Abstract
PURPOSE
To analyze the normal peripapillary choroidal thickness utilizing a commercial spectral domain optical coherence tomography (OCT) device and determine the inter-grader reproducibility of this method.
DESIGN
Retrospective, non-comparative, non-interventional case series.
PARTICIPANTS
Thirty-six eyes of 36 normal patients seen at the New England Eye Center between April and September 2010.
METHODS
All patients underwent high-definition scanning with the Cirrus HD-OCT. Two raster scans were obtained per eye, a horizontal and a vertical scan, both of which were centered at the optic nerve. Two independent graders individually measured the choroidal thickness. Choroidal thickness was measured from the posterior edge of the retinal pigment epithelium to the choroid-scleral junction at 500 μm intervals away from the optic nerve in the superior, inferior, nasal and temporal quadrants. Statistical analysis was conducted to compare mean choroidal thicknesses. Inter-grader reproducibility was assessed by intraclass correlation coefficient and Pearson’s correlation coefficient. Average choroidal thickness in each quadrant was compared to retinal nerve fiber layer (RNFL) thickness in their respective quadrants.
MAIN OUTCOME MEASURES
Peripapillary choroidal thickness, intraclass coefficient, Pearson’s correlation coefficient.
RESULTS
The peripapillary choroid in the inferior quadrant was significantly thinner compared to all other quadrants (p< 0.001). None of the other quadrants were significantly different from each other in terms of thickness. The inferior peripapillary choroid was significantly thinner compared to all other quadrants at all distances measured away from the optic nerve (p< 0.001). Generally, the peripapillary choroid increases in thickness the farther it was away from the optic nerve and eventually approaching a plateau. Intraclass correlation coefficient ranged from 0.62 to 0.93 and Pearson’s correlation coefficient ranged from 0.74 to 0.95 (p< 0.001). Neither RNFL thickness nor average age was significantly correlated with average choroidal thickness.
CONCLUSIONS
Manual segmentation of the peripapillary choroidal thickness is reproducible between graders suggesting that this method is accurate. The inferior peripapillary choroid was significantly thinner than all other quadrants (p< 0.001).
Introduction
Optical coherence tomography (OCT) is a device designed to perform non-invasive structural imaging or “optical biopsy” of the eye.1 The introduction of the OCT has changed the way that ophthalmologists diagnose and treat many ocular disorders. Currently the utility of OCT is primarily in diseases of the retina2,3, glaucoma4,5, and anterior segment6,7. In the past, choroidal imaging with OCT was not possible due to attenuation of OCT signal from the retinal pigment epithelium (RPE) layer and the sensitivity roll off across the imaging range, defined as a progressive loss of OCT signal occurring with increased distance from the zero-delay line. However, more recently, visualization of the choroid has become possible on commercial spectral domain OCT instruments. This new capability is attributable to advancements in OCT technology and imaging protocols, including using the instrument in a mode where the zero delay is positioned posterior to the retina in order to enhance sensitivity to deeper structures, along with improved scanning speed and software advancements, which has allowed for multiple OCT scan averaging to increase signal levels. Spaide, et al demonstrated the capability of commercial spectral domain OCT for choroidal imaging using the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany), averaging up to 100 B-scans at the same location to increase signal.8,9 In addition, Spaide, et al physically moved the OCT device closer to the patient so that the OCT image inverts, allowing the choroid to be closer to the zero-delay line, hence increasing sensitivity to deeper retinal structures. Ikuno, et al utilized the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA), a 5 μm axial resolution spectral domain OCT device capable of averaging 20 B-scans at a time with selective pixel profiling for improved visualization of the choroid, to measure the thickness of the macular choroid in highly myopic eyes at the fovea, and also at the superior, inferior, nasal and temporal quadrants.10 Our group recently demonstrated a simple and reproducible technique to measure the thickness of the normal macular choroid also utilizing the Cirrus HD-OCT.11 Commercially available OCT devices use light sources with short wavelengths at around 840 nm; which have been inadequate for examination of the choroid without the previously mentioned modifications. Several groups have utilized prototype long wavelength OCT with light sources at 1050 nm, thus allowing for enhanced depth penetration necessary for choroidal imaging.12–15 The focus of current choroidal investigations by our group and others has been mainly on macular pathologies, including age-related macular degeneration14,16, central serous choroidal retinopathy17 and myopic degeneration15,18. One group utilized a prototype 1060 nm wavelength OCT to generate 2-dimentional enface images of the macular and peripapillary choroid. However, to our knowledge, quantitative investigations have yet to be undertaken in the peripapillary choroidal region.
