence orthologue to mammalian cationic amino acid transporter 4 (CAT-4) and is therefore a candidate member of the system y+ (Na+ and pH-independent) cationic amino acid-preferring transport activities [28].
CAT proteins form a branch of the solute carrier family 7 (SLC7) [29]. Murine CAT-1, -2 and -3 all display arginine transporter activity when heterologously expressed, but to date, CAT-4 does not [30]. Notably, the Drosophila orthologue of the CAT-1 protein is the transporter SLIMFAST, which mediates arginine transport in fat body, and as a nutrient sensor [31]. In murine pancreatic acinar cells (which are regulated by the DIMM orthologue MIST1, [32], CAT-4 is a membrane-associated protein of secretory granules [33]. Future pursuit of the exact mechanisms and pathways in which CAT-4 operates in DIMM-expressing neurons will help illuminate fundamental neuroendocrine cell physiology.
Additional direct DIMM targets
Regarding the other direct DIMM targets, we mention a few for potential novel insights into mechanisms of neuroendocrine cell regulation. CG11254 (mael): By transcript profiling, Kula-Eversole et al. [26] report that mael is highly enriched in the DIMM-positive l-LNv's. In germ cells, MAEL localizes components of the microRNA pathway and contributes to cellular polarization [19]. CG17293 encodes a protein highly related to mammalian WDR82, and CG7785 encodes a protein highly related to CCLD6 – both of which suggest a connection of DIMM mechanisms to chromatin-modifying properties [34].
There were two genes we concluded likely to be directly targeted (Table 1: “Maybe Direct”) - CG6522 encodes a member of the Testin/ Prickle family of proteins. Notably, the Prickle-like protein RILP interacts with REST and acts as a nuclear translocation factor [35]. The significance of potential Prickle-REST interactions is that REST displays a suppressive effect on neurosecretory properties of PC12 cells [e.g., 36]. In addition, CG32850 encodes a protein orthologous to Ring Finger protein 11, which is a membrane-associated E3 ligase that is expressed widely in brain [37]. Finally, in the larger list of 18 genes representing the intersection of the embryonic and adult DIMM-regulated transcripts (Figure 1), there was sizable representation of genes encoding proteins previously implicated in regulated neuropeptide secretion (Rph) and probable elements of the secretory pathway (PPADC1, RCN2 and Rabx4).
DIMM directs a core program for neuroendocrine cells
These results define principal elements of what we anticipate will be a core program for neuroendocrine cell organization. Among mammalian bHLH proteins, DIMM is most similar to MIST1 [32]. Mills and colleagues have identified several candidate MIST1 targets, including RAB3D [38]. We note that five of the six DIMM targets that responded to DIMM in the trans-activation assay contained E boxes in their first exon-intron regions. The importance of 1st intron E boxes was already established for the case of Phm [12] and is also true for MIST1 target genes so far identified [38]. Furthermore, studies of Phm and CG13248 suggest they can define a consensus DIMM binding profile: they both contain three boxes within the first intron, two of which have the sequence CATATG, all of which contribute synergistically, and one of which appears to have the strongest contribution to DIMM transactivation. We predict many other DIMM targets will display a similar E box profile. Furthermore, how individual target gene products contribute to the DIMM program, and how many more genes are involved, are now pertinent questions that will require additional studies. We anticipate that further analysis of this core DIMM program will help explain the regulatory organization of neuroendocrine cells and their evolution in different phyla. Because DIMM protein persists for the life of neuroendocrine cells in Drosophila, this work may also inform studies of neuroendocrine cell physiology and plasticity.
Developmental Generation of Peptidergic Phenotypes
In the case of neurons that utilize fast conventional neurotransmitters, transcriptional regulatory systems typically exert direct control over genes that encode biosynthetic enzymes, as well as ones for key transporter proteins that retrieve and recycle transmitters back into the lumen of synaptic vesicles [39]. For example, PET-1 supports serotonergic differentiation and directly targets genes that encode the critical biosynthetic enzyme TBH-1 and the serotonin transporter SERT1 [6]. It is striking therefore that our limited but highly validated list of DIMM targets similarly includes genes essential for neuropeptide biosynthesis (Phm and Cyt-b561-1) as well as a transporter that is specifically expressed by neuroendocrine cells (CAT-4). We propose that there may exist an unexpected but essential parallelism in the developmental regulation of secretory systems for small transmitters and for small amidated peptides. This hypothesis can help design experiments to further illuminate the mechanisms that underlie the developmental generation of peptidergic phenotypes.
