Abstract
Objective
Bone morphogenetic proteins (BMPs) are potently proangiogenic, however the mechanisms underlying the regulation of vessel development by BMPs are not fully understood. To assess the significance of BMP endothelial cell precursor-derived regulator (BMPER) in blood vessel formation in vivo we investigated its role in retinal angiogenesis.
Methods and Results
In a model of oxygen-induced retinopathy, Bmper mRNA expression and protein levels are downregulated, correlating with the initiation of SMAD phosphorylation in endothelial cells. Moreover, Bmper haploinsufficiency results in an increased rate of retinal revascularization, with retinas from Bmper +/− mice displaying increased numbers of branching points and angiogenic sprouts at the leading edge of the newly formed vasculature. Furthermore, although Bmper haploinsufficiency does not alter Bmps expression, it does lead to an increase in BMP signaling, as evidenced by increased pSMAD levels in endothelial cells and increased expression of known BMP target genes.
Conclusions
These observations provide compelling evidence that BMPER is important in the regulation of BMP signaling and revascularization in the hypoxic retina. These bring forth the possibility of novel therapeutic approaches for pathological angiogenesis based on manipulation of BMP signaling.
Keywords: angiogenesis, BMP, BMPER, hypoxia, retina
Angiogenesis is the process by which new blood vessels are formed from pre-existing vascular networks. Although it is a more active process during development than in adult life, angiogenesis can be reactivated in situations such as wound healing or tumor growth.1 Several factors (e.g. vascular endothelial growth factor (VEGF)2 and angiopoietins3) have been identified as major regulators of the angiogenic process. However, many more signaling pathways (such as Notch4 and Wnt5) are now emerging as critical coordinators of the myriad of events required for proper blood vessel generation6.
Bone morphogenetic proteins (BMPs) belong to the tumor growth factor-β (TGFβ) superfamily of extracellular signaling proteins. BMPs were initially described by their ability to induce ectopic bone and cartilage formation,7 however recent evidence reveals an important role for BMPs in the development of blood vessels during embryonic development and adult life.8, 9 The relevance of BMPs in angiogenesis is illustrated by the association of mutations of BMP receptors and SMADs with several human vascular genetic diseases, such as hemorrhagic hereditary telangiectasia (linked to mutations in ENG, ACTVRL1, SMAD4), pulmonary artery hypertension (BMPR2, ACTVRL1, SMAD8), and preeclampsia (levels of soluble endoglin).1 Whereas TGFβs are secreted in a latent or inactive form, BMPs are secreted as active molecules, with their bioavailability regulated at the extracellular level through reversible interactions with extracellular regulators.10 One such protein is BMP endothelial cell precursor-derived regulator or BMPER, the vertebrate homolog of Drosophila Crossveinless-2 (CV2). BMPER, originally identified in a screen for differentially-expressed proteins in embryonic endothelial precursor cells 11, is an extracellular regulator of BMPs required for proper BMP signaling.12–14 Interestingly, BMPER has been ascribed both pro- and anti-BMP signaling effects in different experimental settings.11, 12, 15–18 Although the mechanism behind this dual action is still uncertain, growing evidence indicates that the concentration of BMPER relative to BMP may determine the nature of its effect on signaling. Initial evidence of this concentration-dependent action came from studies examining Drosophila posterior crossvein (PCV) formation. BMPER/CV2 is required for PCV formation and moderately increasing expression of a BMPER transgene, while BMP remains unaltered, results in a slight gain in signaling with occasional formation of additional veins. However, continuing to increase expression levels eventually blocks PCV formation, similar to the effect of knocking out BMPER in this system.19 We have observed similar dose-dependent effects of BMPER in several angiogenic assay systems.20 where the addition of BMPER at low concentrations enhances sprouting and vasculature formation, whereas at high concentrations BMPER inhibits these processes. In addition, silencing endogenous BMPER or BMP-4 inhibits HUVEC cell sprouting and migration, indicating that the individual pro-angiogenic function of each of these proteins in these assays is dependent on the other protein also being present.20
Recently, we demonstrated that the ratio of BMP4 to BMPER modifies the intracellular response to BMP-4 in cultured endothelial cells.13 In this instance, low BMPER:BMP-4 ratios led to an increase in signaling evidenced by p-SMAD1/5/8 levels, whereas excess BMPER inhibits BMP4-mediated phosphorylation of SMADS. Here we extend those findings to the in vivo setting by describing the functional significance of BMPER in the regulation of angiogenesis using the oxygen-induced retinopathy (OIR) mouse model.21 The mouse retina is a well-characterized, readily accessible tissue in which to research angiogenesis, both from a developmental and a pathological standpoint. Using this model, we have discovered that BMPER expression is downregulated in vivo in response to sudden hypoxia, correlating with the initiation of BMP signaling in endothelial cells and the commencement of revascularization of the retina. These events occur even though Bmp4 levels do not change during the course of hypoxia. Furthermore, Bmper haploinsufficiency results in increased SMAD phosphorylation and faster revascularization of the retina, highlighting the importance of BMPER levels in regulating BMP mediated angiogenesis.
METHODS
A more detailed version of these Methods can be found in the Supplemental Data.
Animals
Bmper+/− mice were generated and maintained as described previously.13 All experimental procedures on mice were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee for the Use of Animals in Research.
Immunostaining
Enucleated eyes were fixed overnight in 4% PFA and dissected whole mounted retinas or frozen sections were stained appropriately.
RNA Isolation and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
RNA was extracted using the RNeasy kit (QIAGEN) and retrotranscribed using the iScript cDNA Synthesis Kit (Bio-Rad). PCR was performed on an ABI 7900HT Sequence Detection System (Applied biosystems). Fold change expression was calculated using the ΔΔCt method using 18S as the internal control.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Comparisons between groups were made by 2-tailed Student’s t test, one-way or two-way ANOVA to determine statistical significance between groups.