A variety of ocular pathologies may have primary or associated pathology located in the peripapillary choroidal region, including glaucoma19,20 and high myopia18. Quantitative and qualitative analysis of this region may aid in elucidating their pathophysiology, tracking disease progression, and potentially measuring response to therapy. Having an understanding of normal baseline choroidal thickness is crucial for such future studies. The purpose of this investigation is to establish the thickness of the normal peripapillary choroid in four different quadrants by utilizing a similar technique established by our group for macular choroidal thickness. The intergrader reproducibility of this method is also addressed. In addition, peripapillary choroidal thicknesses are correlated to retinal nerve fiber layer (RNFL) thicknesses and average patient age.
Methods
Subjects and OCT Scan Protocol
A retrospective spectral domain OCT scan review was conducted for normal patients, who underwent scanning of the optic nerve region with the Cirrus HD-OCT at the New England Eye Center, Tufts Medical Center, between April and September 2010. Patients’ medical records were reviewed to exclude patients with glaucoma, retinal or choroidal pathologies. In addition, patients with refractive error of greater than −6.0 diopters were also excluded. This study was approved by the Tufts Medical Center Institutional Review Board and was conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki.
All patients were imaged using the high-definition scan protocol, which was composed of a single 6 mm raster scan consisting of 4096 A-scans. Images were taken in the normal fashion, with the choroid farther away from the zero-delay line, since unlike the Heidelberg Spectralis, the Cirrus HD-OCT processing software does not compensate dispersion when the scans are inverted, resulting in decreased axial image resolution. A horizontal and a vertical scan were taken in each eye utilizing the high-definition scan protocol: both scans were taken through the optic nerve, each one bisecting the optic nerve into approximately equal halves (Figure 1, left panel). Line placement was aided by charge coupled device camera fundus visualization and anatomic landmarks such as where the retinal vessels emerged from the optic nerve. For inclusion in the study, image signal strengths must be least 6 out of 10. One eye per patient was chosen as the study eye, based upon which eye had the greater average signal strength of the horizontal and vertical scans. If scans in both eyes had the same average signal strength, then the eye with better overall visualization of the choroid-scleral junction was selected.
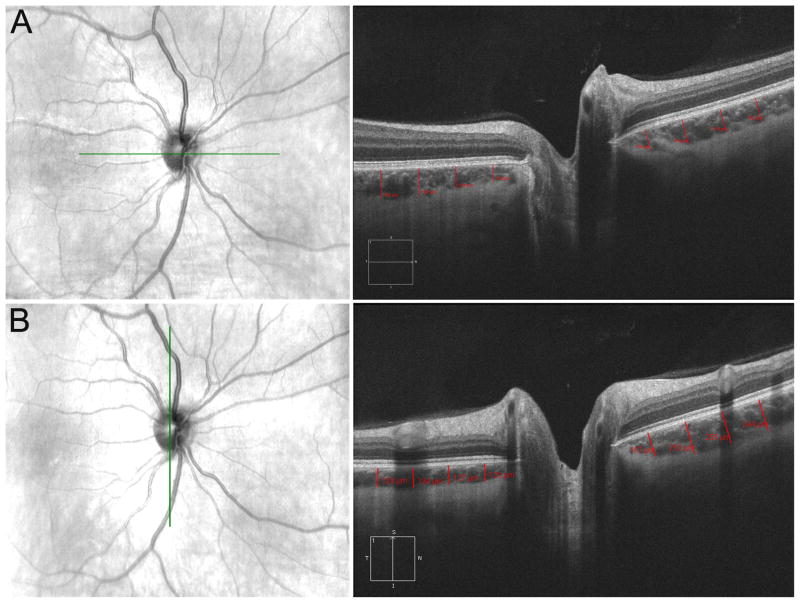
Figure 1.
