Abstract
Individual differences in dopamine D2 receptor (D2R) expression in the brain are thought to influence motivation and reinforcement for ethanol and other rewards. D2R exists in two isoforms, D2 long (D2LR) and D2 short (D2SR), produced by alternative splicing of the same gene. The relative contributions of D2LR versus D2SR to ethanol and sugar water drinking are not known. Genetic engineering was used to produce a line of knockout (KO) mice that lack D2LR and consequently have increased expression of D2SR. KO and wild-type (WT) mice of both sexes were tested for intake of 20% ethanol, 10% sugar water and plain tap water using established drinking-in-the-dark procedures. Mice were also tested for effects of the D2 antagonist eticlopride on intake of ethanol to determine whether KO responses were caused by lack of D2LR or over-representation of D2SR. Locomotor activity on running wheels and in cages without wheels was also measured for comparison. D2L KO mice drank significantly more ethanol than WT in both sexes. KO mice drank more sugar water than WT in females but not in males. Eticlopride dose- dependently decreased ethanol intake in all groups except male KO. KO mice were less physically active than WT in cages with or without running wheels. Results suggest that over-representation of D2SR contributes to increased intake of ethanol in the KO mice. Decreasing wheel running and general levels of physical activity in the KO mice rules out the possibility that higher intake results from higher motor activity. Results extend the literature implicating altered expression of D2R in risk for addiction by delineating the contribution of individual D2R isoforms. These findings suggest that D2LR and D2SR play differential roles in consumption of alcohol and sugar rewards.
Keywords: Dopamine, D2 receptor, knockout, ethanol drinking, alcoholism, drinking-in-the-dark
Introduction
Dopamine is widely recognized as a key neurotransmitter signal involved in the perception and motivation for reward, but the details have not been worked out (Flagel et al., 2010). One prominent hypothesis is that genetic or environmentally induced variation in specific components of the dopamine signaling system in the brain could underlie individual differences in predisposition to consume rewarding substances such as drugs of abuse or high caloric foods or drinks (Volkow et al., 1999; Wang et al., 2004). However, the dopamine system itself is extraordinarily complex. At least 5 different dopamine receptor subtypes are known to exist, and each probably makes contributions individually and in combination with other subtypes through their abundance and distribution in the brain (Bergson et al., 1995). In addition, variation in the abundance or distribution of the dopamine transporter protein that clears dopamine from extracellular spaces, the enzyme monoamine oxidase that degrades dopamine in the terminals, or the rate limiting enzyme that synthesizes dopamine, tyrosine hydroxylase, within the dopamine cell bodies themselves, could make contributions (Dietz et al., 2005; Gulley et al., 2003). Furthermore, the abundance or structural variants of the numerous downstream signaling molecules within the cells receiving the dopamine signal including adenylyl cyclase, cyclic AMP, protein kinase A, protein phosphatases, DARPP-32 etc., could influence perception or motivation for reward (Stipanovich et al., 2008). Given the complexity of the dopamine signaling pathway, a reductionist approach is useful for isolating individual components.
Along with all the other dopamine receptor subtypes, the dopamine D2 receptor (D2R) appears to have an important function in motivation for reward. For example, evidence from the literature suggests that human alcoholics tend to have fewer D2R than non-alcoholics (Hietala et al., 1994; Tupala et al., 2001; Volkow et al., 2006; Volkow et al., 1996). The difference does not go away after 1–4 months of detoxification suggesting that reduced D2R either predisposes alcoholism or is a persistent consequence of repeated alcohol intoxication (Volkow et al., 2002). Genetic association studies, while controversial and inconsistent, generally support the hypothesis that polymorphisms which reduce D2R expression increase alcoholism risk (Kraschewski et al., 2009; Noble et al., 1991; Pato et al., 1993). Taken together, the human studies suggest that reduced expression of D2R expression in brain reward circuits predisposes excessive alcohol consumption.
Results from animal studies on the association between D2R and ethanol drinking behavior have been less consistent. Quantitative trait locus (QTL) analysis has established a QTL for ethanol consumption using the two-bottle choice test on mouse chromosome 9 approximately at the D2R locus. However, in 23 Recombinant Inbred strains derived from C57BL/6J and DBA/2J (BXD), D2R expression in the nucleus accumbens was not correlated with ethanol preference or consumption (Hitzemann et al., 2003). D2R null mice (which lack both D2LR and D2SR) display reduced alcohol consumption in the two-bottle choice test which is the opposite result one would expect if reduced D2R is associated with increased drinking (Phillips et al., 1998). However, when D2R was partially restored in the nucleus accumbens using a viral vector approach, ethanol intake in the D2R null mice increased whereas in wildtype, D2R over-expression decreased ethanol intake. Hence, the relationship between ethanol drinking and accumbens D2R appears to follow an inverted U-shape curve (Thanos et al., 2005). In selectively bred lines of mice, the high alcohol preferring line (HAP) displayed reduced expression of D2R in the nucleus accumbens as compared to the low alcohol preferring line (LAP) (Bice et al., 2008). Hence, results have been inconsistent and the interpretation would benefit from identification of key variables or underlying mechanisms that could contribute to the discrepancies.