RESULTS
Hypoxia downregulates BMPER expression in endothelial cells
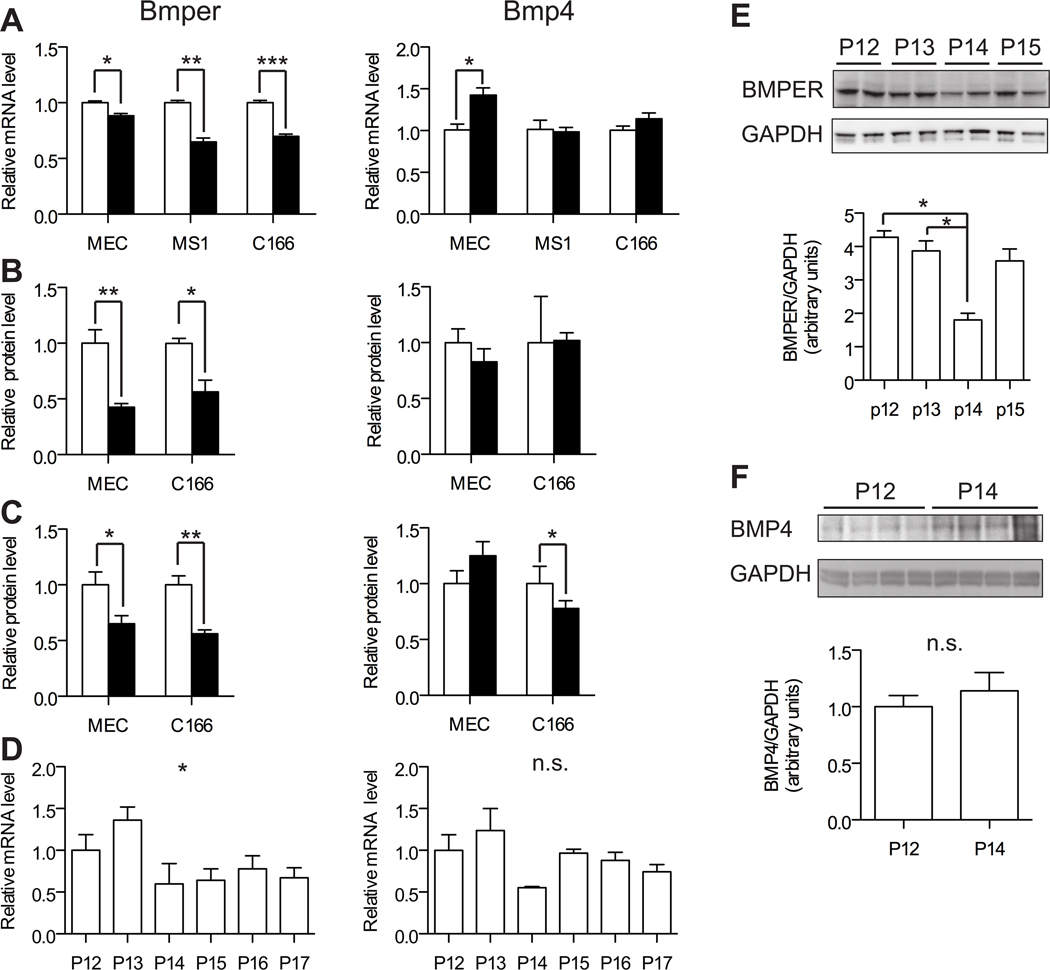
Since both hypoxia and BMP signaling are linked to angiogenesis, we postulated that hypoxic conditions could affect the expression of BMPs and BMPER in endothelial cells. In order to test this theory, we first examined the regulation of Bmp4 and Bmper mRNA levels in various endothelial cells cultured under hypoxic (1% oxygen) or normoxic (21% oxygen) conditions for 24 hours. We chose to focus on Bmp4 as our previous studies demonstrated that BMPER can regulate BMP4-mediated angiogenesis in endothelial cells20. Under these conditions, Bmper levels were significantly downregulated in response to hypoxia in MEC (mouse myocardial endothelial cells), C166 (mouse embryonic yolk sac endothelial cells) and MS1 (mouse pancreas microvascular endothelial cells; Figure 1A). In contrast, little to no change in Bmp4 levels were seen in C166 and MS1 cells under hypoxic conditions, whereas hypoxic MECs exhibited a 42% increase in Bmp4 levels compared to the same cells cultured under normoxic conditions (Figure 1A). ELISA analysis confirmed that, while BMP4 protein was not affected, BMPER protein levels were significantly reduced in both the cell lysate (Figure 1B) and extracellular media (Figure 1C) of both MEC and C166 cells under hypoxic conditons.
Figure 1. BMPER expression is downregulated in endothelial cells in response to hypoxia.
A-C, MEC, MS1 and C166 cells were cultured for 24 hours in normoxic (open bars) or hypoxic (closed bars) conditions. Fold change in mRNA expression (A) or protein levels of BMP4 and BMPER from cell lysates (B) or extracellular media (C) is represented relative to corresponding levels under normoxic conditions. D-F, Wild type mice were subjected to OIR and BMPER and BMP4 mRNA (D) and protein (E, F) expression was determined from the retina of different animals at each time point. The change in expression at each time point is represented relative to the levels measured at P12 (0 days in hypoxia) (n= 3 to 4). * p,0.05, ** p<0.01, *** p<0.001, n.s. not significant.