Example of peripapillary choroid thickness measurements (right eye). (A) Right: Lines denote placements for the nasal (left) and temporal (right) quadrants. Left: Line denotes where scan was taken relative to the fundus. (B) Right: Lines denote placements for the superior (right) and inferior (left) quadrants. Left: Line denotes where scan was taken relative to the fundus.
Peripapillary choroidal thickness measurements
Two independent graders (JH, LB) individually measured peripapillary choroidal thicknesses at the superior, inferior, nasal and temporal quadrants utilizing measurement tools found on the Cirrus HD-OCT analysis software. Four measurements were made in each of the four quadrants at 500 μm intervals along the line of the RPE (Figure 1). For example, the first measurement was made close to the optic nerve at 500 μm distal to the beginning of the RPE, and the fourth measurement was taken at 2000 μm away from the beginning of the RPE. Each thickness measurement was made perpendicular to the RPE going from the posterior RPE edge to the choroid-scleral junction.
Choroidal thicknesses obtained from the two graders were averaged for use in statistical analysis. Analysis of variance with post-hoc Scheffé testing was conducted to compare mean choroidal thicknesses among the four quadrants. All statistical analyses were conducted via Microsoft Excel (version 12.1, Microsoft Corporation, Redmond, WA) and SPSS Statistics (version 19, IBM Corporation, Chicago, IL).
Additional statistical analyses
The reproducibility of choroidal thickness measurements between graders was calculated via intraclass correlation coefficient and Pearson’s correlation coefficient. Additionally, average patient age and average RNFL thickness were both plotted against average choroidal thickness to calculate the correlation coefficient and statistical significance.
Results
Of the 36 patients recruited, 15 were male and 21 were female. 23 right and 13 left eyes were used. The average age of the patients was 48 ± 16 year old (range: 28 to 79 years old). 20 patients were Caucasian, 10 were African American and 6 patients were Asian. The choroid-scleral junction was clearly delineated in 36 out of 40 patients.
Peripapillary choroidal thickness measurements
One way analysis of variance was used to determine whether average choroidal thickness differed among each of the 4 peripapillary regions. The analysis showed significant differences among the groups (p< 0.001; Table 1). Geographic differences existed among the four quadrants. The superior, nasal and temporal quadrants had thicker average choroidal thickness at 229.41 ± 50.96 μm (mean ± standard deviation), 227.17 ± 81.40 μm and 208.84 ± 55.70 μm, respectively. The inferior quadrant had the thinnest peripapillary choroidal thickness among all four quadrants (149.90 ±5 0.14 μm). Post-hoc analysis utilizing the Scheffé method demonstrated that the inferior peripapillary choroid was significantly thinner compared to all other quadrants (p≤ 0.001; Table 2). However, none of the other quadrants (superior, nasal or temporal) demonstrated significant differences with each other.
Table 1.
Average and standard deviation of peripapillary choroidal thickness grouped by quadrant and by distance from the optic nerve.
| 500 μm | 1000 μm | 1500 μm | 2000 μm | Average by quadrant | |
|---|---|---|---|---|---|
| Superior | 187.72±46.26 | 236.17±54.87 | 250.79±58.01 | 256.06±67.28 | 229.41±50.96 |
| Inferior | 139.75±47.19 | 151.03±55.86 | 155.28±55.92 | 157.19±46.87 | 149.90±50.14 |
| Nasal | 209.85±77.05 | 234.53±89.43 | 232.94±86.97 | 232.56±80.86 | 227.17±81.40 |
| Temporal | 168.08±50.34 | 204.56±56.08 | 229.85±65.62 | 242.26±62.30 | 208.84±55.70 |
|
| |||||
| Average* | 176.35±61.74 | 206.57±73.64 | 217.22±76.46 | 221.55±75.04 | 203.83±68.36 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Intervals represent increasing distance from optic nerve. Significance obtained by analysis of variance. Thicknesses denote mean±standard deviation. All values are in micrometers.