One limitation of most previous studies is that they did not differentiate between the two distinct subtypes of D2R, the D2LR and D2SR, which are generated by alternative splicing of the same gene. D2LR has a 29 amino acid insertion in the third cytoplasmic loop of the protein which is absent in D2SR (Dal Toso et al., 1989; Monsma et al., 1989). The two isoforms coexist in most brain tissue analyzed and the ratio of D2LR versus D2SR mRNA expression varies from region to region (Mack et al., 1991; Neve et al., 1991). The differences in protein structure, expression pattern, and previous studies using D2LR knockout (KO) mice, suggest that the two D2R isoforms have different functions (Smith et al., 2002; Usiello et al., 2000; Wang et al., 2000; Xu et al., 2002). It is possible that altered expression of both D2L and D2S, altered expression of one or the other, or the ratio that matters for influencing brain reward pathways.
In an effort to delineate the contribution of D2LR and D2SR in ethanol consumption, we generated mutant mice lacking D2LR and expressing only D2SR by removing exon 6 on the D2R gene (Wang et al., 2000). The goal of the present study was to determine how manipulating one component of the dopamine signaling system, namely removing D2LR or subsequent compensatory increased expression of D2SR, would affect motivation for reward as measured by intake of 20% ethanol, 10% sugar water and level of voluntary wheel running behavior. Hence, we compared D2LR KO mice to wild-type (WT) control mice for intake of these solutions and display of wheel running. For comparison we also measured intake of plain tap water and baseline physical activity in cages without wheels.
The mutant mice were backcrossed onto the C57BL/6 strain (from Taconic, Germantown, NY) for 7 generations so that a majority of their genome is comprised of DNA from C57BL/6 (Wang et al., 2000). This is an important attribute of the model because C57BL/6, unlike most other strains, will drink ethanol to the point of intoxication (Rhodes et al., 2007). Hence, by studying the mutation in a C57BL/6 background we are investigating the role of D2LR and D2SR in a background strain that is already motivated to drink ethanol solutions.
Methods
Animals
All mice were bred and raised at the Beckman Institute animal facility. The D2LR KO mice were generated as described in Wang et al. (2000). In brief, a DNA construct containing the dopamine D2 receptor gene lacking exon 6 that transcribes mRNA for the third cytoplasmic loop of the protein was incorporated into an embryonic stem (ES) cell of 129/terSv origin and placed into a C57BL/6 (Taconic, Germantown, NY) blastocyst to create the chimera. Chimeric mice containing ES cells in the germ line were crossed with C57BL/6 mice (Taconic, Germantown, NY) to produce heterozygous mice. Heterozygotes were then backcrossed onto C57BL/6 (Taconic, Germantown, NY) for 7 generations, each time genotyping offspring and selecting only those that are heterozygous for the mutation for the next backcross. Following 7 generations of backcrossing, heterozygotes for the mutation were crossed together. From the heterozygote crosses, approximately 10 pairs of mice that were homozygous for the mutation and 10 pairs that were homozygous for the wildtype D2 allele were used to found 2 homozygous lines, referred to hereafter as KO or WT, respectively. These 2 lines were bred separately for 6 generations each time maintained by approximately 10 breeder pairs, deliberately avoiding sibling matings. The offspring of the 6th generation were used in this study. Genotyping was conducted periodically and in mice used for this study to confirm that the KO line was homozygous for the mutation whereas WT was homozygous for the intact D2 receptor gene. WT mice display both D2LR and D2SR in varying ratios depending on the brain region, dominated by D2LR (the long form of the protein), approximately 85–95% in most regions (Mack et al., 1991; Usiello et al., 2000; Wang et al., 2000). D2LR KO mice display only D2SR (the short form of the protein), in increased abundance relative to the WT, such that total levels of D2R are similar between KO and WT (Wang et al., 2000).
Husbandry
Mice were individually housed for one week before the start of the experiment, and remained singly housed throughout the duration of the studies. Standard polycarbonate shoebox cages (dimensions 29×19×13 cm L×W×H) were used with corncob bedding (Harlan Teklad, Madison, Wisconsin, USA). Rooms were controlled for temperature (21 °C) and photo-period (12-h L:D; lights on at 7 am and off at 7 pm). Food (Harlan Teklad 7012) and water were provided ad libitum, except when 20% ethanol or 10% sugar water was substituted for water for 2 or 4 hours as described below. Red lights were illuminated continuously (mice cannot see red light) for data collection during the dark phase of the light-dark cycle. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All efforts were made to minimize the number of animals used and their suffering.