BMPER expression is downregulated in vivo in the mouse retina during OIR
To study the role of BMPER in the regulation of angiogenesis in vivo, we turned to the retina since it provides an easily accessible system in which to study vessel development and because we had identified this tissue as an enriched location of Bmper transcript levels (Supplemental Figure I). We examined whether BMPER could modulate angiogenesis under hypoxia-induced re-vascularization using the OIR model. Using this model, we observed that Bmper levels decreased approximately two fold soon after the onset of hypoxia (P14 and subsequent time points) when compared to the level of retinal Bmper at P12 and P13 (Figure 1D). This difference was confirmed by ANOVA analysis and post-test for linear trend. BMPER protein levels were also reduced in whole retina extracts at P14 (Figure 1E). Next the regulation of Bmp4 during this period of hypoxic-revascularization of the retina was examined. Bmp4 transcript levels decreased briefly at P14 (not statistically significant as determined by ANOVA analysis) but then returned to normal levels by P15 and subsequent time points (Figure 1D). Analysis of BMP4 protein levels at P12 and P14 in independent samples demonstrated a slight (but not statistically significant) increase in expression (Figure 1F). In addition, mRNA levels of other Bmps analyzed showed no significant changes during retinal revascularization (Supplemental Figure 2). These findings indicate that exposure of retinal tissue to hypoxia in vivo induces downregulation of Bmper levels without significantly affecting Bmps expression, similar to our observations from in vitro experiments with endothelial cells.
BMPER downregulation correlates with the initiation of BMP signaling in hypoxic retinas
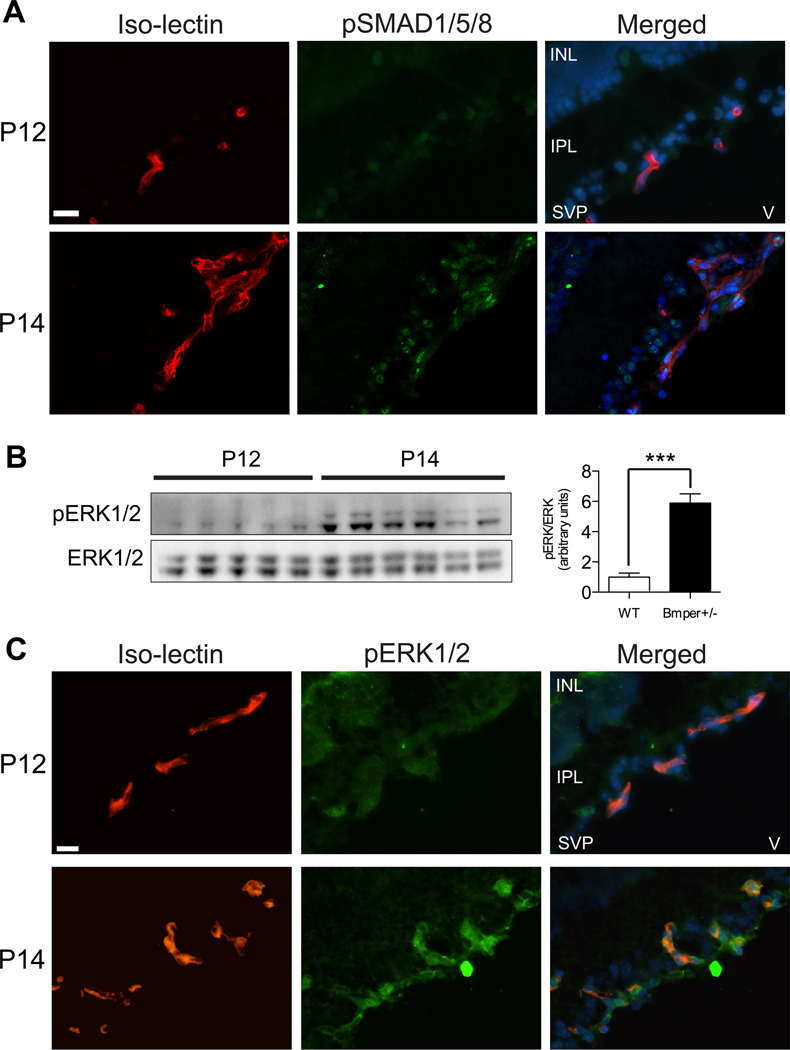
Our previous observations that reduced concentrations of BMPER correlate with an increase in BMP activity13 raised the possibility that the decrease in BMPER levels seen in the hypoxic retinas following OIR might correlate with an increase in endogenous BMP signaling within the retina. To test this theory, we examined the levels of phosphorylated SMADS 1/5/8 in retinas before (P12) and after (P14) the onset of hypoxia. Whereas no SMAD phosphorylation could be detected at P12, strong phospho-SMAD1/5/8 was detected at P14 in nuclei from both endothelial cells (iso-lectin positive) and astrocytes (round nuclei in close proximity to endothelial cells, Figure 2A), even though Bmp4 expression itself was not increased at this time point (Figure 1D). Since BMPs can also signal via ERK1/2 in endothelial cells,22 we next examined whether ERK1/2 stimulation was also affected in response to hypoxic stimulation in the retinas. Western blot analysis of retina lysates revealed that ERK1/2 was also strongly phosphorylated in response to hypoxia (P14; Figure 2B). Immunofluorescence staining of frozen sections from P12 and P14 retinas confirmed that the location of the pERK1/2 signal was indeed endothelial cells and some closely adjacent iso-lectin negative cells, most likely pericytes. No pERK1/2 staining was detected in astrocytes (Figure 2C). Our data imply that, in the hypoxic retina, BMP signaling within the endothelium is controlled through Bmper regulation rather than through changes in Bmp4 expression.
Figure 2. Hypoxia-induced retinal revascularization is accompanied by SMAD1/5/8 and ERK1/2 phosphorylation in endothelial cells.
Retinal sections from OIR-subjected mice at P12 (0 days hypoxia) and P14 (2 days hypoxia) were stained with fluorescently-labeled iso-lectin (red), anti-pSMAD1/5/8 (green in A) or anti-pERK1/2 (green in C), and DAPI (blue) or total lysates were probed by western blot analysis with antibodies to pERK1/2 and total ERK1/2. INL: inner nuclear layer, IPL: inner plexiform layer, SVP: superficial vascular plexus, V: vitreous. *** p<0.001. Scale bars: 20μm.