Quantitative mass spectrometry
Fly heads of the control (GMR>UAS-ppMII; UAS-dimm) and experimental (GMR>UAS-ppMII; UAS-dimm; UAS-RNAi) transgenic lines were collected in frozen state. MS-based quantitation was based on prior quantitative measurement approaches [22–23,43]. Further technical details are provided in Supplemental Information.
Supplementary Material
ACKNOWLEDGMENTS
We thank our laboratory members for helpful discussions and Jason Mills for his comments on an earlier draft of this manuscript. We thank Jennifer Trigg and Weihua Li for excellent technical assistance. We also thank the Bloomington Stock Center, the DGRC and the VDRC for flies, and the Drosophila Genome Center for providing information. This work was supported by NIH P30-DA018310 and a NIDA grant NS031609 (to JVS), by a grant from the Dana Foundation and by NIE grant EY016807 (to SP), by NINDS grant NS036570 and NSF IOS-0744261 (to JBS), by NIH P01 NS044232 and P30-NS045713 and the Howard Hughes Medical Institute (to MR), and NINDS grant NS21749 (to PHT), and by a P30-NS057105 to Washington University. Imaging was performed at the Washington University Bakewell Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
EXPERIMENTAL PROCEDURES. All procedures are found in the Supplemental Information. All microarray data are publically available (GEO accession #GSE31113).
REFERENCES
- 1.Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr. Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- 2.Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiol. 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- 3.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 4.Yue C, Mutsuga N, Verbalis J, Gainer H. Microarray analysis of gene expression in the supraoptic nucleus of normoosmotic and hypoosmotic rats. Cell. Mol. Neurobiol. 2006;26:959–978. doi: 10.1007/s10571-006-9017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hindmarch C, Yao S, Beighton G, Paton J, Murphy D. A comprehensive description of the transcriptome of the hypothalamoneurohypophyseal system in euhydrated and dehydrated rats. Proc. Nat'l. Acad. Sci. U S A. 2006;103:1609–1614. doi: 10.1073/pnas.0507450103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature. 2009;458:885–889. doi: 10.1038/nature07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewes RS, Park D, Gauthier SA, Schaefer AM, Taghert PH. The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Develop. 2003;130:1771–1781. doi: 10.1242/dev.00404. [DOI] [PubMed] [Google Scholar]
- 9.Allan DW, Park D, Pierre SE, Taghert PH, Thor S. Regulators acting in combinatorial codes also act independently in single differentiating neurons. Neuron. 2005;45:689–700. doi: 10.1016/j.neuron.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Hewes RS, Gu T, Brewster JA, Qu C, Zhao T. Regulation of secretory protein expression in mature cells by DIMM, a basic helix-loop-helix neuroendocrine differentiation factor. J. Neurosci. 2006;26:7860–7869. doi: 10.1523/JNEUROSCI.1759-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park D, Veenstra JA, Park JH, Taghert PH. Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS ONE. 2008a;3:e1896. doi: 10.1371/journal.pone.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park D, Shafer OT, Shepherd SP, Suh H, Trigg JS, Taghert PH. The Drosophila bHLH protein Dimmed directly activates Phm, a gene encoding a neuropeptide amidating enzyme. Mol. Cell. Biol. 2008b;28:410–421. doi: 10.1128/MCB.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park D, Taghert PH. Peptidergic neurosecretory cells in insects: Organization and control by the bHLH protein DIMMED. Gen. Comp. Endocrinol. 2009;162:2–7. doi: 10.1016/j.ygcen.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Gumbiner B, Kelly RB. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982;28:51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- 15.Hamanaka Y, Park D, Yin P, Annangudi SP, Edwards TN, Sweedler J, Meinertzhagen IA, Taghert PH. Transcriptional orchestration of the regulated secretory pathway in neurons by the bHLH protein DIMM. Curr. Biol. 2010;20:9–18. doi: 10.1016/j.cub.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verelst W, Asard H. A phylogenetic study of cytochrome b561 proteins. Genome Biol. 2003;4:R38. doi: 10.1186/gb-2003-4-6-r38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Ann. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 18.Perin MS, Fried VA, Slaughter CA, Südhof TC. The structure of cytochrome b561, a secretory vesicle-specific electron transport protein. EMBO J. 1988;7:2697–2703. doi: 10.1002/j.1460-2075.1988.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Findley SD, Tamanaha M, Clegg NJ, Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Develop. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- 20.Ui K, Nishihara S, Sakuma M, Togashi S, Ueda R, Miyata Y, Miyake T. Newly established cell lines from Drosophila larval CNS express neural specific characteristics. In Vitro Cell Dev. Biol. Anim. 1994;30:209–221. doi: 10.1007/BF02632042. [DOI] [PubMed] [Google Scholar]