Drugs and Drinking Solutions
The 20% ethanol drinking solution was prepared from 200 proof absolute anhydrous ethanol (Pharmco-Aaper brand, Brookfield, CT) diluted to 20% (v/v) using tap water. 20% ethanol was used because previous studies established that maximal intake of ethanol occurs at this concentration resulting in behavioral intoxication in the C57BL/6J genotype (Rhodes et al., 2005; Rhodes et al., 2007). Similarly, the 10% sucrose drinking solution was prepared from sucrose (Sigma Aldrich, St. Louis, MO) dissolved in tap water at 10% (w/v) concentration. 10% sucrose was used because previous studies established that the C57BL/6J genotype drink large quantities of this solution, presumably because they find it rewarding (Gupta et al., 2008; Kamdar et al., 2007). Eticlopride (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline and administered via intraperitoneal (IP) injections in a volume of 10 ml/kg. Eticlopride was administered at doses 0.1, 0.3 and 1.0 mg/kg corresponding to a low, medium and high dose, respectively. Eticlopride was chosen because our previous study showed that eticlopride effectively blocked dopamine agonist-induced behaviors in both KO and WT mice (Fetsko et al., 2003). Studies have established that eticlopride binds with very high affinity and selectivity to dopamine D2 receptors, as shown by low IC50 values for the displacement of both [3H]spiperone (1.0 nM) and [3H]domperidone (1.6 nM) in rat striatal homogenates (Hall et al., 1985), and greater potency as an antagonist against D2 receptors as compared to D3 and D4 receptors in transfected cell lines (Tang et al., 1994).
Experiment 1: Intake of ethanol, sucrose and plain tap water
Experiment 1 was conducted in two separate batches (total n=42, 7 WT and 7 KO in the first batch, 14 WT and 14 KO in the second batch). In the first batch, animals were tested for intake of ethanol first, then sugar water, then plain water using 3 repetitions of the 4 day drinking-in-the-dark procedure over a three week period following Rhodes et al. (2005). In the second batch, animals were tested for intake of the three solutions in the reverse order, plain water first, then sugar water then ethanol (see Experiment 2 batch 1). All females were used in the first batch, whereas sexes were equally represented in the second batch. Mice were 100 ± 2 days old at the start of testing. For a complete list of the total sample sizes per group collapsed across batches see Figure 1.
Figure 1.
Consumption of alternative fluids in KO and WT mice. A) Average volume of 20% ethanol (ml) consumed during the 4 hr test on the 4th day of access in females and males. Average grams of ethanol consumed per kilogram body mass ± SE are reported within bars, along with sample sizes. B) Average volume of 10% sugar water consumed. C) Average volume of plain tap water consumed. Standard error bars are shown. More females were used than males because the variance in drinking behavior was greater in females than males possibly due to the estrus cycle in females. WT are represented as black bars, KO as gray bars.
Experiment 1a: 20% Ethanol
The purpose of Experiment 1a was to examine the effects (if any) of D2LR deficiency on ethanol consumption. The drinking-in-the-dark procedure was conducted as described by Rhodes et al. (2005). Mice were presented with 20% ethanol drinking solutions over 4 consecutive days. Starting 3 hours after lights shut off, the water bottles were replaced with 10 ml graduated cylinders fitted with double ball bearing sipper tubes (to prevent leakage) containing a 20% ethanol drinking solution. This was done in the home cages where animals were singly housed. Presentations lasted 2 hours on the first 3 days and 4 hours on the final day. Intakes were recorded every 30 minutes. After the 2- or 4-hour periods, the cylinders were replaced with the original water bottles. The purpose of the initial 3 days of 2 hr access was to acclimate the animals to the new solutions. The dependent variable of interest is the total fluid intake expressed in ml and g/kg ethanol, over the 4 hours of access on the 4th day following Rhodes et al. (2005).
Experiment 1b: 10% Sucrose
The purpose was to determine the specificity of the responses observed in Experiment 1a when a sweet sugar solution is used as the reward instead of ethanol. The drinking in the dark procedure was identical to that of Experiment 1a, except substituting the 20% ethanol with 10% sucrose solution.
Experiment 1c: Plain Tap Water
The purpose was to determine whether results in Experiments 1a and b were specific to the high appetitive value of the ethanol and sugar water rather than related to general intake of fluids. The drinking in the dark procedure was identical to that of Experiment 1a, except substituting the 20% ethanol solutions with plain tap water.
Experiment 2: Intake of 20% Ethanol with Eticlopride
The purpose of Experiment 2 was to determine whether the ethanol responses observed in Experiment 1a were caused by the lack of D2LR or the presence or over-representation of D2SR. To accomplish this objective, the dopamine D2 antagonist eticlopride was administered prior to ethanol presentation. The logic was as follows. If the antagonist has no effect on KO behavior, then KO responses are most likely a consequence of D2LR deficiency. On the other hand, if the antagonist alters KO behavior, particularly in the direction toward WT behavior, then responses are most likely a consequence of the presence or over-representation of D2SR relative to D2LR.