Denotes average measured by distance from the optic nerve.
Table 2.
Analysis of variance with post-hoc testing.
| Quadrant A | Quadrant B | Mean Difference (A–B) | P-value | 95% Confidence Interval (μm)
|
|
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Superior | Inferior | 79.50 μm | <0.001 | 38.88 | 120.13 |
| Nasal | 2.23 μm | 0.999 | −38.40 | 42.86 | |
| Temporal | 20.57 μm | 0.563 | −20.06 | 61.19 | |
|
| |||||
| Inferior | Superior | −79.50 μm | <0.001 | −120.13 | −38.88 |
| Nasal | −77.27 μm | <0.001 | −117.90 | −36.64 | |
| Temporal | −58.93 μm | 0.001 | −99.56 | −18.31 | |
|
| |||||
| Nasal | Superior | −2.23 μm | 0.999 | −42.86 | 38.39 |
| Inferior | 77.27 μm | <0.001 | 36.64 | 117.90 | |
| Temporal | 18.34 μm | 0.653 | −22.29 | 58.96 | |
|
| |||||
| Temporal | Superior | −20.57 μm | 0.563 | −61.19 | 20.06 |
| Inferior | 58.93 μm | 0.001 | 18.31 | 99.56 | |
| Nasal | −18.34 μm | 0.653 | −58.96 | 22.29 | |
One way analysis of variance was used to compare the mean thickness among the four quadrants. It demonstrated overall significant differences between the groups (p< 0.001).
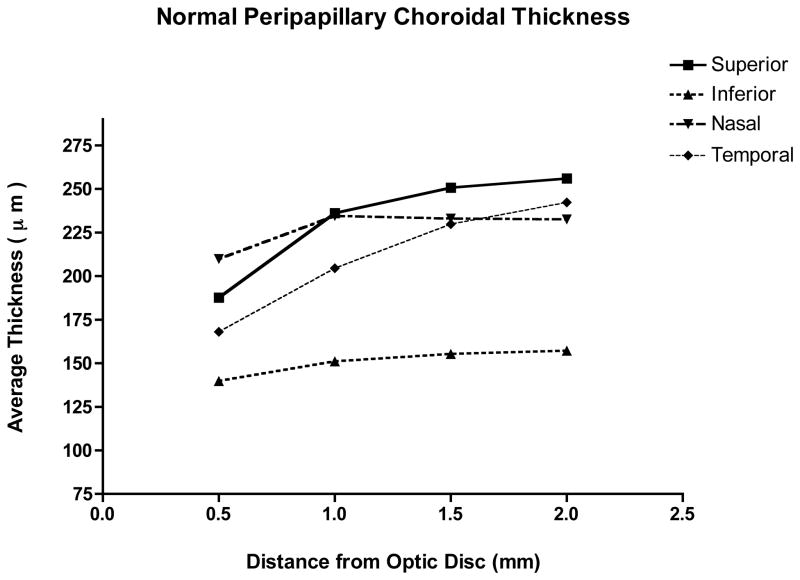
The thickness of the peripapillary choroid generally increased moving distally from the optic nerve, and then approaching a plateau (Figure 2). The exception was in the nasal quadrant, where the choroidal thickness actually decreased at 1500 μm away from the optic nerve and then reached a plateau. The average thickness of the inferior quadrant was significantly thinner than all others at all distances measured away from the optic nerve (p< 0.001).
Figure 2.
Distance from optic nerve (in mm) versus choroidal thickness (in μm). Mean thickness in each quadrant at 500-micrometer intervals from the optic nerve.
Inter-grader reproducibility
The intraclass correlation coefficients were calculated to assess inter-grader reproducibility (Table 3). Intraclass correlation coefficients ranged from moderate (0.62, superior quadrant) to very high (0.93, nasal quadrant). Agreeability was also assessed via the Pearson’s correlation coefficient, which showed moderate (0.74, superior quadrant) to very strong (0.95, nasal quadrant) agreement between the two observers (p< 0.001).