Bmper haploinsuficiency accelerates retinal revascularization in OIR
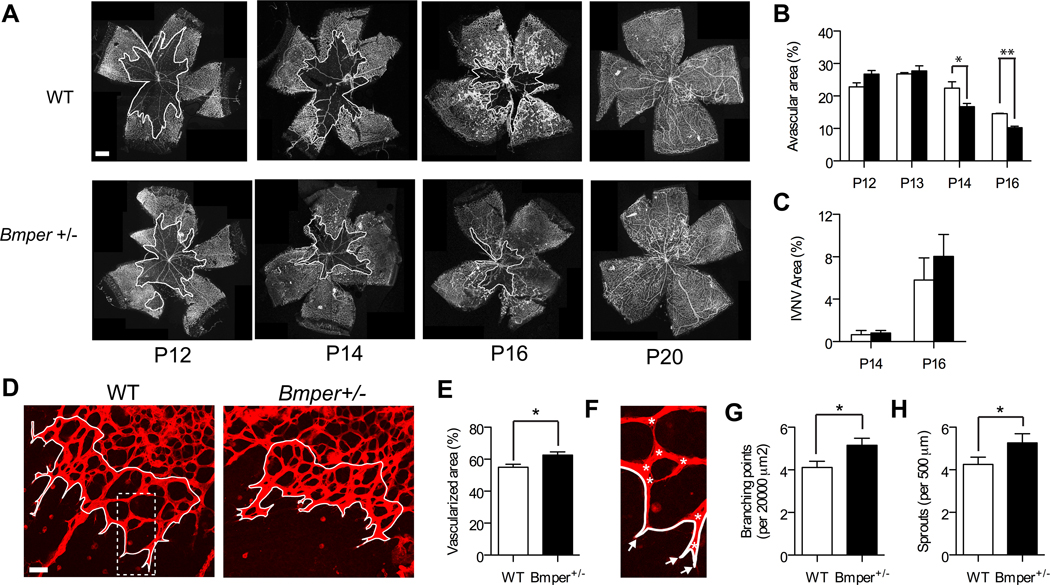
To further understand the physiological role of BMPER in the development of retinal vasculature, we measured the pattern and rate of blood vessel formation in genetically modified mice. Because Bmper-/- animals die at birth,13 we examined the differences between WT and Bmper+/− mice. Following five days of hyperoxia (i.e., P12), Bmper+/− animals exhibited a similar degree of retinal avascularity as compared with WT littermate controls (Figure 3A, B). However, once hypoxia was established, Bmper haploinsufficiency correlated with a faster recovery of the retinal vascular bed as indicated by a reduced percentage of avascularized area in the retinas of the Bmper+/− mice at later time points. At two (P14) and four (P16) days following the onset of hypoxia, Bmper+/− mice displayed 25% and 30% more retinal vascularization than the respective WT littermate controls (Figure 3A, B), with no differences in intravitreous neovascularization, an indication of pathological aberrant angiogenesis (Figure 3C). The increase in intraretinal vascularization seen in the Bmper+/− mice following the onset of hypoxia was accompanied by a more dense and complex network of newly formed vessels. The leading edge of the newly formed vasculature in Bmper+/− mice revealed approximately 20% more vascularization compared with the littermate controls (Figure 3D and E) with an increase in the number of connections between the new vessels (Figure 3G) and increased numbers of sprouts compared to WT littermates (Figure 3H). (Online supplement). Together, these observations reveal a role for low concentrations of BMPER in the angiogenic response of endothelial cells from hypoxic retinas, which is consistent with our previous in vitro data13.
Figure 3. Bmper+/− retinas display increased intraretinal re-vascularization during the course of hypoxia.
A, Iso-lectin-stained retinal whole mounts from WT and Bmper+/− mice at different time points during hypoxia-induced revascularization. B-C, Avascularized (white outline) (B) and intravitreous neovascularization (IVNV) (C) areas were quantified from whole-mounted retinas (n= 3 to 8 mice per time point and genotype). D, Confocal micrographs of retinal flat mounts stained with iso-lectin from P14 WT or Bmper+/− OIR mice. E-H, Vascularized area (E), branching points (asterisks) (G) and vessels sprouts (arrows) (H) were quantified within the leading edge of re-vascularization area (white outline) in at least 5 random fields of view per retina (n= 3 mice per genotype). WT (open bars), Bmper+/− (closed bars). * p,0.05, ** p<0.005. Scale bars: 500 μm (A) and 50 μm (D).