Experiment 2 was performed in three separate batches (total n=70). The first batch used the same mice as were used in Experiment 1 (batch 2; 14 WT and 14 KO equally represented by sex) following sugar water intake measurements and 5 weeks when they were left undisturbed between experiments. The second batch included females only (n=20 total, 9 WT and 11 KO). The third batch included males only (n=22, 11 WT and 11 KO). For a complete list of the total sample sizes per group collapsed across batches see Figure 2.
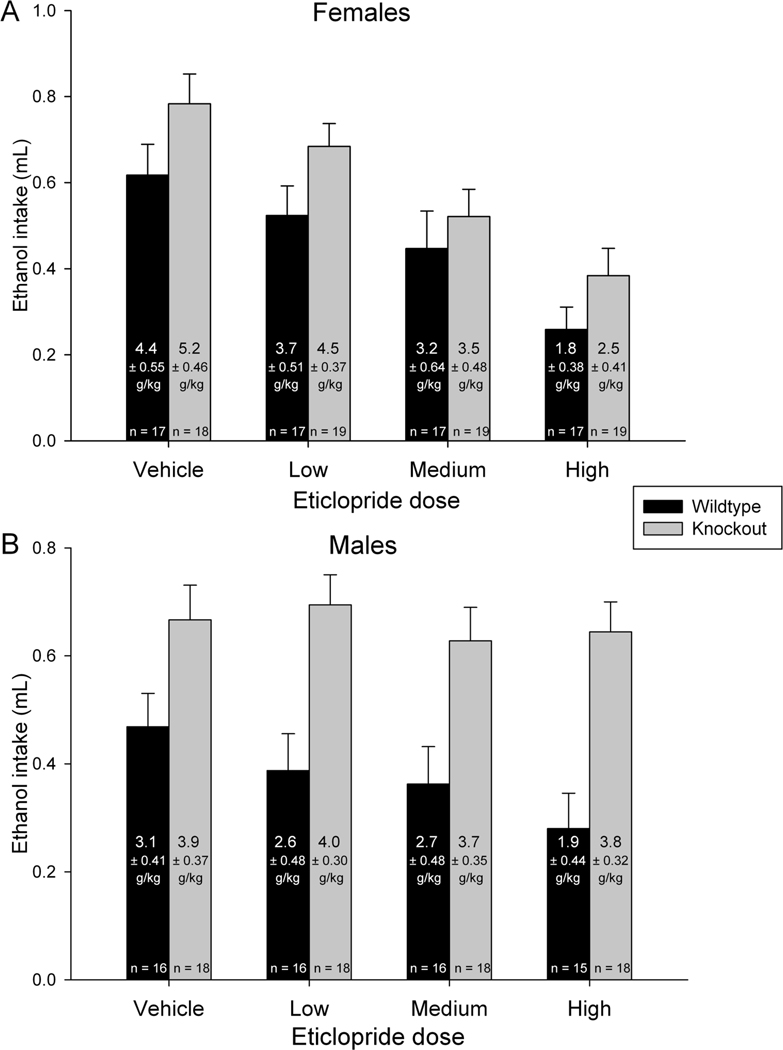
Figure 2.
Consumption of 20% ethanol after eticlopride administration in KO and WT mice. A) Average volume of 20% ethanol (ml) consumed during the 4 hr test after administration of vehicle, low (0.1 mg/kg), medium (0.3 mg/kg) or a high (1.0 mg/kg) dose of eticlopride in females. Grams of ethanol consumed per kilogram body mass ± SE are reported within bars, along with sample sizes. B) Same as A for males. Standard error bars are shown. WT are represented as black bars, KO as gray bars.
The original drinking in the dark procedure was modified in order to implement a within subjects design where a number of doses of a drug can be examined within the same individual within a short amount of time (see Gupta et al., 2008; Kamdar et al., 2007). This modified procedure used 8 days of ethanol presentations over a 2-week time period. Each week mice were presented with 20% ethanol drinking solutions for 2 consecutive days for 4 hrs each day, followed by 1 day off, and then again 2 consecutive days of 4-hr ethanol access (Monday-Tuesday, and Thursday-Friday, with Wednesday off). On the later of the consecutive days (Tuesday and Friday) mice were given one of four injections: a low (0.1 mg/kg), medium (0.3 mg/kg) or high (1.0 mg/kg) dose of eticlopride, or saline. Thus, before mice received injections they always had 1 day of access to a drinking solution without injections, and 1 or 2 days where they were left undisturbed. The rationale for leaving the animals undisturbed was to separate episodes of the 2-day cycle and to reduce the chance for carry over effects of the injection following presentations of drinking solutions. The order in which the four injections were administered in each animal was permuted to maximize number of combinations among the mice within a batch and was randomized across the groups. This was done so that order of injections would not need to be considered in the statistical analysis. By the end of the second week all mice had received each of the four injections. The dependent variable of interest is the intake of ethanol in ml and g/kg on each of the 4 injection days.