Table 3.
Assessment of inter-grader reproducibility.
| Intraclass Correlation Coefficient | 95% Confidence Interval | Inter-observer Correlation Coefficient (r) | P-value of Correlation | |
|---|---|---|---|---|
| Superior | 0.62 | 0.17 to 0.82 | 0.74 | <0.001 |
| Inferior | 0.88 | 0.71 to 0.94 | 0.90 | <0.001 |
| Nasal | 0.93 | 0.82 to 0.97 | 0.95 | <0.001 |
| Temporal | 0.76 | 0.42 to 0.89 | 0.85 | <0.001 |
Correlations with choroidal thickness
Average choroidal thickness by quadrant was plotted against RNFL thickness from the same region to calculate the Pearson correlation coefficients. Correlations of determination (r2) for the superior, inferior, nasal and temporal quadrants were 0.16, 0.03, 0.02, 0.0006, respectively. The age of the patient did not correlate with the average thickness of the peripapillary choroid (r2= 0.06). None of the correlations reached statistical significance.
Discussion
The choroid is composed mainly of vessels and connective tissue.21 Investigations of the choroid in the past relied primarily upon histological sections. These studies had significant limitations, since it was difficult to accurately maintain the fidelity of the choroid during fixation because of its highly vascular nature and the numerous histological processing artifacts that may arise.22 Recently, Rosen, et al correlated OCT and indocyanine green angiography C-scan data to describe various macular pathologies.23 However, this study did not quantitatively describe choroidal parameters such as thickness. With the advent of spectral domain OCT, improvements in processing software, and the ability for high levels of scan averaging, it is now possible to conduct in vivo cross-sectional studies of the choroid, allowing for a much more accurate depiction of this dynamic tissue. Currently, the main focus of spectral domain OCT choroidal studies is in the macular region. However efforts in studying the peripapillary choroid may also be revealing for both primary retinal disorders and also for conditions such as glaucoma or high myopia.
Peripapillary choroidal thickness
Recent studies have established the thickness of the normal macular choroid.8,9,11 The present study described a simple and reproducible method to accurately measure the thickness of the normal choroid across four different quadrants around the optic nerve. Several similarities could be drawn between macular and peripapillary choroidal thicknesses. First, in both macular and peripapillary regions, choroidal thicknesses differs by a large magnitude depending on the location in the eye. For example, the macular choroidal thicknesses ranged from 157 μm nasally to 272 μm central foveally, while the peripapillary choroidal thicknesses ranged from 149 μm (inferior quadrant) to 229 μm (superior quadrant). Additionally, a significant level of variability existed in the choroid thickness. For example, the standard deviation of the central foveal choroid was 81 μm while the average peripapillary choroid was also comparable, ranging from 50–81 μm. In terms of the general trends in choroidal thicknesses, the peripapillary choroid gradually increased further away from the optic nerve, eventually approaching a plateau. The macular choroid also demonstrated a similar trend, with the thinnest choroid closer to the optic nerve, and thicker choroid farther away.
It is unclear why the inferior quadrant of the peripapillary choroid is thinner than the other quadrants. This regional difference observed in choroidal thickness may be attributable to the development of the eye. Since the optic fissure is located in the inferior aspect of the optic cup, and it is the last part of the globe to close, this regional difference in ocular development may also contribute to the thinner choroid found in the inferior quadrant.
Inter-observer reproducibility
This study measured the thickness of the peripapillary choroid via manual segmentation by two independent operators. The interclass correlation coefficients between the two operators were strong. The high reproducibility of this method showed promise for its use in future studies. Manual measurements of the peripapillary choroid took on average 5 minutes per scan. Given the potential clinical applications of this measurement, along with the time pressures and limited availability of trained operators for choroid segmentation that exist in real clinical settings, future studies in this area should focus on development of automated choroidal segmentation software.
Correlations with choroidal thickness
Macular choroid thickness was found to have a weak negative correlation to age in previous studies.9,12 However, the same association was not found in the present study analyzing the peripapillary choroid. This may be due to the small sample size used in this study. Additionally, peripapillary choroidal thickness was not significantly correlated to RNFL thickness— which may also be due to the small sample size used. Thus, future larger scale studies should be conducted to confirm these observations.