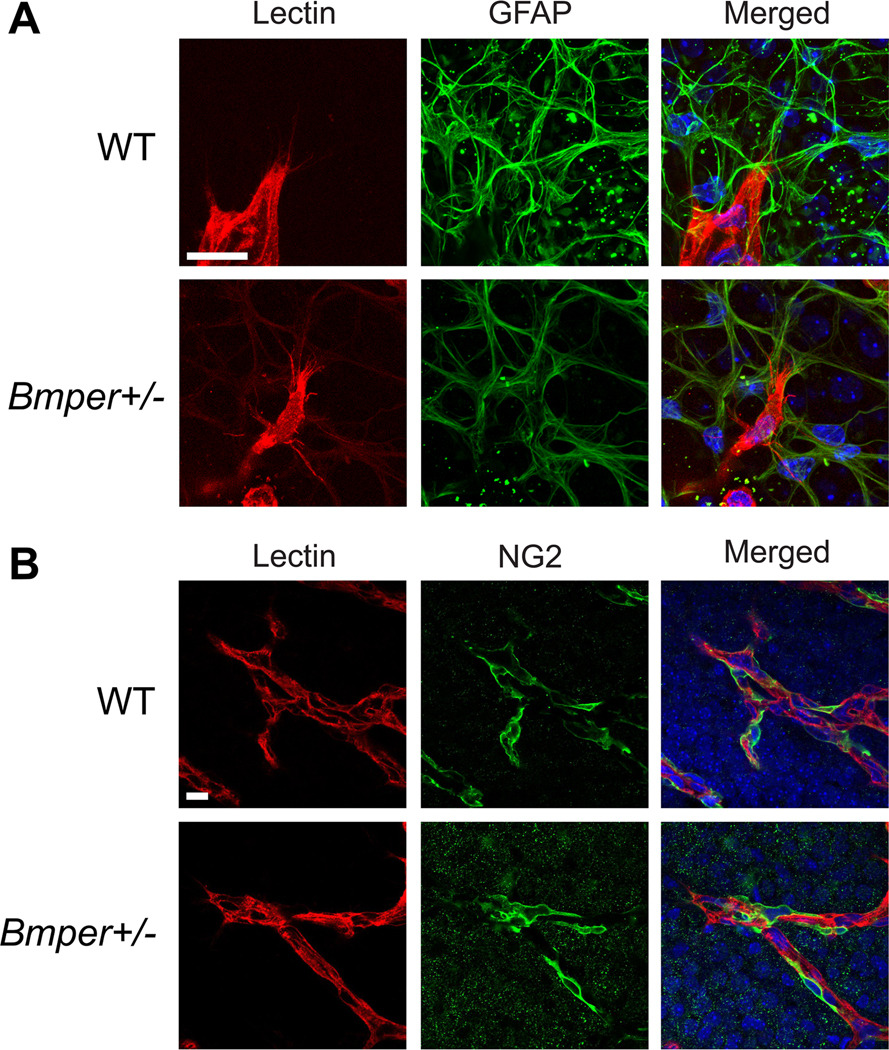
New vessel formation in Bmper +/− retinas follows astrocytic scaffolding and recruits pericytes in a normal fashion
One important aspect of vascular development in the retina is the interaction of endothelial cells with the pre-existing scaffolding laid down by the astrocytic network.23 To determine whether the changes observed in the retinal vasculature of Bmper +/− mice are related to alterations in guidance cues provided by other cells, we examined the relationship between these newly formed vessels and the resident astrocytic network in both Bmper +/− and WT mice. Immunostaining of retinas at two days following the onset of hypoxia (P14) with iso-lectin (endothelial cells) and GFAP (astrocytes) revealed that the pattern of the astrocytic network was not significantly different between Bmper +/− and WT animals (Figure 4A). Closer examination of the filopodia of the endothelial cells at the tip of the growing vascular sprouts in Bmper +/− mice revealed that these cells followed the astrocytic network similarly to what was found in WT animals (Figure 4A), indicating a stable interaction between the endothelial cells and adjacent astrocytes in the Bmper +/− retinas. The proper formation of a stable vasculature also requires the recruitment of pericytes to the wall of the new vessels.25 In order to study the presence of pericytes in the neovascularized region of the retinas we co-stained P14 retinas with iso-lectin and NG2 (pericytes), and discovered that both WT and Bmper +/− miceBmper+/− retinas displayed appropriate coverage of new vessels with pericytes, in a ratio of 3:1 (endothelial cell:pericyte; Figure 4B).24 Thus, although Bmper haploinsufficiency leads to an increased growth rate of retinal vasculature, this vasculature is both normal in appearance and arranged so as to be fully functional once completely laid down.
Figure 4. Angiogenesis in Bmper+/− mice indicates normal astrocyte-filopodia interactions and pericyte recruitment.
Flat mounted whole retinas from P14 WT or Bmper+/− mice subjected to OIR were co-stained with iso-lectin (red), DAPI (blue) and GFAP (A) or NG2 (B) (green) and z-stacked images were acquired on a confocal microscope. For endothelial cell:pericyte ratio calculations, nuclei from pericytes (green) and endothelial cells (red) were quantified. Scale bars: 20 μm.
Bmper haploinsuficiency increases BMP signaling in endothelial cells in response to hypoxia
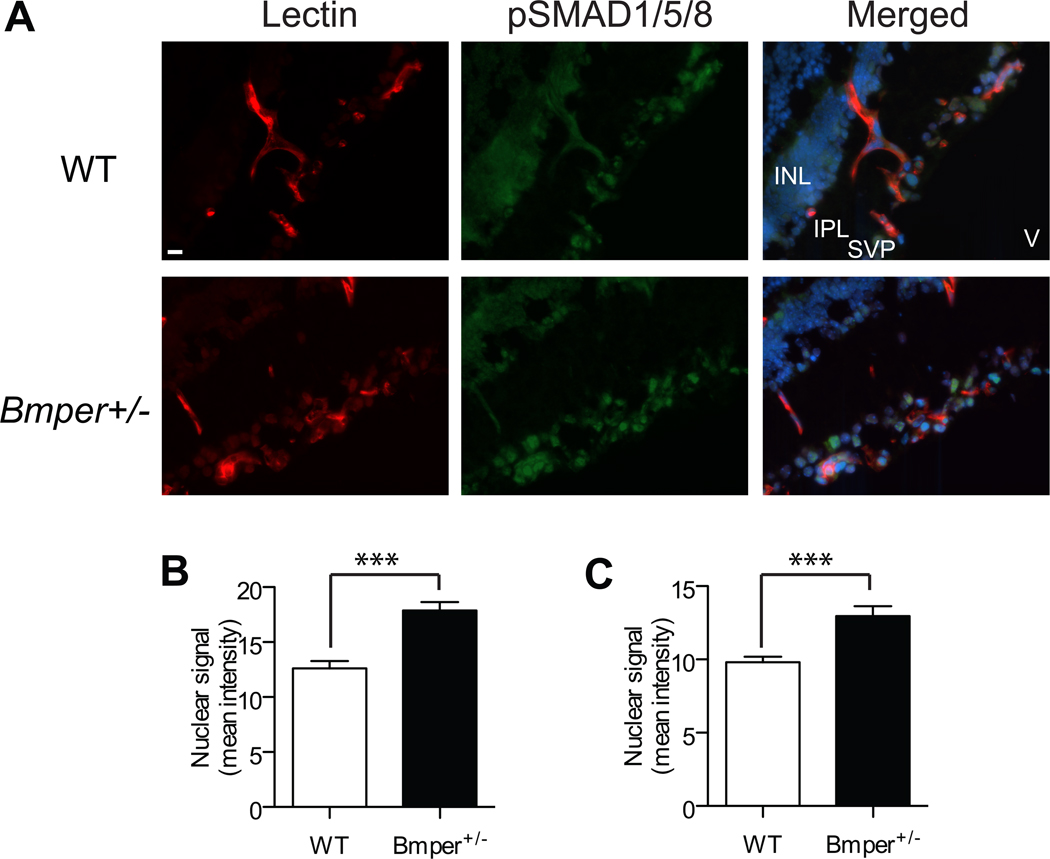