Experiment 3: Physical activity
The same mice used in Experiment 2, batch 2, were used again in Experiment 3, following the ethanol eticlopride measurements and 5 days when they were left undisturbed between experiments. Mice were first measured for wheel running over 18 days and then placed in home cages designed for video tracking from above for an additional 8 days.
Experiment 3a: Running wheels
The purpose was to determine whether results in Experiments 1 and 2 were specific to the high appetitive value of the ethanol and sugar water or whether they also generalize to wheel running behavior. Mice were placed on running wheels for 18 days. Dimensions of running wheel cages were 36×20×14 cm (L×W×H) with a 23 cm diameter wheel mounted in the cage top (Respironics, Bend, OR). Wheel rotations were monitored continuously in 1 min increments throughout the experiment via magnetic switches interfaced to a computer.
Experiment 3b: Home cages
The purpose was to determine whether results in previous experiments could be explained by differences in general arousal or baseline levels of movement in their home cages without running wheels. Immediately after the wheel running, the mice in Experiment 3a were transferred to custom made home cages for video tracking. Dimensions of the cages were 18.5×33.5×16 cm, constructed of clear plastic with food and water access mounted on the side (see Zombeck et al.). Horizontal distance traveled was recorded continuously using TopScan (Clever Sys, Vienna, VA, USA) video tracking software.
Statistical Analysis
Data were analyzed using SAS Proc Mixed procedures. P<0.05 was considered statistically significant. In experiment 1, fluid intake (ml or g/kg for ethanol) over the 4-hour test on day 4 was analyzed by two-way ANOVA including genotype (WT or KO), sex (male or female), and the interaction between genotype and sex as factors. Two-hour intakes on days 1–4 were analyzed using 3-way repeated measures ANOVA with day as the repeated measures factor, and genotype and sex as between-subjects factors, or as 2-way repeated measures ANOVA, separately by sex. For the plain water trials in batch 2, due to leakage in some of the drinking tubes, we lost data for 5 animals, hence the reduced sample sizes for the plain water results. In experiment 2, fluid intake over the 4-hr tests during injection trials was analyzed by three-way repeated measures ANOVA including genotype, sex, dose (veh, low, med, high dose of eticlopride; within-subjects factor) and all interactions as factors. In experiment 3, distance traveled in running wheels or home cages without wheels (km/day) were compared between KO and WT female mice using unpaired t-tests.
Results
Body mass
KO animals were on average 3 grams heavier than WT (F1,38=19.4, P<0.0001), and males 4 grams heavier than females (F1,38=23.7, P=0.0005). The interaction between sex and genotype for body mass was significant (F1,38=4.3, P=0.04) because the difference in body mass between KO and WT was approximately 2 grams for females and 5 grams for males. The following were the average body masses of the animals at approximately 100 ± 2 days old: WT females 22.2 ± 0.27 g, WT males 24.2 ± 0.17 g, KO females 23.7 ± 0.47 g, KO males 28.9 ± 1.05 g.
Experiment 1a: 20% Ethanol
See Figure 1a. Results of the 2-way ANOVA indicated that KO mice drank approximately 30% more ethanol as compared to WT mice (main effect of genotype, F1,38=17.7, P=0.0002). Sex and the interaction between sex and genotype were not significant. When ethanol was analyzed as gram per kilogram body mass, results were similar, KO drank more than WT (F1,38=9.4, P=0.004) and no other factor was significant. An analysis of 2 hour intakes on days 1–4 indicated that KO animals drank more ethanol than WT starting on the first day and that the difference was consistent on all 4 days. This was indicated by a significant effect of genotype (F1,112=15.1, P=0.0002), but no other factors in the 3-way repeated measures ANOVA.
Experiment 1b: 10% Sucrose
See Figure 1b. Results of the 2-way ANOVA indicated a significant effect of genotype (F1,38=8.1, P=0.007) and a significant interaction between sex and genotype (F1,38=4.9, P=0.03). Posthoc analysis indicated that female KO mice drank approximately twice as much sucrose solution as compared to female WT (P=0.005), but no other differences were significant. An analysis of 2-hour intakes on days 1–4 indicated that KO females drank approximately twice as much sucrose solution than WT starting on the first day and that the difference was consistent on all 4 days. This was indicated by a significant effect of genotype (F1,80=8.7, P=0.004) but no other factors in the 2-way repeated measures ANOVA. KO males drank similar levels of sucrose as compared to WT on all 4 days as indicated by no significant factors in the 2-way repeated measures ANOVA.