Potential glaucoma correlation
A number of studies noted abnormally reduced choroidal circulation in glaucomatous eyes compared to normal eyes.19,24,25 More recently, Schwartz, et al found that inferior peripapillary choroid had smaller degrees of fluorescein slopes (a proxy for blood flow when evaluated with vessel diameter) compared with the nasal and temporal regions.26 They hypothesized that this decrease may predispose the inferior region of the optic nerve to glaucomatous ischemic damage.
The findings of this study may suggest a possible explanation for the well-known observation that glaucoma typically affects the inferior optic nerve region first. There are significant regional differences in the peripapillary choroidal thicknesses among different quadrants that exist in normal eyes. Specifically, the inferior peripapillary choroid was significantly thinner than all other regions (p< 0.001). It may be possible that regions with thinner choroid may represent areas of lower blood supply. However, this is purely hypothetical and Doppler and other additional investigational studies comparing the peripapillary choroidal thicknesses of normal to glaucomatous eyes will have to be conducted to confirm this proposition. Another possible explanation may be that regional differences in choroid thickness may affect the stress forces to the RNFL, optic nerve head or lamina cribosa— and these forces may contribute to glaucomatous damage. This is also purely hypothesis generating, and further studies will have to be done to validate this hypothesis as well.
Limitations
Several limitations are present in this study. First, while the majority of the scans delineated the choroid clearly, 10% of the scans could not be analyzed due to an inability to identify the choroid-scleral junction precisely. Previous reports of macular choroidal thicknesses by our group reported that around 25% of the scans could not be analyzed due to indistinct choroid-scleral junction.11 However, given that the average peripapillary choroidal thicknesses were thinner (ranging from 149 μm inferiorly to 229 μm superiorly) compared to macular choroidal thicknesses (from 157 μm nasally to 272 μm central foveally), the depth penetration necessary for adequate visualization of the peripapillary choroidal region may be less than that for the macular choroidal region. The prevalence of poor choroidal visualization may also be minimized by limiting the scans to ones with signal strengths greater than or equal to 6/10.
While every attempt was made to place the B-scans in a manner that bisects the optic nerve, since this was a manual process, the scan registration was inevitably subject to some variability. The subjectivity may be decreased by the use of retinal vessels as guides for segmentation, however, in some instances, there were variations to where the retinal vessels may exit the optic nerve, which would also lead to variations as to where the horizontal and vertical scans were taken.
Another limitation to the study is the relatively small number of patients enrolled. However, given the highly significant difference in thicknesses observed between the quadrants and the significant level of agreement seen between the observers, the overall results of the study are most likely to generalize. The retrospective study design is another limitation since there may be some baseline factors in the study participants that were not fully accounted for. However, given the strict inclusion and exclusion criteria used, the effect of this limitation is likely to be minimal.
Lastly, since only one view in each of the four quadrants was obtained, analysis of data obtained from this method gave a general idea of what the thickness in each of the region was. However, more scans around the peripapillary region will have to be taken to generate more accurate two-dimensional maps of the peripapillary choroid. Overcoming this limitation will require increased OCT scanning speed to allow for greater scan averaging and better retinal coverage, or the use of longer wavelength OCT imaging12–15, which has deeper choroidal penetration. Thus long wavelength imaging and increasing scan speed may be a potential area for the future development of OCT devices.
Acknowledgments
Financial Support
This work was supported in part by a Research to Prevent Blindness Challenge grant to the New England Eye Center/Department of Ophthalmology -Tufts University School of Medicine, NIH contracts RO1-EY11289-24, R01-EY13178-10, R01-EY013516-07, Air Force Office of Scientific Research FA9550-07-1-0101 and FA9550-07-1-0014.
Footnotes
Financial Disclosures
James G. Fujimoto, P, receives royalties from intellectual property owned by M.I.T. and licensed to Carl ZeissMeditech, Inc.; O, has stock options in Optovue, Inc..