Given our in vitro findings that lower concentrations of BMPER increase BMP-mediated SMAD signaling in endothelial cells, we next sought to determine if the mechanism underlying the accelerated revascularization in the retinas of Bmper+/− mice was also linked to an increase in BMP signaling. Frozen sections of WT and Bmper+/− retinas taken at P15 (72 hours in hypoxia) were stained with an anti-pSMAD1/5/8 antibody (Figure 5A) and the intensity of the nuclear signal in both endothelial cells and astrocytes was quantified. As expected, retinas from Bmper+/− animals exhibited increased nuclear SMAD signaling in both endothelial cells and astrocytes (Figure 5B, C).
Figure 5. BMP signaling increases in in retinal endothelial cells and astrocytes from OIR-treated Bmper+/− mice.
A, Retinal sections from P15 (3 days hypoxia) WT and Bmper+/− mice were stained with iso-lectin (red), anti-pSMAD1/5/8 (green) and DAPI (blue). B-C, The intensity of nuclear pSMAD1/5/8 signal was quantified in endothelial cells (iso-lectin positive) (B) and astrocytes (C) using ImageJ software. Nuclear mean intensity was measured from at least 10 random fields in sections 70μm apart from two mice per genotype group. INL: inner nuclear layer, IPL: inner plexiform layer, SVP: superficial vascular plexus, V: vitreous. *** p<0.001. Scale bar: 11 μm.
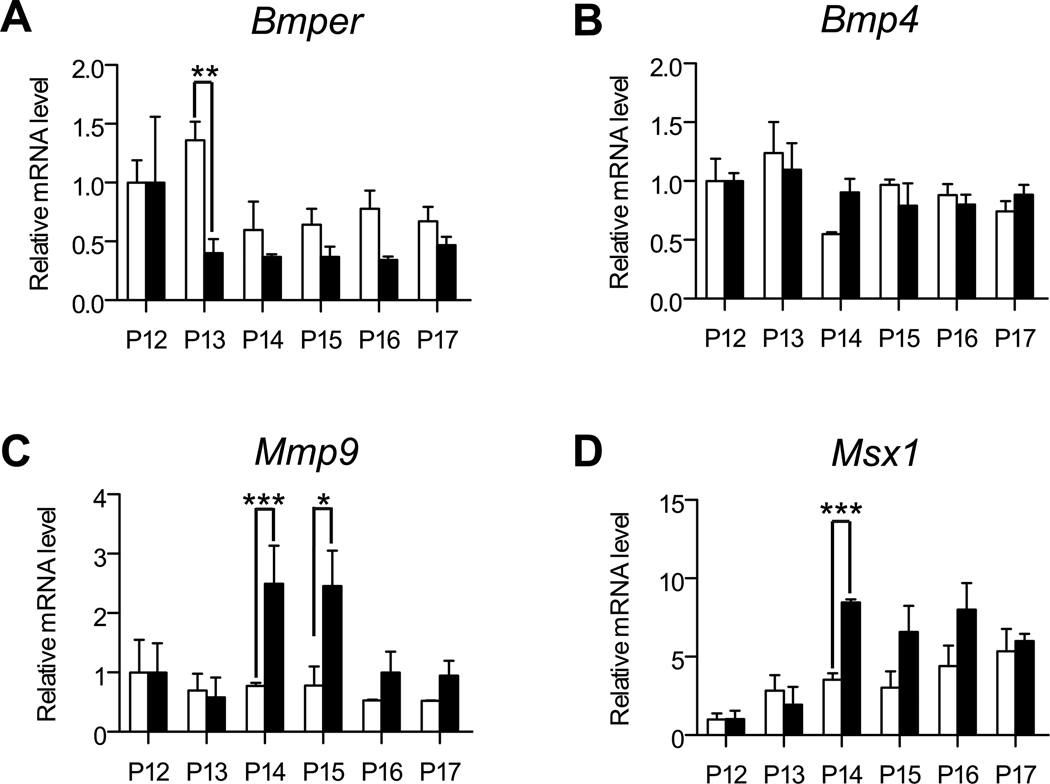
In an effort to understand the mechanism of this altered BMP regulation in the Bmper+/− retinas we compared the time course of Bmper mRNA regulation in the retinas following the onset of hypoxia. We discovered that Bmper downregulation occurred earlier in the Bmper+/− retinas compared to WT retinas (Figure 6A). Whereas WT controls demonstrated a reduction in Bmper mRNA at 48 hours following the start of hypoxic treatment (i.e. P14), in Bmper+/− retinas Bmper mRNA was already decreased after only 24 hours of hypoxia (i.e. P13), even though at this point in the experiment no differences in vascularization patters could be detected. In addition, Bmp4, Bmp2, Bmp6 or Bmp7 levels did not demonstrate significant changes during the course of hypoxia in Bmper+/− retina, similar to what was seen in WT controls (Figure 6B and Supplemental Figure II). Finally, we confirmed the altered modulation of BMP signaling in Bmper+/− retinas by investigating the expression of BMP target genes in the OIR-treated retinas. As revascularization occurs Bmper+/− retinas displayed an increase in the expression of the ERK1/2-regulated gene Mmp925 compared to WT retinas (Figure 6C). MMP9 has been linked to the regulation of angiogenesis in various contexts6 and specifically in the ordered revascularization of the mouse retina26. In addition, quantitative RT-PCR of the SMAD regulated gene Msx1 confirmed an increased response to BMP signaling in Bmper+/− retinas (Figure 6D). Msx1, which is a known BMP target gene expressed by pericytes and endothelial cells,27, 28 was steadily upregulated during the course of hypoxia in WT retinas, which was in contrast to Bmper+/− retinas which exhibited an accelerated increase in Msx1 levels following the onset of hypoxia (Figure 6D).