Experiment 1c: Plain Tap Water
See Figure 1c. Results of the 2-way ANOVA indicated a significant effect of sex (F1,33=12.1, P=0.002), but no significant effects of genotype were detected. Posthoc analysis indicated that males drank approximately twice as much plain water as compared to females (P=0.002). An analysis of 2 hour intakes on days 1–4 indicated that KO animals drank similar quantities of plain water as compared to WT on all 4 days. As noted in the methods, in batch 1, animals received ethanol first, then sugar water, then plain water whereas in batch 2 the presentation of the solutions was reversed. Females were tested in both batches allowing us to test the influence of presentation order on intake of the three solutions in females, but no significant effect of presentation order was detected.
Experiment 2: 20% Ethanol with Eticlopride
See Figure 2. Results of the 3-way ANOVA for ethanol intake expressed in ml indicated a significant effect of genotype (F1,262=39.3, P<0.0001), dose (F3,262=10.7, P<0.0001), and the interaction between sex and dose (F3,262=3.4, P=0.02). For ethanol expressed as g/kg, results were similar. Significant effects of genotype (F1,262=16.4, P<0.0001), dose (F3,262=10.3, P<0.0001), and the interaction between sex and dose (F3,262=3.7, P=0.01) were detected. From inspection of Figure 2, it is apparent that KO mice drank more ethanol than WT at all doses of eticlopride in both males and females. As dose of eticlopride increased from low to high, ethanol intake tended to decrease in all groups by a similar magnitude except in the male KO mice where it apparently was not affected by the antagonist treatment.
Experiment 3a: Physical activity on running wheels
See Figure 3a. On the first day when running wheels were novel, WT mice ran approximately twice as far as KO (t18=3.4, P=0.003), but on day 2, levels of running dropped in WT and no differences were detected between the genotypes. On days 3–10 levels of running began to gradually increase in WT and KO reaching a plateau by approximately day 10, and on each of these days KO ran approximately 30% less than WT. Average distance traveled on wheels over the 18 days was significantly lower in KO mice as compared to WT (t18=2.4, P=0.03; 4.6 km/day ± 0.39 SE for WT versus 3.2 km/day ± 0.59 for KO mice).
Figure 3.
Physical activity in KO and WT female mice. A) Average distance traveled on running wheels in km/day as measured by the number of wheel rotations multiplied by the circumference of the wheel are shown for KO and WT female mice. B) Average distance traveled in home cages without wheels as measured by video tracking in km/day are shown for KO and WT female mice. Standard error bars are shown. WT are represented as filled circles, KO as open circles.
Experiment 3b: Physical activity in home cages
See Figure 3b. On the first day when the mice were transferred into the new cages, level of activity was approximately 60% higher than on remaining days, and although average distance was farther for WT than KO on the first day, the difference was not statistically significant. However, on days 2–8, distance traveled in the home cages began to gradually increase in WT and KO, and on each day KO moved approximately 30% less than WT. Average distance traveled in home cages without wheels over the 8 days was significantly lower in KO mice as compared to WT (t14=4.6, P=0.0004; 239 m/day ± 14.1 SE for WT versus 172 m/day ± 3.0 for KO mice).
Discussion
The main finding of this study is that D2LR KO mice, which lack D2LR but compensate by over-expressing D2SR, displayed increased consumption of ethanol. The excessive drinking behavior displayed by female D2LR KO mice likely results from the increased expression of D2SR rather than loss of D2LR. This assertion is based on the following results: (1) In females, results demonstrated that the D2R antagonist eticlopride attenuated ethanol consumption in both KO and WT in a similar manner (Fig. 2A). If the increased ethanol intake in KO mice were due to D2LR deficiency alone, then the antagonist should have had no effect on drinking behavior in the D2LR KO mice, and should have increased rather than decreased drinking in WT; (2) Previous studies have established that D2R null mice (which are deficient in both D2LR and D2SR) display reduced ethanol consumption (Phillips et al., 1998). Taken together, these data supports the hypothesis that D2LR deficiency alone unlikely accounts for increased ethanol intake observed in D2LR KO mice.
Our conclusion does not exclude the possibility that reduced expression of D2R is associated with increased consumption of ethanol as suggested in the literature (McBride et al., 1993; Stefanini et al., 1992; Volkow et al., 1996). Instead, the present study suggests that the critical variable impacting reward could be the ratio of long versus short forms of D2R expression in the brain (Wang et al., 2000). Given that D2LR typically dominates expression in brain tissue (Fetsko et al., 2003), an overall reduction in D2R could easily result in relatively greater representation of D2SR relative to D2LR, and that shift in ratio in favor of D2SR could be the critical factor predisposing increased alcohol consumption.