- 21.Baggerman G, Cerstiaens A, Loof AD, Schoofs L. Peptidomics of the Larval Drosophila melanogaster Central Nervous System. J. Bio. Chem. 2002;277:40368–40374. doi: 10.1074/jbc.M206257200. [DOI] [PubMed] [Google Scholar]
- 22.Brockmann A, Annangudi SP, Richmond TA, Ament SA, Xie F, Southey BR, Rodriguez-Zas SR, Robinson GE, Sweedler JV. Quantitative Peptidomics Reveal Brain Peptide Signatures of Behavior. Proc. Natl. Acad. Sci., U.S.A. 2009;106:2383–2388. doi: 10.1073/pnas.0813021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos-Ortolaza DL, Bushlin I, Abul-Husn N, Annangudi SP, Sweedler J, Devi LA. Quantitative neuroproteomics of the synapse. Methods Mol. Biol. 2010;615:227–46. doi: 10.1007/978-1-60761-535-4_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taghert PH, Hewes RS, Park JH, O'Brien MA, Han M, Peck ME. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J. Neurosci. 2001;21:6673–6686. doi: 10.1523/JNEUROSCI.21-17-06673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park D, Han M, Kim YC, Han KA, Taghert PH. Ap-let neurons—a peptidergic circuit potentially controlling ecdysial behavior in Drosophila. Dev. Biol. 2004;269:92–108. doi: 10.1016/j.ydbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Kula-Eversole E, Nagoshi E, Shang Y, Rodriguez J, Allada R, Rosbash M. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. U S A. 2010;107:13497–13502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helfrich-Förster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Nat'l. Acad. Sci. U S A. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White MF, Christensen HN. The two-way flux of cationic amino acids across the plasma membrane of mammalian cells is largely explained by a single transport system. J. Biol. Chem. 1982;257:10069–10080. [PubMed] [Google Scholar]
- 29.Closs EI, Boissel JP, Habermeier A, Rotmann A. Structure and function of cationic amino acid transporters (CATs) J. Membr. Biol. 2006;213:67–77. doi: 10.1007/s00232-006-0875-7. [DOI] [PubMed] [Google Scholar]
- 30.Wolf S, Janzen A, Vékony N, Martiné U, Strand D, Closs EI. Expression of solute carrier 7A4 (SLC7A4) in the plasma membrane is not sufficient to mediate amino acid transport activity. Biochem. J. 2002;364:767–775. doi: 10.1042/BJ20020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 32.Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat. Rec. 2000;259:157–167. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Rindler MJ, Xu CF, Gumper I, Smith NN, Neubert TA. Proteomic analysis of pancreatic zymogen granules: identification of new granule proteins. J. Proteome Res. 2007;6:2978–92. doi: 10.1021/pr0607029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Skalnik DG. Wdr82 is a C-terminal domain-binding protein that recruits the Setd1A Histone H3-Lys4 methyltransferase complex to transcription start sites of transcribed human genes. Mol. Cell Biol. 2008;28:609–618. doi: 10.1128/MCB.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimojo M, Hersh LB. REST/NRSF-interacting LIM domain protein, a putative nuclear translocation receptor. Mol. Cell Biol. 2003;23:9025–9031. doi: 10.1128/MCB.23.24.9025-9031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Alessandro R, Klajn A, Stucchi L, Podini P, Malosio ML, Meldolesi J. Expression of the neurosecretory process in pc12 cells is governed by rest. J. Neurochem. 2008;105:1369–1383. doi: 10.1111/j.1471-4159.2008.05259.x. [DOI] [PubMed] [Google Scholar]
- 37.Anderson LR, Betarbet R, Gearing M, Gulcher J, Hicks AA, Stefánsson K, Lah JJ, Levey AI. PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J. Neuropathol. Exp. Neurol. 2007;66:955–964. doi: 10.1097/nen.0b013e3181567f17. [DOI] [PubMed] [Google Scholar]
- 38.Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Błazewska KM, McKenna CE, Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol. Cell Biol. 2010;30:1269–1284. doi: 10.1128/MCB.01328-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amara SJ, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.