Figure 6. Bmper haploinsufficieny leads to enhanced expression of BMP and ERK target genes.
Mice were subjected to OIR and RNA extracted from the retina of WT (open bars) or Bmper+/− (closed bars) littermate animals. Bmper (A), Bmp4 (B), Mmp9 (C) and Msx1 (D) mRNA expression at each time point was determined by quantitative RT-PCR. For comparison purposes the relative expression of Bmper and Bmp4 in WT animals from Figure 1 is reproduced here. Expression at every time point is represented relative to the corresponding P12 expression (0 days hypoxia) for each genotype group (n= 3 to 4 mice per genotype) in B-E. * p,0.05, ** p<0.01, *** p<0.001.
DISCUSSION
The role that BMPs play in the processes of angiogenesis and vasculogenesis is widely accepted. Still, despite the progress made in recent years, the mechanisms of BMP-mediated vessel formation are not fully understood.8, 9 The BMP signaling pathway comprises a complex network of extracellular, membrane and intracellular regulators29. Recently we discovered two new BMP-regulated genes, Cox2 and MyoX,30, 31 that mediate the downstream angiogenic properties of BMP. In the current study, we turned our focus to regulatory elements upstream of BMP in the angiogenesis signaling cascade and investigated the role that BMPER, an extracellular modulator of BMP signaling, plays in the regulation of BMP-mediated angiogenesis in vivo. Previously, we have shown BMPER to modify the angiogenic response of endothelial cells to BMPs in in vitro experiments.13, 20 In addition, BMPER has been implicated, albeit indirectly, in the vascular phenotypes associated with the FoxO knock-out mouse,32 in a thoracic aortic aneurysm mouse model33 and in hypoplastic left heart syndrome.34 However, until now, no studies have demonstrated the modulation of endogenous BMBER in response to physiological stimuli, nor has BMPER been shown to be able to regulate angiogenic processes in vivo. Our data provide the first evidence that endogenous BMPER levels are regulated in response to hypoxia both in endothelial cells in vitro and in vivo and that this regulation of BMPER levels has a direct effect on angiogenic processes in a mouse model of OIR.
Previously we had demonstrated that BMPER regulates BMP4 action in vitro via a concentration-dependent mechanism, switching from a pro- to an anti-BMP effector, depending on the concentration of exogenously added BMPER.13, 20 Until now, however, the physiological significance of this concentration-dependent relationship between BMPER and BMP was not known. Using endothelial cell cultures and a mouse model of OIR to induce revascularization in the retina, we discovered that hypoxia and the start of retinal revascularization coincides with a physiological down-regulation of BMPER and a concurrent increase in BMP signaling in retinal endothelial cells and astrocytes (Figures 1 and 2). Interestingly, however, measurement of the in vivo levels of Bmps in retinas from mice exposed to the OIR model demonstrated no change in Bmps expression (Figure 1 and Supplemental Figure II). If our hypothesis that lower BMPER:BMP concentration ratios drives BMPER’s pro-BMP effects is correct, our results here suggest that the endogenous level of BMPER in the revascularizing retina is decreased such that it is lower than the local concentration of BMPs. Indeed, Bmper+/− retinas (in which Bmper expression is decreased by approximately 50% compared to WT retinas; Supplemental Figure III), exhibit enhanced physiologic BMP signaling in vivo, evidenced by an increase in SMAD phosphorylation (Figure 5) and BMP target gene expression in retinal lysates (Figure 6). These findings underscore the role of extracellular regulators such as BMPER in modulating BMP signaling at the protein level and not through enhanced transcriptional regulation of BMPs after angiogenic stimuli.