The specificity of D2LR deficiency on intake of ethanol and sugar rewards differed for males and females and the explanation for this sex difference is not clear. KO females showed increased ethanol and sugar water intake, whereas KO males specifically showed increased ethanol consumption, not sugar water intake (Fig. 1A,B). In addition, results of experiment 2 showed that eticlopride attenuated ethanol intake in KO females but not in males (Fig 2). The implication is that in males where the effect is specific to ethanol, the absence of D2LR rather than the over-representation of D2SR increases ethanol intake. However, this is inconsistent with the previous findings by other investigators that D2R null mice showed reduced ethanol preference (Phillips et al., 1998). Moreover, the conclusion for males should be interpreted with caution because had we used higher doses of eticlopride, we might have seen dose dependent reductions in ethanol intake in D2LR deficient male mice as we observed in females.
The increased intake of the 10% sugar water solution in KO females as compared to WT females (Fig. 1B) could reflect greater motivation to consume calories or greater motivation for the sweet taste. The calorie hypothesis is plausible given that the KO mice weighed more than WT mice which could reflect increased feeding or decreased energy expenditure. Moreover, evidence from the literature suggests that dopamine D2-like antagonists increase appetite and body weight in humans and rodent models although the cellular mechanisms for this effect on energy balance is not known (e.g., Fell et al., 2007; Graham et al., 2005). The purpose of including the sugar reward in this study was to examine the specificity of the KO effect on ethanol consumption as compared to an alternative natural reward. One way to separate the contribution of calories from taste in the motivation for the drinking behavior would be to measure intake of an artificial sweetener such as saccharin (e.g., Phillips et al., 1994). Future studies using saccharin or other artificial sweeteners are needed to arbitrate between the calorie versus taste alternative hypotheses.
One limitation of all knockout studies is the difficulty in attributed differences to the null mutation rather than compensatory changes that occur during development to compensate for the loss of the deleted protein. In our case, we know from previous studies, that removal of D2LR results in increased expression of D2SR (Wang et al., 2000), and we found evidence that at least in females, the presence of D2SR is required for increased ethanol intake (Fig 2A). However, in addition to increased expression D2SR, other components of the monoamine neurotransmitter systems could have changed to compensate for the D2LR deficiency and these unknown changes could also have contributed to the observed differences between WT and KO in this study.
Another limitation of the present study is that we used separate homozygous lines of KO and WT mice rather than using littermates from heterozygote crosses due to logistical constraints. Hence, it is possible that some segregating 129/terSv and C57BL/6 alleles in the founding populations fixed from genetic drift in different directions (i.e., toward 129/terSv or C57BL/6 origin) in the two lines after 6 generations. If divergent fixation occurred at locations in the genome that influence drinking, then allelic differences other than the mutation could have contributed to drinking differences between the lines. However, we believe this possibility is unlikely because after 7 generations of backcrossing, less than 1% of the genome is expected to contain 129/terSv origin (Silver, 1995). As with all knockout models that employ backcrossing, 129/terSv DNA will flank the mutation due to linkage disequilibrium (Gerlai, 1996). However, given that 129 derived strains drink very little ethanol as compared to C57BL/6 (Rhodes et al., 2007), it is unlikely 129/terSv DNA flanking the mutation caused the KO mice to drink more ethanol than WT. Hence, we conclude that the probability that divergent fixation of 129/terSv DNA in the two lines contributed to the differences between the KO and WT mice in this study is vanishingly small.
The increased intake of ethanol and sugar water in the KO females relative to WT females cannot be attributed to increased overall arousal or levels of locomotor activity because the KO mice were approximately 30% less active than WT in cages with or without running wheels (Fig. 3). The wheel running data also establish specificity for reward in KO mice, because wheel running is thought to be rewarding and reinforcing (Greenwood et al., 2011; Rhodes et al., 2003). Hence, the increased consumption of ethanol and sugar water observed in Figures 1 and 2 in KO mice are specific for the ingested rewards and do not extend to wheel running (Fig. 3). The present results that KO mice displayed reduced levels of physical activity relative to WT are consistent with our previous findings indicating that D2LR plays a prominent role in motor function (Fetsko et al., 2005; Wang et al., 2000).
In summary, we observed that in a background of C57BL/6, mice expressing D2SR and lacking D2LR drank more ethanol than WT. The implication is that over-representation of D2SR relative to D2LR could contribute to enhanced ethanol intake in genetically predisposed individuals. Future studies will determine the signaling pathways associated with D2SR and D2LR and the role of individual D2R isoforms in excessive intake of other drugs of abuse.