Jay S. Duker, S, receives research support from Carl ZeissMeditech, Inc., Optovue, Inc., and Topcon Medical Systems, Inc..
Disclosures
The sponsors had no role in the design or conduct of this research. Authors with financial/conflicting interests are listed after references.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coker JG, Duker JS. Macular disease and optical coherence tomography. Curr Opin Ophthalmol. 1996;7:33–8. doi: 10.1097/00055735-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103:1260–70. doi: 10.1016/s0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 4.Schuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Pieroth L, Schuman JS, Hertzmark E, et al. Evaluation of focal defects of the nerve fiber layer using optical coherence tomography. Ophthalmology. 1999;106:570–9. doi: 10.1016/s0161-6420(99)90118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado MJ, Ruiz-Oblitas L, Munuera JM, et al. Optical coherence tomography evaluation of the corneal cap and stromal bed features after laser in situ keratomileusis for high myopia and astigmatism. Ophthalmology. 2000;107:81–7. doi: 10.1016/s0161-6420(99)00022-6. discussion 88. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith JA, Li Y, Chalita MR, et al. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology. 2005;112:238–44. doi: 10.1016/j.ophtha.2004.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:3876–80. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- 11.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–9. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–6. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 13.Povazay B, Hermann B, Hofer B, et al. Wide-field optical coherence tomography of the choroid in vivo. Invest Ophthalmol Vis Sci. 2009;50:1856–63. doi: 10.1167/iovs.08-2869. [DOI] [PubMed] [Google Scholar]
- 14.Yasuno Y, Miura M, Kawana K, et al. Visualization of sub-retinal pigment epithelium morphologies of exudative macular diseases by high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:405–13. doi: 10.1167/iovs.08-2272. [DOI] [PubMed] [Google Scholar]
- 15.Povazay B, Bizheva K, Hermann B, et al. Enhanced visualization of choroidal vessels using ultrahigh resolution ophthalmic OCT at 1050 nm. [Accessed February 27, 2011];Opt Express [serial online] 2003 11:1980–6. doi: 10.1364/oe.11.001980. Available at: http://www.opticsinfobase.org/abstract.cfm?URI=oe-11-17-1980. [DOI] [PubMed]
- 16.Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147:644–52. doi: 10.1016/j.ajo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010;117:1792–9. doi: 10.1016/j.ophtha.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara T, Imamura Y, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Yin ZQ, Vaegan, Millar TJ, et al. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6:23–32. [PubMed] [Google Scholar]
- 20.Spraul CW, Lang GE, Lang GK, Grossniklaus HE. Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res. 2002;42:923–32. doi: 10.1016/s0042-6989(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 21.Dohrmann GJ. The choroid plexus: a historical review. Brain Res. 1970;18:197–218. doi: 10.1016/0006-8993(70)90324-0. [DOI] [PubMed] [Google Scholar]
- 22.Anger EM, Unterhuber A, Hermann B, et al. Ultrahigh resolution optical coherence tomography of the monkey fovea: identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res. 2004;78:1117–25. doi: 10.1016/j.exer.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Rosen RB, Hathaway M, Rogers J, et al. Simultaneous OCT/SLO/ICG imaging. Invest Ophthalmol Vis Sci. 2009;50:851–60. doi: 10.1167/iovs.08-1855. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki S, Inoue Y, Yoshikawa K. Peripapillary fluorescein angiographic findings in primary open angle glaucoma. Br J Ophthalmol. 1996;80:812–7. doi: 10.1136/bjo.80.9.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollo G, Greve EL, van den Berg TJ, Vargha P. Evaluation of the peripapillary circulation in healthy and glaucoma eyes with scanning laser Doppler flowmetry. Int Ophthalmol. 1996–1997;20:71–7. doi: 10.1007/BF00212949. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz B, Harris A, Takamoto T, et al. Regional differences in optic disc and retinal circulation. Acta Ophthalmol Scand. 2000;78:627–31. doi: 10.1034/j.1600-0420.2000.078006627.x. [DOI] [PubMed] [Google Scholar]