Despite demonstrating that Bmper haploinsufficiency correlates with increased BMP signaling and increased angiogenesis (Figures 3, 5 and 6), we were surprised to discover that the retinas of Bmper+/− mice were anatomically indistinguishable from the retinas of their WT siblings in terms of basal vascular development (P6 and P12; Supplemental Figure III). Given our previous observations, we would have predicted that the hypoxic interludes that occur throughout retinal vasculature development, teamed with the already decreased level of Bmper expression would lead to a heightened degree of BMP activation in the Bmper+/− retinas and result in an increase in vascularization. One possible explanation for the lack of increased angiogenesis in the Bmper+/− retinas during development could be that signaling through other pathways (such as VEGF) could be sufficient to drive retina vascularization during normal development but not in a stress-induced situation such as the OIR model. This could be explained by the nature of the hypoxia induced in the OIR model compared to the hypoxia experienced naturally during development. The developing retina, although still hypoxic in comparison to the mature retina does have a balance between oxygen supply (hyaloid vessels regress around P7) and demand (immature neural retina), whereas the obliterated zone of the retina in mice exposed to the OIR model suffers a sudden oxygen undersupply.35, 36 In fact, the OIR mouse model recapitulates many of the hallmarks of retinopathy of prematurity in humans,37 and thus BMPER-mediated effects on BMP-induced angiogenesis may have more relevance to angiogenesis that is re-activated in pathologic conditions where sudden hypoxia drives neovascularization, such as wound healing, cardiovascular disorders or tumor growth.1 This is not to say that BMPER is irrelevant during developmental processes. During embryonic development, Bmper is expressed in the ribs, skull, long bones and limb girdle bones during the period of bone maturation. Consistent with this pattern of expression, Bmper knock-out animals have a variety of bone defects characterized by reduced ossification.12, 14 Invasion by endothelial cells and vascularization of the ossification centers is a critical step in bone maturation.38 Therefore, given our observations of BMPER’s role in angiogenesis in the retina, it is possible that the bone phenotype of Bmper null animals could be attributed, in part, to a defect in proper vascular invasion of the cartilage template.
Organized vascularization, both through stimulation of angiogenesis and remodeling of existing vasculature, is essential not only for physiologic development of the retina but also for the normal development of most other tissues. The developing mouse retina is an excellent canvas in which to study the induction of vascularization and revascularization, the findings of which can apply equally to developmental angiogenesis and pathological conditions. In spite of the progress made in recent years, the full mechanisms by which BMPs induce vascular development still remain relatively unknown, especially in terms of the regulators that monitor and determine the outcome of BMP-signaling. Our observations in hypoxia-driven revascularization of the retina offer an insight into the physiological regulation of BMP signaling by BMPER. Our data offers an exciting revelation of BMPER-mediated regulation of BMP signaling that could end up being a potential target for therapeutic intervention in pathological conditions involving aberrant angiogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Andrea Portbury for her invaluable help writing and editing this paper.
SOURCES OF FUNDING
This work was supported by NIH GRANT R01 HL61656 (CP), R01 EY017011 (MEH PI) and R01 EY015130 (MEH PI), and Deutsche Forschungsgemeinschaft DFG Mo973/6-1 (MM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 4.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 5.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Miralles I, Schisler JC, Patterson C. New insights into bone morphogenetic protein signaling: focus on angiogenesis. Curr Opin Hematol. 2009;16:195–201. doi: 10.1097/MOH.0b013e32832a07d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- 11.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133:4463–4473. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- 13.Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation. Dev Biol. 2008;323:6–18. doi: 10.1016/j.ydbio.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- 17.Coles E, Christiansen J, Economou A, Bronner-Fraser M, Wilkinson DG. A vertebrate crossveinless 2 homologue modulates BMP activity and neural crest cell migration. Development. 2004;131:5309–5317. doi: 10.1242/dev.01419. [DOI] [PubMed] [Google Scholar]
- 18.Binnerts ME, Wen X, Cante-Barrett K, Bright J, Chen HT, Asundi V, Sattari P, Tang T, Boyle B, Funk W, Rupp F. Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem Biophys Res Commun. 2004;315:272–280. doi: 10.1016/j.bbrc.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O'Connor MB, Blair SS. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–812. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 21.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 22.Zhou Q, Heinke J, Vargas A, Winnik S, Krauss T, Bode C, Patterson C, Moser M. ERK signaling is a central regulator for BMP-4 dependent capillary sprouting. Cardiovasc Res. 2007;76:390–399. doi: 10.1016/j.cardiores.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Kubota Y, Suda T. Feedback mechanism between blood vessels and astrocytes in retinal vascular development. Trends Cardiovasc Med. 2009;19:38–43. doi: 10.1016/j.tcm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, Karlo JC, Landreth GE, Leone G, Ostrowski MC. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS One. 2009;4:e8283. doi: 10.1371/journal.pone.0008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundkvist A, Lee S, Iruela-Arispe L, Betsholtz C, Gerhardt H. Growth factor gradients in vascular patterning. Novartis Foundation symposium. 2007;283:194–201. doi: 10.1002/9780470319413.ch15. discussion 201-196, 238-141. [DOI] [PubMed] [Google Scholar]
- 27.Kiyono M, Shibuya M. Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol Cell Biol. 2003;23:4627–4636. doi: 10.1128/MCB.23.13.4627-4636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goupille O, Saint Cloment C, Lopes M, Montarras D, Robert B. Msx1 and Msx2 are expressed in sub-populations of vascular smooth muscle cells. Dev Dyn. 2008;237:2187–2194. doi: 10.1002/dvdy.21619. [DOI] [PubMed] [Google Scholar]
- 29.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren R, Charles PC, Zhang C, Wu Y, Wang H, Patterson C. Gene expression profiles identify a role for cyclooxygenase 2-dependent prostanoid generation in BMP6-induced angiogenic responses. Blood. 2007;109:2847–2853. doi: 10.1182/blood-2006-08-039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones JA, Barbour JR, Stroud RE, Bouges S, Stephens SL, Spinale FG, Ikonomidis JS. Altered transforming growth factor-beta signaling in a murine model of thoracic aortic aneurysm. J Vasc Res. 2008;45:457–468. doi: 10.1159/000127437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricci M, Mohapatra B, Urbiztondo A, Birusingh RJ, Morgado M, Rodriguez MM, Lincoln J, Vatta M. Differential Changes in TGF-beta/BMP Signaling Pathway in the Right Ventricular Myocardium of Newborns With Hypoplastic Left Heart Syndrome. J Card Fail. 16:628–634. doi: 10.1016/j.cardfail.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast Precursors, but Not Mature Osteoblasts, Move into Developing and Fractured Bones along with Invading Blood Vessels. Dev Cell. 19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.