Acknowledgements
This research was funded by NIH grants DA027487 and MH083807 to JSR and a Trust-Private fund to YW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J. Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice PJ, Liang T, Zhang L, Strother WN, Carr LG. Drd2 expression in the high alcohol-preferring and low alcohol-preferring mice. Mamm. Genome. 2008;19:69–76. doi: 10.1007/s00335-007-9089-2. [DOI] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH. The dopamine D2 receptor: two molecular forms generated by alternative splicing. Embo. J. 1989;8:4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol. Behav. 2005;86:347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Anjum N, Dickinson K, Marshall KM, Peltola LM, Vickers S, Cheetham S, Neill JC. The distinct effects of subchronic antipsychotic drug treatment on macronutrient selection, body weight, adiposity, and metabolism in female rats. Psychopharmacology (Berl) 2007;194:221–231. doi: 10.1007/s00213-007-0833-9. [DOI] [PubMed] [Google Scholar]
- Fetsko LA, Xu R, Wang Y. Alterations in D1/D2 synergism may account for enhanced stereotypy and reduced climbing in mice lacking dopamine D2L receptor. Brain Res. 2003;967:191–200. doi: 10.1016/s0006-8993(02)04277-4. [DOI] [PubMed] [Google Scholar]
- Fetsko LA, Xu R, Wang Y. Effects of age and dopamine D2L receptor-deficiency on motor and learning functions. Neurobiol. Aging. 2005;26:521–530. doi: 10.1016/j.neurobiolaging.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2010;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Graham KA, Perkins DO, Edwards LJ, Barrier RC, Jr, Lieberman JA, Harp JB. Effect of olanzapine on body composition and energy expenditure in adults with first-episode psychosis. Am. J. Psychiatry. 2005;162:118–123. doi: 10.1176/appi.ajp.162.1.118. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol. Clin. Exp. Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hall H, Kohler C, Gawell L. Some in vitro receptor binding properties of [3H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. Eur. J. Pharmacol. 1985;111:191–199. doi: 10.1016/0014-2999(85)90756-3. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- Hitzemann R, Hitzemann B, Rivera S, Gatley J, Thanos P, Shou LL, Williams RW. Dopamine D2 receptor binding, Drd2 expression and the number of dopamine neurons in the BXD recombinant inbred series: genetic relationships to alcohol and other drug associated phenotypes. Alcohol. Clin. Exp. Res. 2003;27:1–11. doi: 10.1097/01.ALC.0000047862.40562.27. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kraschewski A, Reese J, Anghelescu I, Winterer G, Schmidt LG, Gallinat J, Finckh U, Rommelspacher H, Wernicke C. Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenet. Genomics. 2009;19:513–527. doi: 10.1097/fpc.0b013e32832d7fd3. [DOI] [PubMed] [Google Scholar]
- Mack KJ, Todd RD, O'Malley KL. The mouse dopamine D2A receptor gene: sequence homology with the rat and human genes and expression of alternative transcripts. J. Neurochem. 1991;57:795–801. doi: 10.1111/j.1471-4159.1991.tb08221.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10:387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, McVittie LD, Gerfen CR, Mahan LC, Sibley DR. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342:926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- Neve KA, Neve RL, Fidel S, Janowsky A, Higgins GA. Increased abundance of alternatively spliced forms of D2 dopamine receptor mRNA after denervation. Proc. Natl. Acad. Sci. USA. 1991;88:2802–2806. doi: 10.1073/pnas.88.7.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Pato CN, Macciardi F, Pato MT, Verga M, Kennedy JL. Review of the putative association of dopamine D2 receptor and alcoholism: a meta-analysis. Am. J. Med. Genet. 1993;48:78–82. doi: 10.1002/ajmg.1320480204. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat. Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol. Clin. Exp. Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav. Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Silver L. Mouse genetics: concepts and applications. New York: Oxford University Press; 1995. [Google Scholar]
- Smith JW, Fetsko LA, Xu R, Wang Y. Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience. 2002;113:755–765. doi: 10.1016/s0306-4522(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Stefanini E, Frau M, Garau MG, Garau B, Fadda F, Gessa GL. Alcohol-preferring rats have fewer dopamine D2 receptors in the limbic system. Alcohol Alcohol. 1992;27:127–130. [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbille AG, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Todd RD, Heller A, O'Malley KL. Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J. Pharmacol. Exp. Ther. 1994;268:495–502. [PubMed] [Google Scholar]
- Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H, Wang GJ, Hitzemann R, Volkow ND. Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. 2005;77:130–139. doi: 10.1016/j.lfs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergstrom K, Sarkioja T, Rasanen P, Mantere T, Callaway J, Hiltunen J, Tiihonen J. Dopamine D(2)/D(3)-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and 2 alcoholics. Mol. Psychiatry. 2001;6:261–267. doi: 10.1038/sj.mp.4000859. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch. Gen. Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am. J. Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, et al. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J. Addict. Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J. Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Hranilovic D, Fetsko LA, Bucan M, Wang Y. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol. Psychiatry. 2002;7:1075–1082. doi: 10.1038/sj.mp.4001145. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Swearingen SP, Rhodes JS. Acute locomotor responses to cocaine in adolescents vs. adults from four divergent inbred mouse strains. Genes Brain Behav. 2010;9:892–898. doi: 10.1111/j.1601-183X.2010.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]